Abstract
Muscles of the vertebrate neck include the cucullaris and hypobranchials. Although a functional neck first evolved in the lobe-finned fishes (Sarcopterygii) with the separation of the pectoral/shoulder girdle from the skull, the neck muscles themselves have a much earlier origin among the vertebrates. For example, lampreys possess hypobranchial muscles, and may also possess the cucullaris. Recent research in chick has established that these two muscles groups have different origins, the hypobranchial muscles having a somitic origin but the cucullaris muscle deriving from anterior lateral plate mesoderm associated with somites 1–3. Additionally, the cucullaris utilizes genetic pathways more similar to the head than the trunk musculature. Although the latter results are from experiments in the chick, cucullaris homologues occur in a variety of more basal vertebrates such as the sharks and zebrafish. Data are urgently needed from these taxa to determine whether the cucullaris in these groups also derives from lateral plate mesoderm or from the anterior somites, and whether the former or the latter represent the basal vertebrate condition. Other lateral plate mesoderm derivatives include the appendicular skeleton (fins, limbs and supporting girdles). If the cucullaris is a definitive lateral plate-derived structure it may have evolved in conjunction with the shoulder/limb skeleton in vertebrates and thereby provided a greater degree of flexibility to the heads of predatory vertebrates.
Keywords: cucullaris, hypobranchial, lateral plate mesoderm, neck musculature, somites, vertebrates
Introduction
The evolution and development of the vertebrate neck have not attracted much interest over the last 150 years. There have been a multitude of studies on the development and evolution of the head and pectoral girdle but little on the region in between. In the last 30 years, however, advances in our understanding of developmental mechanisms, and an increased ability to study these with new techniques, has led to an awakened interest. The muscles of the head differ from those of the trunk not only in the contribution of cranial neural crest cells to the connective tissue and tendons, but also in the genetic pathways employed during development (Bismuth & Relaix, 2010; Kelly, 2010; Sambasivan et al. 2011). The muscles that connect the head and the pectoral girdle cross this important developmental boundary. Unfortunately, many gene expression studies on head and trunk muscle development lack data regarding the muscles of the neck. There are, however, numerous histological and cell-tracing studies describing the development of the neck muscles. For example, the developmental origin of the ventral hypobranchial muscles has been shown to be in the anterior somites in all vertebrates examined, but the dorsal cucullaris muscle has been described as derived either from paraxial cranial mesoderm or anterior somites (Fig. 1), usually varying with the techniques used and the organism (e.g. Edgeworth, 1911, 1935; Piatt, 1938; Noden, 1983b; Piekarski & Olsson, 2007). A recent study of chick, however, traced the origin of the cucullaris to the lateral plate mesoderm (Theis et al. 2010; Fig. 1). The same study also showed similarities in the developmental genetic pathway between the cucullaris and the head muscles rather than the trunk muscle pathway.
Fig. 1.
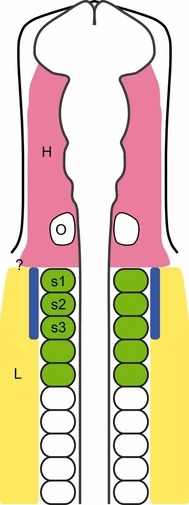
Diagram of stage 10 chick embryo. Pink represents the head mesoderm and yellow, lateral plate mesoderm. Somites in green contribute to the hypobranchial muscles (Couly et al. 1993). The blue area indicates the lateral plate mesoderm which gives rise to the cucullaris muscle and its derivatives according to Theis et al. (2010). H, head mesoderm; L lateral plate mesoderm; O, otic vesicle; s1–s3, somites one to three; ?, indicates the uncertainty of the border between the head and lateral plate mesoderm.
The evolutionary origin of the neck muscles occurs well before the appearance of a functional neck. While some of the muscles, like the hypobranchial muscles, appear in cyclostomes lacking a pectoral girdle (Kuratani, 2008; Kusakabe et al. 2011), others, like the cucullaris muscle, may have first appeared with the ventral and lateral separation of the pectoral girdle from the skull in fossil gnathostomes such as the placoderms (Figs 2 and 3; but see Kusakabe et al. 2011). Separation also occurs in chondrichthyans, due to the lack of a bony skeleton connecting the skull and girdle. In tetrapods, the functional neck involves the complete separation of the head from the girdle. However, this separation also occurs in the sarcopterygian fish relatives Tiktaalik roseae (Daeschler et al. 2006) and coelacanths (Millot & Anthony, 1958). Chondrichthyans possess a cucullaris musculature (Fig. 4) but, curiously, coelacanths do not (Millot & Anthony, 1958).
Fig. 3.
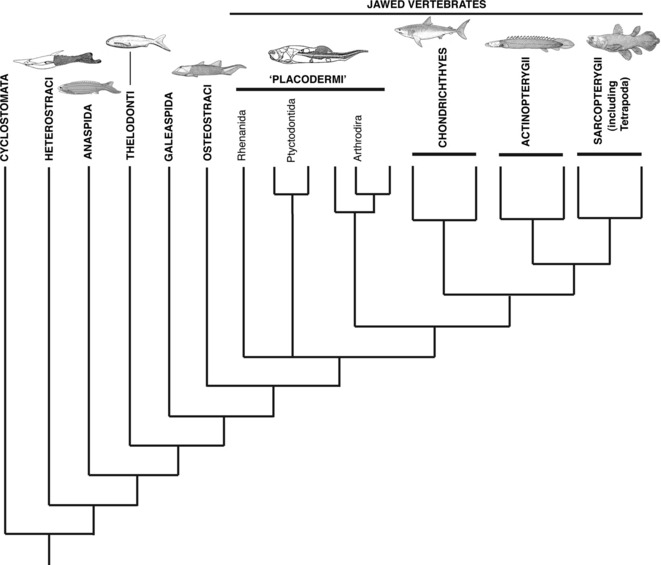
Phylogenetic relationships of the Vertebrata (adapted from Brazeau, 2009).
Fig. 4.
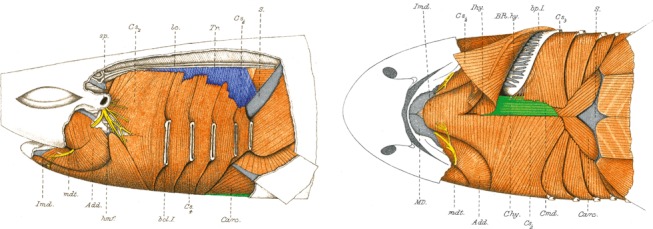
Muscles of the branchial and pectoral region of a shark, Scyllium canicula. The muscle in blue is derived from the cucullaris and the muscles in green are from the hypobranchial group (modified from Allis, 1917). Chy, coracohyoideus; Cmd, coracomandibularis; S, shoulder girdle; Tr, trapezius.
Fig. 2.

Eastmanosteus sp. (Placodermi; Australian Museum AMF82185). The headshield and trunkshield are ventrally and laterally separated. Originally, the shields were connected dorsally (see text) but due to damage, this region appears separated (modified from Johanson & Smith, 2003).
Although the development of neck muscles has been studied in amniotes and anamniotes, virtually nothing is known about the origins of, and gene pathways associated with, the neck muscles in more basal vertebrates such as chondrichthyans and osteichthyans [actinopterygian (ray-finned) fishes such as the zebrafish; sarcopterygian (lobe-finned) fishes including the lungfishes]. Although it would have been predicted that the cucullaris in these taxa was somite-derived, the work of Theis et al. (2010) questions these assumptions. This review will discuss the development and evolution of the vertebrate neck region, beginning with a summary of previous research regarding the development of the neck musculature, continuing with a description of the relevant gene pathways involved, and concluding with a discussion of neck musculature in the fossil record, and an attempt to place the results of Theis et al. (2010) in a wider context.
