Abstract
‘Evo-devo’, an interdisciplinary field based on developmental biology, includes studies on the evolutionary processes leading to organ morphologies and functions. One fascinating theme in evo-devo is how fish fins evolved into tetrapod limbs. Studies by many scientists, including geneticists, mathematical biologists, and paleontologists, have led to the idea that fins and limbs are homologous organs; now it is the job of developmental biologists to integrate these data into a reliable scenario for the mechanism of fin-to-limb evolution. Here, we describe the fin-to-limb transition based on key recent developmental studies from various research fields that describe mechanisms that may underlie the development of fins, limb-like fins, and limbs.
Keywords: fin-to-limb evolution, paired appendage development, repression mode of apical fold
Introduction
Vertebrates that have adapted evolutionarily to aquatic or terrestrial environments show characteristic phenotypic changes. These characteristics include the adjustment of biological calcium homeostasis (gills or parathyroid gland; Okabe & Graham, 2004), breath control (swim bladder or lung; Zheng et al. 2011), skull and body shape (streamlined or flattened), structures for supporting the body (well developed girdles and firm joints in terrestrials as an adjustment to gravity; Matsuoka et al. 2005), and locomotive organs (fins or limbs; Sordino et al. 1995). Vertebrates that possess organs adapted for an aquatic environment are fish; terrestrial ones are tetrapods.
Organs that share a common developmental origin and evolutionary ancestry are defined as ‘homologous organs’. Fish and tetrapods have many homologous organs, which are sometimes altered for the organism’s adaptation to a specific environment. The paired fins in fish and limbs in tetrapods are a good example of homologous organs. They were derived from locomotive organs in common ancestors of vertebrates, and they share many developmental processes and genetic networks. The limb bud, the embryonic primordium of tetrapod limbs, develops to form a three-dimensional pattern for the limb along three axes: the proximo-distal (PD) axis, which is regulated by apical ectodermal ridge (AER) signals such as Fgf8 and Wnt3a (Kengaku et al. 1998; Fernandez-Teran & Ros, 2008; Lu et al. 2008); the antero-posterior (AP) axis, regulated by zone-polarizing activity (ZPA) signals such as Shh (Harfe et al. 2004; Zeller et al. 2009); and the dorso-ventral (DV) axis, controlled by several ectodermal molecules such as Wnt7a and En1 (Loomis et al. 1998). For fin development, the formation, maintenance, and function of the AER are as essential as in limb development (Grandel et al. 2000), and Shh, expressed in the posterior fin bud (Neumann et al. 1999; Yonei-Tamura et al. 2008), functions in AP patterning (Dahn et al. 2007).
Although the vertebrate paired appendages are homologous and develop through similar genetic networks, fins and limbs have obvious morphological differences. In fact, it can be difficult to find corresponding elements between the fin skeleton and limb skeleton in extant vertebrates. Whereas the limb skeleton is composed of endochondral bones (endoskeleton), the fin skeleton consists almost entirely of fin rays (exoskeleton), with a poor underpinning endoskeleton (Tamura et al. 2008). Because of the vast morphological differences between fins and limbs, some regard them not as homologous organs in the classical (morphological) sense but as organs with ‘deep homology’, which means they arose by the modification of pre-established genetic regulatory circuits (Shubin et al. 2009). In this review, we clarify the morphological distinction between fins and limbs, and present several possible candidate mechanisms for the differential developmental process. Fins and limbs must have different developmental mechanisms that generate their morphological characteristics, as well as shared basic mechanisms for the initiation and outgrowth of the appendage primordia.
Materials and methods
Construction of Prx1-GFP transgenic fish
A 2.4-kb genomic sequence upstream of mouse prx1 (Suzuki et al. 2007) was excised from pCS2-Mprx1-GFP3 vector using SalI and HindIII, and was subcloned into the SalI and HindIII sites of pBluescript SK+. To generate a Tol2 construct harboring the insertion of Mprx1-GFP, T2AL200R150G (a kind gift from Dr. Koichi Kawakami; Urasaki et al. 2006; accession no. AB262452) was digested with XhoI and BamHI, and the XhoI-BamHI fragment of Mprx1-GFP was inserted in pBluscript SK+ vector. Transposase mRNA was synthesized as described previously (Kawakami, 2004; Kawakami et al. 2004a). Tol2-based Mprx1-GFP vector and transposase mRNA were co-injected into wild-type fertilized eggs, and F1 embryos were analyzed under an SP6 fluorescent microscope (Olympus) (Fig. 3).
Fig. 3.

Proportion, location, and orientation of the pectoral fin bud in zebrafish. (A–D) Developmental changes of the pectoral fin bud, visualized using Mprx1-GFP transgenic fish (lateral view, anterior is to the left). The stage is shown at the top-right in hpf (hours post-fertilization). The Mprx1 enhancer (5′ upstream regulatory element of the Prx1 gene in mouse genome) was activated in the pectoral fin mesenchyme and pharyngeal arch. See also Hernandez-Vega & Minguillon (2011) as a reference for the Mprx1 enhancer. GFP-fluorescence and bright-field images are merged, and the shape of the fin bud is outlined in blue (A′–D′). (E) Visualization of the fin skeletal mesenchyme by Mprx1-GFP. High-magnification view of the pectoral fin bud at 74 hpf in the Mprx1-GFP transgenic zebrafish. In the endoskeletal region, mesenchymal cells (ed, endoskeletal disc; mm, migrating fin mesenchyme; sco, scapulacoracoid) show GFP fluorescence. cl, cleithrum bone. Scale bars: 200 μm (A–D); 100 μm (E). (F) Schematic representation of the orientation of the fin bud and proportions of the endo- and exo-skeletal regions. The orientation of the pectoral fin bud changes drastically during fin development (Ant., anterior; Post., posterior; V, ventral; D, dorsal). Note that the lateral view of the fin (also in A–D) always displays the ventral surface of the fin. Each fin bud is divided into the apical fold (AF) region (light blue) and endoskeletal region (dark blue).
In situ hybridization
Frem2a (accession no. BK006471) was isolated (an 894-bp fragment) with the primers 5′-ACCCTTTGAGTTGACCGTTG-3′ and 5′-TCGTATTTCCCATCCGAGAG-3′ using RT-PCR on 24-hpf embryos. The RNA antisense probe was synthesized with T7 RNA polymerase from the amplified frem2a clone following linearization by SpeI. Whole-mount and section in situ hybridizations were performed as described previously (Abe et al. 2007). For preparation of delicate cryosections, fixed embryos were soaked in gelatin-embedded solution [fish gelatin (Sigma): 30% sucrose: DDW = 3 : 2 : 1] for 6 h (Fagotto & Gumbiner, 1994; Suzuki et al. 2010) (Fig. 4).
Fig. 4.
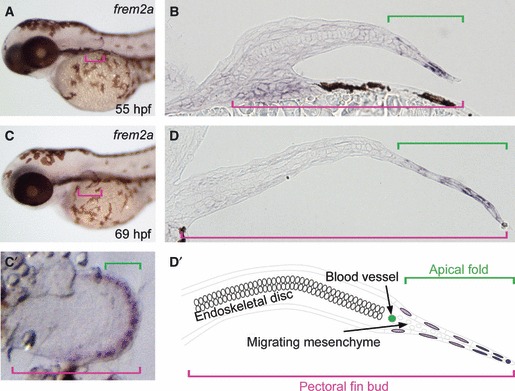
Frem2a mRNA in situ hybridization of the pectoral fin bud in zebrafish. (A,B) Frem2a is expressed in the apical fold (AF) of the pectoral fin bud at 55 hpf (pink brackets). In the AF shown by a transverse section (green bracket), frem2a is strongly expressed in the distal edge but only weakly in the proximal region. (C,D) At 69 hpf, the pectoral fin bud grows out distally, and the expression pattern of frem2a is still similar to (A) and (B). A high-magnification view of the pectoral fin bud at 69 hpf is shown in (C′). Schematic representation of the transverse section (D) of the fin bud is shown in (D′). Frem2a expression in the distal AF epidermis is stronger (dark purple) than in the proximal AF epidermis (light purple).
Anatomy of the fin and limb skeletons
Tetrapod limbs can be clearly divided into three domains: stylopod, zeugopod, and autopod. The stylopod consists of a single long bone (humerus/femur) and the proximal epiphysis of the bone is articulated to the pectoral/pelvic girdle. There are two long bones in the zeugopod (radius/tibia and ulna/fibula). The autopod includes many bone elements that can be subdivided into carpal/tarsal bones, metacarpal/metatarsal bones, and phalanges; the number of bones varies among tetrapod taxa (Tamura et al. 2008). The entire limb skeleton is endoskeleton (endochondral bones), which is first formed as cartilage followed by replacement with mineralized bone (see also the next chapter).
