Abstract
Our research on the evolution of the vertebrate head focuses on understanding the developmental origins of morphological novelties. Using a broad comparative approach in amphibians, and comparisons with the well-studied quail-chicken system, we investigate how evolutionarily conserved or variable different aspects of head development are. Here we review research on the often overlooked development of cranial muscles, and on its dependence on cranial cartilage development. In general, cranial muscle cell migration and the spatiotemporal pattern of cranial muscle formation appears to be very conserved among the few species of vertebrates that have been studied. However, fate-mapping of somites in the Mexican axolotl revealed differences in the specific formation of hypobranchial muscles (tongue muscles) in comparison to the chicken. The proper development of cranial muscles has been shown to be strongly dependent on the mostly neural crest-derived cartilage elements in the larval head of amphibians. For example, a morpholino-based knock-down of the transcription factor FoxN3 in Xenopus laevis has drastic indirect effects on cranial muscle patterning, although the direct function of the gene is mostly connected to neural crest development. Furthermore, extirpation of single migratory streams of cranial neural crest cells in combination with fate-mapping in a frog shows that individual cranial muscles and their neural crest-derived connective tissue attachments originate from the same visceral arch, even when the muscles attach to skeletal components that are derived from a different arch. The same pattern has also been found in the chicken embryo, the only other species that has been thoroughly investigated, and thus might be a conserved pattern in vertebrates that reflects the fundamental nature of a mechanism that keeps the segmental order of the head in place despite drastic changes in adult anatomy. There is a need for detailed comparative fate-mapping of pre-otic paraxial mesoderm in amphibians, to determine developmental causes underlying the complicated changes in cranial muscle development and architecture within amphibians, and in particular how the novel mouth apparatus in frog tadpoles evolved. This will also form a foundation for further research into the molecular mechanisms that regulate rostral head morphogenesis. Our empirical studies are discussed within a theoretical framework concerned with the evolutionary origin and developmental basis of novel anatomical structures in general. We argue that a common developmental origin is not a fool-proof guide to homology, and that a view that sees only structures without homologs as novel is too restricted, because novelties must be produced by changes in the same framework of developmental processes. At the level of developmental processes and mechanisms, novel structures are therefore likely to have homologs, and we need to develop a hierarchical concept of novelty that takes this into account.
Keywords: extirpation, FOXN3 morpholino, GFP-transgenic axolotl, long-term fate-mapping, novelties, somites
Introduction
The development of the vertebrate head is a classical subject in comparative morphology that has a rich older literature (Gegenbaur, 1888; Goodrich, 1930; de Beer, 1937 for review), and a modern three-volume treatise on the vertebrate skull (Hanken & Hall, 1993) devotes an entire volume to development. Among the many research questions currently being asked in the field of vertebrate head development and evolution, we will in this review concentrate on the development of cranial muscles, which is less studied in comparison to the skeletal elements of the head. Even though muscles and most of the skeletal tissues of the head are derived from two different embryological sources (head mesoderm vs. neural crest), their development is tightly intermingled with each other. Our experimental approaches include classical extirpation techniques, long-term fate-mapping and gene knock-down experiments using morpholinos in amphibians, evaluated using both classical (histology, clearing-and-staining) and novel methods such as micro-CT, as well as documenting development using immunostaining combined with confocal microscopy.
The vertebrate head has undergone drastic anatomical changes during evolution. Major radiations might have been possible only in connection with the origin of novelties, as for example the jaws and the cranial muscles associated with them, which enabled gnathostomes to become efficient predators. Our work is mainly focused on the developmental origin of such novel anatomical structures that entail important evolutionary consequences. Therefore, we first need to introduce how developmental studies at the cellular and molecular levels are connected to the more general questions of how novel structures emerge during vertebrate development and evolution.
The general problem: the origin of evolutionary novelties
An important aspect of evolution is the generation of novelties, but what is a novelty? Pigliucci (2008) has reviewed different uses of this concept. One extreme view is that ‘Novelties and apomorphies are essentially the same’ (Arthur, 2000), but this goes against the notion that novelties should refer to something of importance for the evolution of major groups, or for adaptive radiations, rather than something that is useful for distinguishing between closely related species. We would, for example, consider the shield (carapax and plastron) of turtles to be a novelty, but hardly the subtle differences in form and coloration of the shield between the different species of Galapagos turtles present on the different islands in the archipelago.
Another possibility is to focus on the function rather than the structure of the novelty. Ernst Mayr wrote that ‘Any newly acquired structure or property that permits the performance of a new function, which, in turn, will open a new adaptive zone’ is a novelty (Mayr, 1963). This allows for relatively subtle changes to be called novelties, and can be contrasted with the much more rigorous view taken by Müller & Wagner (1991), who maintain that a novelty must lack homologous structures in the ancestral species. Thus, structures often considered to be important novelties, such as the wings of birds, pterosaurs and bats, are not novelties because they are homologous to the forelimbs of other tetrapods and the pectoral fins of all gnathostomes. In this view feathers are a novelty but wings are not (Wagner & Lynch, 2009).
We think it would be incongruous to ignore the functional importance of a structure when deciding whether to call it a novelty or not. We need to work towards a concept of novelty that navigates between the Scylla of making all apomorphies into novelties and the Charybdis of defining the concept so narrowly that it does not take into account that novelties have to refer to evolutionarily important characters. Also, the hierarchical structure of organisms needs to be taken into account, so that a novel structure can be understood as the result of changes to a gene regulatory network that produces anatomical structures. In that way, novelties can be understood mechanistically and can be built into a general explanation of the evolution of developmental processes. Furthermore, there seems to be no way around using our value judgments of the evolutionary importance of a character to recognize novelties. From an EvoDevo point of view, novelties can be seen as the subset of apomorphic characters that are worthy of further investigation by scientists interested in seeking a general explanation for the evolution of development of these characters.