Neck musculature and associated skeleton
Neck muscle homology: cucullaris and hypobranchial
As mentioned earlier, the muscles that connect the head with the pectoral girdle can be divided into two main groups of muscles, the dorsal cucullaris and its derivatives, which attach to the back of the skull, the neck vertebrae and to the pectoral girdle (Edgeworth, 1935), and the ventral hypobranchial group (Figs 4–7). These comprise most of the muscles of the tongue and the muscles attaching the branchial or hyoid apparatus and their derivatives to the pectoral girdle (Edgeworth, 1935). The cucullaris muscle is present in all major clades of gnathostome vertebrates, with the exceptions of some Actinopterygii (Winterbottom, 1974; Greenwood & Lauder, 1981), caecilians (Edgeworth, 1935) and snakes (Edgeworth, 1935). Some batoids (skates and rays), sturgeons (Edgeworth, 1935) and coelacanths (Millot & Anthony, 1958) also lack the cucullaris muscle. It is primitively innervated by the ramus accessorius of the Vagus (X) nerve in anamniotes, whereas in amniotes the Accessorius (XI) nerve provides most of the nerve supply (Edgeworth, 1935). The cucullaris is generally considered to be homologous between vertebrates (Edgeworth, 1935; Greenwood & Lauder, 1981; Diogo & Abdala, 2010), based on development, innervation and anatomy; however, the nomenclature is highly variable. It has been described under many different names, e.g. as trapezius in shark (Vetter, 1874; Edgeworth, 1911; Allis, 1917), amphibians (Piatt, 1938) and chick (Noden & Francis-West, 2006), dorsoclavicularis in lungfish (Greil, 1913), protractor pectoralis in osteichthyan fish (Winterbottom, 1974; Greenwood & Lauder, 1981; Diogo & Abdala, 2010) and amphibians (Diogo & Abdala, 2010). In textbooks (e.g. Homberger & Walker, 2004; Kardong, 2009) and studies on cranial muscle development, the term ‘cucullaris’ is commonly used to describe the single muscle of anamniotes such as sharks (Edgeworth, 1935; Kuratani, 2008), amphibians (Edgeworth, 1935; Kesteven, 1944; Piekarski & Olsson, 2007) and the single muscle of amniotes prior to its developmental subdivision into the trapezius and sternocleidomastoideus complex, as in birds (Huang et al. 1997; Noden et al. 1999; Theis et al. 2010). For more extensive reviews on the nomenclature and homology of cranial muscles see Edgeworth (1935) and Diogo & Abdala (2010). In amniotes, the cucullaris commonly develops into two parts: the trapezius and sternocleidomastoideus complex, which may be subdivided further (Diogo & Abdala, 2010). The nomenclature of these muscles is also highly variable (Fürbringer, 1902; Edgeworth, 1935; Diogo & Abdala, 2010).
Fig. 7.
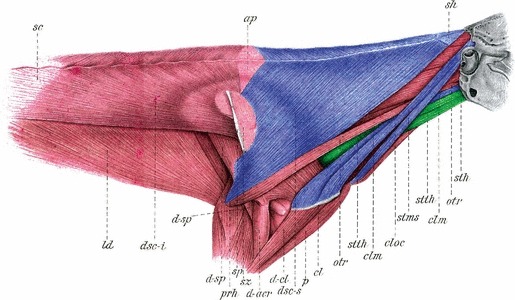
Anatomy of the neck and shoulder region of a rabbit. The muscles in blue are derived from the cucullaris and the muscles in green are from the hypobranchial group. Please note that although dsc-i, musculus dorsoscapularis inferior, is also considered to be part of the cucullaris group, there is currently no description of its development (modified from Streissler, 1900). clm, cleidomastoideus; cloc, cleidooccipitalis; dsc-s, dorsoscapularis superior; sh, tendon; stms, sternomastoideus superficialis; sth, sternohyoideus; stth, sternothyroideus.
Fig. 5.
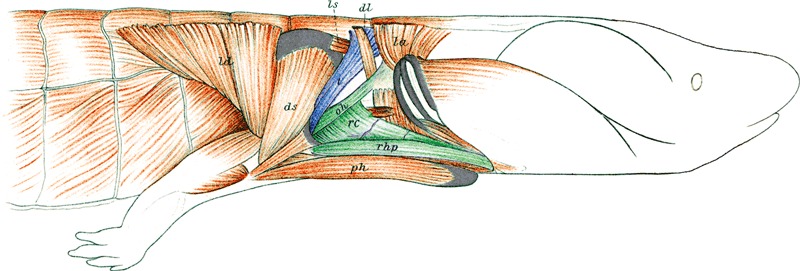
Muscles of the neck and pectoral region of a salamander, Necturus maculosus. The muscle in blue is derived from the cucullaris and the muscles in green are from the hypobranchial group (modified from Wilder, 1912). oh, omohyoideus; rc, rectus cervicis; rhp, rectus superficialis hypobranchialis posterior; t, trapezius.
The cucullaris muscle has traditionally been regarded as a part of the posterior branchial arch musculature of the head, based on the innervation and anatomy, e.g. Vetter’s comparative work on the anatomy of the jaw and branchial muscles of fish (Vetter, 1874). Unfortunately, very few early studies on muscle development mention the cucullaris. Greil (1913), in his extensive study of embryos of Australian lungfish (Neoceratodus forsteri), considered it to be derived from the second somite, whereas Edgeworth (1935) described it as derived from the posterior edge of the caudalmost branchial muscle plate in the same species. Piatt (1938) mentioned that apart from being derived from the caudalmost branchial levator muscle, the cucullaris in Ambystoma maculatum potentially also receives a contribution from ‘the dorsolateral spinal muscle primordium’, which would imply a partial somitic origin. McKenzie (1962) noted that in some mammals, the ‘deep’ part (relative to the position of the Accessorius nerve) of the sternomastoideus was likely to have a somitic contribution. This condition was observed in humans and pig but not in rabbit. Edgeworth (1911, 1935), on the other hand, considered the cucullaris to be derived from the caudalmost branchial arch mesoderm in all gnathostomes with exception of sharks, where it was derived from the dorsal edge of all of the five branchial muscle plates; this was contested by Allis (1917). Edgeworth also considered birds to lack a cucullaris, instead having a cranio-cervicalis muscle derived entirely from the first four somites rather than non-segmented cranial mesoderm. Although Edgeworth’s idea of a cranio-cervicalis muscle was not widely accepted, support for a somitic contribution to the cucullaris muscle in birds increased with the discovery of the heterochromatin condensations in quail cells by Le Douarin in 1969; and the quail-to-chick chimaera system for tracing embryonic cell fate. Noden traced both neural crest cells (1983a) and mesoderm (Noden, 1983b, 1986, 1988; Noden et al. 1999) in chicken, showing that the cucullaris received its muscle cells and connective tissue from somitic mesoderm. This was confirmed by an extensive study by Couly et al. (1993), showing that the first six somites provide cells to the cucullaris in chick, and was re-examined in studies by Huang et al. (1997, 2000), who mapped the origin of the cucullaris in chick to mainly the first three somites. Using Wnt1 and Sox10 constructs in mouse to drive expression of GFP in neural crest cells, Matsuoka et al. (2005) showed that the connective tissue of the trapezius and sternocleidomastoideus, and also the cartilage at the attachment points on the pectoral girdle, were derived from neural crest cells. Using another genetic construct, the myofibres were shown to be derived from HoxD4-expressing mesoderm and this was presumed to represent a somitic origin for these muscles. Valasek et al. (2010), also using a Wnt1 transgenic construct, confirmed the presence of neural crest cells in the connective tissue. In anamniotes, the somitic contribution to the cucullaris muscle was confirmed by Piekarski & Olsson (2007), using FITC-dextran injections in Ambystoma mexicanum embryos. In view of this large body of evidence suggesting a somitic origin of the cucullaris, the results from Theis et al. (2010) were surprising. They modified the somite transplantation technique used by Huang et al. (2000), and showed that the cucullaris muscle of chick was primarily derived from the occipital lateral plate mesoderm lateral to somites one to three (Fig. 1) and not from the somites. Added support came from molecular data showing that the development of the cucullaris muscle utilized the same genetic pathways as the other pharyngeal arch muscles (with the exception of the hypobranchial and extra-ocular muscles), rather than pathways associated with the trunk musculature.