The paired appendages of chondrichthyans (skates and sharks), actinopterygians (paddlefish, sturgeons, amias, gars, and teleosts), and sarcopterygian fish (lungfish and coelacanths) are fins (pectoral/pelvic fins). In actinopterygians, the fin skeleton can be divided into three domains: proximal radials, distal radials, and fin rays (Grandel & Schulte-Merker, 1998; Davis et al. 2004b) (Fig. 1). Four or more proximal radials, which are columnar bones at the proximal-most domain, are located distal to the pectoral/pelvic girdle. At the distal end of the proximal radials, there is a line of pea-like distal radials. These two layers of skeleton are formed as endoskeleton, like the limb skeleton, but they occupy only a small portion of the entire fin structure. Thus, it is difficult to directly compare the skeletal domains between fins and limbs. The major component of the fin skeleton is the fin rays, located in the distal-most domain. Fin rays are thin, paper-like structures supported by rod-like bones (called lepidotrichia) radiating from the distal end of the distal radials. The lepidotrichia form as exoskeleton (membrane bone), in which mesenchymal cells directly differentiate into mineralized bone tissue (Geraudie & Landis, 1982; Landis & Geraudie, 1990).
Fig. 1.

Skeletal domains (patterns) in fins and limbs. The proximal end of the appendages is to the left in all pictures. (A–D) Pectoral fin skeletons of the paddlefish (A), zebrafish (B), bamboo shark (C) and dogfish (D). In these actinopterygians (A,B) and chondrichthyans (C,D), there is a radial domain (consisting of several radial bones, proximal to the red broken line) and a fin-ray region (lepidotrichia or ceratotrichia, distal to the red broken line). (E–H) Pectoral fin skeletons of Sauripterus (E), Eustenopteron (F), Panderichthys (G), and Tiktaalik (H). These sarcopterygian fish have the three parts of the endoskeletal domain (stylopod, zeugopod, and multi-patterned radial bones in the distal domain: roughly separated by blue lines) and a fin-ray region (distal to the red broken line). (I–K) Forelimb skeletons of Acanthostega (I), chicken (J), and mouse (K). The distal-most endoskeletal domain is the autopod domain (distal to the right blue line), and there is no fin-ray region. (A–C) were redrawn from Dahn et al. (2007), (D) from an article (Yonei-Tamura et al. 2008), (E) and (I) from a review (Raff, 2007), and (F–H) from an article (Boisvert et al. 2008).
Chondrichthyans, which are derived from a common ancestor of actinopterygians and sarcopterygians, as well as of tetrapods, are extant species in which the common developmental processes and similar genomic sequences of the fin and limb can be investigated. The skeleton of the chondrichthyan fin consists of several cartilaginous elements, but their pattern only slightly resembles that in the fin endoskeleton of actinopterygians; however, it still does not correspond to any obvious subdivision of the tetrapod limb (Dahn et al. 2007; Freitas et al. 2007; Yonei-Tamura et al. 2008). The skeletal pattern of pectoral fins in the skate is peculiar, and fin structures combine with the head at later stages of development. Moreover, in the fin-ray region of chondrichthyans, there are no minerarized bone elements, and the beams of the fin ray are composed of a collagenous matrix filling (ceratotrichia; Goodrich, 1904). Thus, comparative analyses of the skeletal pattern among extant species seems insufficient to reveal the fin-to-limb transition in evolution. However, fortunately, paleontological analyses of the fossils of extinct sarcopterygian fish can fill in many of the gaps.
Tetrapods are thought to have evolved from sarcopterygian fish, with the fin-to-limb transition occurring during this process. Many fossils of basal sarcopterygian fish have been discovered and the skeletal pattern of their appendages provides invaluable evidence regarding the fin-to-limb transition. Sauripterus, one of the most basal sarcopterygian fish (Davis et al. 2004a), has a fin skeletal pattern similar to that of chondrichthyans. However, Eustenopteron and Panderichthys, also sarcopterygian fish, have limb-like sequential domains along the PD axis (Vorobyeva, 1992; Cote et al. 2002). A recent study using CT scans revealed the precise skeletal pattern of a Panderichthys fin (Boisvert et al. 2008), showing that the pectoral fin endoskeleton of Panderichthys consists of a humerus, radius, ulna, and distal radial bones (meaning digit-like structures) along the PD axis, as well as fin rays at the surrounding of the endoskeleton. Tiktaalik is considered to be the most tetrapod-like sarcopterygian fish (Shubin et al. 2006). Unlike the fin skeleton of Panderichthys, radial bones in Tiktaalik are articulated with adjacent bones. The ulna articulates with the ulnare and the intermediate, and the ulnare and the intermediate joint with five proximal radial bones and three distal radial bones.
Thus, sarcopterygian fish (Eustenopteron, Panderichthys, and Tiktaalik) appear to possess incomplete versions of the three endoskeletal domains (related to the stylopod, zeugopod, and autopod), although they have unsettled skeletal patterns, fin rays and no digits; they therefore have a fin-limb mixture pattern. Taking this evidence together, the process of fin-to-limb transition can be categorized into four steps.
the formation of two proximal domains (stylopod, zeugopod) that exist in sarcopterygian appendages but not in actinopterygian fins;
the formation of the autopod region during the evolution of sarcopterygian fish;
the determination of bone numbers in the appendages, including digit numbers;
loss of the fin ray, which happens not in the sarcopterygian fish appendage, but in the tetrapod limb.
Cellular origin of fin rays
The skeletal differences between fins and limbs arise not only in pattern (domain) formation, but also in the process of cell differentiation during bone maturation. The vertebrate skeleton can be classified into cartilage and mineralized bones, both of which are supporting tissues of the body. Whereas cartilage is formed by mesenchymal condensation and the subsequent deposition of collagenous matrix, mineralized bones are formed by physiologic calcification. In chondrichthyans, the fin skeletons consist of cartilaginous bones and ceratotrichia (collagen fibrils). The fins of other gnathostomes (actionopterygians and sarcopterygians) mainly consist of mineralized bones in adults. The calcification of mineralized bones comes about by one of two formation processes: endochondral ossification and membranous ossfication.
In endochondral ossification, cartilage is used as a template or model for the final product. The chondrogenic cells (chondrocytes) are replaced by osteocytes, starting with the attachment of blood vessels to the middle of the cartilage and invasion of osteoblasts toward both ends of the bone (Hartmann & Tabin, 2000; Karsenty & Wagner, 2002; Maes et al. 2010). The endoskeleton of tetrapod limbs and actinopterygian fins is formed by endochondral ossification. Fin rays, by contrast, are formed by membranous ossification, as are some craniofacial and clavicle bones; in this case, mesenchymal cells directly differentiate into osteoblasts without a chondrogenic step. In fin-ray formation, collagenous fibrils called actinotrichia are formed first; mesenchymal cells then move along these fibrils and differentiate into membrane bones called lepidotrichia (Grandel & Schulte-Merker, 1998). In the evolution of tetrapods, the lepidotrichia of fin rays are lost in both the paired appendages and the median fin rays (step 4 in the previous chapter); the larvae of amphibians have a continuous line of median fin, but the structure includes no bones (Tucker & Slack, 2004). Therefore, a disorder of the skeletogenesis in membrane bones in the whole trunk body may be related to the loss of fin rays.
An interesting hypothesis for the cause of fin-ray loss during the fin-to-limb transition was proposed by Hall (2005). In this scenario, lepidotrichia are considered ‘neural crest-derived ornaments’, and neural crest cells, which are the originating cells of lepidotrichia in actinopterygian and sarcopterygian fins, lose the ability to undergo skeletogenesis in the fin rays of the paired and median appendages during an evolutionary step in the fin-to-limb transition. There are two types of cellular origin for skeleton: mesoderm (somitic mesoderm or lateral plate mesoderm, LPM) and neural crest cells. For example, tetrapod limb skeleton and actinopterygian fin endoskeleton originate from LPM cells (Gibert et al. 2006), whereas odontoblasts in tooth organs and some craniofacial bones are of neural-crest origin (Graham et al. 2004; Chai & Maxson, 2006). Thus, neural-crest cells can contribute to membrane bone formation. In tetrapods, neural crest cells do not contribute to bone formation in the trunk region (Noden, 1978; Nakamura & Ayer-le Lievre, 1982), with some exceptions, such as in some shoulder girdle bones (Matsuoka et al. 2005). In actinopterygians and sarcopterygian fish, the neural crest-derived ornaments are hypothesized, and the membrane bones in the fin rays are proposed to originate from neural crest cells by analogy to the membrane bone formation in the tetrapod head region (Smith et al. 1994). In support of this idea, the dorsal fins of larval amphibians and fish contain mesenchyme of neural crest origin (Dushane, 1935; Bodenstein, 1952; Eisen & Weston, 1993), and fin rays express some marker genes for neural crest cells (Smith et al. 1994; Freitas et al. 2006).
