Abstract
The cephalic neural crest produces streams of migrating cells that populate pharyngeal arches and a more rostral, premandibular domain, to give rise to an extensive ectomesenchyme in the embryonic vertebrate head. The crest cells forming the trigeminal stream are the major source of the craniofacial skeleton; however, there is no clear distinction between the mandibular arch and the premandibular domain in this ectomesenchyme. The question regarding the evolution of the gnathostome jaw is, in part, a question about the differentiation of the mandibular arch, the rostralmost component of the pharynx, and in part a question about the developmental fate of the premandibular domain. We address the developmental definition of the mandibular arch in connection with the developmental origin of the trabeculae, paired cartilaginous elements generally believed to develop in the premandibular domain, and also of enigmatic cartilaginous elements called polar cartilages. Based on comparative embryology, we propose that the mandibular arch ectomesenchyme in gnathostomes can be defined as a Dlx1-positive domain, and that the polar cartilages, which develop from the Dlx1-negative premandibular ectomesenchyme, would represent merely posterior parts of the trabeculae. We also show, in the lamprey embryo, early migration of mandibular arch mesenchyme into the premandibular domain, and propose an updated version of the heterotopy theory on the origin of the jaw.
Keywords: gnathostomes, hypophysis, jaw evolution, lamprey, mandibular arch, neural crest, prechordal cranium, polar cartilage, premandibular region, trabecula
Introduction
The vertebrate body plan is distinctly different from those of other animal phyla and subphyla, mainly because it possesses complex structural patterns that make the understanding of its embryonic development and evolutionary origin very difficult. One main reason for this is the involvement of the neural crest – the vertebrate-specific cell lineages with extensive migratory capabilities and pluripotency in differentiation into a wide variety of cell types (reviewed by Le Douarin, 1982; Noden, 1988). Secondly, the vertebrate embryo exhibits multiple types of segmental patterns along the body axis, such as somitomerism (the repetitive arrangement of somite-derived and somite-associated structures), branchiomerism (represented by the serially arranged pharyngeal arches and their associated structures) and neuromerism (the subdivision of the neural tube into neuromeric compartments). The developmental relationships among these are not yet fully understood, rendering the vertebrate morphology extremely complicated. Thirdly, there are some enigmatic structures in the developing head of vertebrates that are sometimes interpreted as representing secondarily lost segments, such as the ‘premandibular arch’ or ‘anterior (mesodermal) cavities’ of ancestral forms (reviewed by Adachi & Kuratani, in press). The trabecular cartilages of the gnathostome chondrocranium, for example, are structures whose evolutionary significance remains unclear.
An understanding of the vertebrate body plan should be accompanied by an understanding of the development and evolutionary origin of the head. Central questions of the ‘head problems’, such as those on the origins of the neurocranium and the jaw, remain to be answered. Since Rathke (1827), it has generally been accepted that the gnathostome jaw was obtained by modification of one of the pharyngeal arches, called the mandibular arch. This notion is clearly supported by developmental data. Modern developmental biology has shown that the Hox code, the antero-posteriorly nested expression of Hox genes, specifies the positional value of this arch as the ‘default’ among the pharyngeal arches, by which the more posterior arches express more Hox genes (see Hunt et al. 1991; Gendron-Maguire et al. 1993; Rijli et al. 1993; Couly et al. 1998; Grammatopoulos et al. 2000; Pasqualetti et al. 2000; Trainor et al. 2002; see also Takio et al. 2004 for agnathans; reviewed by Kuratani et al. 1997a). Thus the Hox code fits the apparent ‘rostralmost’ nature of the mandibular arch in a developmental context. However, how can we define this arch as opposed to the more rostral ectomesenchymal compartment?
The above question is intimately connected with the definition of the mandibular arch in vertebrates: among the serially homologous pharyngeal arch derivatives, the mandibular arch apparently represents the rostralmost element, exhibiting a highly modified development to serve as a part of the oral apparatus. Similar difficulties are associated with the rostralmost segmental element of the arthropod head (Rempel, 1975; Scholtz, 1995; Eriksson et al. 2003). The most apparent argument against the idea of a ‘rostralmost mandibular arch’ would be the assumption of premandibular arch(es), discussed since Huxley (1874) (reviewed by de Beer, 1937). Independent of whether this classical morphological idea holds true or not, it is a fact that an extensive neural crest-derived ectomesenchymal cell population was demonstrated to be present rostral to the mandibular arch (Couly et al. 1993; Kuratani et al. 2001). Understanding the evolution and development of this ‘premandibular ectomesenchyme’ will be most important in understanding the vertebrate head.
To understand the evolutionary acquisition of the vertebrate jaw, it will be especially important to integrate classical embryological knowledge and modern developmental biological data, to enable us to see the mechanism behind this evolutionary novelty as a series of evolutionary changes in developmental programmes. Comparative embryological observations give us hints as about truly relevant changes in spatiotemporal developmental patterns in embryos among various animals, and developmental biology can relate these changes to the changes in gene expression patterns, tissue interactions and gene regulatory networks. In the evo-devo research on evolutionary novelties, one crucial step is to identify which changes in a developmental programme result in which effects in morphogenesis, rather than trying to homologize all the embryonic characters and their associated gene expression patterns. Additionally, each element is connected spatiotemporally and causally to others, through numerous interactions.
The vertebrate head is a particularly challenging topic for evo-devo studies. It has long been realized that the skeletogenic mesenchyme and its embryonic environment, especially structures such as the nasal and hypophyseal placodes and the rostral endoderm, do have significance as factors in creating the oral apparatus and the craniofacial pattern as a whole (Piotrowski & Nüsslein-Volhart, 2000; Couly et al. 2002; Dickinson & Sive, 2007, 2009; Benouaiche et al. 2008; also see Haeckel, 1891; Janvier, 1996, for the evolutionary significance of the positions of nostrils and hypophysis). In this review, we will examine the development and the evolution of the mandibular arch as the major component of the vertebrate craniofacial region together with surrounding structures, especially those found in the rostral part of the head. Among the latter, we will characterize and explain the premandibular domain of vertebrate embryos, a topic that, so far, has been largely neglected.
Specifically, we approach the following questions:
What are the developmental sources and what is the evolutionary identity of the trabeculae?
What are the so-called polar cartilages found in the caudal part of the trabeculae?
How can we define the mandibular arch?
Which ectomesenchymal modules can be seen in the trigeminal crest cells and the mandibular arch?
Do we have to revise the heterotopy theory that was put forth to explain the evolution of the gnathostome jaw by Shigetani et al. (2002) a decade ago?
These questions are inherently connected and cannot be dealt with one after the other.
Nature of the premandibular domain
The cranium in gnathostomes consists of the dorsal ‘neurocranium’, which encapsulates the central nervous system and sensory organs, and the ventral ‘viscerocranium’, which surrounds and supports the pharynx (Goodrich, 1930; de Beer, 1937; Portmann, 1969; Moore, 1981). Developmentally, the neurocranium is further composed of at least two different parts. One of these consists of parachordal cartilages that arise on both sides of the notochord (Fig. 1A, left), often continuing rostrally into the orbital (postorbital) cartilage (Fig. 1B). Further rostrally, there is another pair of rod-like cartilages called ‘trabeculae’ rostral to the notochord (Fig. 1A). Morphologically, the paired trabeculae appear as a direct rostral continuation of the paired parachordals, especially in embryos in which an orbital cartilage does not develop (Fig. 1A). Being located lateral to the hypophysis, the trabeculae form the hypophyseal foramen (Fig. 1A). The basic morphological configuration of the neurocranium as above is highly conserved among gnathostomes (de Beer, 1937), implying that there has been almost no significant or extensive shifts in developmental programmes during their evolution. In comparative morphological studies, the trabeculae were often described as being connected to the more posterior part of the neurocranium by means of another pair of cartilaginous elements, called the polar cartilages (Fig. 1B; see Allis, 1923, 1931, 1938; de Beer, 1937 for reviews). The latter cartilages are explained as representing either the elements belonging to the mandibular arch, or as parts of the trabeculae (see below).
Fig. 1.
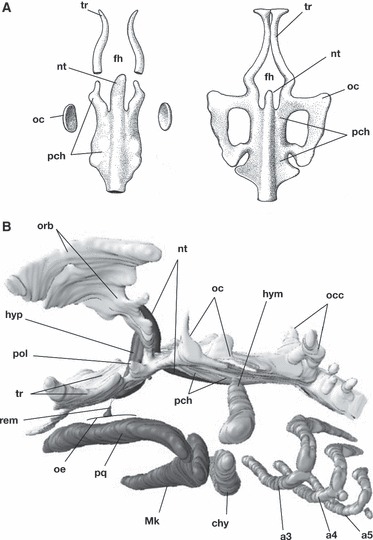
Development of the chondrocranium of modern gnathostomes. (A) Early chondrocrania of a teleost species, Salmo salar (11 and 13 mm), representing a gnathostome neurocranium with no orbital cartilage. In this type of chondrocrania, the cranial base begins to form from two pairs of rod-like cartilages arranged antero-posteriorly: trabeculae and parachordals. (B) Reconstruction of a chondrocranium of a shark, Scyliorhinus torazame. Some cartilaginous structures in the ethmoidal region such as nasal capsules are not reconstructed here. Along the course of the notochord (nt), the chordal cranium consists of the parachordal (pch) and the orbital cartilages (orb). The trabecula, a prechordal cranial element, connects the chordal cranium by means of a cartilaginous nodule called the polar cartilage (pol). Note that the trabecula is located rostral to the position of the hypophysis (hyp), and the polar cartilage caudal to it. The remnant of the hypophyseal duct (rem) represents the original position of Rathke’s pouch. a3–a5, pharyngeal arches; chy, ceratohyal; fh, hypophyseal fenestra; hym, hyomandibular; hyp, hypophysis; Mk, Meckel’s cartilage; nt, notochord; oc, otic capsule; occ, occipital arch; oe, oral ectoderm; orb, orbital cartilage; pch, parachordal; pol, polar cartilage; pq, palatoquadrate; rem, remnant of the hypophyseal duct; tr, trabecula.