In contrast to the cucullaris muscle and its derivatives, the study of the hypobranchial group of muscles has been less controversial. They are innervated by the spinal Hypobranchialis nerve in anamniotes and the Hypoglossus nerve (XII) in amniotes, and fall into two main groups, the rostral geniohyoideus and the caudal rectus cervicis muscles (Edgeworth, 1935). It should be noted that there are a number of other muscles in the same area but those are derived from unsegmented paraxial head mesoderm and are innervated by Trigeminus (V), Facialis (VII), Glossopharyngeus (IX), Vagus (X) or spinal nerves (Edgeworth, 1935). In gnathostomes, cells from the ventro-lateral part of a varying number of anterior somites migrate ventral to the pharyngeal arches in a structure called the hypoglossal chord (Fig. 8; Hunter, 1935). The geniohyoideus gives rise to most of the muscles of the tongue in tetrapods, whereas the rectus cervicis gives rise to muscles connecting the branchial and hyoid apparatus to the ventral pectoral girdle and sternum (Edgeworth, 1935). In the shark, geniohyoideus and rectus cervicis are derived from several of the more caudal somites and give rise to the geniocoracoideus (van Wijhe, 1882; Dohrn, 1884; Edgeworth, 1935; Allis, 1917 called this muscle coracomandibularis) and rectus cervicis (Edgeworth, 1935; Allis, 1917 called this muscle coracohyoideus; Diogo & Abdala, 2010 described it as sternohyoideus). The somitic contribution to these was confirmed in Australian lungfish (Greil, 1913; Edgeworth, 1935) and amphibians (Platt, 1897; Lewis, 1910; Edgeworth, 1935; Piatt, 1938; Martin & Harland, 2006; Piekarski & Olsson, 2007). However, most of the available data on the development of the hypobranchial muscles is, as in the case of the cucullaris, derived from studies of birds. Edgeworth (1935) considered that only one somite contributed cells to the hypobranchial muscles in chick, but most other studies list a varying number of somites. Hunter (1935) described the tongue muscles as derived from the first seven somites based on histology, which was partly confirmed by Deuchar’s (1958) study of the fate of the first three somites using carbon particles, and Hazelton’s (1970) study using tritium-labelled thymidine in the first four somites. Noden (1983b, 1986) found a contribution from somites two to five, using quail-chick chimaeras. Couly et al. (1993), using the same method, showed that the hypobranchial muscles were derived from the first five somites. Huang et al. (1999) re-examined the developmental fate of single somites in the neck region and found that somites two to six give rise to all the hypobranchial tongue muscles in chick. The connective tissue component of the rostral hypobranchial muscles was shown by Le Lièvre & Le Douarin (1975) to be neural crest cell-derived in chick. This was later confirmed and expanded by Noden (1983a) and Kontges & Lumsden (1996). The first description of neural crest cell contribution to the caudal attachment of the hypobranchial muscles to the pectoral girdle was provided by McGonnell et al. (2001), tracing a population of neural crest cells to the attachment of the cleidohyoid muscle (derived from rectus cervicis; Edgeworth, 1935) to the clavicle in chick. This was confirmed in all the hypobranchial muscles of the mouse by the study of Matsuoka et al. (2005). In summary, the major neck muscles are all connected by neural crest-derived connective tissues; the hypobranchial muscles are clearly somatic-derived and controversy exists whether the cucullaris anlagen is of somitic or cranial origin.
Fig. 8.
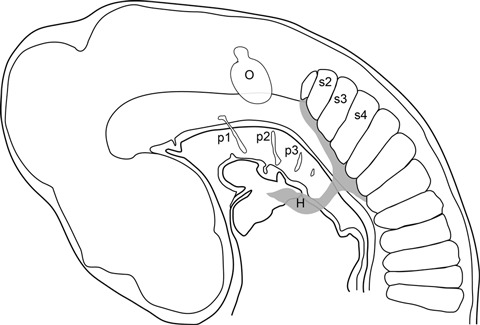
Diagram of stage 19 chick embryo, showing the cells migrating from the ventral somites into the hypoglossal chord (grey) and further anterior into the head region (modified from Hunter, 1935). H, hypoglossal chord; O, otic vesicle; p1–p3, pharyngeal pouches; s2–s4, somites two to four.
Neck musculature: skeletal attachments
The skeletal elements involved in the neck region include the skull or braincase, branchial arches, and the pectoral or shoulder girdle. The latter girdles have changed substantially through vertebrate evolution with a reduction in the dermally derived elements and increase in the endochondral structures, including the scapula and coracoid, which also change through evolution (Kardong, 2009). The dermally derived bones include the cleithrum, clavicle and interclavicle, as well as a series of small bones that connect the skull to the pectoral girdle in many fish: anocleithrum, supracleithrum, post-temporal. As dermal bones, these structures develop in the dermis via epithelial–mesenchymal interactions, whereas endochondral bones form from a mesodermally derived cartilaginous precursor. Neural crest contributes to most cranial dermal bones and postcranially, as far as is currently known, to the turtle plastron, alligator gastralia and zebrafish dermal caudal fin rays (Couly et al. 1993; Smith et al. 1994; Gilbert et al. 2007). However, given the predominance of dermal bone in the head and trunk skeletons of early fossil vertebrates, Smith & Hall (1990, 1993) suggested that neural crest also contributed to these bony skeletons. Dermal and endochondral ossifications were considered developmentally separate until work by Matsuoka et al. (2005) which indicated that the mouse scapula had a dual origin, based on the attached musculature and, more specifically, the muscle connective tissue. In this study, it was observed that most of the scapula was mesodermally derived but neural crest-derived muscle connective tissue attached to the scapular spine, and the anterior part of the spine itself was said to be neural crest-derived, rather than from mesoderm. Thus it is muscle connectivity that determined developmental origin of these bones. However, this was questioned in a subsequent paper by Valasek et al. (2010: p. 487), who observed that neural crest cells were only ‘scattered on the surface’ of the scapular spine. Other researchers have demonstrated that the dorsal part of the tetrapod scapula is derived from anterior somites rather than lateral plate mesoderm, including the region where the cucullaris muscle would attach (summarized by Piekarski & Olsson, 2011; Fig. 6; Shearman et al. 2011).
Fig. 6.