In our research, we have investigated several novel structures, the most drastic being the rostralia in frog tadpoles and the novel organization of cranial muscles that has evolved in parallel to operate the rostralia. The rostralia are cartilage elements that form a crucial part of a novel feeding apparatus, and the new arrangement of cranial muscles is necessary for their proper function. The rostralia can be considered as important novelties because of the evolutionary success (measured as species number) of frogs in comparison with other recent amphibians, which is likely to be associated with the evolution of this novel feeding apparatus in tadpoles. The evolutionary origin of the rostralia is unresolved (Svensson & Haas, 2005). They are normally situated in front of Meckel’s cartilage in the lower jaw (infrarostrals) and in front of the trabecular horns in the upper jaw (suprarostrals), and as a result most tadpoles have an ‘extra mouth’ in front of the structures that will form the mouth in the adult frog. Maybe the real evolutionary novelties are not the rostral cartilages as such, but the articulation between Meckel’s cartilage and the infrarostrals, and the articulation between the trabecular horns and the suprarostrals. If so, the infrarostrals are partitioned off from Meckel’s cartilage and the suprarostrals from the trabecular horns and would then have homologs.
The cells that make up the novel head skeletal structures have been shown to be neural crest-derived (see review by Gross & Hanken, 2008a). Associated muscles consist of muscle fibers of mesodermal origin, but the muscle attachment points and other connective tissues surrounding the muscle fibers are often neural crest derived (Köntges & Lumsden, 1996; Olsson et al. 2001). Below we describe this developmental system and our research on the cellular origin of muscles and skeleton in the head region in amphibians.
Cranial muscle patterns and development in salamander and frog larvae
The cranial muscles in amphibian larvae are traditionally divided into groups based on their innervation. The mandibular arch group, which includes muscles that close the mouth, are innervated by the fifth (trigeminal) cranial nerve, and the hyoid group, which includes muscles that open the jaw by the seventh (facial) cranial nerve. Further posteriorly, we find muscle groups innervated by the glossopharyngeus, vagus and hypoglossal nerves. Ambystoma mexicanum (the Mexican axolotl) shows a relatively basal larval head anatomy in relation to most other amphibians, and we will use it for comparisons with frog tadpoles such as Xenopus laevis (the African clawed frog) and Bombina orientalis (the Oriental fire-bellied toad), which show many specialized novel structures not present in salamander larvae. Most of our discussion will focus on a comparison between A. mexicanum and X. laevis for the simple reason that these are the major amphibian model systems that have been used, although some fate-mapping and extirpation data are available for B. orientalis and will be discussed as well. We exclude the third group of extant amphibians, the caecilians, entirely from the discussion because very little is known about cranial muscle development in these animals. Studies of caecilian larvae have shown a pattern of cranial muscles similar to that seen in salamanders such as the axolotl (Kleinteich & Haas, 2007, 2011), but the early development and important aspects such as fate-mapping are completely unknown.
Salamander larvae are carnivores that feed like an adult animal by suction feeding, whereas most frog tadpoles, unlike adults, are vegetarians that either scrape algae from the substrate, such as B. orientalis, or are mid-water suspension feeders like X. laevis larvae. There is reason to believe that the salamander mode is basal evolutionarily, and thus it becomes important to understand how the derived anatomy seen in frog tadpoles might have evolved.
In order to compare the pattern of cranial muscles, we can use A. mexicanum as a general example. Figure 1 shows lateral (A,C) and ventral (B,D) views of a stage 41 larva shortly before hatching of A. mexicanum stained for muscles (desmin, A and B) or cartilage (collagen II) and axons (acetylated alpha-tubulin, C and D) in whole-mount and scanned with a confocal microscope. The levator mandibulae muscles (lml and lme) are clearly separated from the depressor mandibulae muscle (dm), which (as can be seen in Fig. 1C), attaches to the posterior end of Meckel’s cartilage (Mc) and is innervated by another cranial nerve (VII) than the levator muscles (V; Fig. 1). Ventral muscles in the mandibular and hyoid arches are the intermandibularis (im) and interhyoideus (ih), and other ventrally located muscles are the geniohyoideus (gh) and the rectus cervicis (rc; Fig. 1B,D). Several differences become obvious when comparing this pattern from the axolotl with that of frog larvae. Frog tadpoles have evolved several novel skeletal elements, such as the rostralia, a pair of extra cartilages used for feeding (Fig. 2). This has led to major changes in the pattern and number of cranial muscles that operate these rostralia. The number of mandibular arch muscles in tadpoles varies, but is higher than in salamander larvae. Xenopus laevis tadpoles have five levator mandibulae muscles, of which one has a new function (to move the tentacle), but more typical tadpoles that scrape algae, such as B. orientalis, have up to seven muscles that move the supra- and infrarostral cartilages. Frog tadpoles have undergone drastic changes also to the hyoid muscles, such that the depressor mandibulae (Fig. 3A,B) has been divided into up to three different angularis muscles (hyo-, quadrato- and suspensorioangularis; am) with a ventral position, and two muscles that function in buccal pumping (the orbitohyoideus and suspensoriohyoideus muscles; osh). From a comparative perspective, it is important to understand the timing of early cranial muscle development, because changes in timing (heterochrony) often go along with changes in anatomy.
Fig. 1.