The idea that the trabecula represents the skeletal element of the premandibular arch was first proposed by Huxley (Huxley, 1874; cited and reviewed by de Beer, 1937). This idea was further strengthened by Allis (1923, 1931, 1938) and has been cited in several textbooks since then (de Beer, 1937; Romer, 1966; Romer & Parsons, 1977; Fig. 2). In classical comparative morphology, several lines of arguments were built to support the presence of premandibular arch(es) in ancestral animals. First, the trigeminal nerve, or the rostralmost member of the branchiomeric nerves, arises as two separate primordia, one for the ophthalmic nerve (first branch of the trigeminal nerve; nV1) and ganglion, and the other for the maxillomandibular (nV2 + 3) components (Goodrich, 1930; de Beer, 1937; also see Sewertzoff, 1931 for different interpretations). This condition is also present in the lamprey (Koltzoff, 1901; Damas, 1944; Kuratani et al. 1997b). Of these, nV2 + 3 was thought to represent the original branchiomeric nerve only for the mandibular arch, whereas nV1 was thought to represent the secondarily degenerated nerve of the reduced premandibular arch. Secondly, the rostral part of the neurocranium is preformed by the paired rod-like cartilage, similar to that found in other visceral arches. Thirdly, the elasmobranch embryos show three or more pairs of epithelial mesodermal cavities in the head, each one of which was assumed to be associated with each pharyngeal arch (Balfour, 1878; van Wijhe, 1882; Bjerring, 1977; Jarvik, 1980). Of these, the rostralmost pair (premandibular cavities) does not have its pharyngeal arch counterpart, leading to the assumption that the ancestral premandibular arch would have developed ventral to this cavity pair.
Fig. 2.
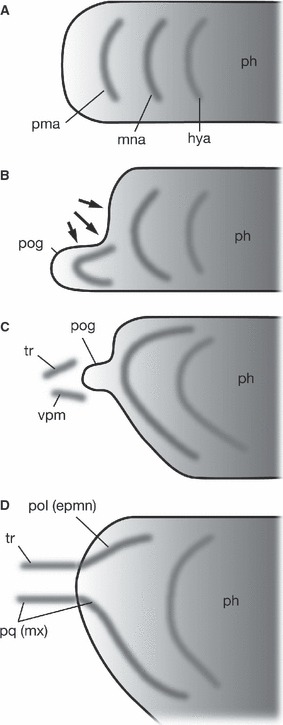
Development of the premandibular and mandibular components in gnathostome embryos by Allis (1938). (A) A generalized concept of the pharynx and pharyngeal arches. Along the antero-posterior axis of the pharyngeal endoderm, three successive pharyngeal arches develop as serial homologues. (B) In the elasmobranch neurula, described in Acanthias by Scammon (1911), because of the growth of the forebrain and formation of the cephalic flexure, the rostralmost part of the pharynx becomes pressed ventrally to form the vestigial preoral gut (pog), associated with the precursor of premandibular arch skeletons. (C) In older embryos, the preoral gut diminishes, leaving the skeletogenic precursor rostral to the mandibular arch. The premandibular skeletal elements consist of dorsal and ventral elements. (D) In the fully grown chondrocranium, the dorsal element of the premandibular arch skeleton differentiates into the major part of the trabecula, whereas the ventral part provides the rostral portion of the maxillary (palatoquadrate) cartilage. Caudal to the trabecula is found the polar cartilage, a mandibular arch-derived element participating in the formation of the medial bar of the cranial base. Thus, the polar cartilage is identified as a dorsal component of the mandibular arch skeleton by Allis. hya, hyoid arch; mna, mandibular arch; ph, pharynx; pma, premandibular arch; pog, preoral gut; pol(epmn), polar cartilage explained as epipharyngo-mandibular element of the mandibular arch; pq(mx), palatoquadrate as the primary skeletal element of the maxillary process; tr, trabecula; vpm, ventral element of the premandibular arch.
A purely morphological understanding of the trabeculae as premandibular arch elements has some clear problems. First, no clear pharyngeal pouch develops in the endoderm rostral to the first pharyngeal pouch (Fig. 3A). This may explain the atypical developmental pattern of the trigeminal ganglia [for the diffused developmental pattern of trigeminal placodes, see Kastschenko (1887) and D’Amico-Martel & Noden (1983)]. Secondly, there is no pharyngeal arch muscle rostral to the mandibular arch. Third, unlike the interpretation by Stensiö (1927), no fossil agnathan is reported to have possessed premandibular arches. Without a typical premandibular ‘arch’, agnathan branchiomeric nerves and pharyngeal arches show similar levels of differentiation to those of gnathostomes (Janvier, 1996). To add further recent molecular evidence, no Dlx genes are expressed in the ectomesenchyme rostral to the mandibular arch of gnathostomes; dorso-ventrally nested expressions of Dlx family genes (Dlx code: Depew et al. 2002) specify the dorso-ventral morphology of pharyngeal arches. Thus, this molecular developmental mechanism does not appear to play a role in any parts of the premandibular ectomesenchyme as components of the pharyngeal arches.
Fig. 3.
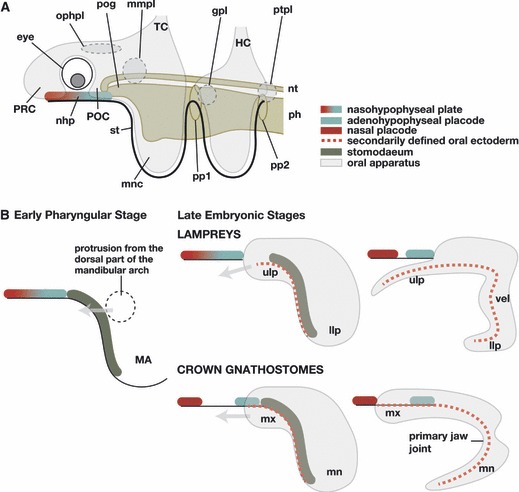
Comparison of craniofacial development between the lamprey and crown gnathostomes. (A) The generalized morphological pattern of the vertebrate head common to the lamprey and modern gnathostomes. (B) The anatomical difference between the lamprey and modern gnathostomes becomes clear in the later developmental pattern. In both animals there is a dorsal part of the mandibular arch growing rostrally to form the dorsal roof of the oral apparatus. Note, in the lamprey, the upper lip contains the premandibular component, at least in its medial part. Because of the late separation of the nasohypophyseal plate, this rostral growth forms an oral roof beneath the nasal and hypophyseal placodes. In the gnathostomes, the dorsal part of the mandibular arch grows rostrally as the maxillary process, lateral to the hypophysis, to form a part of the upper jaw. The medially located premandibular structure (trabecula, not shown) also grows rostrally lateral to the hypophysis, separate from the maxillary process. gpl, Geniculate placode; HC, hyoid crest cells; llp, lower lip; MA, mandibular arch; mmpl, maxillomandibular placode; mn, mandibular process; mnc, mandibular arc crest cells; mx, maxillary process; nhp, nasohypophyseal plate or placodes; nt, notochord; ophpl, ophthalmic placode; ph, pharynx; pog, preoral gut; pp1–2, pharyngeal pouches; POC, postoptic crest cells; PRC, preoptic crest cells; ptpl, petrosal placode; st, stomodaeum; TC, trigeminal crest cells; ulp, upper lip; vel, velum.
For the above reasons, the presence of the premandibular arch cannot be accepted now, but several facts should still be borne in mind. For example, since Platt (1893), a large part of the cranium, especially the viscerocranium in gnathostomes, has been shown to arise from the neural crest, unlike the more caudal part of the neurocranium (Le Lièvre, 1978; Noden, 1983; reviewed by Le Douarin, 1982; Noden, 1988; and by Hall & Hörstadius, 1988). According to Couly et al. (1993), who constructed chimeric embryos between chicken and Japanese quail, the trabeculae and their derivatives develop from the neural crest-derived ectomesenchyme similar to pharyngeal arch skeletal elements. The rostral neurocranium has been called the ‘prechordal cranium’ because it arises rostral to the notochord, in contrast to the more caudal ‘chordal cranium’, which arises lateral to the notochord and chondrifies under the instruction of notochord-derived signals (Couly et al. 1993). Recently, Wada et al. (2011) reported the central stem of the prechordal cranium originated from a rostral unpaired intertrabecula and a caudal moiety, the paired trabeculae arising lateral to the hypophysis (unlike the pattern depicted in Fig. 1A, right; Wada et al. 2005, 2011 and references therein). These two parts arise from different crest cell populations and with distinct genetic programmes (Wada et al. 2005, 2011; Eberhart et al. 2006).
Another point to be noted is the rostral end of the endoderm. In the history of classical comparative embryology, the position of the hypothetical premandibular arches was assumed to be rostral to the mouth opening (Sewertzoff, 1931; de Beer, 1937). In this line of reasoning, the skeletal parts of the premandibular arches are thought to be represented by minor cartilages around the margin of the mouth opening, as noted in Sewertzoff (1931). The mouth, however, does not represent the rostral end of the endoderm, but secondarily opens in the ventral pharynx by rupturing of the oropharyngeal membrane that forms between the rostro-ventral pharyngeal endoderm and the surface ectoderm (Fig. 3; Kupffer, 1900; reviewed by Stadmüller, 1938; Kuratani, 2012). The depression on the ventral head ectoderm prefiguring the mouth is the stomodaeum (Fig. 3B). Thus, the premandibular ectomesenchymal component should be found towards the direction of the preoral gut where the primordium of the trabecula is found, not towards the mouth (Kuratani, 2012).