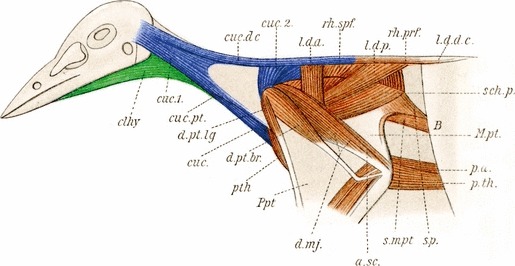
Muscles of the neck and pectoral region of a bird, Cyanocorax cyanopogon. The muscles in blue are derived from the cucullaris and the muscles in green are from the hypobranchial group (modified from Fürbringer, 1902). clhy, cleidohyoideus; cuc, cucullaris and sternocleidomastoideus; cuc.1, cranial part of cucullaris; cuc.2, cervical part of cucullaris; cuc.dc, cucullaris dorsocutaneus; cuc.pt, cucullaris propatagialis.
Genetic pathways in neck muscle development
Genetic differences in head vs. trunk muscle development
As noted above, Theis et al. (2010) showed that the cucullaris muscle followed a cranial rather than trunk developmental program. However, these results were primarily from the chick and so it still needs to be determined whether these findings apply to gnathostomes as a whole. Cranial mesoderm is defined as the mesoderm anterior to the somites and will give rise to all the muscles of the head (with the exception of the hypobranchial muscles) as well as the basal occipital part of the skull (Noden, 1983a; Couly et al. 1992). Analyses of early cranial mesoderm development in the chick have revealed three stages of patterning (Bothe et al. 2011). These involve an early demarcation of anterior and posterior cranial mesoderm by the expression of the transcription factors Pitx2 vs. Tbx1, respectively, which then become refined under the control of Fgf and Retinoic acid signalling. Later, Pitx2 expression becomes restricted to eye and mandibular arch muscle progenitors, whereas Tbx1 is then co-expressed with the bHLH factor MyoR in all pharyngeal arch muscle precursors. This expression of Pitx2, Tbx1 and MyoR in distinct subsets of head muscles is conserved in mouse and likely reflects an ancient patterning mechanism (Chapman et al. 1996; Gage et al. 1999; Kitamura et al. 1999; Lu et al. 2002). A loss of Pitx2 function in mouse reflects its early expression in anterior cranial mesoderm: eye and mandibular arch muscles are lost or severely hypoplastic, but hyoid and branchial arch muscles are mostly unaffected (Dong et al. 2006). Mice lacking Tbx1 function show a number of craniofacial abnormalities such as losses of posterior pharyngeal arch-derived muscles (Lindsay et al. 2001). A conditional loss of Tbx1 function in the mesoderm using the Mesp1:Cre line causes a loss of the branchial and hyoid arch-derived muscles but does not severely affect the mandibular arch muscles (Zhang et al. 2006; Dastjerdi et al. 2007). A loss of function of both MyoR and the related Tcf21 gene result in a loss of several mandibular arch muscles but was not reported to overtly affect hyoid or branchial arch-derived muscles (Lu et al. 2002). Interestingly, the limited muscle phenotypes observed in Tbx1 and MyoR/Tcf21 mutant mice do not correlate with the wider expression of Tbx1 and MyoR/Capsulin in all pharyngeal arch muscle precursors. This implies that molecular differences between the pharyngeal arch muscles revealed by loss of gene function, reflects a more complicated regulation of muscle differentiation than that suggested by the overlapping expression patterns observed. We refer readers to an excellent review detailing the genes important for head muscle development (Sambasivan et al. 2011) but note that this is principally based on studies in amniotes and so does not necessarily hold for more basal vertebrates.
Myogenic differentiation in the pharyngeal arch muscles is regulated by Pitx2 and Tbx1; these two transcription factors act to regulate expression of the myogenic regulatory factors Myf5 and Myod (Kelly et al. 2004; Sambasivan et al. 2009). In contrast, Myf5 and Myod expression in the somitic mesoderm are regulated by Pax3 (Tajbakhsh et al. 1997). Mice lacking Pax3 function show a loss of several trunk and limb muscles, but head muscles are unaffected (Bober et al. 1994; Goulding et al. 1994; Relaix et al. 2005). Pax3:Myf5 double mutant mice lack all somitic-derived muscles, including some tongue and infrahyoid muscles, but pharyngeal arch muscles and the trapezius and sternocleidomastoid muscles are still present (Tajbakhsh et al. 1997). This suggests that some of the neck muscles are not developing under the myogenic program functioning in the somites but instead are following a head muscle program. Similar evidence for a head muscle program operating in some neck muscles is observed in Tbx1 mutant mice. Posterior pharyngeal arch-derived muscles are absent, as are the trapezius and sternocleidomastoid muscles (Kelly et al. 2004; Theis et al. 2010). In contrast, these mice have been reported to not have any muscle defects in somite-derived muscles of the limb (Grifone et al. 2008). Human patients with DiGeorge syndrome (also called 22q deletion syndrome and velocardiofacial syndrome), have point mutations in the TBX1 gene and show many similar features to those observed in the mouse Tbx1 mutants (Lindsay et al. 2001; Scambler, 2010). Intriguingly, these patients also display sloping shoulders due to small shoulder and pectoral muscles, suggesting that TBX1 is also important for the development of these somitic-derived muscles in humans. It will be necessary to assess the role of Tbx1 in muscle development of other species to determine whether Tbx1 function is differentially functioning in cranial mesoderm and somitic-derived neck and shoulder muscles.
One matter of controversy relating to the origins of distinct muscles at the neck region is where the exact boundary between the head and somitic mesoderm is defined. At the paraxial level, a clear boundary becomes defined between the somitic and cranial mesoderm at the level of the first somite. However, several genes expressed in the somites show a tapering expression anteriorly into the most posterior cranial mesoderm prior to somitogenesis, including Paraxis and Pax3 (Bothe et al. 2011). Likewise, several genes expressed in the posterior cranial mesoderm, including Alx4 and Twist, show a graded expression posteriorly into the anterior somites. This is not seen for other genes such as Myf5 and Myod, which show a sharp anterior boundary of expression in the first somite. One implication of this is that the molecular graduation between cranial and somitic mesoderm is not strictly demarcated by patterning genes, but the process of somitogenesis and regulation of myogenesis is differentially regulated between the two tissues. This may then limit the relative contributions of cells at the boundary to somitic vs. cranial mesodermally derived tissues.
Other genes show a more lateral expression at the posterior extent of the cranial mesoderm: these include Isl1 and Nkx2.5 (Bothe & Dietrich, 2006). Fate mapping of these lateral cells expressing Isl1 in the mouse has revealed that they contribute to both pharyngeal arch muscles and to the outflow tract of the heart and the right ventricle (Nathan et al. 2008). Similarly, the trapezius and sternocleidomastoid receive cells from this Isl1-expressing lineage, providing further evidence that these muscles are following a head muscle program (Theis et al. 2010). The location of boundary in the lateral mesoderm between the most posterior cranial and most anterior trunk cells is not well defined. The posterior boundary of Isl1 and Nkx2.5 occurs at the level of somite 2 and implies that the transition zone between head and trunk in the lateral plate may occur at a different axial level to that of the somites. This has ramifications for determining whether a muscle is derived from cranial or trunk mesoderm, as many of the markers used to demarcate mesoderm in the trunk do not show similar boundaries in the lateral plate and paraxial mesoderm (Burke, 2007). These include HoxD4 and Pax3, which have been primarily used to determine which cells are derived from the somites (Matsuoka et al. 2005; Theis et al. 2010). It will be important to define at which axial level, lateral plate possesses a cranial identity and can potentially contribute to head muscles; variations to this boundary may explain the perceived differences in trunk vs. cranial mesoderm contributions to the cucullaris that have been described for various fate maps from frogs and chicks (Huang et al. 2000; Piekarski & Olsson, 2007).