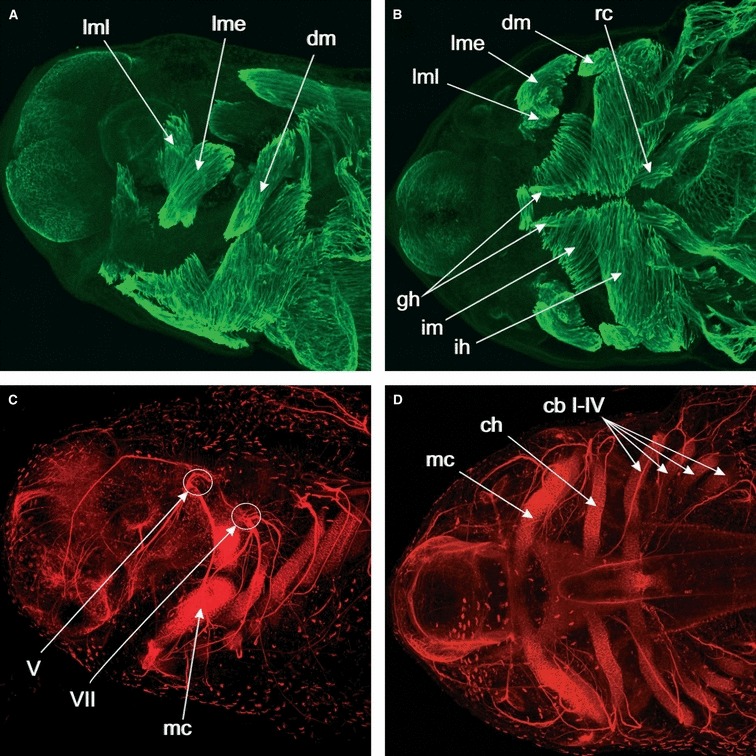
Whole-mount immunolabeled stage 41 Ambystoma mexicanum embryo. Max-projections of CLSM-image stacks. (A) Lateral view of the head. Desmin is used as a muscle marker and is expressed in the differentiating cranial muscles. The levator mandibulae longus (lml) is extending its origin dorsally and the levator mandibulae externus (lme) is extending caudally. The depressor mandibulae muscle (dm) is extending close to its insertion. (B) Ventral view of the head. Desmin expression in the differentiating cranial muscles. In addition to the muscles visible in lateral view, we now also see ventral muscles such as the intermandibularis (im) and interhyoideus (ih) muscles, and how they insert on the ventral midline. Also visible are the hypobranchial muscles geniohyoideus (gh) and rectus cervicis (rc). (C) Lateral view of the head. Nerve axons are marked by antibodies towards acetylated alpha-tubulin, cartilage is marked by a collagen II antibody. A comparison with (A), which shows the same specimen viewed in another channel to show the muscle staining, shows that the levator muscles are innervated by cranial nerve V and insert on the dorsal side of Meckel’s cartilage (mc), whereas the depressor mandibulae is innervated by cranial nerve VII and inserts at the caudal end of Meckel’s cartilage. (D) Markers as in (C). Ventral view of the head. By comparing with (B), we can see how cartilages and muscles match up. Levator and depressor mandibulae muscles, for example, attach to Meckel’s cartilage (mc), and the interhyoideus muscle (ih) to the ceratohyal (ch). Gill muscles are associated with the ceratobrachials I–IV (cb I–IV).
Fig. 2.
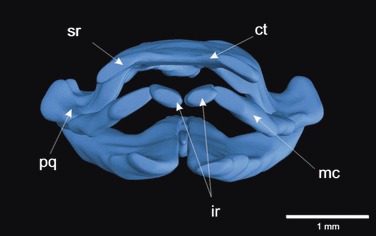
Three-dimensional reconstruction of the cranial cartilages of a stage 46 Xenopus laevis larva in frontal view. The unique infrarostral (ir) and suprarostral (sr) cartilages support the lower and upper jaw, which are composed of Meckel’s cartilage (mc) and the palatoquadrate (pq). The infrarostral cartilages articulate over a novel intramandibular joint with Meckel’s cartilage in the lower jaw and the suprarostral cartilages are fused with the cornua trabeculae (ct) in the upper jaw of X. laevis. In other frog larvae (such as Bombina orientalis), the suprarostral cartilages form separate cartilages that articulate rostrally with the trabecular horns. Cartilage: ct, cornua trabecula (trabecular horn); ir, infrarostral; mc, Meckel’s cartilage; pq, palatoquadrate; sr, suprarostral.
Fig. 3.
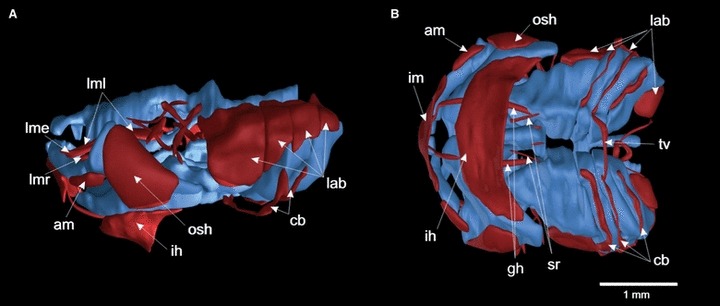
(A, B) Three-dimensional reconstruction of the cranial cartilages and muscles of a Xenopus laevis larva at stage 46, depicted in lateral (left) and ventral views. The cranial muscles are colored red and the cartilages blue. Xenopus laevis larvae have five levator mandibulae muscles, the levator longus group, which share a common origin (levator mandibulae longus superificalis, profundus and internus; lml), the levator mandibulae articularis (lmr) and the levator mandibulae externus (lme). The levator muscles extend diagonally from their origin to their insertion at Meckel’s cartilage (lml, lmr, lme) or the tentacle (lml pars profundus). They support the elevation of the lower jaw to close the mouth, and the pars profundus effect the retraction of the tentacular cartilage. The hyoid muscles have also undergone drastic evolutionary changes, such that the depressor mandibulae is divided into three different angularis muscles (hyo-, quadrato- and suspensorioangularis; am) with a more ventral position compared with Ambystoma mexicanum, and two lateral portions, the orbitohyoideus and suspensoriohyoideus muscles (osh). The ventral muscles also show differences to those in A. mexicanum. The interhyoideus and intermandibularis muscles (ih, im) form broad transverse bands and have no insertion on the midline. In addition, the hypobranchial muscle m.geniohyoideus, which functions in opening the mouth, inserts on the infrarostral cartilage in X. laevis, not on Meckel’s cartilage as it does in A. mexicanum. Muscles: am, angularis muscles; cb, mm. constrictores branchiales II–IV; gh, m. geniohyoideus; ih, m. interhyoideus; im, m. intermandibularis; lab, mm. levator arcuum branchialium I–IV; lme, m. levator mandibulae externus; lml, m. levator mandibulae longus; lmr, m. levator mandibulae anterior; osh, m. orbitohyoideus; rc, m. rectus cervicis; sr, m. subarcualis rectus I; tv, m. transversus.