The roof of the preoral gut is known as the prechordal plate, which is also found as a rostral continuation of the notochord (Adelmann, 1922, 1926, 1927; Seifert et al. 1993; Sauka-Spengler et al. 2003; reviewed by Adachi & Kuratani, in press). As the definitive rostral end of the notochord is formed, the prechordal plate differentiates into the premandibular mesoderm that grows bilaterally (de Beer, 1924; Kuratani et al. 1999). Thus, the rostral pole of the endoderm forms the rostral end of the notochord and the rostralmost mesodermal component (reviewed by Adachi & Kuratani, in press). The premandibular ectomesenchymal component is found in the vicinity of the preoral gut, in front of the mandibular arch (Fig. 3A). Consistent with this, Wada et al. (2011) found that the postoptic crest (POC) cells, or the source of the paired part of the trabecula, are found lateral to the preoral gut (Fig. 3A). The primordium of the more rostral element, the intertrabecula, is found rostral to the eye (Wada et al. 2011). The latter mesenchyme is called the preoptic crest (PRC) cells (Fig. 3A).
Even more curious is another recent finding in developmental biology; Couly et al. (2002) found that removal of the rostral head endoderm (including the domain of the future preoral gut) in a Hamburger and Hamilton’s stage 8 (HH stage 8; Hamburger & Hamilton, 1951) chicken embryo leads to the absence of the prechordal cranium. Removal of a slightly more posterior endodermal region results in the loss of the rostral part of the mandibular arch skeleton, and removal of an even more caudal region leads to the absence of jaw joints. Thus, the morphological patterning of the neural crest-derived skeletal elements, including the trabeculae and their derivatives, requires interaction with the endodermal epithelium, on which the skeletal morphology is spatially mapped (Ruhin et al. 2003). Whether the premandibular/mandibular (pmm) boundary in such an endodermal mapping coincides with the prechordal/chordal (pcc) boundary poses an important question for understanding of the gnathostome neurocranium (Fig. 4).
Fig. 4.
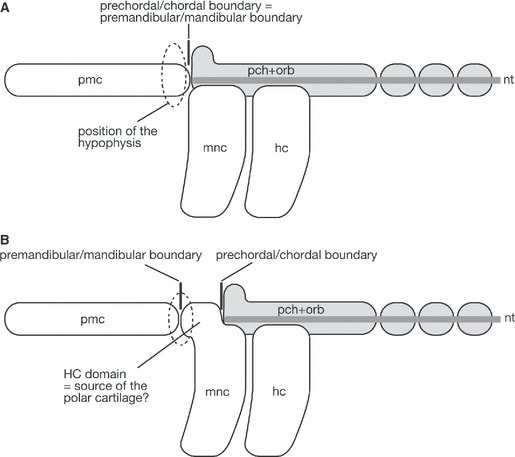
Diagram to explain the question regarding the nature of the polar cartilage. (A) It is generally accepted that the rostral, prechordal part of the neurocranium is formed of premandibular ectomesenchyme. Thus, the boundary between the premandibular and mandibular ectomesenchyme is expected to be found at the same level as the boundary between the prechordal and chordal cranium, and the polar cartilage is simply understood as the posterior part of the trabecula. In this scheme, the hypophysis is found between the caudal portions of trabeculae, the prechordal cranial element. (B) If the polar cartilage represents the ‘prechordal’ mandibular arch-derivative, we will have to assume the presence of an enigmatic ectomesenchymal domain corresponding to the ‘HC domain’. This diagram is based on the understanding of Haller (1923) and Allis (1938) shown in Fig. 5A. In this scheme, the hypophysis represents the boundary between the ectodermal portions covering the forebrain and the pharynx. hc, hyoid arch crest cells; mnc, mandibular arch crest cells; nt, notochord; pch+orb, parachordal and orbital cartilages; pmc, premandibular crest cells.
The boundary between the prechordal and chordal cranium
There is a boundary in the neurocranial base between the neural crest-derived prechordal cranium and mesodermally derived chordal cranium (Couly et al. 1993). This boundary was first shown in avian embryos and was also recently shown in transgenic mice (McBratney-Owen et al. 2008; see also Yoshida et al. 2008). This represents the difference in cell lineages and the inductive events for chondrification: the chordal cranium requires signals emanated from the notochord, whereas the prechordal cranium is induced by other embryonic environmental cues such as signals from the endoderm. The high levels of environmental dependency in the prechordal cranium were demonstrated by Noden (1978).
In the chicken and mouse chondrocrania, the prechordal-chordal cranial boundary coincides approximately with the rostral limit of the notochord, which is close to the hypophysis (Couly et al. 1993; McBratney-Owen et al. 2008). Then, can we regard the entire prechordal cranium as being derived from the premandibular ectomesenchyme? In other words, when viewed from the anteroposterior distribution of the ectomesenchyme and paraxial mesoderm, will the pcc boundary coincide with the premandibular-mandibular boundary (Fig. 4)? In this connection, a pair of small, nodule-like cartilages, called the polar cartilages, has been recognized between the trabeculae and chordal cranium (Fig. 1B; de Beer, 1937 and references therein). This pair may appear simply as the posteriormost part of the trabecula that shows semi-independent chondrification from the rest of the trabeculae, but some authors regarded it as an independent cartilaginous element. The argument about the identity of the polar cartilages is thus tightly linked to the definition of the mandibular arch.
The boundary between the mandibular arch and the premandibular domain
As to the identity of the polar cartilages, Allis (1923, 1931, 1938) published a series of reviews in which he dealt with the development of some skeletal elements developing in the rostral part of the neurocranium together with the development of the hypophyses, the mandibular arch and the oral cavity.
Allis (1938) defined the premandibular domain as part of the head where the forebrain is originally directly surrounded by the ventral surface ectoderm (Fig. 5A). This corresponds to the area of secondarily expanded forebrain, where the trabeculae later form the floor of the brain [Allis (1923) first called this the ‘cerebral surface’; see also de Beer (1937) for expansion of the forebrain]. Allis also recognized the pharynx as the part surrounded by the ‘visceral ectoderm’, although this term sounds rather contradictory (Fig. 5A). This ectoderm covers the entire visible mandibular arch including the stomodaeum. Between this and the cerebral ectoderm, we can find Rathke’s pouch, or the anlage for the adenohypophysis. According to Allis (1938) and Haller (1923), the hypophysis of gnathostomes arises not simply as a dorsal pocket of the oral cavity, as first recognized by Rathke (1839), but rather is specified at the boundary of the cerebral and visceral ectoderm. In the shark embryo, before the maxillary process has formed, the early hypophyseal anlage appears exactly as originally described by Haller (1923) (Fig. 5A). In other words, the position of the hypophysis is thought to represent the pmm boundary that simultaneously delineates the polar cartilage and the trabecula antero-posteriorly (Fig. 4B).
Fig. 5.
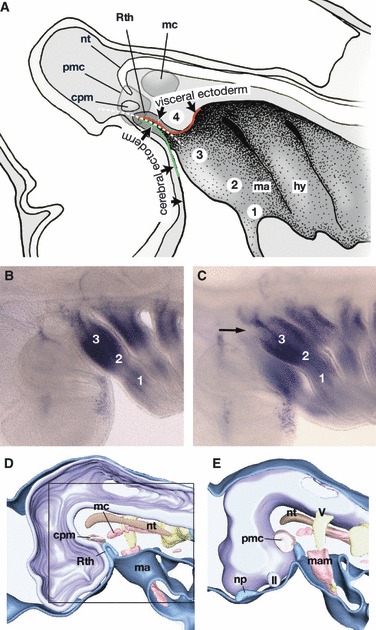
Topographical relationship between the mandibular arch and the hypophyseal anlage. (A) A medial view of the early pharyngula of a shark. Redrawn from Haller (1923). According to Haller, Rathke’s pouch does not arise as a part of the oral ectoderm, which is not defined at the stage of initial hypophyseal development, but at the junction of two different ectoderms: the cerebral ectoderm that covers the forebrain, and the visceral ectoderm that covers the mandibular arch and forms the stomodaeum. Haller (1923) divided the mandibular arch along the dorso-ventral axis into four subdivisions (1–4), termed, from a ventral to a dorsal direction, ‘Unterkieferstück (lower jaw region)’ (1), ‘Zwischenstück (intermediate or jaw joint region)’ (2), ‘Oberkieferstück (maxillary region)’ (3), and ‘Kieferaugenspaltstück (hypophyseal cushion region, abbreviated as HC region in the text)’ (4). The HC region is found just caudal to the hypophyseal anlage, and the polar cartilage is suggested to develop in this domain. Thus, Allis (1938) regarded the polar cartilage as the mandibular arch derivative. (B) Dlx1 expression pattern in a pharyngula of Scyliorhinus torazame. Lateral view. (C) The same embryo as B, slightly tilted to view the embryo ventrally. The HC region of this embryo does not appear to express Dlx1. (D,E) Partially reconstructed early pharyngula of an elephant fish, Callorhynchus milii. Medial (D) and lateral (E) views (E is left–right inverted for comparison). Square in (D) indicates the equivalent region shown in (A). These figures show that the embryonic pattern is highly conserved in chondrichthyans and lamprey, in which nasal and hypophyseal placodes are more closely associated with each other. cpm, Commissure part of the premandibular cavity; hy, hyoid arch; II, optic nerve; ma, mandibular arch; mam, mandibular arch mesoderm; mc, mandibular cavity; np, nasal placode; nt, notochord; pmc, premandibular cavity; Rth, Rathke’s pouch; V, trigeminal nerve anlage.
Curiously, although the trabecula is found in the premandibular domain as usually understood, the polar cartilage arises in the domain of the above-defined mandibular arch (Fig. 5A). In the early chondrocranium of shark embryos, this distinction appears to hold true (Fig. 1B). This is the reason Allis (1923, 1938) identified the polar cartilages as elements belonging to the mandibular arch (Fig. 2).