Evidence for a neck and associated musculature in the fossil record
The early phylogenetic history of vertebrates is dominated by fossil jawless forms (Donoghue et al. 2006). Living jawless vertebrates include the lampreys and hagfish, currently assigned to the Cyclostomata (Delarbre et al. 2002; Heimberg et al. 2010). Fossil groups include such taxa as the Heterostraci, Anaspida, Thelodonti, Galaeaspida and Osteostraci (Janvier, 1996). Of these groups, only the Osteostraci have been shown to have a pectoral fin supported by an internal skeleton that articulates to a pectoral girdle (Janvier, 1985; Janvier et al. 2004). However, this region forms part of the extensive bony headshield of the Osteostraci, so that there is no separation of the pectoral fin and girdle from the head region (Fig. 9). Besides this, the scapulocoracoid is positioned within an embayment of the headshield and is isolated from the braincase by the postbranchial wall; there was no space for connecting musculature between these, including the cucullaris (Janvier, 1985).
Fig. 9.
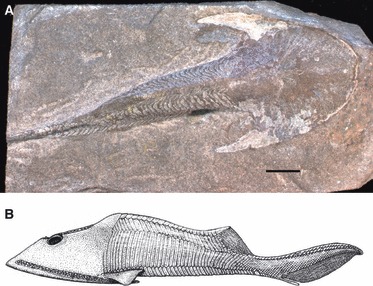
Osteostraci (A) Cephalaspis lyelli (NHM UK OR 20087). The headshield is fused with the pectoral girdle and no neck region can be observed. (B) Reconstruction of a zenaspidid osteostracan (modified from Janvier, 1996).
The first indication of a separation of the skull and pectoral girdle is in the phylogenetically basal jawed vertebrates, the Placodermi (Fig. 4). In this group, there are two points of connection between the skull/braincase and bones homologous to the pectoral girdle of more derived groups (e.g. Osteichthyes). The braincase articulates to the anterior vertebral column, while the skull or headshield also articulates at two lateral points to the anterior bones of the trunkshield (homologized to the osteichthyan cleithrum; Zhu & Schultze, 2001).
The placoderms represent a crucial group in vertebrate phylogeny because they effectively bridge the transition between jawless and jawed vertebrates. Moreover, the Placodermi have recently been resolved as a non-monophyletic group (Brazeau, 2009). As such, the various placoderm taxa are resolved to nodes along the basal jawed vertebrate phylogeny, allowing character evolution to be more finely delineated, node by node. The anterior face of the placoderm trunkshield shows various embayments or discrete areas ventrally which were suggested to be for the attachment of branchial arch musculature, but only in more derived placoderms (Johanson, 2003). In more basal taxa such as the Rhenanida, the entire anterior margin of the trunkshield is covered in dermal denticles, with no smoother surfaces present for muscle attachment (Ørvig, 1975; Johanson & Smith, 2003). Comparable distinct or discrete areas were not observed along the dorsal margin of the trunkshield in any placoderm taxa (Johanson, 2003). Despite this, several placoderm taxa show large lateral embayments in the braincase, identified as attachment areas for a cucullaris musculature (e.g. Anderson, 2008). If this identification is correct, then one would expect the cucullaris to attach to the dorsal trunkshield, at least in more derived placoderms where dermal denticles are largely absent from this area.
Therefore, it is not entirely certain when, phylogenetically, a cucullaris musculature evolved in early vertebrates, or, in the context of the results of Theis et al. (2010), whether these muscles derived from somites or lateral plate mesoderm. This is also important with respect to the timing of the evolution of the lateral plate mesoderm and its association with the appendicular skeleton (fins and supporting girdles). Lampreys show an intriguing combination of putative somite-derived cucullaris homologues (infraoptic muscles; Kusakabe et al. 2011) and lateral plate mesoderm which has not separated into splanchnic and somatic layers (Onimaru et al. 2010). The somatic layer contributes to fin/limb development in jawed vertebrates. Lampreys lack paired fins, and this lack of mesodermal separation may have also affected development of a cucullaris muscle from the lateral plate mesoderm.
The homology of the infraoptic muscles to the cucullaris was based on the position of the muscle (Kusakabe et al. 2011), but a postcranial attachment skeleton is absent. In early vertebrates that possess this skeleton, cucullaris muscles were suggested above to be absent due to incorporation of the head and pectoral girdle in a massive bony shield in the Osteostraci, and lack of muscle attachment surfaces in the most basal placoderms. Fins and supporting girdles are generally believed to derive from the lateral plate mesoderm, which would contradict suggestions above that the evolution of a lateral plate mesoderm-derived cucullaris musculature could have been coincident with the evolution of these other lateral plate mesoderm-derived structures early in vertebrate evolution. At this point in early vertebrate evolution (more derived placoderms and other jawed vertebrates), the cucullaris could have been somite-derived (as in lampreys, Kusakabe et al. 2011) or derived from lateral plate mesoderm (following results of Theis et al. 2010). Data from living fishes are urgently needed in this regard in order to close the wide phylogenetic gap between amniote and anamniote tetrapods, and lampreys. One interesting corollary of this discussion is that fin- and girdle-producing (and cucullaris-producing) lateral plate mesoderm may have been absent not only in lampreys but also in osteostracans and basal placoderms. Most tetrapod shoulder girdles have a somitic component dorsally (Piekarski & Olsson, 2011) and it is possible that the osteostracan and basal placoderm pectoral fin/girdle derived entirely from somitic tissues (cf. Shearman & Burke, 2009).
A fully functional neck, involving the separation of the skull from the shoulder girdle, is often considered a character of land-living tetrapods. In most fish relatives of the tetrapods, the skull and girdle are connected by a series of dermal bones (Fig. 10). However, these bones are absent in chondrichthyans, and a complete series is absent in coelacanths, resulting in the separation of the skull from the pectoral girdle (Forey, 1998). Although cucullaris muscles are present in chondrichthyans, they are absent in the living coelacanth Latimeria, with muscles to the anterodorsal parts of the dermal pectoral girdle attaching to the branchial arches alone (Millot & Anthony, 1958; Forey, 1998). Coelacanths belong to the group Sarcopterygii, which also includes the Dipnoi (cucullaris present; protractor pectoralis, Diogo & Abdala, 2010) and Tetrapodomorpha, the most closely related fish to the Tetrapoda. These taxa have a full series of bones connecting the skull to the girdle, but in the recently discovered Tiktaalik roseae (Daeschler et al. 2006), the skull and girdle have become separated. Tiktaalik retains the bones that connect the skull to the girdle in other sarcopterygians, but has lost the bones at the rear of the skull, as well as the operculum and suboperculum covering the gill arches. These bones are also absent in the first digited tetrapods from the Devonian, e.g. Acanthostega (Daeschler et al. 2006), but are present in coelacanths (Forey, 1998). We presume that the cucullaris was present in Tiktaalik and the Devonian tetrapods, but the absence of the cucullaris in coelacanths coupled with the separation of the skull and pectoral girdle is puzzling.
Fig. 10.