Studies of cranial muscle development have shown that the beginning of development is highly conserved, with muscle plates (anlagen) forming in the same position and number in both salamanders and frogs. Thus, in both types of larvae a mandibular muscle plate is formed that becomes associated with cranial nerve V, a hyoid muscle plate forms in association with cranial nerve VII, and so on. Differences start to appear in later development, so that in frog tadpoles the cranial muscle anlagen become divided up into a higher number of distinct muscles than in the salamander larvae. Changes in developmental mechanisms that underlie the evolution of the cranial muscle diversity seen in frog tadpoles today, however, remain almost completely unknown, and this is an important field for future studies.
Despite the anatomical differences in cranial muscle arrangements between the larvae of salamanders and tadpoles, aspects of the timing of their development are quite similar. Ericsson & Olsson (2004) showed that the cranial muscles in the axolotl develop in a conserved spatio-temporal pattern. The muscle anlagen are all close to their future origins, and fibers develop before they reach their respective insertions. Muscle anlagen divide up into the different elements, which constitute the larval cranial musculature. Ziermann & Olsson (2007) documented the timing of cranial muscle development in X. laevis, and could show a pattern similar to that found in A. mexicanum. In both species, cranial muscle anlagen grow from their origins towards their insertions, a pattern that seems to be conserved between urodeles and anurans.
Developmental origins of cranial muscle cells
Cranial mesoderm development
The development of the cranial mesoderm remains enigmatic in most animals. The quail-chick chimeric system has been used to show that the paraxial mesoderm gives rise to the myofibers in most cranial muscles (Noden, 1983a, b, 1986b; Noden et al. 1999; Noden & Francis-West, 2006). A number of investigations by Huang and co-workers (Huang et al. 1997, 1999, 2000; Huang & Christ, 2000) have confirmed and extended Noden’s work. In particular, data at a new level of detailed resolution are now available for somites 1–10 in the chicken. However, the development of the pre-otic paraxial mesoderm remains less well-studied, except in the quail-chick system, and no modern fate-mapping data are available for amphibians. Earlier claims that the pre-otic mesoderm is segmented into so-called ‘somitomeres’ (Jacobson & Meier, 1984; Meier & Packard, 1984; Jacobson, 1988) have been proven wrong. The head mesoderm in this region is unsegmented (Freund et al. 1996; Noden et al. 1999; Jouve et al. 2002; Kuratani, 2005). Because the pre-otic mesoderm is not organized into clearly defined units, it is very difficult to study the migration and fate of cells in this area. It is parsimonious to assume that the paraxial mesoderm in the head gives rise to the muscle fibers in most mandibular and hyoid arch muscles in amphibians, because this has been shown to be the case in birds (Noden, 1986a). Although we are working on the development of the pre-otic mesoderm, our focus is on the fate of post-otic paraxial mesoderm cells, which are organized into somites.
The fate of somitic mesoderm cells
Somite long-term cell fate has not been analyzed from a comparative perspective with modern cell-marking methods before our work in A. mexicanum. The quail-chick system has provided very good baseline data for use in comparison with other species. Almost everything we know about somitic cell migration and fate is based on work using quail-chick chimeras. Consequently, to what degree cell fate has been conserved despite the drastic anatomical differences among vertebrates is poorly known.
In A. mexicanum, both transplantations of single somites using GPF-transgenic animals as donors, and injection of the tracer dye fluorescein dextran (Gross & Hanken, 2004) into the center of single somites was used to map the fate of somitic cells from the first six somites (Piekarski & Olsson, 2007, 2011; Piekarski, 2009). We could confirm and extend earlier studies in salamanders based on classical histology, and produce a detailed fate-map of both skeletal and muscular derivatives from individual somites. Although most cranial muscles are derived from pre-otic paraxial mesoderm, somites 2–4 contribute to the two hypobranchial muscles mm. geniohyoideus and rectus cervicis that reach into the ventral anterior head (Piekarski, 2009). The geniohyoideus muscle is derived from somite 2 only, whereas the rectus cervicis muscle has a mixed origin from somites 2, 3 and 4 (summarized in Fig. 4). These results are consistent with the findings of Piatt (1938) in Ambystoma maculatum, who used classical histology to predict the formation of the hypobranchial muscles.
Fig. 4.
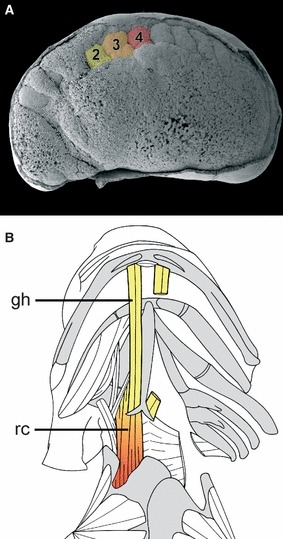
Summary of the contributions from somites 2–4 to the hypobranchial muscles in the axolotl. (A) An SEM of an early tailbud stage embryo. The epidermis is partly removed to visualize underlying structures. Somites 2–4 are color coded. (B) Anatomical drawing of the ventral side of an axolotl. Muscles on the right side are removed to reveal the skeleton. The two muscles of the hypobranchial apparatus, mm. geniohyoideus (gh) and rectus cervicis (rc) have a different embryonic origin. Whereas the geniohyoideus muscle (gh) is derived only from cells originating from somite 2, the rectus cervicis muscle (rc) has a mixed origin of somites 2, 3 and 4.
A comparison between data obtained from quail-chick chimeras and from A. mexicanum revealed three differences in the contributions of the somites to the hypobranchial musculature. The first is that the number of contributing somites is less in the axolotl; three instead of five in the chicken. Furthermore, in the axolotl somites do not contribute regularly to the contralateral side as found in the quail-chick chimeras. The third difference is that unlike in the chicken, the participating somites do not contribute equally to the hypobranchial muscles. This is caused by differences in the migratory behavior of somitic cells in the two species. In A. mexicanum, cells in the ventral processes of each somite that will form the hypobranchial muscles stay separate longer than in the chicken, where they fuse at earlier stages (Piekarski, 2009). Thus, only cells from somite 2 reach the anlage of the geniohyoideus muscle, and there is no contribution from more caudal somites. These results show that homologous muscle groups can develop in an overall similar way, but when examined in more detail, the modi of development can be quite different.