Allis’s argument depends largely on Haller (1923), who described the early development of the hypophysis in shark embryos (Fig. 5A). Haller divided the mandibular arch into four subdomains called, from the dorsal to ventral direction, ‘Kieferaugenspaltstück (jaw-optic fissure region)’, ‘Oberkieferstück (maxillary region)’, ‘Zwischenstück (intermediate region)’, and ‘Unterkieferstück (lower jaw region)’. Of these, ‘jaw-optic fissure region’ appears to be a misnomer, and will be called the hypophyseal cushion (Polster), hereafter abbreviated to the HC region (Fig. 5A). Based on this scheme, Allis (1938) pointed out that the polar cartilage arises in this HC region (Fig. 5A) and he thought that the fusion of the trabecula and polar cartilage represents the junction between two successive visceral arches (Fig. 2). Furthermore, as the polar cartilage originates from the HC region of the mandibular arch, Allis (1923, 1931, 1938) thought that it represented the pharyngo-mandibular element, which had been found rather unacceptable in classical comparative morphology (de Beer, 1937).
Experimental and molecular embryology and the origin of the polar cartilage
Can evolutionary developmental biology solve the above question? If we look at recent labelling results in fate mapping studies addressing the craniofacial ectomesenchyme, we readily find reasons to deny the above hypothesis about the mandibular arch origin of the polar cartilage, or even the existence of this cartilage as an independent entity.
For example, Shigetani et al. (2000) found that, in early chicken embryos (HH stage 13), when the mandibular arch has not yet completely formed, there is already a distinction in the trigeminal crest cells caudal to the eye, of cells destined to become the premandibular domain and mandibular arch, and that the latter domain roughly corresponds to the Fgf8-expressing domain in the ventral surface ectoderm. In other words, the rostral limit of the Fgf8 expression prefigures the pmm boundary. At HH stage 12, the Rathke’s pouch is definitely located rostral to the mandibular arch domain (Fig. 4B), and the maxillary process arises from the mandibular arch domain, not from the premandibular domain. The latter finding is inconsistent with Haller’s concept noted above.
Although a different terminology is employed, Cerny et al. (2004) have also shown a similar result in their fate-mapping study using axolotl embryos. They showed that the maxillary process is derived from a part of the mandibular arch, and that the POC cells will form the trabeculae. The latter study clearly showed that the rostromedial and lateral portions of the dorsal oral apparatus are developmentally specified in anterior and posterior domains of apparently continuous trigeminal crest cells in earlier developmental stages. Importantly, however, this experiment did not show that the posterior part of the prechordal cranium has an independent origin (as a separate entity) as the polar cartilage. Furthermore, Wada et al. (2011) have recently shown clearly that the posterior part of the trabecula, which is expected to develop as the polar cartilages, also has its origin in the POC cells. Thus, even if there is a cartilaginous module to be called the polar cartilage, it seems very likely that it shares the same developmental origin with the rest of the trabecula. If this is correct, there is even a possibility that the polar cartilage per se may be an artefact created by some comparative embryologists.
However, the solution to the question may not be so simple, as the labelling experiments performed in zebrafish embryos do not even support the notion of separate developmental origins for the trabecula and mandibular arch derivatives. By single-cell labelling methods, Eberhart et al. (2006) have shown that there are crest cells that differentiate into a part of the palatoquadrate and the trabecula. In this connection, Lee et al. (2004) have also come to the conclusion that parts of the trabecula and maxillary process are derived from the same domain as the trigeminal crest cells, based on focal injection of vital dyes, similar to the method employed by Shigetani et al. (2000). It has not been clarified yet why the latter two groups obtained different results. At least it is obvious that fate-mapping of the boundary between the premandibular and mandibular domains will take a very delicate and fine-tuned labelling experiment. Simultaneously, the process of cell lineage specification may possibly shift spatiotemporally through evolution and can vary in each species, even if the basic morphological pattern of the chondrocranium is conserved.
We also have to consider regulatory gene expression patterns that are associated with specific morphological domains. For example, Dlx genes have been shown to be expressed in the pharyngeal arch ectomesenchyme with a nested pattern reminiscent of Haller’s scheme, but not expressed in the premandibular domain of some gnathostome embryos (Qiu et al. 1997; Shigetani et al. 2000, 2002; Depew et al. 2002). Then, will the HC region express any Dlx genes as the dorsalmost part of the molecularly defined mandibular arch? In a late pharyngula stage of gnathostomes, the HC region is laterally covered by the maxillary region, the anlage of the upper jaw (Figs 1B and 3B). Therefore, whether it is a part of the mandibular arch or not, the HC region does not develop as a part of the ‘functional jaw’, as correctly suggested by Haller (1923) and Allis (1923, 1931, 1938).
According to our observation in the shark embryo, Dlx1 is not expressed in the HC domain (Fig. 5B,C). Among the Dlx genes, as in mouse and chicken, this gene in the shark is expressed most dorsally and in the most widespread ectomesenchymal domain of the mandibular arch ectomesenchyme. If the gnathostome mandibular arch is to be defined by Dlx1 expression, the HC region does not represent a part of the mandibular arch but more likely to corresponds with the POC cells, a part of the premandibular domain. As noted above, Fgf8 is already expressed to specify the mandibular arch domain and the stomodaeum, and Rathke’s pouch is developing rostral to the Fgf8-positive domain even before the influx of crest cells (HH stage 10). In other words, the hypophysis in the chick embryo (and possibly the shark) does not appear to arise at the pmm boundary (see Fig. 4A). Instead, it arises rostral to the mandibular arch, as defined by the expression of Fgf8/Dlx1, leading us to the possibility that the HC region of Haller (1923) represents the POC cells.
The above contradiction appears to have originated from the distinction between the cerebral and visceral ectoderm, which is likely to be an artificial definition. Rather, the position of the hypophyseal placode is more likely to be specified by the induction from the hypothalamic anlage (Takuma et al. 1998; reviewed by Zhu et al. 2007), not from the interface of cerebral/pharyngeal domains pre-specified in the ectoderm (Fig. 5A). In this context, it would be worth considering that, among the genes known to be expressed in domains of rostral ectoderm such as Pitx, Pax6, and Sp8 (Boorman & Shimeld, 2002; Uchida et al. 2003; Kawakami et al. 2004; Jeong et al. 2008; Sjodal & Gunhaga, 2008; Sugahara et al. 2011), so far no gene reported was expressed differentially between the cerebral and visceral ectodermal domains recognized by Allis (1923) and Haller (1923).
As discussed above, the polar cartilage is at present most likely to represent simply the posterior part of the trabecula as the premandibular ectomesenchymal derivative. Thus, the polar cartilage does not exist as an individual entity. Furthermore, the polar cartilage is now mentioned less than before, especially in descriptions based on the whole-mount staining method using alcian blue. This raises the possibility that the polar cartilage was an artefact based on biased observations, particularly based on the transverse histological sections of older embryos when the trabecula is bent laterally at the level of the hypophysis, for example because of the growth of the internal carotid artery.
In the present review, we propose a developmental definition of the mandibular arch ectomesenchyme as follows: it is the part of the trigeminal ectomesenchyme that expresses Dlx1 and its cognates. It is also associated with the mandibular arch mesoderm, and separated from the hypophyseal anlage by a certain distance. The HC domain, or the POC cell population, does not express Dlx genes and is located lateral and caudal to the hypophyseal anlage as the major source of the paired trabeculae. Finally, the rostral limit of the chordal cranium is to be found in the orbital cartilage, or in the crista sellaris in those animals that do not develop an orbital cartilage. The entire prechordal cranium rostral to this limit can be regarded as the neural crest-derived premandibular cranium, which is also rostral to the mandibular arch. Thus the pmm boundary is most likely to be found at the same level as the pcc boundary (Fig. 4A).
On the lamprey trabecula
To understand the origins of the prechordal cranium and the jaw in gnathostomes, the developmental fate has to be compared for each subpopulation of the trigeminal crest cells between gnathostomes and agnathan animals. To investigate the agnathan condition, embryos of lampreys have been frequently employed (reviewed by Kuratani, 2012; for the anatomy of the lamprey head, see Hardisty, 1981; Hardisty & Rovainen, 1982; Marinelli & Strenger, 1954). Similar to gnathostomes, the premandibular ectomesenchyme of the lamprey embryo can also be divided into PRC and POC cells (Fig. 3A; for lamprey neural crest cell development and migration, see Horigome et al. 1999; Tomsa & Langeland, 1999; Kuratani et al. 2001; McCauley & Bronner-Fraser, 2003, 2006; for development of the lamprey viscerocranium, see Johnels, 1948; Mallatt, 1984; Ohtani et al. 2008; Martin et al. 2009; Yao et al. 2011). Before the differentiation of the oral apparatus, developmental patterns of trigeminal ectomesenchyme are not fundamentally different between gnathostome and lamprey embryos, especially when the nasal and hypophyseal placodes are close to each other in gnathostomes (Figs 3 and 5). The latter placodes form one median plate in the lamprey as the nasohypophyseal plate (Fig. 3; von Kupffer, 1894, 1900; de Beer, 1923; reviewed by Haller, 1898). In both taxa, the prechordal plate is initially seen as the roof of the preoral gut, with no clear boundary with the rostral part of the notochord.
Lamprey embryos also have a cartilaginous element called the trabecula that serves as a floor of the forebrain (Parker, 1883; Johnels, 1948). As in gnathostomes, the lamprey trabeculae also appear as a pair of rod-like cartilages whose rostral ends are united with their counterparts by means of a transverse commissure. These structures together form a cartilaginous ring that surrounds the hypothalamus and hypophysis. In addition, there were studies suggesting the neural crest origin of the lamprey trabecula (Langille & Hall, 1988; also see Newth, 1951, 1956). It was shown by embryological observations and labelling experiments, however, that the lamprey trabecula is of mandibular mesodermal origin and, therefore, this cartilage is more likely to be homologous with the gnathostome parachordal that has secondarily grown and shifted anteriorly (Johnels, 1948; Kuratani et al. 2004). The POC cells in the lamprey appear to develop into the mesenchyme of the upper lip of ammocoete larvae (Shigetani et al. 2002).