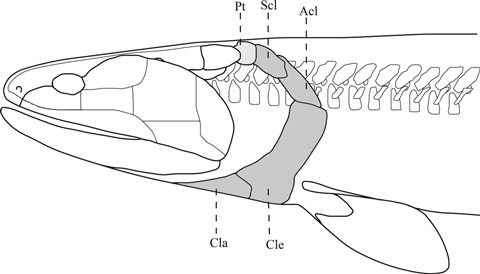
Lateral view of the head and pectoral girdle of Eusthenopteron foordi. Opercular bones have been removed to emphasize the ventral and lateral separation of the pectoral girdle from the head. Modified from Andrews & Westoll (970). Reproduced by permission of The Royal Society of Edinburgh from Transactions of the Royal Society of Edinburgh volume 68 (1970), pp. 207–329. Acl, anocleithrum; Cla, clavicle; cle, cleithrum; Pt, post-temporal bone; Scl, supracleithrum.
Concluding remarks
The comparative anatomists of the 19th century listed the cucullaris as part of the head musculature, based mainly on the anatomy and innervation pattern (e.g. Vetter, 1874). This was further supported by the embryologists using histological methods in the first half of the 20th century (Edgeworth, 1911, 1935; Greil, 1913; Piatt, 1938). Experiments using cell-tracing in chick at the end of the 20th century, however, concluded that its origin lay in the anterior somites (Noden, 1983b, 1986; Couly et al. 1992; Huang et al. 1997, 2000). With the study of Theis et al. (2010), the pendulum has swung back, such that the cucullaris can again be considered part of the head musculature, at least in chick and, based on genetic evidence, in mouse. While the study of Theis et al. (2010) provided new answers to questions of head and muscle development it also raised several new ones that should be the focus of future research. For example, where is the border between the head and the trunk mesoderm in the somites and lateral plate mesoderm? The genetic network used by the developing cucullaris muscle indicates that at least the lateral plate mesoderm lateral to the first three somites in chick could be considered to be part of the head mesoderm (Theis et al. 2010). Is this the same in all vertebrate species, including the cyclostome lamprey? Most older studies on cucullaris development only mention one mesodermal source (reviewed by Edgeworth, 1935), but studies of salamander (Piatt, 1938) and mammals (McKenzie, 1962) indicate a possible dual contribution of somitic and head mesoderm to the cucullaris. At the time of writing there is no other study of cell migration and differentiation comparable to that of Theis et al. (2010). Despite being formed largely from occipital lateral plate mesoderm, Theis et al. (2010) showed a ∼ 10% contribution of somitic cells to the cucullaris in chick. Do these somitic cells acquire the head muscle genetic differentiation pathway or do they keep their original trunk muscle system? This combination of somite and lateral plate mesoderm is also reflected in the contribution of these to the shoulder girdle of all major groups of tetrapods, influenced by the position of the girdle relative to the lateral somitic frontier (reviewed in Piekarski & Olsson, 2011; Shearman & Burke, 2009). Is the contribution of somite and lateral plate mesoderm to the cucullaris muscle also related to the lateral somitic frontier? Future research on the cucullaris should follow the work of Matsuoka et al. (2005) in considering both the muscle and its skeletal attachments.
As noted, the discovery of the contribution of lateral plate mesoderm to the cucullaris (and pectoral/shoulder girdle) requires corroboration from taxa other than birds, particularly fish and more phylogenetically basal tetrapods. Fossil vertebrates can also be investigated by searching for evidence of muscle attachment on parts of the shoulder girdle where the hypobranchials and cucullaris attach in extant vertebrates (e.g. Johanson, 2003). Exciting new research is finding evidence of preserved muscles in Devonian placoderms (370 million years old; Ahlberg et al. 2009), although not yet in the neck region. A substantial segment of vertebrate phylogeny comprises the jawless vertebrates; taxa that either lack strong evidence for pectoral fins and girdles (Johanson, 2010), or have girdles and fins continuous with the headshield, with no apparent space for a neck musculature. Future research should focus on the developmental issues described above, but also on these phylogenetically important fossil taxa.
Acknowledgments
We would like to thank the Editors inviting us to contribute to this special issue of Journal of Anatomy and the two anonymous reviewers. Rolf Ericsson is supported by a Marie Curie International Incoming Fellowship. We would also like to thank the Palaeontology Department at the Natural History Museum, London, for funding.
References
- Ahlberg PE, Trinajstic K, Long JA. The body musculature of arthrodire placoderms. Soc Vert Paleo Prog Abst. 2009;29:52A. [Google Scholar]
- Allis EP. The homologies of the muscles related to the visceral arches of the gnathostome fishes. Q J Microsc Sci. 1917;62:308–406. [Google Scholar]
- Anderson PSL. Cranial muscle homology across modern gnathostomes. Biol J Linn Soc. 2008;94:195–216. [Google Scholar]
- Bismuth K, Relaix F. Genetic regulation of skeletal muscle development. Exp Cell Res. 2010;316:3081–3086. doi: 10.1016/j.yexcr.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Bober E, Franz T, Arnold HH, et al. Pax-3 is required for the development of limb muscles: a possible role for the migration of dermomyotomal muscle progenitor cells. Development. 1994;120:603–612. doi: 10.1242/dev.120.3.603. [DOI] [PubMed] [Google Scholar]
- Bothe I, Dietrich S. The molecular setup of the avian head mesoderm and its implication for craniofacial myogenesis. Dev Dyn. 2006;235:2845–2860. doi: 10.1002/dvdy.20903. [DOI] [PubMed] [Google Scholar]
- Bothe I, Tenin G, Oseni A, et al. Dynamic control of head mesoderm patterning. Development. 2011;138:2807–2821. doi: 10.1242/dev.062737. [DOI] [PubMed] [Google Scholar]
- Brazeau M. The braincase and jaws of a Devonian ‘acanthodian’ and modern gnathostome origins. Nature. 2009;457:305–308. doi: 10.1038/nature07436. [DOI] [PubMed] [Google Scholar]
- Burke AC. Development and evolution of the vertebrate mesoderm. Dev Dyn. 2007;236:2369–2370. doi: 10.1002/dvdy.21290. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Garvey N, Hancock S, et al. Expression of the T-box family genes, Tbx1-Tbx5, during early mouse development. Dev Dyn. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- Daeschler E, Shubin N, Jenkins F. A Devonian tetrapod-like fish and the evolution of the tetrapod body plan. Nature. 2006;440:757–763. doi: 10.1038/nature04639. [DOI] [PubMed] [Google Scholar]
- Dastjerdi A, Robson L, Walker R, et al. Tbx1 regulation of myogenic differentiation in the limb and cranial mesoderm. Dev Dyn. 2007;236:353–363. doi: 10.1002/dvdy.21010. [DOI] [PubMed] [Google Scholar]
- Delarbre C, Gallut C, Barriel V, et al. Complete mitochondrial DNA of the hagfish, Eptatretus burgeri: the comparative analysis of mitochondrial DNA sequences strongly supports the cyclostome monophyly. Mol Phylogenet Evol. 2002;22:184–192. doi: 10.1006/mpev.2001.1045. [DOI] [PubMed] [Google Scholar]
- Deuchar EM. Experimental demonstration of the tongue muscle origin in chick embryos. J Embryol Exp Morphol. 1958;6:527–529. [PubMed] [Google Scholar]