The cranial neural crest and its role for cranial muscle development
As important as the knowledge of the developmental origin of cranial muscles, is the combination of this knowledge with a thorough understanding of the mechanisms that regulate cranial muscle development if we are ever to reach a comprehensive understanding of muscle development and evolution in the vertebrate head region. It is necessary to view cranial muscle development in connection with cranial skeleton development, because muscles in the head always develop in close connection with skeletal elements, which tend to carry patterning information also for the muscles (Tokita & Schneider, 2009). Thus, novelties in cranial muscle patterning must be coordinated with cranial skeletal novelties.
Most of the cranial skeleton is derived from neural crest cells (LeDouarin & Kalcheim, 1999; Hall, 2009), also the novel structures present in frog tadpole heads (Olsson & Hanken, 1996). Much of the classical research during the late 19th and early 20th centuries assessing the embryonic origins of cranial tissues in vertebrates, including derivatives of the neural crest, was based on work done in amphibians (reviewed in Hall & Hörstadius, 1988; Hall, 2009). These early studies precisely and accurately defined the extensive contribution of neural crest to many cranial tissues in both frogs and salamanders (e.g. cranial cartilages; Stone, 1927, 1929). Later studies have extended and refined the classical work. Among amphibians, the contribution of neural crest cells to adult head structures has been mapped in great detail in X. laevis (Gross & Hanken, 2005, 2008a, b; Hanken & Gross, 2005; Gross et al. 2006), and the detailed migratory routes of cranial neural crest cells are now perhaps better known in an amphibian (A. mexicanum) than in any other species (Epperlein et al. 2000; Cerny et al. 2004a, b). Neural crest cells were believed to contribute to cranial skeletal tissues but not to muscles, so when a direct contribution of neural crest to cranial musculature was first reported in 1975 by LeDouarins’s group (LeLièvre & LeDouarin, 1975), this came as quite a surprise. In the 1980s this was elaborated upon by Noden (1983a, b), and later by Couly et al. (1992, 1993), who described the neural crest derivation of connective tissue components of several visceral arch muscles in quail-chick chimaeras. The muscle fibers themselves are not derived from neural crest cells, but seem to always originate from mesoderm. The role of the neural crest in muscle development is part of a system in which the original compartmentalization of hindbrain segments (rhombomeres), the neural crest and musculoskeletal derivatives are maintained throughout neural crest cell migration, pattern formation and histogenesis (Köntges & Lumsden, 1996; Schilling, 1997; Schilling & Kimmel, 1997). This results, for example, in the connective tissue components of a given muscle and its skeletal attachment site(s) being derived from the same migratory crest stream. It can also lead to ‘cryptic’ segmental boundaries, which may lie within individual connective tissue elements, and thus need not correspond to discrete anatomical landmarks (Köntges & Lumsden, 1996; Olsson et al. 2001). The only way to detect such segmental boundaries is to have permanently labeled cells, i.e. to do long-term fate-mapping. Studies are only available for a few species (chicken, mouse, X. laevis, A. mexicanum), so generalizations remain tentative. However, in the species studied the cranial neural crest cells were found to play an important role in integrating the pattern formation during head development. Segmentation in early development, and the migratory routes of cranial neural crest cells, with a division into mandibular, hyoid and branchial streams are conserved. Patterning information from the developing neural crest derivatives is crucial for proper development of cranial muscles. In order to understand how the anatomical differences originate during development, and how the evolutionary novelties characteristic of each vertebrate group are produced, we need to move to the molecular level. In the next section, we describe recent and ongoing work on the regulation of early head development in X. laevis, in particular an analysis of the role(s) of a specific gene, FoxN3, for the proper development of the novel rostral cartilages in frog tadpoles, and the effect of a knock-down of this gene on both skeletal and muscle morphogenesis in the tadpole head.
Molecular mechanisms of cranial neural crest development: the fork head box transcription factors
Several factors, and even gene classes, have been shown to be involved in migration, determination and maintenance of cranial neural crest cells, including the Sox family (Sox8; Sox9; Sox10, Spokony et al. 2002; Hong & Saint-Jeannet, 2005), the Pax family (Pax3), the fork head family (FoxD1; FoxD3, Gomez-Skarmeta et al. 1999; Dottori et al. 2001), and zinc finger proteins like Zic3 and slug. Additionally, several signaling cascades, for example, the FGF, BMP and Wnt pathways were shown to be necessary for determination, migration and/or differentiation of the neural crest (Barembaum & Bronner-Fraser, 2005). Furthermore, it is known that the migratory ability of neural crest cells is reflected in a difference of cell adhesion modulated by cadherins (Taneyhill, 2008). Nevertheless, our knowledge about the detailed mechanisms is limited.
The molecular mechanisms of incipient chondrogenesis and of the morphogenesis of the cranial neural crest-derived jaw and skull also remain incomplete. This is partially due to the anatomical divergence between species. Several genes and gene families that provide insights into the ontogenetic differences of jaw development are known (reviewed in e.g. Francis-West et al. 1998; Richman & Lee, 2003). The most prominent examples are Hox and Dlx genes, where knockouts can create homeotic transformations. For example, simultaneous inactivation of Dlx5 and Dlx6 results in the lower jaw taking on upper jaw identity in mice (Depew et al. 2002, 2005). For regulating cranial cartilage and muscle development, the winged-helix/forkhead class of transcription factors is potentially interesting. They are known to be involved in regulating numerous cellular processes, such as metabolism, proliferation, cell cycle arrest, DNA damage repair, apoptosis and differentiation, but also the maintenance of an undifferentiated state and cell homeostasis. A common DNA-binding structure, the winged-helix DNA-binding domain, which contains 110 conserved amino acids, characterizes this widespread gene family. This domain folds into a variant of the helix–turn–helix motif, and is made up of three α helices and two characteristic large loops, or ‘wings’. According to amino acid variation within this domain, the fork head family is divided into several subclasses, and a unified symbol Fox (Forkhead box) has been adopted for all chordate winged helix/Forkhead transcription factors (A-S, Kaestner et al. 2000). More than 100 members have been identified, in species ranging from yeast to human (Kaestner et al. 2000). Loss of Fox gene function or misregulation has recently been identified in several human diseases (Hannenhalli & Kaestner, 2009).