Rostral to the above noted ring, a pair of cartilaginous nodules provides origins for the trigeminal-nerve-innervated muscles in the upper lip, and thus the cartilages may represent mandibular arch derivatives that have shifted their positions rostrally (see below). A homologue of intertrabecula may exist in the lamprey as a certain ectomesenchymal derivative found caudal (dorsal) to the external nostril. Although it develops from the PRC cells, or the mesenchyme that forms the cushion rostral to the nasohypophyseal plate, it cannot be called a frontonasal process (FN)-derivative because lampreys do not have paired nostrils.
The origin of the gnathostome jaw – revising the heterotopy theory
In the early embryonic head of gnathostomes and lampreys, the prechordal plate and its derivatives, nasohypophyseal placodes, first pharyngeal pouch and mandibular arch are arranged in a very similar topographical relationship (Fig. 3A; see Scott, 1883, 1887; Leach, 1951; for hypophyseal development in the lamprey). In the ventro-lateral part of the head of the early lamprey embryo (Tahara’s stage 20.5; Tahara, 1988), there is a pair of conspicuous processes, called the cheek processes (Fig. 6A,B). This process initially contains mandibular mesoderm and the first pharyngeal pouch, and the prechordal plate (roof of the preoral gut) is protruding rostral to the mandibular mesoderm (Fig. 6A,B). The prechordal plate expresses LjHh together with the notochord and rostral endoderm (Sugahara et al. 2011; Fig. 7A–D, compare with Fig. 6). Thus, the anlage of the premandibular mesoderm is clearly rostral to the mandibular arch around this stage (Fig. 6A,F; also see Fig. 7D). By stage 21, however, trigeminal crest cells have started to cover the rostral head, and thereafter the prechordal plate is covered by the growing ectomesenchyme (Fig. 6; also see Claydon, 1938). Therefore, the cheek process no longer consists only of the mandibular mesoderm and the first pharyngeal pouch but also contains the anlage for the premandibular mesoderm. It is around this stage that the cheek process begins to divide antero-posteriorly to differentiate into upper and lower lips (Fig. 7E–G), indicating that these structures are not purely of the mandibular arch. From this stage onward, it therefore becomes difficult to define the POC domain in the lamprey.
Fig. 6.
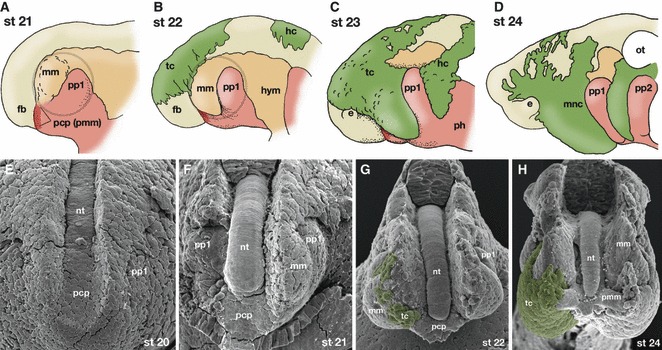
Development of the cheek process and the prechordal plate in the lamprey. (A–D) Diagrammatic representation of the rostral head part of the lamprey embryos based on the development of Lethenteron japonicum. At Tahara’s stage 21, the prechordal plate, or the developmental source of the premandibular mesoderm is seen rostral to the mandibular arch mesoderm. Grey circle indicates the cheek process. (B) At stage 22, cephalic crest cells begin to migrate. The mandibular arch mesoderm has grown ventrally and rostrally. (C) By stage 23, the trigeminal crest cells have covered the entire mandibular arch and also a part of the prechordal plate. (D) The mandibular arch and the prechordal plate are totally covered by the trigeminal crest cells. (E–H) Scanning electron micrographs of the developing head of L. japonicum. The surface ectoderm of the embryos and part of the neural tube have been removed. (E) Stage 20. The rostral part of the notochord continues rostrally into the roof of the preoral gut as the prechordal plate (pcp). The rostralmost head mesoderm appears as the mandibular mesoderm rostral to the first pharyngeal pouch (pp1) and lateral to the prechordal plate. (F) Stage 21. The prechordal plate is found rostral to the level of the mandibular mesoderm (mm). (G) Stage 22. Trigeminal crest cells (tc: coloured green) have begun to cover the rostral head. (H) Stage 24. The prechordal plate-derived premandibular mesoderm (pmm) is penetrating the rostral subpopulation of the trigeminal crest cells. From the position of the mandibular mesoderm, this rostral cell population of the trigeminal crest cells appears to be located in a premandibular position from this photograph. e, eye; fb, forebrain; hc, hyoid crest cells; hym, hyoid arch mesoderm; mm, mandibular arch mesoderm; mnc, mandibular arch crest cells; ot, otocyst; pcp, prechordal plate; pmm, premandibular mesoderm; pp1–2, pharyngeal pouches; tc, trigeminal crest cells. Photographs for (E–H) by Naoto Horigome.
Fig. 7.
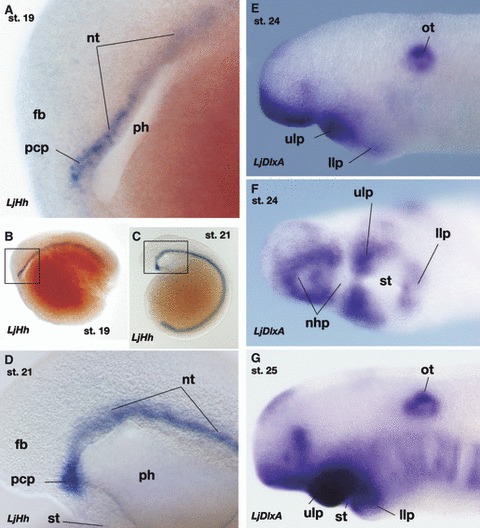
Expression of LjHh and LjDlxA in embryos of the lamprey, Lethenteron japonicum. (A,B) Expression of LjHh in Tahara’s stage 19 embryo. Box in (B) is enlarged in (A). (C,D) Stage 21 embryo. Box in (C) is enlarged in (D). During these stages, LjHh is expressed in the prechordal plate that appears as the rostral continuation of the notochord. Compare with Fig. 5A,E,F. (E,F) LjDlxA expression in stage 24 embryo. (F) shows an oblique ventral view. (G) LjDlxA expression at stage 25. Note that this gene is upregulated during the pharyngular development in the ectomesenchyme in upper and lower lips. Whether the DlxA-positive ectomesenchyme in the upper lip consists only of the premandibular POC cells or contains mandibular arch ectomesenchyme remains unknown. Compare with Fig. 5H. fb, forebrain; nhp, nasohypophyseal plate; nt, notochord; ot, otocyst; pcp, prechordal plate; ph, pharynx; st, stomodaeum; ulp, upper lip.
Unlike in gnathostomes, the lamprey POC cells have been reported to express the Dlx gene (Neidert et al. 2001; Shigetani et al. 2002; Cerny et al. 2010; Kuraku et al. 2010). In the heterotopy theory (Shigetani et al. 2002), the most conspicuous difference between the lamprey and the gnathostome oral patterning is the extent of Dlx-expressing ectomesenchyme in the trigeminal crest cells: in the whole-mount lamprey embryos, Dlx-negative POC ectomesenchyme is apparently absent, and the entire ectomesenchyme caudal to the eye appears to be Dlx-positive (Fig. 7E–G; Neidert et al. 2001; Shigetani et al. 2002; Cerny et al. 2010; Kuraku et al. 2010). The POC ectomesenchyme in the lamprey appears to differentiate into the upper lip, not into the trabecula as in gnathostomes (Fig. 6; Shigetani et al. 2002). The heterotopy theory predicts, in the agnathan–gnathostome transition, a heterotopic, caudal shift of ectodermal FGF8 domain restricted Dlx upregulation to the domain caudal to the pmm boundary, thus limiting the oral patterning domain into the mandibular arch (and secondarily, FN derivatives participated in its formation; reviewed by Kuratani, 2012). From a morphological point of view, the above shift can also be viewed as a shift of the position of the mouth, leading to the shift of pharyngeal endoderm-induced Fgf8 expression. However, the above scenario suggests that the association between Dlx regulation and pmm boundary differs between cyclostomes and gnathostomes. Otherwise, if this association were conserved through vertebrate evolution, this would challenge the heterotopy theory, as will be shown below.
As shown in Fig. 6H, the premandibular mesoderm does grow into the rostral part of the cheek process ectomesenchyme, defining the lamprey POC cells that differentiate into the upper lip (Kuratani et al. 1999). However, the mandibular mesoderm-derived muscle anlage reaches this domain just after this stage (Kuratani, 2012). It has not been clarified yet whether this change can be ascribed to the rostral growth of the mandibular arch mesoderm described above (Fig. 6) or to the secondary migration after stage 25 as hypothesized previously (Kuratani et al. 2004). Although these upper lip muscle primordia are thought to be accompanied by mandibular arch crest cells as the source of connective tissue (Noden, 1983; Köntges & Lumsden, 1996), it has not been clearly observed whether the Dlx-positive upper lip ectomesenchyme represents such secondarily immigrating crest cells or the original POC cells (Sugahara & Kuratani, unpublished observation).
From the above discussion, would it be possible to homologize the lamprey upper lip and gnathostome maxillary process? Prior to the establishment of the oral ectoderm, the topographical relationships among the stomodaeum, nasohypophyseal placodes, and the mandibular arch are largely conserved between the lamprey and gnathostomes (Fig. 3). A part of the lamprey upper lip and the gnathostome maxillary process are derived from the dorsal part of the mandibular arch and grow rostrally to meet with its counterparts on the contralateral side, defining the oral cavity. In the latter developmental process of the gnathostomes, Rathke’s pouch is secondarily incorporated in the oral ectoderm, whereas in the lamprey, fusion of the upper lips on both sides takes place caudal to the nasohypophyseal plate, and thus the hypophysis does not open in the oral cavity as in gnathostomes. The upper lip also contains at least POC cells, which is not seen in the gnathostome maxillary process.