- Diogo R, Abdala V. Muscles of Vertebrates - Comparative Anatomy, Evolution, Homologies and Development. Enfield, New Hampshire: Science Publishers; 2010. [Google Scholar]
- Dohrn A. Studien zur Urgeschichte des Wirbelthierkörpers. Mittheil Zool Stat Neapel. 1884;5:102–195. [Google Scholar]
- Dong F, Sun X, Liu W, et al. Pitx2 promotes development of splanchnic mesoderm-derived branchiomeric muscle. Development. 2006;133:4891–4899. doi: 10.1242/dev.02693. [DOI] [PubMed] [Google Scholar]
- Donoghue P, Sansom I, Downs J. Early evolution of vertebrate skeletal tissues and cellular interactions, and the canalization of skeletal development. J Exp Zool B Mol Dev Evol. 2006;306B:278–294. doi: 10.1002/jez.b.21090. [DOI] [PubMed] [Google Scholar]
- Edgeworth FH. On the morphology of the cranial muscles in some vertebrates. Q J Microsc Sci. 1911;56:167–316. [Google Scholar]
- Edgeworth FH. The Cranial Muscles of Vertebrates. Cambridge: University Press; 1935. [Google Scholar]
- Forey P. History of the Coelacanth Fishes. London: Chapman & Hall; 1998. [Google Scholar]
- Fürbringer M. Zur vergleichenden Anatomie des Brustschulterapparates und der Schultermusklen. Jena Zeit Med Naturwiss. 1902;36:289–736. [Google Scholar]
- Gage PJ, Suh H, Camper SA. Dosage requirement of Pitx2 for development of multiple organs. Development. 1999;126:4643–4651. doi: 10.1242/dev.126.20.4643. [DOI] [PubMed] [Google Scholar]
- Gilbert S, Bender G, Betters E, et al. The contribution of neural crest cells to the nuchal bone and plastron of the turtle shell. Int Comp Biol. 2007;47:401–408. doi: 10.1093/icb/icm020. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ. Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development. 1994;120:957–991. doi: 10.1242/dev.120.4.957. [DOI] [PubMed] [Google Scholar]
- Greenwood PH, Lauder GV. The protractor pectoralis muscle and the classification of teleost fishes. Bull Br Mus Nat Hist (Zool) 1981;41:213–234. [Google Scholar]
- Greil A. Entwicklungsgeschichte des Kopfes und des Blutgefässsystemes von Ceratodus forsteri II. Die epigenetischen Erwerbungen während der Stadien 39–48. Denkschr Med-Naturwiss Ges Jena. 1913;4:935–1492. [Google Scholar]
- Grifone R, Jarry T, Dandonneau M, et al. Properties of branchiomeric and somite-derived muscle development in Tbx1 mutant embryos. Dev Dyn. 2008;237:3071–3078. doi: 10.1002/dvdy.21718. [DOI] [PubMed] [Google Scholar]
- Hazelton RD. A radioautographic analysis of the migration and fate of cells derived from the occipital somites in the chick embryo with specific reference to the development of the hypoglossal musculature. J Embryol Exp Morphol. 1970;24:455–466. [PubMed] [Google Scholar]
- Heimberg AM, Cowper-Sallari R, Sémon M, et al. microRNAs reveal the interrelationships of hagfish, lampreys, and gnathostomes and the nature of the ancestral vertebrate. Proc Natl Acad Sci U S A. 2010;107:19379–19383. doi: 10.1073/pnas.1010350107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberger DG, Walker WF. Vertebrate Dissection. 9th edn. Belmont: Thomson-Brooks/Cole; 2004. [Google Scholar]
- Huang RJ, Zhi QX, Ordahl CP, et al. The fate of the first avian somite. Anat Embryol. 1997;195:435–449. doi: 10.1007/s004290050063. [DOI] [PubMed] [Google Scholar]
- Huang RJ, Zhi QX, Izpisua-Belmonte JC, et al. Origin and development of the avian tongue muscles. Anat Embryol. 1999;200:137–152. doi: 10.1007/s004290050268. [DOI] [PubMed] [Google Scholar]
- Huang RJ, Zhi QX, Patel K, et al. Contribution of single somites to the skeleton and muscles of the occipital and cervical regions in avian embryos. Anat Embryol. 2000;202:375–383. doi: 10.1007/s004290000131. [DOI] [PubMed] [Google Scholar]
- Hunter RP. The early development of the hypoglossal musculature in the chick. J Morph. 1935;57:473–498. [Google Scholar]
- Janvier P. Les Céphalaspides du Spitsberg. Paris: Centre national de la Recherche scientifique; 1985. [Google Scholar]
- Janvier P. Early Vertebrates. Oxford: Oxford University Press; 1996. [Google Scholar]
- Janvier P, Arsenault M, Desbiens S. Calcified cartilage in the paired fins of the osteostracan Escuminaspis laticeps (Traquair 1880), from the Late Devonian of Miguasha (Quebec, Canada), with a consideration of the early evolution of the pectoral fin endoskeleton in vertebrates. J Vert Paleo. 2004;24:773–779. [Google Scholar]
- Johanson Z. The clavobranchialis musculature in sarcopterygian fishes, and contribution to osteichthyan feeding and respiration. Contrib Zool. 2003;72:17–37. [Google Scholar]
- Johanson Z. Evolution of paired fins and the lateral somitic frontier. J Exp Zool B Mol Dev Evol. 2010;314:347–352. doi: 10.1002/jez.b.21343. [DOI] [PubMed] [Google Scholar]
- Johanson Z, Smith MM. Placoderm fishes, pharyngeal denticles and the vertebrate dentition. J Morph. 2003;257:289–307. doi: 10.1002/jmor.10124. [DOI] [PubMed] [Google Scholar]
- Kardong KV. Vertebrates: Comparative Anatomy, Function, Evolution. Boston: McGraw-Hill Higher Education; 2009. [Google Scholar]
- Kelly R. Core issues in craniofacial myogenesis. Exp Cell Res. 2010;316:3034–3041. doi: 10.1016/j.yexcr.2010.04.029. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Jerome-Majewska LA, Papaioannou VE. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Hum Mol Genet. 2004;13:2829–2840. doi: 10.1093/hmg/ddh304. [DOI] [PubMed] [Google Scholar]
- Kesteven LH. The Evolution of the Skull and the Cephalic Muscles: A Comparative Study of their Development and Adult Morphology. Part II. The Amphibia. Sydney: Australian Museum; 1944. [Google Scholar]
- Kitamura K, Miura H, Miyagawa-Tomita S, et al. Mouse Pitx2 deficiency leads to anomalies of the ventral body wall, heart, extra- and periocular mesoderm and right pulmonary isomerism. Development. 1999;126:5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- Kontges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Kuratani S. Evolutionary developmental studies of cyclostomes and the origin of the vertebrate neck. Dev Growth Diff. 2008;50:S189–S194. doi: 10.1111/j.1440-169X.2008.00985.x. [DOI] [PubMed] [Google Scholar]
- Kusakabe R, Kuraku S, Kuratani S. Expression and interaction of muscle-related genes in the lamprey imply the evolutionary scenario for vertebrate skeletal muscle, in association with the acquisition of the neck and fins. Dev Biol. 2011;350:217–227. doi: 10.1016/j.ydbio.2010.10.029. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. Particularités du noyau interphasique chez la Caille japonaise (Coturnix coturnix japonica). Utilisation de ces particularités comme ‘marquage biologique’ dans les recherches sur les interactions tissulaires et les migrations cellulaires au cours de l’ontogenese. Bull Biol France Belg. 1969;103:435–452. [PubMed] [Google Scholar]
- Le Lièvre CS, Le Douarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Lewis WH. The relation of the myotomes to the ventro-lateral musculature and to the anterior limbs in Amblystoma. Anat Rec. 1910;4:183–190. [Google Scholar]