While the expression of most Fork head genes are well characterized and several Fox genes or even subclasses, and their interaction partners, molecular mechanisms and target genes have been extensively studied, little is known about the roles of the FoxN subclass genes, especially in developmental processes. FoxN3 (also known as CHES1) was first isolated from checkpoint mutation strains of Saccharomyces cerevisiae (Pati et al. 1997). FoxN3 presence results in G2/M cell cycle arrest after DNA damage. Additionally putative interaction partners were identified like Sin3 or RPD3, which were found as main components in histone deacetylase complexes (HDAC; Scott & Plon, 2005; Schuff et al. 2007). While the mechanism of FoxN3 signaling is still unclear, it might act as a transcription repressor (Scott & Plon, 2005). This is supported by the fact that FoxN3 binds co-regulators like SKIP (Ski-interacting protein; Scott & Plon, 2005), which is also a repressor in the HDAC and in the Notch signaling, or MEN-1 (menin; Busygina et al. 2006), a tumor suppressor gene.
FoxN3 is required for cranial cartilage and muscle morphogenesis
HDACI (RPD3), SKIP and MEN-1 gain special interest because they are involved in cranial crest development (Pillai et al. 2004; Engleka et al. 2007). There is less data on the role of FoxN3 during embryogenesis. Functional knock-down of FoxN3 in X. laevis leads to abnormal formation of the jaw cartilages and reduced eye size, accompanied by an increase in apoptotic cell number. Moreover, an absence or malformation of distinct cranial nerves and reduced tadpole size can be observed (Schuff et al. 2007). A gene-trap-based Foxn3 mutant mouse also exhibits severe craniofacial defects during embryogenesis, reduced expression of several osteogenic genes in craniofacial tissues and postnatal lethality (Samaan et al. 2011). Notably, described deletions of the chromosomal region 14q32 in human (Schlade-Bartusiak et al. 2008) shows similar abnormalities in comparison to this mouse mutant. These results indicate an indispensable role of FoxN3 in the craniofacial development of vertebrates in general.
Effects of FoxN3 knock-down on cranial muscle development in X. laevis
Following a morpholino-based knock-down of FoxN3, cranial muscle development is affected in a way that resembles the situation when classical extirpation techniques are used to remove cranial neural crest cells (Hall, 1950; Olsson et al. 2001; Ericsson et al. 2004). In general, the early development is normal, muscles start to differentiate at their normal origins but fail to extend towards their insertions. The muscle fibers also do not differentiate normally, often making the muscles shorter and giving them a frayed appearance (Schmidt et al. 2011). Mandibular, hyoid and branchial muscles show abnormal development, but muscles of the larynx and ocular muscles remain more or less normal. This makes sense if the direct effect of a functional knock-down of FoxN3 is to slow down or inhibit cranial neural crest development, because the connective tissue of the affected muscles is neural crest-derived, whereas larynx muscles and external ocular muscles have mesoderm-derived connective tissue (Piatt, 1938; Noden, 1983a, b; Noden & Francis-West, 2006). Muscle shape and size are defined by the surrounding neural crest cells and neural crest-derived cartilages (Rinon et al. 2007; Tokita & Schneider, 2009). Cell differentiation in the mesodermal anlagen is regulated through enhanced proliferation of neural crest cells. Additionally, myogenesis is also maintained by neural crest cells through segregation of BMP antagonists like gremlin or chordin (Noden & Francis-West, 2006). Therefore, the smaller size of the muscle anlagen and the shortened muscles in FoxN3-depleted tadpoles might be caused indirectly by disturbances in the development of neural crest-derived cells. Further genetic investigations are necessary to identify the affected part(s) of myogenesis, but the anlagen are formed in the correct position and, therefore, a proper migration of cranial paraxial mesoderm cells seems to have taken place.
The timing of cranial muscle development in FoxN3-depleted X. laevis specimens is also affected. In comparison to normal development, in which cranial muscle developmental timing has been determined (Ziermann & Olsson, 2007), FoxN3-depleted larvae are delayed by two developmental stages (Schmidt et al. 2011). This delay in cranial muscle development is probably the main reason for some of the malformations seen, for example the fusion of the mm. geniohyoideus and subarcualis rectus I (Fig. 5). When timing is normal, these muscles become separated by the ceratobranchial cartilage, which differentiates between them (Ziermann & Olsson, 2007). However, because FoxN3 knock-down causes a reduction in size of the branchial basket as well as a delay in development by two stages, apparently no connective tissue migrates in to separate these muscles from each other. Also, the failure of the m. geniohyoideus to reach its normal insertion on the infrarostral cartilage often seen in bilaterally FoxN3-depleted larvae (Fig. 5) is likely to be a result of the delayed outgrowth of the muscle. The geniohyoideus muscle stretches from its origin on the first ceratobranchial all the way to the most rostral part of the mouth (Fig. 5), and it is easy to imagine how a slowdown of development can cause it to fail to reach its normal insertion. The same argument can be made for the m. quadrato-hyoangularis, which takes its origin from the palatoquadrate and often fails to reach its normal insertion on Meckel’s cartilage in FoxN3-depleted specimens.
Fig. 5.
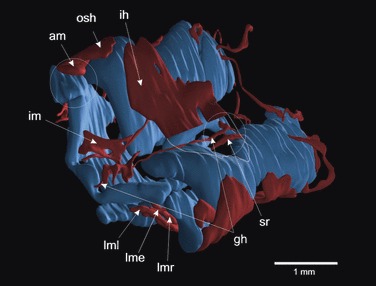
Three-dimensional reconstruction of the cranial cartilages and muscles of a bilaterally FoxN3-MO injected Xenopus laevis larva at stage 46 in ventro-lateral view. Morpholino-based knock-down of FoxN3 produced a delay in cranial muscle development by two developmental stages. The functional knock-down caused a wide variety of muscle malformations. All the muscles are smaller and show a frayed and disordered appearance (i.e. im). Some muscles start to differentiate at their normal origin but fail to extend to their insertion during muscle development, which effects a shifts of insertions (am, gh), and some muscles are anastomosed at their origin (gh + sr). Muscles: am, angularis muscles; gh, m. geniohyoideus; ih, m. interhyoideus; im, m. intermandibularis; lme, m. levator mandibulae externus; lml, m. levator mandibulae longus (comprising two parts; superficialis and profundus); lmr, m. levator mandibulae anterior; osh, m. orbitohyoideus; sr, m. subarcualis rectus I.