In median fin development, however, some studies have suggested that somite-derived cells are involved in the fin rays of chondrichthyans (Neyt et al. 2000; Freitas et al. 2006; Cole & Currie, 2007), and others suggested an LPM origin for lateral fin rays (Schaeffer, 1987). Further analysis should clarify the cellular origin of the lepidotrichia in fish fins and its relationship to the changes in the developmental processes between fins and limbs. Long-term cell tracing during fin-ray formation is technically very difficult and has not been reported, but recent transgenic techniques using zebrafish, medaka, and frogs (Gargioli & Slack, 2004; Deguchi et al. 2009) should help us trace specific cell populations for long periods.
A recent study examined the relationship between the loss of fin rays and the genomic loss of the Actinodin (And) family genes, which are involved in the construction of actinotrichia (Zhang et al. 2010). The knockdown of And genes causes the loss of actinotrich formation, followed by failure in the migration of mesenchymal cells that form lepidotrichia. Interestingly, And genes have not been found in database searches of any tetrapod species (described in Zhang et al. 2010). The final destination of mesenchymal cell differentiation by the loss of And genes has not been reported; nevertheless, a disorder of actinotrich formation might have triggered the loss of fin rays during the fin-to-limb transition as suggested by Zhang et al. (2010).
Fin development and fin-to-limb evolution
Pattern formation in fin development
The four steps (as described in the ‘Anatomy of the fin and limb skeletons' chapter) involved in the fin-to-limb transition described above could have occurred successively, synchronously, or independently. Step 4 is mainly a matter of cell differentiation, as described in the ‘Cellular origin of fin rays' chapter, and the first three steps are related to pattern formation during fin/limb development. The functions of the developmental mechanisms underlying the differences in morphology between fins and limbs should be as follows: step 1, to determine the proximal region (stylopod and zeugopod); step 2, to establish the autopod region; and step 3, to determine the number of bones, e.g. the number of digits. For all three steps, HoxA, HoxD, and Shh are common but important factors in the basic mechanisms of pattern formation in fins and limbs.
In limb development, PD patterning is mediated by Meis1, Hoxa11, and Hoxa13, which are expressed sequentially along the PD axis (Tamura et al. 1997, 2008; Mercader et al. 1999; Zeller, 2010). Meis1 expression is restricted to the most proximal region, equivalent to the stylopod, and it functions in stylopod formation (Capdevila et al. 1999; Mercader et al. 1999, 2000; Yashiro et al. 2004). For proximal domain formation (stylopod and zeugopod), a mutually exclusive boundary of Meis1 and Hoxa11 expression is regulated by retinoic acid (RA) and AER signals (Cooper et al. 2011; Rosello-Diez et al. 2011). Meis genes are also expressed at the proximal-most region in the developing pectoral fin bud of zebrafish (Waskiewicz et al. 2001), and Hoxa9 and Hoxa10, which are also expressed in the proximal region of the fin bud, are co-localized within the Hoxa11/Hoxa13 expression domain (Grandel et al. 2000). In chondrichthyan fins, however, the Meis1 expression domain is distinct from the Hoxa11/Hoxa13 expression domain (Sakamoto et al. 2009). It is possible that the mechanism for creating the boundary between the Meis1 and Hoxa11 expression domains is also involved in the proximal domain (stylopod and zeugopod) formation of step 1, although the function of Meis in fish fins remains unclear.
In the early stage of limb development, the Hoxa13-expressing domain completely overlaps with the Hoxa11 domain in the limb mesenchyme, but these domains are gradually separated from each other by the negative regulation of Hoxa11 expression by Hoxa13. Hoxa11 is eventually expressed only in the zeugopod region, whereas Hoxa13 expression becomes restricted distally, to the autopod region, at later stages (Yokouchi et al. 1991; Nelson et al. 1996; Stadler et al. 2001; Sato et al. 2007). Ectopic expression of Hoxa11 in the autopod disrupts the formation of a normal skeletal pattern (Mercader et al. 1999), and ectopic expression of Hoxa13 in the zeugopod region causes an abnormal skeletal pattern in that region (Yokouchi et al. 1995).
During patternless limb regeneration in adult Xenopus, which gives rise to a spike-like shaft of bone instead of digits, Hoxa11 and Hoxa13 are expressed in the regenerating limb mesenchyme, but not in separated domains, along the PD axis (Ohgo et al. 2010; Tamura et al. 2010), suggesting that the appropriate expression pattern of these Hox genes is related to the appropriate limb skeleton pattern in tetrapods as well. In the fin development of the actinopterygian zebrafish, the fin buds express hoxa11b and hoxa13b, whose expression domains overlap, and never separate along the PD axis of the fin (Grandel et al. 2000; Metscher et al. 2005). Consistent with expression patterns of the Hoxa11 and Hoxa13 genes, the endochondral bones in the fish fins derived from hoxa11b and hoxa13b double-positive mesenchyme do not correspond to any skeletal element in tetrapod limbs. In chondrichthyan fins, Hoxa11 and Hoxa13 are expressed in the same region but there is a narrow region where Hoxa13 is expressed but not Hoxa11 (Sakamoto et al. 2009).
Collectively, these observations suggest that morphological differences between fins and limbs are correlated with the mechanisms for separating the expression domains of hoxa11 and hoxa13 (Sordino et al. 1995) and this may have been critical for step 2 in the fin-to-limb transition. Thus, it is likely that differences between fins and limbs are associated with the expression pattern of meis1-hoxa11-hoxa13 along the PD axis. To our knowledge, there is no information on hoxa expression in extant sarcopterygian fins; these data will be important for understanding how the separation of hoxa expression is regulated, and what role it plays in defining fins vs. limbs.
The 5′Hoxd genes have been well analyzed as candidate regulators of limb skeletal formation along the PD and AP axes. These genes are expressed in the posterior region at early stages of tetrapod limb development (the early phase of 5′Hoxd expression). The posterior-biased domains expand anteriorly as limb development proceeds and at the late phase, the expanded 5′Hoxd domain comes to correspond largely with the autopod region (Nelson et al. 1996; Zakany & Duboule, 2007). In actinopterygians, the hoxd domain never expands anteriorly and the genes continue to be expressed in the posterior region of the fin bud (Sordino et al. 1995; Grandel et al. 2000; Davis et al. 2007). These findings suggest that the anterior expansion of 5′Hoxd is involved in the morphological changes from fins to limbs (Shubin et al. 2009).
The evidence described above suggests that fish fins do not possess the mechanism for step 2, but recent studies show that developing fish fins may have at least partial mechanisms for distal and late-phase Hox gene expression. Zebrafish hoxa13a and hoxa13b are teleost-specific duplicated gene sets resulting from whole-genome duplication, and their hoxa13a expression is restricted to the distal fin mesenchyme at later stages of development (Ahn & Ho, 2008). The expression domain of the evx2 gene, which corresponds to the autopod region in the tetrapod limb, is restricted to the posterior-distal region of the zebrafish fin bud (Sordino et al. 1996; Tarchini & Duboule, 2006).
A tetrapod-like late-phase 5′hoxd gene expression pattern has been demonstrated in the developing zebrafish (Ahn & Ho, 2008), paddlefish (Davis et al. 2007), and catshark (Freitas et al. 2007). In tetrapod limb development, the late-phase 5′hoxd expression in the autopod is controlled by a cis-regulatory element distinct from the early phase regulator (Woltering & Duboule, 2010). In an interspecies transgenic analysis, a green fluorescent protein (GFP) reporter gene regulated by the late-phase enhancer of mouse 5′Hoxd genes was activated in a small portion of the late-phase 5′hoxd expression domain of the zebrafish pectoral fin (Schneider et al. 2011). Schneider et al. (2011) suggest that fins have a mechanism for late-phase 5′Hoxd expression that is insufficient for anterior expansion, and the transformation into tetrapod limbs might have arisen from a modification of conserved cis- and trans-acting mechanisms of Hox regulation. Even early tetrapods (e.g. Acanthostega and Ichthyostega) might have incomplete mechanisms for regulating 5′Hoxd expression in the autopod, as suggested by Zakany et al. (1997), because they show polydactyly in their limbs that looks like the phenotypes of mutant mice with a loss of 5′Hoxd function: e.g. the Hoxd11/12/13-knockout mouse.