To explain the morphology of the lamprey larva, Haller (1923) stated that the median fusion of dorsal domains takes place much earlier than in gnathostomes, and the upper lip has to grow not laterally, but underneath the hypophysis. It is true that heterochronic differences exist between the lamprey and gnathostomes, especially in the separation of nasal and hypophyseal placodes, which may partly explain the above morphological difference. This idea, however, does not consider the involvement of the POC cells in the upper lip formation. Otherwise, there are the following developmental differences between the lamprey upper lip and the gnathostome upper jaw:
The lamprey upper lip is likely to be formed by a dorsal part of the mandibular arch and POC cells, whereas in the gnathostomes, the upper jaw is formed from the dorsal part of the mandibular arch with secondary involvement of the FN-derived structures.
In general, the gnathostome upper jaw primordium does not contain a muscle primordium.
In the lamprey embryo, the junction of the upper and lower lip primordia does not express Bapx cognates, which in gnathostomes defines the primary jaw joint.
For the above reasons, the lamprey upper lip cannot be directly homologized with the gnathostome upper jaw. Rather, the upper lip primordium in the lamprey appears to represent a complex structure corresponding to the maxillary process and the posterior portion of the trabecula primordia in gnathostomes, fused medio-laterally. In a way, it may be possible to understand the evolution of the jaw as a separation of this compound primordium medio-laterally to form the maxillary process and trabecula separately. In this regard, it may be worth mentioning that the pterygoid process, a part of the palatoquadrate developing in the maxillary process in gnathostomes (Goodrich, 1930; de Beer, 1937), is always morphologically and developmentally closely associated with the trabecula (Olsson & Hanken, 1996; Reiss, 1997; Eberhart et al. 2006; see de Beer, 1937 for topographical relationships between the upper jaw and the prechordal cranium). In genetic experiments also, this rostral part of the upper jaw element behaves differently from the rest of the mandibular arch skeleton (Miller et al. 2004; Zuniga et al. 2010). It belongs to future studies to determine whether this ambiguity or inconsistency regarding the definition of the mandibular arch represents a secondary condition associated only with certain gnathostome lineages, or reflects an ancestral developmental state in which the premandibular and mandibular components are used to build together a common morphological unit.
To facilitate the above separation of pterygoid process and trabecula, the hypophyseal placode has to be separated from the nasal placode early in development, to allow the ectomesenchyme to grow rostrally to the hypophysis. Thus, leaving aside the ambiguity of the expression pattern of Dlx in POC cells of the lamprey, the heterotopic shift remains the key to understanding jaw evolution.
The previous version of the heterotopy theory assumed that the ectodermally derived FGF/BMP signals define the anterior and posterior limits of the oral domain in the ectomesenchyme, whose extent was assumed to differ between the lamprey and gnathostomes (Shigetani et al. 2005). To test this original hypothesis, the role of the lamprey POC cells has to be clarified (the medial subpopulation of the crest cells in the upper lip primordium). It also has to be tested whether the stomodaeum is specified in an equivalent part of the head ectoderm with an equivalent inductive event in both animal lineages.
The heterotopy theory is still valid in that different parts of the ectomesenchyme differentiate into the dorsal portion of the oral apparatus between lampreys and gnathostomes, by a different pattern of growth and distribution of the trigeminal crest cells in late developmental stages. In this connection, a recent observation of a fossil group, Galeaspida, has suggested that this stem group of (jawless) gnathostomes appears to have possessed a hypophysis that opens into the oral cavity, not confluent with the nasal opening (Gai et al. 2011). Such a condition is much closer to the condition in modern gnathostomes than to the condition observed in the lamprey. It has not been clarified whether this fossil species had trabeculae, but the external nostril is unpaired. The most plausible scenario, therefore, would be that the separation of the nasal and hypophyseal placodes took place first, and then the nasal placode became paired during evolution, which should have allowed the dorsal oral process, including the posterior trabecula and maxillary process, to grow rostrally towards the hypophysis (see Scott, 1883; Haller, 1923; and Kuratani, 2004 for the evolutionary sequence of mono- to diplorhiny or vice versa). It would have been after the split of the nasal placode into a paired structures that the FN-derived component could be incorporated to participate in the formation of the upper jaw, as the intertrabecula and premaxilla.
Acknowledgments
We are grateful to Christian Mitgutsch for his critical reading of the manuscript. We also thank Naoto Horigome for the scanning electron micrographs of lamprey embryos. Our sincere gratitude is extended to Susumu Hyodo, Justin Bell, Tes Toop, and John A. Donald for their advice and assistance in obtaining Callorhinchus milii embryos.
References
- Adachi N, Kuratani S. Development of head and trunk mesoderm in a dogfish, Scyliorhinus torazame. I. Embryology and morphology of the head cavities and related structures. Evol Dev. doi: 10.1111/j.1525-142X.2012.00542.x. (in press), in press. [DOI] [PubMed] [Google Scholar]
- Adelmann HB. The significance of the prechordal plate: an interpretative study. Am J Anat. 1922;31:55–101. [Google Scholar]
- Adelmann HB. The development of the premandibular head cavities and the relations of the anterior end of the notochord in the chick and robin. J Morphol Physiol. 1926;42:371–439. [Google Scholar]
- Adelmann HB. The development of the eye muscles of the chick. J Morphol Physiol. 1927;44:29–87. [Google Scholar]
- Allis EP. Are the polar and trabecular cartilages of vertebrate embryos the pharyngeal elements of the mandibular and premandibular arches? J Anat. 1923;58:37–51. [PMC free article] [PubMed] [Google Scholar]
- Allis EP. Concerning the homologies of the hypophysial pit and the polar and trabecular cartilages of fishes. J Anat. 1931;65:247–265. [PMC free article] [PubMed] [Google Scholar]
- Allis EP. Concerning the development of the prechordal portion of the vertebrate head. J Anat. 1938;72:584–607. [PMC free article] [PubMed] [Google Scholar]
- Balfour FM. The development of the elasmobranchial fishes. J Anat Physiol. 1878;11:405–706. [Google Scholar]
- de Beer GR. Some observations on the hypophysis of Petromyzon and Amia. Q J Microsc Sci. 1923;67:257–292. [Google Scholar]
- de Beer GR. Studies on the vertebrate head. Part I. Fish. Q J Microsc Sci. 1924;1924:287–341. [Google Scholar]
- de Beer GR. The Development of the Vertebrate Skull. London: Oxford University Press; 1937. [Google Scholar]
- Benouaiche L, Gitton Y, Vincent C, et al. Sonic hedgehog signalling from foregut endoderm patterns the avian nasal capsule. Development. 2008;135:2221–2225. doi: 10.1242/dev.020123. [DOI] [PubMed] [Google Scholar]
- Bjerring HC. A contribution to structural analysis of the head of craniate animals. Zool Scrpt. 1977;6:127–183. [Google Scholar]
- Boorman CJ, Shimeld SM. Cloning and expression of a Pitx homeobox gene from the lamprey, a jawless vertebrate. Dev Genes Evol. 2002;212:349–353. doi: 10.1007/s00427-002-0249-9. [DOI] [PubMed] [Google Scholar]
- Cerny R, Lwigale P, Ericsson R, et al. Developmental origins and evolution of jaws: new interpretation of ‘maxillary’ and ‘mandibular’. Dev Biol. 2004;276:225–236. doi: 10.1016/j.ydbio.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Cerny R, Cattell M, Sauka-Spengler T, et al. Evidence for the prepattern/cooption model of vertebrate jaw evolution. Proc Natl Acad Sci U S A. 2010;107:17262–17267. doi: 10.1073/pnas.1009304107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claydon GJ. The premandibular region of Petromyzon planeri Part 1. Proc Zool Soc B. 1938;1938:1–16. [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- Couly G, Grapin-Botton A, Coltey P, et al. Determination of the identity of the derivatives of the cephalic neural crest: incompatibility between Hox gene expression and lower jaw development. Development. 1998;125:3445–3459. doi: 10.1242/dev.125.17.3445. [DOI] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, et al. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning facial skeleton in the vertebrate head. Development. 2002;129:1061–1073. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- Damas H. Recherches sur le développement de Lampetra fluviatilis L. – contribution à l’étude de la cephalogénèse des vertébrés. Arch Biol Paris. 1944;55:1–289. [Google Scholar]
- D’Amico-Martel A, Noden DM. Contributions of placodal and neural crest cells to avian peripheral ganglia. Am J Anat. 1983;166:445–468. doi: 10.1002/aja.1001660406. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381–385. doi: 10.1126/science.1075703. [DOI] [PubMed] [Google Scholar]
- Dickinson AJ, Sive HL. Positioning the extreme anterior in Xenopus: cement gland, primary mouth and anterior pituitary. Semin Cell Dev Biol. 2007;18:525–533. doi: 10.1016/j.semcdb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Dickinson AJ, Sive HL. The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth. Development. 2009;136:1071–1081. doi: 10.1242/dev.032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart JK, Swartz ME, Crump JG, et al. Early hedgehog signaling from neural to oral epithelium organizes anterior craniofacial development. Development. 2006;133:1069–1077. doi: 10.1242/dev.02281. [DOI] [PubMed] [Google Scholar]
- Eriksson BJ, Tait NN, Budd GE. Head development in the onychophoran Euperipatoides kanangrensis with particular reference to the central nervous system. J Morphol. 2003;255:1–23. doi: 10.1002/jmor.10034. [DOI] [PubMed] [Google Scholar]
- Gai Z, Donoghue PC, Zhu M, et al. Fossil jawless fish from China foreshadows early jawed vertebrate anatomy. Nature. 2011;476:324–327. doi: 10.1038/nature10276. [DOI] [PubMed] [Google Scholar]
- Gendron-Maguire M, Mallo M, Zhang M, et al. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell. 1993;75:1317–1331. doi: 10.1016/0092-8674(93)90619-2. [DOI] [PubMed] [Google Scholar]