Evolutionary changes in cranial muscle patterning within amphibians
As noted above, the detailed origin of the cells that turn into muscle fibers in the mandibular and hyoid arches has not yet been mapped in any amphibian, in fact only the quail-chick system has allowed this level of detail. The effects of extirpation of single streams (mandibular, hyoid, branchial) of cranial neural crest cells on cranial muscle development and their normal fate have, however, been investigated in both a salamander (A. mexicanum; Ericsson et al. 2004) and in a frog (B. orientalis; Olsson et al. 2001). This gives us the opportunity to compare the more basal muscle pattern and its formation in the larva of A. mexicanum and most other salamanders (Fig. 1) with the very derived state found even in a relatively unspecialized tadpole such as that of B. orientalis (Fig. 6; for the more specialized X. laevis see Fig. 3), as well as the importance of the neural crest for proper cranial muscle development. In order to operate the novel supra- and infrarostral cartilages, frog tadpoles have changed the patterning of mandibular and hyoid muscles drastically. In A. mexicanum, the larval muscle–cartilage connections follow a simple pattern in that muscles of the mandibular arch (such as the levator mandibulae group) have both their origins and insertions on cartilages derived from the mandibular neural crest stream. The levator mandibulae muscles, for example, insert on Meckel’s cartilage (Fig. 1). In comparison, the frog tadpole has a more elaborate set of levator mandibular muscles (Fig. 6), and although they both originate and insert on mandibular arch cartilages, the necessity of moving both infra- and suprarostral cartilages have led to some muscles inserting directly on the suprarostrals, with the new function of depressing the suprarostrals during mouth closure. The levator mandibulae externus profundus (Fig. 6; mlmep) and one part of the levator mandibulae longus (Fig. 6; mlml) have undergone this change. In the hyoid arch, the patterning changes are even more radical. The depressor mandibulae muscle in salamanders (Fig. 1; dm) has evolved into the angularis group (Fig. 6; ang) and the orbito- and suspensoriohyoideus muscles (Fig. 6; osh). Changes to cranial muscle anatomy in tadpoles include muscles inserting at novel positions, for example the m. quadratoangularis, which is a larval-specific muscle unique to frogs. While it is appropriately considered a hyoid arch muscle based on its pattern of innervation and its fate in the adult frog, the m. quadratoangularis in larvae is associated exclusively with mandibular arch skeletal elements (De Jongh, 1968; Cannatella, 1999). These skeletal elements, the palatoquadrate and Meckel’s cartilages, are both derived from mandibular stream neural crest (Olsson & Hanken, 1996), but the connective tissue attachments for the m. quadratoangularis on the same cartilages are derived from hyoid stream neural crest, and only affected by hyoid crest extirpation, and not by extirpation of other streams (Olsson et al. 2001). The same relation exists for the m. orbitohyoideus at its origin from the palatoquadrate cartilage. Thus, cranial neural crest cells from a given migratory stream are not the sole source of skeletal and other connective tissues that come to be associated anatomically with the corresponding branchial arch. Rather, the pattern of neural crest derivation of individual cartilages and muscle connective tissues corresponds to their respective sites of embryonic origin, regardless of the extent to which these muscles and cartilages are anatomically and functionally linked at later stages. We now need a proper long-term fate-map of the pre-otic paraxial mesoderm in a frog to really be able to determine how the complicated architecture of this major novelty only present in tadpoles, the rostralia and the muscles that operate them, is built during normal development. This would also form a foundation for further research into the molecular mechanisms that regulate rostral head morphogenesis.
Fig. 6.
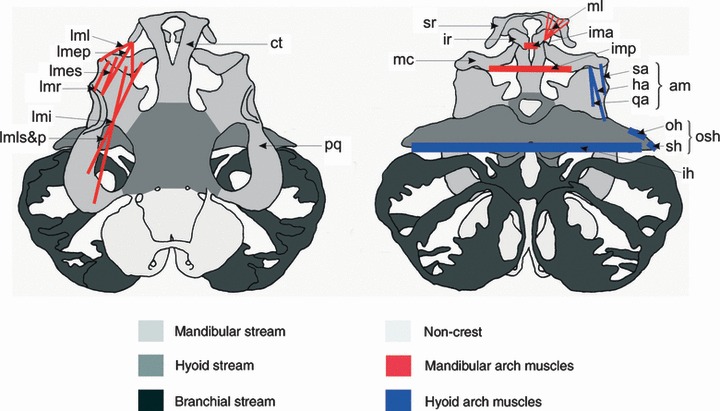
Larval skull and cranial musculature of Bombina orientalis, depicted in dorsal (left) and ventral (right) views. Neural crest-derived cartilages are shaded according to the migratory stream from which they originate (redrawn from Olsson & Hanken, 1996): light gray, mandibular stream; medium gray, hyoid stream; dark gray, branchial stream. The few non-crest-derived cartilages are lightly shaded. Cranial muscles are depicted schematically; only muscles of interest for the discussion in this paper are shown. Mandibular (first) arch muscles are red, hyoid (second) arch muscles are blue. Paired muscles are depicted on one side only. Cartilages: ct, cornua trabecula; ir, infrarostral; mc, Meckel’s cartilage; pq, palatoquadrate; sr, suprarostral. Muscles: mandibular arch muscles: ima, m. intermandibularis anterior; imp, m. intermandibularis posterior; lmep, m. levator mandibulae externus profundus; lmes, m. levator mandibulae externus superficialis; lmi, m. levator mandibulae internus; lml, m. levator mandibulae lateralis; lmls&p, m. levator mandibulae longus superficialis andt profundus; lmr, m. levator mandibulae articularis; ml, m. mandibulolabialis. Hyoid arch muscles: am, angularis group; ha, m. hyoangularis; ih, m. interhyoideus; oh, m. orbitohyoideus; osh, m. orbito- and suspensoriohyoideus; qa, m. quadratoangularis; sa, m. suspensorioangularis; sh, m. suspensoriohyoideus. Redrawn based on Olsson et al. (2001). Anatomical nomenclature follows Haas (2001).