Shh is a morphogen for AP patterning in limb development. Shh-deficient mice have limb buds with a tapered shape: they become increasingly narrow along the AP axis, resulting in severe abnormalities in the zeugopod (lack of ulna) and autopod (only single-digit formation) (Litingtung et al. 2002). In contrast, knockout mice of Gli3, a negative regulator of Shh expression and function, have wide limb buds and show polydactyly (te Welscher et al. 2002). Thus, the relative width of the field for AP patterning in the limb bud may determine the number of bones (Zhu et al. 2010) and Shh appears to be involved determining the size of the field as well as the skeletal identity along the AP axis. Zhu et al. (2010) made an interesting prediction, based on an in silico analysis, that the skeletal patterns of the fins in sarcopterygian fish can also be explained by the relative width of the AP-patterning field. Shh is known to be involved in the patterning of developing fins (Dahn et al. 2007; Yonei-Tamura et al. 2008; Sakamoto et al. 2009) and it is therefore possible that the mechanism for forming limb pattern is not its tetrapod-specific function of Shh signaling but a modification of a common function in determining the bone number of vertebrate appendages (Fig. 2).
Fig. 2.
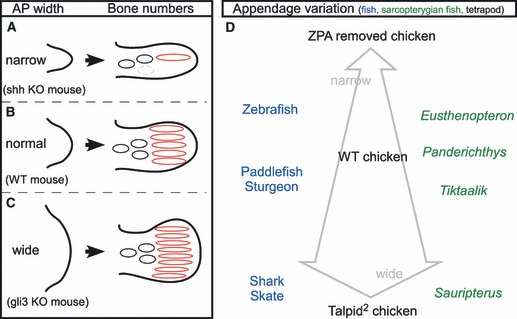
Model for diversification of the appendage skeleton: relationship between the AP width of the appendage primordia and the bone number. (A–C) The number of digits (red) is regulated by AP determinants such as ZPA and AER. In mouse limbs, a deficiency of Shh (Litingtung et al. 2002) causes narrow limb buds and single digit formation (A), whereas normal-sized limb buds give rise to five digits (B). In contrast, a lack of Gli3 (te Welscher et al. 2002) leads to wider limb buds and polydactyly (C). (D) Diagram showing how limb-bud width relates to the type of appendage skeleton in a variety of animals. The tapered arrow provides reference for the effect of limb-bud width on the appendage skeleton in a tetrapod (chicken) embryo: Talpid2 mutants have wider limb buds, which cause extra digits to form, owing to ectopic expression of Shh. ZPA removal results in narrower limb buds and fewer digits. To the left of the arrow are the names of extant fish (in blue) showing skeletal variations that could be predicted to arise from narrow (top) to wide (bottom) fin buds. Note that chondrichthyans (e.g. shark, skate) have many more radial bones than do actinopterygians (e.g. paddlefish, sturgeon). To the right (in green) the names of different sarcopterygian fish species are shown. Among these fish, believed to be direct ancestors of tetrapods, appendage skeletal variations are species-specific. As described by Zhu et al. (2010), the skeletal variations can be explained by the differences in limb-bud width along the AP axis.
According to paleontological explanations of fossil evidence that sarcopterygian fish had incomplete sets of limb skeletal elements along the PD and AP axes, the developmental mechanisms of limb skeletal formation, which could be incomplete, may have provided the bases for the development of fins in sarcopterygian fish. Embryological data from fin development support this idea, as described above. The complicated pattern of bones along the AP axis in the distal fin of Panderichthys and Tiktaalik is still not equivalent to digits or carpal bones. The skeletal patterning in sarcopterygian fish seems to be more appropriate for stylopods and zeugopods than for autopods. Autopod formation may have been hindered as well by fin-ray formation, which is a fin-specific trait. As a characteristic difference between fins and limbs, we cannot help but focus on the loss of fin-ray formation (step 4). In the last section of this article, we will further examine the developmental process of the fin ray, focusing on a special epithelial structure, the apical fold.
Apical fold formation in fin development
In zebrafish, the first visible difference between fin development and limb development is the emergence of the apical fold, the fin ray-forming envelope. The developing limb bud contains a thick region (the AER) at the distal margin of the ectodermal jacket that is essential for successive pattern formation along the PD axis. The developing fin bud also contains a functional AER structure at the apex of the bud at an early stage (Norton et al. 2005); this fin AER soon lifts and starts to elongate (Grandel & Schulte-Merker, 1998). This elongated structure, called the apical fold (AF), is never seen in the limb bud, and continues to elongate along the PD axis after the AER–AF transition (Dane & Tucker, 1985) (Fig. 3). A similar structure can be seen in the developing median fin, in which the AER forms transiently and then is transformed into the median fin fold (MFF; Abe et al. 2007). The AF and MFF are back-to-back sheets of epidermis lined with double-layered basement membranes, and mesenchymal collagenous fibrils, actinotrichia, form in the space between these epidermal sheets during development (Wood & Thorogood, 1984; Zhang et al. 2010). Precursor cells of membrane bone invade the AF region, move distally along actinotrichia, and differentiate into lepidotrichia. Thus, the AF provides the space in which the fin ray bones are made, and whether the AF exists, that is, whether or not the AER–AF transition occurs, is thought to be a key determinant for the difference between fins and limbs (Thorogood, 1991). Thorogood (1991) proposed an interesting model, the ‘clock model’, in which variation of the endoskeletal pattern is caused by variation of the timing of the AER–AF transition; a less-patterned endoskeleton is formed by short exposure to AER signals, and a limb-like pattern is formed by longer exposure to AER signals than that of the less-patterned skeleton. To verify this hypothesis, we need to understand molecular functions of the AER and AF.
The AER and AF have obvious structural and functional differences. Frem/Fras family genes, which encode extracellular matrix components and are involved in cell–cell adhesion, are expressed in the AER/MFF (Gautier et al. 2008). Frem2a is expressed in the AER of the early-stage fin. In the AF, frem2a is expressed strongly in the distal region (as strongly as in the early AER) and weakly in the proximal region (the presumptive fin ray-forming region) (Fig. 4). These observations suggest that the entire AF does not correspond to the AER but that the distal AF may be equivalent to the AER. Correspondingly, laminin α5, a basement membrane-associated protein that is important for the transition from the AER to MFF, is strongly distributed at the distal edge of the AF (Webb et al. 2007).
The AER marker genes in the limb bud wnt2b, dlx2, dlx5a, sp8, and sp9, are also expressed in the AER and AF of the fin bud (Neumann et al. 1999; Ng et al. 2002; Kawakami et al. 2004b). The knockdown of sp8 and sp9 causes complete loss of the fin bud (Kawakami et al. 2004b), suggesting these genes are involved in AER/AF formation or maintenance. Fgf signals are pivotal for fin outgrowth and limb development, although the expression patterns of fgfs are more complicated in the fin bud. Fgf24, which exists only in actinopterygian and chondrichthyan genomes, is expressed in the fin mesenchyme (at fin initiation stages) and then in the AER/AF (at fin outgrowth stages) (Fischer et al. 2003). In the limb bud, no fgfs show this kind of transference of expression domain from the mesenchyme to the epidermis. Fgf24 acts upstream of fgf10, and an fgf24 mutant (ikarus) exhibits complete fin loss with lack of fgf10 expression, indicating that Fgf24 acts like Fgf10 in limb initiation (Draper et al. 2003; Harvey & Logan, 2006). Moreover, fgf24 is a member of the fgf8/17/18 subfamily and is expressed in the AER/AF (Draper et al. 2003), indicating that Fgf24 may also act like Fgf8 in limb outgrowth. In addition, some studies have shown that Fgf8 and Fgf4, crucial AER factors for limb development (Sun et al. 2000; Mariani et al. 2008), start to be expressed in the AF after the AER–AF transition (Nomura et al. 2006; Jovelin et al. 2007). However, Fischer et al. (2003) reported that these genes are expressed earlier, at the AER formation stage. In any case, Fgf signals are important for fin development because fish mutants of Ext2 [which acts in heparan sulfate proteoglycan (HSPG) synthesis and is essential for Fgf10 signaling] (dackel) and Fgf10 (daedalus) lack pectoral fins (Grandel et al. 2000; Lee et al. 2004; Norton et al. 2005).