- Goodrich ES. Studies on the Structure and Development of Vertebrates. London: Macmillan; 1930. [Google Scholar]
- Grammatopoulos GA, Bell E, Toole L, et al. Homeotic transformation of branchial arch identity after Hoxa2 overexpression. Development. 2000;127:5355–5365. doi: 10.1242/dev.127.24.5355. [DOI] [PubMed] [Google Scholar]
- Haeckel E. Anthropogenie oder Entwickelungsgeschichte des Menschen. Keimes- und Stammesgeschichte. 4th edn. Leipzig: Wilhelm Engelmann; 1891. [Google Scholar]
- Hall BK, Hörstadius S. The Neural Crest. New York: Oxford University Press; 1988. [Google Scholar]
- Haller B. Untesuchungen über die Hypophyse und die Infundibularorgane. Morphol Jahrb. 1898;25:31–114. [Google Scholar]
- Haller G. Über die Bildung der Hypophyse bei Selachiern. Morphol Jahrb. 1923;53:95–135. [Google Scholar]
- Hamburger V, Hamilton H. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Hardisty MW. The Skeleton. In: Hardisty W, Potter IC, editors. The Biology of the Lampreys. 3M. London: Academic Press; 1981. pp. 333–376. [Google Scholar]
- Hardisty MW, Rovainen CM. Morphological and functional aspects of the muscular system. In: Hardisty MW, Potter IC, editors. The Biology of the Lampreys. 4a. London: Academic Press; 1982. pp. 137–231. [Google Scholar]
- Horigome N, Myojin M, Hirano S, et al. Development of cephalic neural crest cells in embryos of Lampetra japonica, with special reference to the evolution of the jaw. Dev Biol. 1999;207:287–308. doi: 10.1006/dbio.1998.9175. [DOI] [PubMed] [Google Scholar]
- Hunt P, Whiting J, Muchamore I, et al. Homeobox genes and models for patterning the hindbrain and branchial arches. Dev Suppl. 1991b;1:187–196. [PubMed] [Google Scholar]
- Huxley TH. On the structure of the skull and of the heart of Menobranchus lateralis. Proc Zool Soc Lond. 1874;1874:186–204. (cited in de Beer 1931) [Google Scholar]
- Janvier P. Early Vertebrates. New York: Oxford Scientific Publications; 1996. [Google Scholar]
- Jarvik E. Basic Structure and Evolution of Vertebrates. Vol. 2. New York: Academic Press; 1980. [Google Scholar]
- Jeong J, Li X, McEvilly RJ, et al. Dlx genes pattern mammalian jaw primordium by regulating both lower jaw-specific and upper jaw-specific genetic programs. Development. 2008;135:2905–2916. doi: 10.1242/dev.019778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnels AG. On the development and morphology of the skeleton of the head of Petromyzon. Acta Zool. 1948;29:139–279. [Google Scholar]
- Kastschenko N. Das Schlundspaltengebiet des Hühnchens. Arch Anat Physiol. 1887;1887:258–300. [Google Scholar]
- Kawakami Y, Esteban CR, Matsui T, et al. Sp8 and Sp9, two closely related buttonhead-like transcription factors, regulate Fgf8 expression and limb outgrowth in vertebrate embryos. Development. 2004;131:4763–7474. doi: 10.1242/dev.01331. [DOI] [PubMed] [Google Scholar]
- Koltzoff NK. Entwicklungsgeschichte des Kopfes von Petromyzon planeri. Bull Soc Nat Moscou. 1901;15:259–289. [Google Scholar]
- Köntges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- von Kupffer C. Die Deutung des Hirnanhanges. Sitzungsberichte der Gesellschaft für Morphologie und Physiologie zu München. 1894 Jahrg. (cited by Haller, 1898) [Google Scholar]
- von Kupffer C. Studien zur vergleichenden Entwicklungsgeschichte des Kopfes der Kranioten. 4. Heft: Zur Kopfentwicklung von Bdellostoma. München u. Leipzig: Verlag von J. F. Lehmann; 1900. [Google Scholar]
- Kuraku S, Takio Y, Sugahara F, et al. Evolution of oropharyngeal patterning mechanisms involving Dlx and endothelins in vertebrates. Dev Biol. 2010;341:315–323. doi: 10.1016/j.ydbio.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Kuratani S. Evolution of the vertebrate jaw: comparative embryology reveals the developmental factors behind the evolutionary novelty. J Anat. 2004;205:335–347. doi: 10.1111/j.0021-8782.2004.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani S. Evolution of the vertebrate jaw from developmental perspectives. Evol Dev. 2012;14:76–92. doi: 10.1111/j.1525-142X.2011.00523.x. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Matsuo I, Aizawa S. Developmental patterning and evolution of the mammalian viscerocranium: genetic insights into comparative morphology. Dev Dyn. 1997a;209:139–155. doi: 10.1002/(SICI)1097-0177(199706)209:2<139::AID-AJA1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Ueki T, Aizawa S, et al. Peripheral development of the cranial nerves in a cyclostome, Lampetra japonica: morphological distribution of nerve branches and the vertebrate body plan. J Comp Neurol. 1997b;384:483–500. [PubMed] [Google Scholar]
- Kuratani S, Horigome N, Hirano S. Developmental morphology of the cephalic mesoderm and re-evaluation of segmental theories of the vertebrate head: evidence from embryos of an agnathan vertebrate, Lampetra japonica. Dev Biol. 1999;210:381–400. doi: 10.1006/dbio.1999.9266. [DOI] [PubMed] [Google Scholar]
- Kuratani S, Nobusada Y, Horigome N, et al. Embryology of the lamprey and evolution of the vertebrate jaw: insights from molecular and developmental perspectives. Philos Trans R Soc Lond B Biol Sci. 2001;356:15–32. doi: 10.1098/rstb.2001.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuratani S, Murakami Y, Nobusada Y, et al. Developmental fate of the mandibular mesoderm in the lamprey, Lethenteron japonicum: comparative morphology and development of the gnathostome jaw with special reference to the nature of trabecula cranii. J Exp Zool B Mol Dev Evol. 2004;302B:458–468. doi: 10.1002/jez.b.21011. [DOI] [PubMed] [Google Scholar]
- Langille RM, Hall BK. Role of the neural crest in development of the trabeculae and branchial arches in embryonic sea lamprey, Petromyzon marinus (L) Development. 1988;102:301–310. [Google Scholar]
- Le Douarin NM. The Neural Crest. Cambridge: Cambridge University Press; 1982. [Google Scholar]
- Le Lièvre CS. Participation of neural crest-derived cells in the genesis of the skull in birds. J Embryol Exp Morphol. 1978;47:17–37. [PubMed] [Google Scholar]
- Leach J. The hypophysis of lampreys in relation to the nasal apparatus. J Morphol. 1951;89:217–255. [Google Scholar]
- Lee SH, Bedard O, Buchtova M, et al. A new origin for the maxillary jaw. Dev Biol. 2004;276:207–224. doi: 10.1016/j.ydbio.2004.08.045. [DOI] [PubMed] [Google Scholar]
- Mallatt J. Early vertebrate evolution: pharyngeal structure and the origin of gnathostomes. J Zool. 1984;204:169–183. [Google Scholar]
- Marinelli W, Strenger A. Vergleichende Anatomie und Morphologie der Wirbeltiere. Vienna: Franz Deuticke; 1954. [Google Scholar]
- Martin WM, Bumm LA, McCauley DW. Development of the viscerocranial skeleton during embryogenesis of the sea lamprey, Petromyzon marinus. Dev Dyn. 2009;238:3126–3138. doi: 10.1002/dvdy.22164. [DOI] [PubMed] [Google Scholar]
- McBratney-Owen B, Iseki S, Bamforth SD, et al. Development and tissue origins of the mammalian cranial base. Dev Biol. 2008;322:121–132. doi: 10.1016/j.ydbio.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley DW, Bronner-Fraser M. Neural crest contributions to the lamprey head. Development. 2003;130:2317–2327. doi: 10.1242/dev.00451. [DOI] [PubMed] [Google Scholar]
- McCauley DW, Bronner-Fraser M. Importance of SoxE in neural crest development and the evolution of the pharynx. Nature. 2006;441:750–752. doi: 10.1038/nature04691. [DOI] [PubMed] [Google Scholar]
- Miller CT, Maves L, Kimmel CB. moz regulates Hox expression and pharyngeal segmental identity in zebrafish. Development. 2004;131:2443–2461. doi: 10.1242/dev.01134. [DOI] [PubMed] [Google Scholar]
- Moore WJ. The Mammalian Skull. London: Cambridge University Press; 1981. [Google Scholar]
- Neidert AH, Virupannavar V, Hooker GW, et al. Lamprey Dlx genes and early vertebrate evolution. Proc Natl Acad Sci U S A. 2001;98:1665–1670. doi: 10.1073/pnas.98.4.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newth DR. Experiments on the neural crest of the lamprey embryo. J Exp Biol. 1951;28:247–260. [Google Scholar]
- Newth DR. On the neural crest of the lamprey embryo. J Embryol Exp Morphol. 1956;4:358–375. [Google Scholar]
- Noden DM. The control of avian cephalic neural crest cytodifferentiation. I. Skeletal and connective tissues. Dev Biol. 1978;67:296–312. doi: 10.1016/0012-1606(78)90201-4. [DOI] [PubMed] [Google Scholar]
- Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983;96:144–165. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- Noden DM. Interactions and fates of avian craniofacial mesenchyme. Development. 1988;103(Suppl):121–140. doi: 10.1242/dev.103.Supplement.121. [DOI] [PubMed] [Google Scholar]
- Ohtani K, Yao T, Kobayashi M, et al. Expression of Sox and fibrillar collagen genes in lamprey larval chondrogenesis with implications for the evolution of vertebrate cartilage. J Exp Zool B Mol Dev Evol. 2008;310B:596–607. doi: 10.1002/jez.b.21231. [DOI] [PubMed] [Google Scholar]
- Olsson L, Hanken J. Cranial neural-crest migration and chondrogenic fate in the oriental fire-bellied toad Bombina orientalis: defining the ancestral pattern of head development in anuran amphibians. J Morphol. 1996;229:105–120. doi: 10.1002/(SICI)1097-4687(199607)229:1<105::AID-JMOR7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Parker WK. On the skeleton of Marsipobranch fishes. Part II. Petromyzon. Philos Trans R Soc Lond B Biol Sci. 1883;1883:411–457. [Google Scholar]
- Pasqualetti M, Ori M, Nardi I, et al. Ectopic Hoxa2 induction after neural crest migration results in homeosis of jaw elements in Xenopus. Development. 2000;127:5367–5378. doi: 10.1242/dev.127.24.5367. [DOI] [PubMed] [Google Scholar]
- Piotrowski T, Nüsslein-Volhart C. The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio. Dev Biol. 2000;225:339–356. doi: 10.1006/dbio.2000.9842. [DOI] [PubMed] [Google Scholar]
- Platt JB. Ectodermic origin of the cartilages of the head. Anat Anz. 1893;8:506–509. [Google Scholar]
- Portmann A. Einführung in die vergleichende Morphologie der Wirbeltiere. Basel: Schwabe & Co; 1969. [Google Scholar]
- Qiu M, Bulfone A, Ghattas I, et al. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: mutations of Dlx-1Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev Biol. 1997;185:165–184. doi: 10.1006/dbio.1997.8556. [DOI] [PubMed] [Google Scholar]
- Rathke H. 1827. Bemerkungen über den innern Bau des Qerders (Ammocoetes branchialis) und des kleinen Neunauges (Petromyzon planeri. Neueste Schriften der Naturf. Gesellsch. Danzog. Bd II.