- Lindsay EA, Vitelli F, Su H, et al. Tbx1 haploinsufficiency in the DiGeorge syndrome region causes aortic arch defects in mice. Nature. 2001;410:97–101. doi: 10.1038/35065105. [DOI] [PubMed] [Google Scholar]
- Lu JR, Bassel-Duby R, Hawkins A, et al. Control of facial muscle development by MyoR and capsulin. Science. 2002;298:2378–2381. doi: 10.1126/science.1078273. [DOI] [PubMed] [Google Scholar]
- Martin B, Harland R. A novel role for lbx1 in Xenopus hypaxial myogenesis. Development. 2006;133:195–208. doi: 10.1242/dev.02183. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, et al. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–355. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonnell I, McKay I, Graham A. A population of caudally migrating cranial neural crest cells: functional and evolutionary implications. Dev Biol. 2001;236:354–363. doi: 10.1006/dbio.2001.0330. [DOI] [PubMed] [Google Scholar]
- McKenzie J. The development of the sternomastoid and trapezius muscles. J Anat. 1962;89:526–531. [PMC free article] [PubMed] [Google Scholar]
- Millot J, Anthony J. Anatomie de Latimeria Chalumnae. Tome I. Squelette et Muscles. Paris: Centre National de la Recherche Scientifique; 1958. [Google Scholar]
- Nathan E, Monovich A, Tirosh-Finkel L, et al. The contribution of Islet1-expressing splanchnic mesoderm cells to distinct branchiomeric muscles reveals significant heterogeneity in head muscle development. Development. 2008;135:647–657. doi: 10.1242/dev.007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983a;96:144–165. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- Noden DM. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat. 1983b;168:257–276. doi: 10.1002/aja.1001680302. [DOI] [PubMed] [Google Scholar]
- Noden DM. Patterning of avian craniofacial muscles. Dev Biol. 1986;116:347–356. doi: 10.1016/0012-1606(86)90138-7. [DOI] [PubMed] [Google Scholar]
- Noden DM. Interactions and fates of avian craniofacial mesenchyme. Development. 1988;103:121–140. doi: 10.1242/dev.103.Supplement.121. [DOI] [PubMed] [Google Scholar]
- Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- Noden D, Marcucio R, Borycki A, et al. Differentiation of avian craniofacial muscles: I. Patterns of early regulatory gene expression and myosin heavy chain synthesis. Dev Dyn. 1999;216:96–112. doi: 10.1002/(SICI)1097-0177(199910)216:2<96::AID-DVDY2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Onimaru K, Shoguchi E, Kuratani S, et al. Development and evolution of the lateral plate mesoderm: comparative analysis of amphioxus and lamprey with implications for the acquisition of paired fins. Dev Biol. 2010;359:124–136. doi: 10.1016/j.ydbio.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Ørvig T. Description, with special reference to the dermal skeleton, of a new radotinid arthrodire from the Gedinnian of Arctic Canada. In: Lehman J-P, editor. Problemes Actuels de Paleontologie: Evolution des Vertebres. Paris: Colloque international, CNRS; 1975. pp. 41–71. [Google Scholar]
- Piatt J. Morphogenesis of the cranial muscles of Amblystoma punctatum. J Morph. 1938;63:531–587. [Google Scholar]
- Piekarski N, Olsson L. Muscular derivatives of the cranialmost somites revealed by long-term fate mapping in the Mexican axolotl (Ambystoma mexicanum. Evol Dev. 2007;9:566–578. doi: 10.1111/j.1525-142X.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- Piekarski N, Olsson L. A somitic contribution to the pectoral girdle in the axolotl revealed by long-term fate mapping. Evol Dev. 2011;13:47–57. doi: 10.1111/j.1525-142X.2010.00455.x. [DOI] [PubMed] [Google Scholar]
- Platt JB. The development of the cartilaginous skull and of the branchial and Hypoglossal musculature in Necturus. Morph Jahr. 1897;25:367–464. [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, et al. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–953. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Gayraud-Morel B, Dumas G, et al. Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev Cell. 2009;16:810–821. doi: 10.1016/j.devcel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Kuratani S, Tajbakhsh S. An eye on the head: the development and evolution of craniofacial muscles. Development. 2011;138:2401–2415. doi: 10.1242/dev.040972. [DOI] [PubMed] [Google Scholar]
- Scambler PJ. 22q11 deletion syndrome: a role for TBX1 in pharyngeal and cardiovascular development. Pediatr Cardiol. 2010;31:378–390. doi: 10.1007/s00246-009-9613-0. [DOI] [PubMed] [Google Scholar]
- Shearman R, Burke A. The lateral somitic frontier in ontogeny and phylogeny. J Exp Zool B Mol Dev Evol. 2009;312:603–612. doi: 10.1002/jez.b.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman R, Tulenko F, Burke A. 3D reconstructions of quail-chick chimeras provide a new fate map of the avian scapula. Dev Biol. 2011;355:1–11. doi: 10.1016/j.ydbio.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Smith M, Hall B. Development and evolutionary origins of vertebrate skeletogenic and odontogenic tissues. Biol Rev. 1990;65:277–373. doi: 10.1111/j.1469-185x.1990.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Smith M, Hall B. A developmental model for evolution of the vertebrate exoskeleton and teeth: the role of cranial and trunk neural crest. Evol Biol. 1993;27:387–448. [Google Scholar]
- Smith M, Hickman A, Amanze D, et al. Trunk neural crest origin of caudal fin mesenchyme in the zebrafish Brachydanio rerio. Proc R Soc Lond B Biol Sci. 1994;256:137–145. [Google Scholar]
- Tajbakhsh S, Rocancourt D, Cossu G, et al. Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell. 1997;89:127–138. doi: 10.1016/s0092-8674(00)80189-0. [DOI] [PubMed] [Google Scholar]
- Theis S, Patel K, Valasek P, et al. The occipital lateral plate mesoderm is a novel source for vertebrate neck musculature. Development. 2010;137:2961–2971. doi: 10.1242/dev.049726. [DOI] [PubMed] [Google Scholar]
- Valasek P, Theis S, Krejci E, et al. Somitic origin of the medial border of the mammalian scapula and its homology to the avian scapula blade. J Anat. 2010;216:482–488. doi: 10.1111/j.1469-7580.2009.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter B. Untersuchungen zur vergleichenden Anatomie der Kiemen- und Kiefermusculatur der Fische. Jena Z Med Naturwiss. 1874;12:431–450. [Google Scholar]
- van Wijhe JW. Ueber die Mesodermsegmente und die Entwickelung der Nerven des Selachierkopfes. Verh Konink Akad Weten. 1882;22:1–50. [Google Scholar]
- Winterbottom R. A descriptive synonymy of the striated muscles of the Teleostei. Proc Natl Acad Sci U S A. 1974;125:225–317. [Google Scholar]
- Zhang Z, Huynh T, Baldini A. Mesodermal expression of Tbx1 is necessary and sufficient for pharyngeal arch and cardiac outflow tract development. Development. 2006;133:3587–3595. doi: 10.1242/dev.02539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Schultze H-P. Interrelationships of basal osteichthyans. In: Ahlberg P, editor. Major Events in Early Vertebrate Evolution: Palaeontology, Phylogeny, Genetics and Development. London: Taylor & Francis; 2001. pp. 289–314. [Google Scholar]