The function of the fin AER is being elucidated, as discussed in the overview above. To understand the functional differences between the AER and AF, it is necessary to investigate the molecular networks associated with appendage development, such as those involved in the ectodermal–mesenchymal interaction. However, little is known about the molecular mechanisms underlying the AER–AF transition or the AF function. Continuous events occurring before and after the AER–AF transition make it difficult to distinguish experimentally the AF and AER functions at this time. Although the clock model (the relationship between the AER and skeletal pattern; Thorogood, 1991) and conventional diagrams of Hox regulation (the relationship between Hox and skeletal pattern; Metscher et al. 2005; Schneider et al. 2011; Woltering & Duboule, 2010) complement each other, it should be noted how the AER/AF directs the change of Hox regulation followed by transformation of skeletal pattern. Since fin-ray formation replaces endoskeleton formation after the AER–AF transition, it is possible that AF formation is an inhibitory factor or hindrance for outgrowth, patterning, and distal addition of the endoskeleton along the PD axis in fin development.
We have integrated ideas proposed to explain the similarities and differences between fins and limbs and explain the fin-to-limb evolution from the viewpoint of developmental process; ‘the repression mode of the AF (Fig. 5)’ bridging the clock model with gene expression (on the basis of Sordino et al. 1995; Freitas et al. 2007). In this diagram, we assume that the developmental mechanisms for the limb endoskeletal pattern (the PD separation of HoxA expression and AP expansion of 5′HoxD expression) are discontinued by AF formation (AER-to-AF transition), even if the mechanisms are latent in the fish fin. Given this assumption, this mode hypothesizes that different timings of the discontinuance of pattern formation produce the three types of appendages: fins, limb-like fins, and limbs: (A) In actinopterygians, fins are formed with less-patterned endoskeleton along the PD axis as the late-phase developmental mechanisms are shut off, because of the earlier timing of the AER–AF transition. (B) In sarcopterygian fish (Eustenopteron, Panderichthys and Tiktaalik) and chondrichthyans (shark and skate), the formation of limb-like fins with proximal domains (stylopod and zeugopod) is regulated by persistent AER functioning. Skeletal variations in the zeugopod and autopod of limb-like fins are due to an incomplete regulation of the PD patterning by HoxA and of the AP expansion by 5′HoxD. This incompleteness may also be caused by the later timing of AF formation. (C) In tetrapods, the AF is never formed, allowing the limbs to develop the endoskeletal pattern fully under the regulation of AER signals. The loss of the AF coincides with the acquisition of the autopod provided by the complete functions of HoxA and 5′HoxD. Hox proteins act to define the appendage types on the macro-scale such as fins, limb-like fins, and limbs, depending on the AF repression. The function of Shh, on the other hand, is more homogeneous, with micro-scale variations among fins, or among limb-like fins, or among limbs, and variations in the Shh system are independent of AF repression.
Fig. 5.
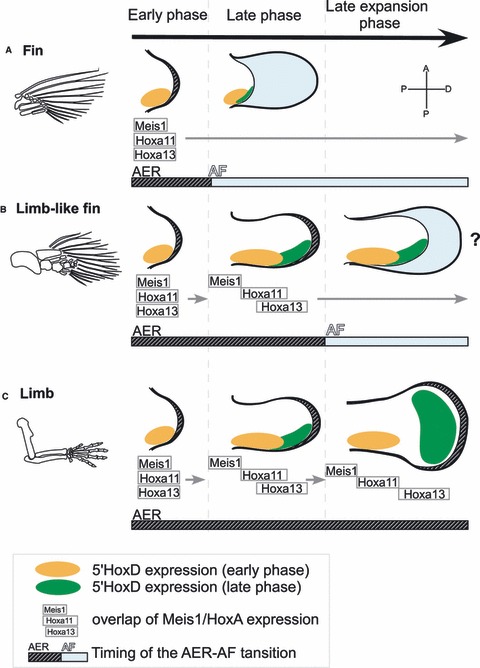
The repression mode of the AF. Morphological differences among fins, limb-like fins, and limbs are explained by a combination of developmental mechanisms: separation of the Meis/HoxA11/HoxA13 expression domains, the degree of 5′HoxD late expansion, and the occurrence and timing of the AER–AF transition. (A) In fin development in actinopterygians, the AER–AF transition occurs at early stages of development, and fin mesenchyme starts differentiating into endoskeleton before the completion of successive change in the gene expression domains (Grandel & Schulte-Merker, 1998). With this timing of transition, the Meis/Hoxa11/Hoxa13 expressions overlap within a domain, and the late phase of 5′Hoxd regulation is not functional (Sordino et al. 1995; Grandel et al. 2000; Metscher et al. 2005). (B) In the limb-like fin development of sarcopterygian fish, the AER–AF transition is speculated to occur later than in other fish (Thorogood, 1991). As a result, distinct Meis/HoxA11 expression domains occur, resulting in formation of the stylopod. When the AER–AF transition occurs, the separation of the HoxA11/HoxA13 domains is still incomplete, and the 5′HoxD domain is posteriorly restricted (Shubin et al. 2009; Woltering & Duboule, 2010; Schneider et al. 2011), resulting in the formation of a zeugopod and dwarfish autopod without any digits (Cote et al. 2002; Shubin et al. 2006; Boisvert et al. 2008). (C) In the limb development of tetrapods, the AF does not form, and the sustained AER promotes the proliferation of undifferentiated mesenchyme (Guo et al. 2003). The expression domain of HoxA13 is separated from that of HoxA11 (Yokouchi et al. 1991; Nelson et al. 1996; Stadler et al. 2001; Sato et al. 2007) and the late-phase 5′HoxD domain is expanded along the AP axis, giving rise to a complete set of autopod elements, including digits. In (A) and (B), the AF formed after the AER–AF transition represses any further progression of molecular mechanisms in the endoskeletal region and discontinues the PD and AP patterning therein. Distal is to the right; anterior is to the top.
The repression mode of the AF can be adapted to all gnathostome appendages. Chondrichthyan fins can be classified as a variation of type (B) appendage, which has a distinct domain of Meis1 expression, incomplete separation of Hoxa11 and Hoxa13 expression domains (Sakamoto et al. 2009), anterior expansion of 5′HoxD late-phase expression (Freitas et al. 2007) and skeletal similarity as seen in the fins of sharks and Sauripterus. Extant crossopterygian (coelacanth and lungfish) fins show too complicated a skeletal pattern to ascertain which types of appendages they should be classified into; for example, the fin endoskeleton of the lungfish consists of a PD series of endoskeleton elements (Johanson et al. 2007) and looks like the hyperphalangy of dolphin or whale limbs, formed by a long exposure to AER signals (Richardson & Oelschlager, 2002). Indeed, the AER–AF transition in lungfish fins is a slow process that includes halfway stages in which the AER and AF co-exist (Hodgkinson et al. 2009). Johanson et al. (2007) suggest that the late phase of hoxd13 expression in the lungfish fin looks like that in tetrapod limbs. Thus, this pattern may be classified as (B) in the repression mode of the AF.
Conclusion
Discoveries and analyses of the fossils of sarcopterygian fish reveal clues to the mystery of the fin-to-limb evolutionary step. With the help of these findings, the mechanisms underlying several types of appendage formation can be examined further from a developmental biology viewpoint, including the molecular networks (trans-acting factors) and/or gene interactions (cis-regulatory elements) involved. Notable achievements have been made in elucidating limb development, and these studies shown that complex interactions of Hox, Shh, AER signals, and other molecules are involved. In addition, studies using classical microsurgeries and genetic inducible knockdown/knockout technologies have also provided clues about the mechanisms underlying skeletal variation (Zeller et al. 2009). On the other hand, mutagenesis and gene knockdown analyses of fish fins have revealed not skeletal variation but rather fin induction (Mercader, 2007). To confirm the repression mode of the AF and to better understand how the differences among fins, limb-like fins, and limbs arose, it will be important not only to compare gene expression patterns but also to examine the spatiotemporal effects of various molecules (e.g. Hox, Shh, and Fgf) and fin-specific genetic relationships in fin development, using microsurgery and transgenic studies (Asakawa et al. 2008; Curado et al. 2008; Hans et al. 2011).