- Rathke H. Entwickelungsgeschichte der Natter (Coluber natrix) Koenigsberg: Verlag der Gebrüder Bornträger; 1839. [Google Scholar]
- Reiss JO. Early development of chondrocranium in the tailed frog Ascaphus truei (Amphibia: Anura): implications for anuran palatoquadrate homologies. J Morphol. 1997;231:63–100. doi: 10.1002/(SICI)1097-4687(199701)231:1<63::AID-JMOR6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Rempel JG. The evolution of the insect head: the endless dispute. Q Entomol. 1975;11:7–25. [Google Scholar]
- Rijli FM, Mark M, Lakkaraju S, et al. Homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993;75:1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- Romer AS. Vertebrate Paleontology. Chicago: The University of Chicago Press; 1966. [Google Scholar]
- Romer AS, Parsons TS. The Vertebrate Body. 5th edn. Philadelphia: Saunders; 1977. [Google Scholar]
- Ruhin B, Creuzet S, Vincent C, et al. Patterning of the hyoid cartilage depends upon signals arising from the ventral foregut endoderm. Dev Dyn. 2003;228:239–246. doi: 10.1002/dvdy.10380. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Baratte B, Lepage M, et al. Characterization of Brachyury genes in the dogfish S. canicula and the lamprey L. fluviatilis Insights into gastrulation in a chondrichthyan. Dev Biol. 2003;263:296–307. doi: 10.1016/j.ydbio.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Scammon RE. Normal plates of the development of Squalus acanthias. In: Keibel F, editor. Normentafeln zur Entwicklungsgeschichte der Wirbeltiere. Vol. 12. Jena: Gustav Fischer; 1911. pp. 1–140. [Google Scholar]
- Scholtz G. Head segmentation in Crustacea – an immunocytochemical study. Zoology. 1995;98:104–114. [Google Scholar]
- Scott WB. On the development of the pituitary body in Petromyzon, and the significance of that organ in other types. Science. 1883;2:184–186. doi: 10.1126/science.ns-2.28.184. [DOI] [PubMed] [Google Scholar]
- Scott WB. On the development of Petromyzon. J Morphol. 1887;1:253–310. [Google Scholar]
- Seifert R, Jacob M, Jacob HJ. The avian prechordal head region: a morphological study. J Anat. 1993;183:75–89. [PMC free article] [PubMed] [Google Scholar]
- Sewertzoff AN. Morphologische Gesetzmässigkeiten der Evolution. Jena: Gustav Fischer; 1931. [Google Scholar]
- Shigetani Y, Nobusada Y, Kuratani S. Ectodermally-derived FGF8 defines the maxillomandibular region in the early chick embryo: epithelial–mesenchymal interactions in the specification of the craniofacial ectomesenchyme. Dev Biol. 2000;228:73–85. doi: 10.1006/dbio.2000.9932. [DOI] [PubMed] [Google Scholar]
- Shigetani Y, Sugahara F, Kawakami Y, et al. Heterotopic shift of epithelial–mesenchymal interactions in vertebrate jaw evolution. Science. 2002;296:1316–1319. doi: 10.1126/science.1068310. [DOI] [PubMed] [Google Scholar]
- Shigetani Y, Sugahara F, Kuratani S. Evolutionary scenario of the vertebrate jaw: the heterotopy theory from the perspectives of comparative and molecular embryology. BioEssays. 2005;27:331–338. doi: 10.1002/bies.20182. [DOI] [PubMed] [Google Scholar]
- Sjodal M, Gunhaga L. Expression patterns of ShhPtc2Raldh3Pitx2Isl1Lim3 and Pax6 in the developing chick hypophyseal placode and Rathke’s pouch. Gene Expr Patterns. 2008;8:481–485. doi: 10.1016/j.gep.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Stadmüller F. Nachtrag zu Band III. Mundöffnung, Lippen, Wangen, Vestibulum oris. In: Bolk L, Göppert E, Kallius E, Lubosch W, editors. Handbuch der vergleichenden Anatomie der Wirbeltiere, Band 5. Berlin: Urban & Schwarzenberg; 1938. pp. 895–1010. [Google Scholar]
- Stensiö EA. The Downtonian and Devonian vertebrates of Spitzbergen 1. Family Cephalaspidae. Skr Svalbard Ishavet. 1927;12:1–391. [Google Scholar]
- Sugahara F, Aota S, Kuraku S, et al. Involvement of Hedgehog and FGF signalling in the lamprey telencephalon: evolution of regionalization and dorsoventral patterning of the vertebrate forebrain. Development. 2011;138:1217–1226. doi: 10.1242/dev.059360. [DOI] [PubMed] [Google Scholar]
- Tahara Y. Normal stages of development in the lamprey, Lampetra reissneri (Dybowski) Zool Sci. 1988;5:109–118. [Google Scholar]
- Takio Y, Pasqualetti M, Kuraku S, et al. Lamprey Hox genes and the evolution of jaws. Nature OnLine. 2004;429 doi: 10.1038/nature02616. 1 p following 262. http://www. nature. com/cgi-taf/DynaPage. [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, et al. Formation of Rathke’s pouch requires dual induction from the diencephalon. Development. 1998;125:4835–4840. doi: 10.1242/dev.125.23.4835. [DOI] [PubMed] [Google Scholar]
- Tomsa J, Langeland JA. Otx expression during lamprey embryogenesis provides insights into the evolution of the vertebrate head and jaw. Dev Biol. 1999;207:26–37. doi: 10.1006/dbio.1998.9163. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Ariza-McNaughton L, Krumlauf R. Role of the isthmus and FGFs in resolving the paradox of neural crest plasticity and prepatterning. Science. 2002;295:1288–1291. doi: 10.1126/science.1064540. [DOI] [PubMed] [Google Scholar]
- Uchida K, Murakami Y, Kuraku S, et al. Development of the adenohypophysis in the lamprey: evolution of the epigenetic patterning programs in organogenesis. J Exp Zool B Mol Dev Evol. 2003;300B:32–47. doi: 10.1002/jez.b.44. [DOI] [PubMed] [Google Scholar]
- Wada N, Javidan Y, Nelson S, et al. Hedgehog signaling is required for cranial neural crest morphogenesis and chondrogenesis at the midline in the zebrafish skull. Development. 2005;132:3977–3988. doi: 10.1242/dev.01943. [DOI] [PubMed] [Google Scholar]
- Wada N, Nohno T, Kuratani S. Dual origins of the prechordal cranium in the chicken embryo. Dev Biol. 2011;356:529–540. doi: 10.1016/j.ydbio.2011.06.008. [DOI] [PubMed] [Google Scholar]
- van Wijhe JW. Über das Visceralskelett und die Nerven des Kopfes der Ganoiden und der Ceratodus. Arch Zool. 1882;5:207–320. [Google Scholar]
- Yao T, Ohtani K, Kuratani S, et al. Development of lamprey mucocartilage and its dorsal-ventral patterning by endothelin signaling, with insight into vertebrate jaw evolution. J Exp Zool B Mol Dev Evol. 2011;314B:339–346. doi: 10.1002/jez.b.21406. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Vivatbutsiri P, Morriss-Kay G, et al. Cell lineage in mammalian craniofacial mesenchyme. Mech Dev. 2008;125:797–808. doi: 10.1016/j.mod.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wang J, Ju BG, et al. Signaling and epigenetic regulation of pituitary development. Curr Opin Cell Biol. 2007;19:605–611. doi: 10.1016/j.ceb.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga E, Stellabotte F, Crump JG. Jagged-Notch signaling ensures dorsal skeletal identity in the vertebrate face. Development. 2010;137:1843–1852. doi: 10.1242/dev.049056. [DOI] [PMC free article] [PubMed] [Google Scholar]