Acknowledgments
We thank Dr. Gembu Abe and Dr. Koichi Kawakami for providing the technique of Tol2-madiated transgenesis. This work was supported by research grants from the Ministry of Education, Science, Sports and Culture of Japan, from KAKENHI (Grant-in-Aid for Scientific Research), and from the ‘Funding Program for the Next Generation of World-Leading Researchers’ from the Cabinet Office, the Government of Japan. T.Y. was supported by JSPS Research Fellowships for Young Scientists, Japan.
References
- Abe G, Ide H, Tamura K. Function of FGF signaling in the developmental process of the median fin fold in zebrafish. Dev Biol. 2007;304:355–366. doi: 10.1016/j.ydbio.2006.12.040. [DOI] [PubMed] [Google Scholar]
- Ahn D, Ho RK. Tri-phasic expression of posterior Hox genes during development of pectoral fins in zebrafish: implications for the evolution of vertebrate paired appendages. Dev Biol. 2008;322:220–233. doi: 10.1016/j.ydbio.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Asakawa K, Suster ML, Mizusawa K, et al. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc Natl Acad Sci U S A. 2008;105:1255–1260. doi: 10.1073/pnas.0704963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenstein D. Studies on the development of the dorsal fin in amphibians. J Exp Zool. 1952;120:213–245. [Google Scholar]
- Boisvert CA, Mark-Kurik E, Ahlberg PE. The pectoral fin of Panderichthys and the origin of digits. Nature. 2008;456:636–638. doi: 10.1038/nature07339. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Tsukui T, Rodriquez Esteban C, et al. Control of vertebrate limb outgrowth by the proximal factor Meis2 and distal antagonism of BMPs by Gremlin. Mol Cell. 1999;4:839–849. doi: 10.1016/s1097-2765(00)80393-7. [DOI] [PubMed] [Google Scholar]
- Chai Y, Maxson RE., Jr Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353–2375. doi: 10.1002/dvdy.20833. [DOI] [PubMed] [Google Scholar]
- Cole NJ, Currie PD. Insights from sharks: evolutionary and developmental models of fin development. Dev Dyn. 2007;236:2421–2431. doi: 10.1002/dvdy.21268. [DOI] [PubMed] [Google Scholar]
- Cooper KL, Hu JK, ten Berge D, et al. Initiation of proximal-distal patterning in the vertebrate limb by signals and growth. Science. 2011;332:1083–1086. doi: 10.1126/science.1199499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cote S, Carroll R, Cloutier R, et al. Vertebral development in the Devonian Sarcopterygian fish Eusthenopteron foordi and the polarity of vertebral evolution in non-amniote tetrapods. J Vertebr Paleontol. 2002;22:487–502. [Google Scholar]
- Curado S, Stainier DY, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc. 2008;3:948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahn RD, Davis MC, Pappano WN, et al. Sonic hedgehog function in chondrichthyan fins and the evolution of appendage patterning. Nature. 2007;445:311–314. doi: 10.1038/nature05436. [DOI] [PubMed] [Google Scholar]
- Dane PJ, Tucker JB. Modulation of epidermal cell shaping and extracellular matrix during caudal fin morphogenesis in the zebra fish Brachydanio rerio. J Embryol Exp Morphol. 1985;87:145–161. [PubMed] [Google Scholar]
- Davis MC, Shubin N, Daeschler EB. A new specimen of Sauripterus taylori (Sarcopterygii, Osteichthyes) from the Famennian Catskill formation of North America. J Vertebr Paleontol. 2004a;24:26–40. [Google Scholar]
- Davis MC, Shubin NH, Force A. Pectoral fin and girdle development in the basal actinopterygians Polyodon spathula and Acipenser transmontanus. J Morphol. 2004b;262:608–628. doi: 10.1002/jmor.10264. [DOI] [PubMed] [Google Scholar]
- Davis MC, Dahn RD, Shubin NH. An autopodial-like pattern of Hox expression in the fins of a basal actinopterygian fish. Nature. 2007;447:473–476. doi: 10.1038/nature05838. [DOI] [PubMed] [Google Scholar]
- Deguchi T, Itoh M, Urawa H, et al. Infrared laser-mediated local gene induction in medaka, zebrafish and Arabidopsis thaliana. Dev Growth Differ. 2009;51:769–775. doi: 10.1111/j.1440-169X.2009.01135.x. [DOI] [PubMed] [Google Scholar]
- Draper BW, Stock DW, Kimmel CB. Zebrafish fgf24 functions with fgf8 to promote posterior mesodermal development. Development. 2003;130:4639–4654. doi: 10.1242/dev.00671. [DOI] [PubMed] [Google Scholar]
- Dushane GP. An experimental study of the origin of pigment cells in Amphibia. J Exp Zool. 1935;72:1–31. [Google Scholar]
- Eisen JS, Weston JA. Development of the neural crest in the zebrafish. Dev Biol. 1993;159:50–59. doi: 10.1006/dbio.1993.1220. [DOI] [PubMed] [Google Scholar]
- Fagotto F, Gumbiner BM. Beta-catenin localization during Xenopus embryogenesis – accumulation at tissue and somite boundaries. Development. 1994;120:3667–3679. doi: 10.1242/dev.120.12.3667. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teran M, Ros MA. The apical ectodermal ridge: morphological aspects and signaling pathways. Int J Dev Biol. 2008;52:857–871. doi: 10.1387/ijdb.072416mf. [DOI] [PubMed] [Google Scholar]
- Fischer S, Draper BW, Neumann CJ. The zebrafish fgf24 mutant identifies an additional level of Fgf signaling involved in vertebrate forelimb initiation. Development. 2003;130:3515–3524. doi: 10.1242/dev.00537. [DOI] [PubMed] [Google Scholar]
- Freitas R, Zhang G, Cohn MJ. Evidence that mechanisms of fin development evolved in the midline of early vertebrates. Nature. 2006;442:1033–1037. doi: 10.1038/nature04984. [DOI] [PubMed] [Google Scholar]
- Freitas R, Zhang G, Cohn MJ. Biphasic Hoxd gene expression in shark paired fins reveals an ancient origin of the distal limb domain. PLoS ONE. 2007;2:e754. doi: 10.1371/journal.pone.0000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargioli C, Slack JM. Cell lineage tracing during Xenopus tail regeneration. Development. 2004;131:2669–2679. doi: 10.1242/dev.01155. [DOI] [PubMed] [Google Scholar]
- Gautier P, Naranjo-Golborne C, Taylor MS, et al. Expression of the fras1/frem gene family during zebrafish development and fin morphogenesis. Dev Dyn. 2008;237:3295–3304. doi: 10.1002/dvdy.21729. [DOI] [PubMed] [Google Scholar]
- Geraudie J, Landis WJ. The fine structure of the developing pelvic fin dermal skeleton in the trout Salmo gairdneri. Am J Anat. 1982;163:141–156. doi: 10.1002/aja.1001630204. [DOI] [PubMed] [Google Scholar]
- Gibert Y, Gajewski A, Meyer A, et al. Induction and prepatterning of the zebrafish pectoral fin bud requires axial retinoic acid signaling. Development. 2006;133:2649–2659. doi: 10.1242/dev.02438. [DOI] [PubMed] [Google Scholar]
- Goodrich ES. On the dermal fin-rays of fishes – living and extinct. Quart J Microscop Sci. 1904;47:465–522. [Google Scholar]
- Graham A, Begbie J, McGonnell I. Significance of the cranial neural crest. Dev Dyn. 2004;229:5–13. doi: 10.1002/dvdy.10442. [DOI] [PubMed] [Google Scholar]
- Grandel H, Schulte-Merker S. The development of the paired fins in the zebrafish (Danio rerio. Mech Dev. 1998;79:99–120. doi: 10.1016/s0925-4773(98)00176-2. [DOI] [PubMed] [Google Scholar]
- Grandel H, Draper BW, Schulte-Merker S. Dackel acts in the ectoderm of the zebrafish pectoral fin bud to maintain AER signaling. Development. 2000;127:4169–4178. doi: 10.1242/dev.127.19.4169. [DOI] [PubMed] [Google Scholar]
- Guo Q, Loomis C, Joyner AL. Fate map of mouse ventral limb ectoderm and the apical ectodermal ridge. Dev Biol. 2003;264:166–178. doi: 10.1016/j.ydbio.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Hall BK. Bones and Cartilage: Developmental and Evolutionary Skeletal Biology. London: Elsevier Academic Press; 2005. [Google Scholar]
- Hans S, Freudenreich D, Geffarth M, et al. Generation of a non-leaky heat shock-inducible Cre line for conditional Cre/lox strategies in zebrafish. Dev Dyn. 2011;240:108–115. doi: 10.1002/dvdy.22497. [DOI] [PubMed] [Google Scholar]
- Harfe BD, Scherz PJ, Nissim S, et al. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell. 2004;118:517–528. doi: 10.1016/j.cell.2004.07.024. [DOI] [PubMed] [Google Scholar]
- Hartmann C, Tabin CJ. Dual roles of Wnt signaling during chondrogenesis in the chicken limb. Development. 2000;127:3141–3159. doi: 10.1242/dev.127.14.3141. [DOI] [PubMed] [Google Scholar]
- Harvey SA, Logan MP. sall4 acts downstream of tbx5 and is required for pectoral fin outgrowth. Development. 2006;133:1165–1173. doi: 10.1242/dev.02259. [DOI] [PubMed] [Google Scholar]
- Hernandez-Vega A, Minguillon C. The Prx1 limb enhancers: targeted gene expression in developing zebrafish pectoral fins. Dev Dyn. 2011;240:1977–1988. doi: 10.1002/dvdy.22678. [DOI] [PubMed] [Google Scholar]
- Hodgkinson VS, Ericsson R, Johanson Z, et al. The apical ectodermal ridge in the pectoral fin of the Australian Lungfish (Neoceratodus forsteri): keeping the fin to limb transition in the fold. Acta Zool. 2009;90:253–263. [Google Scholar]
- Johanson Z, Joss J, Boisvert CA, et al. Fish fingers: digit homologues in sarcopterygian fish fins. J Exp Zool B Mol Dev Evol. 2007;308:757–768. doi: 10.1002/jez.b.21197. [DOI] [PubMed] [Google Scholar]
- Jovelin R, He X, Amores A, et al. Duplication and divergence of fgf8 functions in teleost development and evolution. J Exp Zool B Mol Dev Evol. 2007;308:730–743. doi: 10.1002/jez.b.21193. [DOI] [PubMed] [Google Scholar]
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- Kawakami K. Transgenesis and gene trap methods in zebrafish by using the Tol2 transposable element. Methods Cell Biol. 2004;77:201–222. doi: 10.1016/s0091-679x(04)77011-9. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Takeda H, Kawakami N, et al. A transposon-mediated gene trap approach identifies developmentally regulated genes in zebrafish. Dev Cell. 2004a;7:133–144. doi: 10.1016/j.devcel.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Esteban CR, Matsui T, et al. Sp8 and Sp9, two closely related buttonhead-like transcription factors, regulate Fgf8 expression and limb outgrowth in vertebrate embryos. Development. 2004b;131:4763–4774. doi: 10.1242/dev.01331. [DOI] [PubMed] [Google Scholar]
- Kengaku M, Capdevila J, Rodriguez-Esteban C, et al. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science. 1998;280:1274–1277. doi: 10.1126/science.280.5367.1274. [DOI] [PubMed] [Google Scholar]
- Landis WJ, Geraudie J. Organization and development of the mineral phase during early ontogeny of the bony fin rays of the trout Oncorhynchus-Mykiss. Anat Rec. 1990;228:383–391. doi: 10.1002/ar.1092280404. [DOI] [PubMed] [Google Scholar]
- Lee JS, von der Hardt S, Rusch MA, et al. Axon sorting in the optic tract requires HSPG synthesis by ext2 (dackel) and extl3 (boxer) Neuron. 2004;44:947–960. doi: 10.1016/j.neuron.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Litingtung Y, Dahn RD, Li Y, et al. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418:979–983. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- Loomis CA, Kimmel RA, Tong CX, et al. Analysis of the genetic pathway leading to formation of ectopic apical ectodermal ridges in mouse Engrailed-1 mutant limbs. Development. 1998;125:1137–1148. doi: 10.1242/dev.125.6.1137. [DOI] [PubMed] [Google Scholar]
- Lu P, Yu Y, Perdue Y, et al. The apical ectodermal ridge is a timer for generating distal limb progenitors. Development. 2008;135:1395–1405. doi: 10.1242/dev.018945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, et al. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani FV, Ahn CP, Martin GR. Genetic evidence that FGFs have an instructive role in limb proximal-distal patterning. Nature. 2008;453:401–405. doi: 10.1038/nature06876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, et al. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–355. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercader N. Early steps of paired fin development in zebrafish compared with tetrapod limb development. Dev Growth Differ. 2007;49:421–437. doi: 10.1111/j.1440-169X.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- Mercader N, Leonardo E, Azpiazu N, et al. Conserved regulation of proximodistal limb axis development by Meis1/Hth. Nature. 1999;402:425–429. doi: 10.1038/46580. [DOI] [PubMed] [Google Scholar]
- Mercader N, Leonardo E, Piedra ME, et al. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- Metscher BD, Takahashi K, Crow K, et al. Expression of Hoxa-11 and Hoxa-13 in the pectoral fin of a basal ray-finned fish, Polyodon spathula: implications for the origin of tetrapod limbs. Evol Dev. 2005;7:186–195. doi: 10.1111/j.1525-142X.2005.05021.x. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Ayer-le Lievre CS. Mesectodermal capabilities of the trunk neural crest of birds. J Embryol Exp Morphol. 1982;70:1–18. [PubMed] [Google Scholar]
- Nelson CE, Morgan BA, Burke AC, et al. Analysis of Hox gene expression in the chick limb bud. Development. 1996;122:1449–1466. doi: 10.1242/dev.122.5.1449. [DOI] [PubMed] [Google Scholar]
- Neumann CJ, Grandel H, Gaffield W, et al. Transient establishment of anteroposterior polarity in the zebrafish pectoral fin bud in the absence of sonic hedgehog activity. Development. 1999;126:4817–4826. doi: 10.1242/dev.126.21.4817. [DOI] [PubMed] [Google Scholar]
- Neyt C, Jagla K, Thisse C, et al. Evolutionary origins of vertebrate appendicular muscle. Nature. 2000;408:82–86. doi: 10.1038/35040549. [DOI] [PubMed] [Google Scholar]
- Ng JK, Kawakami Y, Buscher D, et al. The limb identity gene Tbx5 promotes limb initiation by interacting with Wnt2b and Fgf10. Development. 2002;129:5161–5170. doi: 10.1242/dev.129.22.5161. [DOI] [PubMed] [Google Scholar]
- Noden DM. The control of avian cephalic neural crest cytodifferentiation. I. Skeletal and connective tissues. Dev Biol. 1978;67:296–312. doi: 10.1016/0012-1606(78)90201-4. [DOI] [PubMed] [Google Scholar]
- Nomura R, Kamei E, Hotta Y, et al. Fgf16 is essential for pectoral fin bud formation in zebrafish. Biochem Biophys Res Commun. 2006;347:340–346. doi: 10.1016/j.bbrc.2006.06.108. [DOI] [PubMed] [Google Scholar]
- Norton WH, Ledin J, Grandel H, et al. HSPG synthesis by zebrafish Ext2 and Extl3 is required for Fgf10 signalling during limb development. Development. 2005;132:4963–4973. doi: 10.1242/dev.02084. [DOI] [PubMed] [Google Scholar]
- Ohgo S, Itoh A, Suzuki M, et al. Analysis of hoxa11 and hoxa13 expression during patternless limb regeneration in Xenopus. Dev Biol. 2010;338:148–157. doi: 10.1016/j.ydbio.2009.11.026. [DOI] [PubMed] [Google Scholar]
- Okabe M, Graham A. The origin of the parathyroid gland. Proc Natl Acad Sci U S A. 2004;101:17716–17719. doi: 10.1073/pnas.0406116101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff RA. Written in stone: fossils, genes and evo-devo. Nat Rev Genet. 2007;8:911–920. doi: 10.1038/nrg2225. [DOI] [PubMed] [Google Scholar]
- Richardson MK, Oelschlager HH. Time, pattern, and heterochrony: a study of hyperphalangy in the dolphin embryo flipper. Evol Dev. 2002;4:435–444. doi: 10.1046/j.1525-142x.2002.02032.x. [DOI] [PubMed] [Google Scholar]
- Rosello-Diez A, Ros MA, Torres M. Diffusible signals, not autonomous mechanisms, determine the main proximodistal limb subdivision. Science. 2011;332:1086–1088. doi: 10.1126/science.1199489. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Onimaru K, Munakata K, et al. Heterochronic shift in Hox-mediated activation of sonic hedgehog leads to morphological changes during fin development. PLoS ONE. 2009;4:e5121. doi: 10.1371/journal.pone.0005121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Koizumi Y, Takahashi M, et al. Specification of cell fate along the proximal-distal axis in the developing chick limb bud. Development. 2007;134:1397–1406. doi: 10.1242/dev.02822. [DOI] [PubMed] [Google Scholar]
- Schaeffer B. Deuterostome monophyly and phylogeny. Evol Biol. 1987;21:179–235. [Google Scholar]
- Schneider I, Aneas I, Gehrke AR, et al. Appendage expression driven by the Hoxd Global Control Region is an ancient gnathostome feature. Proc Natl Acad Sci U S A. 2011;108:12782–12786. doi: 10.1073/pnas.1109993108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubin NH, Daeschler EB, Jenkins FA., Jr The pectoral fin of Tiktaalik roseae and the origin of the tetrapod limb. Nature. 2006;440:764–771. doi: 10.1038/nature04637. [DOI] [PubMed] [Google Scholar]
- Shubin N, Tabin C, Carroll S. Deep homology and the origins of evolutionary novelty. Nature. 2009;457:818–823. doi: 10.1038/nature07891. [DOI] [PubMed] [Google Scholar]
- Smith M, Hickman A, Amanze D, et al. Trunk neural crest origin of caudal fin mesenchyme in the Zebrafish Branchydanio rerio. Proc R Soc Lond B. 1994;256:137–145. [Google Scholar]
- Sordino P, van der Hoeven F, Duboule D. Hox gene expression in teleost fins and the origin of vertebrate digits. Nature. 1995;375:678–681. doi: 10.1038/375678a0. [DOI] [PubMed] [Google Scholar]
- Sordino P, Duboule D, Kondo T. Zebrafish Hoxa and Evx-2 genes: cloning, developmental expression and implications for the functional evolution of posterior Hox genes. Mech Dev. 1996;59:165–175. doi: 10.1016/0925-4773(96)00587-4. [DOI] [PubMed] [Google Scholar]
- Stadler HS, Higgins KM, Capecchi MR. Loss of Eph-receptor expression correlates with loss of cell adhesion and chondrogenic capacity in Hoxa13 mutant limbs. Development. 2001;128:4177–4188. doi: 10.1242/dev.128.21.4177. [DOI] [PubMed] [Google Scholar]
- Sun X, Lewandoski M, Meyers EN, et al. Conditional inactivation of Fgf4 reveals complexity of signalling during limb bud development. Nat Genet. 2000;25:83–86. doi: 10.1038/75644. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Satoh A, Ide H, et al. Transgenic Xenopus with prx1 limb enhancer reveals crucial contribution of MEK/ERK and PI3K/AKT pathways in blastema formation during limb regeneration. Dev Biol. 2007;304:675–686. doi: 10.1016/j.ydbio.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Hara Y, Takagi C, et al. MID1 and MID2 are required for Xenopus neural tube closure through the regulation of microtubule organization. Development. 2010;137:2329–2339. doi: 10.1242/dev.048769. [DOI] [PubMed] [Google Scholar]
- Tamura K, Yokouchi Y, Kuroiwa A, et al. Retinoic acid changes the proximodistal developmental competence and affinity of distal cells in the developing chick limb bud. Dev Biol. 1997;188:224–234. doi: 10.1006/dbio.1997.8627. [DOI] [PubMed] [Google Scholar]
- Tamura K, Yonei-Tamura S, Yano T, et al. The autopod: its formation during limb development. Dev Growth Differ. 2008;50:S177–S187. doi: 10.1111/j.1440-169X.2008.01020.x. [DOI] [PubMed] [Google Scholar]
- Tamura K, Ohgo S, Yokoyama H. Limb blastema cell: a stem cell for morphological regeneration. Dev Growth Differ. 2010;52:89–99. doi: 10.1111/j.1440-169X.2009.01144.x. [DOI] [PubMed] [Google Scholar]
- Tarchini B, Duboule D. Control of Hoxd genes’ collinearity during early limb development. Dev Cell. 2006;10:93–103. doi: 10.1016/j.devcel.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Thorogood P. The development of the teleost fin and implications for our understanding of tetrapod evolution. In: Hinchliffe J, Hurle J, Summerbell D, editors. Developmental Patterning of the Vertebrate Limb. London: Plenum Press; 1991. pp. 347–354. [Google Scholar]
- Tucker AS, Slack JMW. Independent induction and formation of the dorsal and ventral fins in Xenopus laevis. Dev Dyn. 2004;230:461–467. doi: 10.1002/dvdy.20071. [DOI] [PubMed] [Google Scholar]
- Urasaki A, Morvan G, Kawakami K. Functional dissection of the Tol2 transposable element identified the minimal cis-sequence and a highly repetitive sequence in the subterminal region essential for transposition. Genetics. 2006;174:639–649. doi: 10.1534/genetics.106.060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobyeva EI. The role of development and function in formation of tetrapod-like pectoral fins. Zh Obshch Biol. 1992;53:149–158. [Google Scholar]
- Waskiewicz AJ, Rikhof HA, Hernandez RE, et al. Zebrafish Meis functions to stabilize Pbx proteins and regulate hindbrain patterning. Development. 2001;128:4139–4151. doi: 10.1242/dev.128.21.4139. [DOI] [PubMed] [Google Scholar]
- Webb AE, Sanderford J, Frank D, et al. Laminin alpha5 is essential for the formation of the zebrafish fins. Dev Biol. 2007;311:369–382. doi: 10.1016/j.ydbio.2007.08.034. [DOI] [PubMed] [Google Scholar]
- te Welscher P, Zuniga A, Kuijper S, et al. Progression of vertebrate limb development through SHH-mediated counteraction of GLI3. Science. 2002;298:827–830. doi: 10.1126/science.1075620. [DOI] [PubMed] [Google Scholar]
- Woltering JM, Duboule D. The origin of digits: expression patterns versus regulatory mechanisms. Dev Cell. 2010;18:526–532. doi: 10.1016/j.devcel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Wood A, Thorogood P. An analysis of in vivo cell migration during teleost fin morphogenesis. J Cell Sci. 1984;66:205–222. doi: 10.1242/jcs.66.1.205. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Zhao X, Uehara M, et al. Regulation of retinoic acid distribution is required for proximodistal patterning and outgrowth of the developing mouse limb. Dev Cell. 2004;6:411–422. doi: 10.1016/s1534-5807(04)00062-0. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y, Sasaki H, Kuroiwa A. Homeobox gene expression correlated with the bifurcation process of limb cartilage development. Nature. 1991;353:443–445. doi: 10.1038/353443a0. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y, Nakazato S, Yamamoto M, et al. Misexpression of Hoxa-13 induces cartilage homeotic transformation and changes cell adhesiveness in chick limb buds. Genes Dev. 1995;9:2509–2522. doi: 10.1101/gad.9.20.2509. [DOI] [PubMed] [Google Scholar]
- Yonei-Tamura S, Abe G, Tanaka Y, et al. Competent stripes for diverse positions of limbs/fins in gnathostome embryos. Evol Dev. 2008;10:737–745. doi: 10.1111/j.1525-142X.2008.00288.x. [DOI] [PubMed] [Google Scholar]
- Zakany J, Duboule D. The role of Hox genes during vertebrate limb development. Curr Opin Genet Dev. 2007;17:359–366. doi: 10.1016/j.gde.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Zakany J, Fromental-Ramain C, Warot X, et al. Regulation of number and size of digits by posterior Hox genes: a dose-dependent mechanism with potential evolutionary implications. Proc Natl Acad Sci U S A. 1997;94:13695–13700. doi: 10.1073/pnas.94.25.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller R. The temporal dynamics of vertebrate limb development, teratogenesis and evolution. Curr Opin Genet Dev. 2010;20:384–390. doi: 10.1016/j.gde.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Zeller R, Lopez-Rios J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet. 2009;10:845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- Zhang J, Wagh P, Guay D, et al. Loss of fish actinotrichia proteins and the fin-to-limb transition. Nature. 2010;466:234–237. doi: 10.1038/nature09137. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang Z, Collins JE, et al. Comparative transcriptome analyses indicate molecular homology of zebrafish swimbladder and mammalian lung. PLoS ONE. 2011;6:e24019. doi: 10.1371/journal.pone.0024019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Zhang YT, Alber MS, et al. Bare bones pattern formation: a core regulatory network in varying geometries reproduces major features of vertebrate limb development and evolution. PLoS ONE. 2010;5:e10892. doi: 10.1371/journal.pone.0010892. [DOI] [PMC free article] [PubMed] [Google Scholar]