Concluding remarks: novelty, identity and homology
We are presently investigating the effect of functional knock-down of FoxN3 and functionally related genes on cranial development in three different species, two frogs (X. laevis, B. orientalis) and one salamander (A. mexicanum). The goal is to gain a better understanding of the evolution of the highly specialized cranial skeletal and muscular arrangements seen in frog tadpoles. The infrarostral and suprarostral cartilages are novel structures that are unique to anuran tadpoles (Svensson & Haas, 2005), as is the unique arrangement of cranial muscles used to operate them when tadpoles scrape algae or filter-feed. The evolutionary origin of this complex system remains unclear, and little is known about the molecular basis for the evolution of such novel structures. One aspect that we are investigating is the expression pattern of candidate genes probably affected by FoxN3 knock-down, for example, bagpipe genes. We are also adapting the FoxN3 morpholino-based knock-down technique for use in amphibian species other than X. laevis. The different hypotheses of the evolutionary origin of the rostral cartilages indicate different changes in development. They could either be novel cartilages, which cannot be homologized with other cartilages in amphibians (and thus also novelties as defined by, e.g. Müller & Wagner, 1991), or duplications of other cartilages. A third possibility is that they are derived from a pre-existing cartilage, but have become individualized (Svensson & Haas, 2005). We hope to be able to discriminate between these alternatives in the case of the rostralia using the approach outlined above. As mentioned in the Introduction, it makes a difference if the infra- and suprarostral cartilages themselves are novelties sensu Müller and Wagner and do not have any homologous structures in other organisms. If our research shows that the articulations between Meckel’s cartilage and the infrarostrals, and between the trabecular horns and the suprarostrals, are the real evolutionary novelties, this would shift the focus of what needs to be explained. Based on our fate-mapping data (Olsson & Hanken, 1996), we believe that the infrarostrals are partitioned off from Meckel’s cartilage and the suprarostrals from the trabecular horns. A mechanistic understanding of their evolutionary origin would focus on how novel articulations are produced (Svensson & Haas, 2005), to which we hope our work on the anuran rostralia and associated musculature will contribute.
Novelties must be produced by changes to developmental processes, such as cell migration and other morphogenetic processes, and ultimately to developmental regulatory mechanisms, including local cell–cell signaling and changes in the dynamics of gene regulatory networks. It does not further our understanding to demand that novelties are without homologs, when we know that they must be produced developmentally by changes in processes that make them deviate and become a novelty. At the level of developmental processes or mechanism, they are likely to be homologous. Several examples have been thoroughly investigated, such as the horns of beetles (see Moczek, 2009; Wasik et al. 2010 for review), which have co-opted some of the patterning genes (such as distal-less) that are responsible for limb (proximodistal) outgrowth in arthropods in general. We might find a similar situation in our ongoing work on the role of FoxN3 for the development and evolution of the rostralia and other novel structures in the tadpole head. FoxN3 and the genetic regulatory network of which it is a part might have been co-opted to regulate the changes of neural crest cell differentiation seen in the development of, for example, the rostralia in frog tadpoles. We hope that our approach, in which salamander development is used for comparison, can shed light on this problem. Salamander larvae lack both the rostralia and the novel arrangement of cranial muscles seen in frog tadpoles, and are therefore ideal for this purpose.
Acknowledgments
Our research was made possible by grants from the Deutsche Forschungsgemeinschaft (OL 134/2-4 and 10-1). Jennifer Schmidt has a Ph.D. stipend from the Konrad-Adenauer-Stiftung, and Nadine Piekarski is supported by the Museum of Comparative Zoology, Harvard University. Lennart Olsson spent part of a sabbatical at NESCent, Duke University, USA, and at the Konrad Lorentz Institute in Altenberg, Austria, where his ideas about evolutionary novelties developed. We thank Drs H. H. Epperlein and E. M. Tanaka for kindly providing embryos of GPF-transgenic A. mexicanum, and for allowing transplantation experiments to be performed in their laboratories. B. Metscher (Vienna) produced the X-ray-based computer microtomography data used to make the 3D reconstructions of X. laevis tadpoles. Rolf Ericsson (NHM, London) took the CSLM micrographs in Fig. 1, and is also thanked for a critical reading of an earlier version of this paper. Constructive criticism from two anonymous reviewers and from Zerina Johanson are gratefully acknowledged. For technical support we thank Benjamin Weiss and Katja Felbel. The monoclonal antibody 12/101, developed by Dr J. P. Brockes and used for muscle staining, was obtained from the Developmental Studies Hybridoma bank, which was developed under the auspices of the NICHD and is maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA.
References
- Arthur W. Intraspecific variation in developmental characters: the origin of evolutionary novelties. Am Zool. 2000;40:811–818. [Google Scholar]
- Barembaum M, Bronner-Fraser M. Early steps in neural crest specification. Semin Cell Dev Biol. 2005;16:642–646. doi: 10.1016/j.semcdb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- de Beer GR. The Development of the Vertebrate Skull. Oxford: Oxford University Press; 1937. [Google Scholar]
- Busygina V, Kottemann MC, Scott KL, et al. Multiple endocrine neoplasia type 1 interacts with forkhead transcription factor CHES1 in DNA damage response. Cancer Res. 2006;66:8397–8403. doi: 10.1158/0008-5472.CAN-06-0061. [DOI] [PubMed] [Google Scholar]
- Cannatella DC. Architecture: cranial and axial musculoskeleton. In: McDiarmid RW, Altig R, editors. Tadpoles: The Biology of Anuran Larvae. Chicago: University of Chicago Press; 1999. pp. 52–91. [Google Scholar]
- Cerny R, Lwigale P, Ericsson R, et al. Developmental origins and evolution of jaws: new interpretation of “maxillary” and “mandibular”. Dev Biol. 2004a;276:225–236. doi: 10.1016/j.ydbio.2004.08.046. [DOI] [PubMed] [Google Scholar]
- Cerny R, Meulemans D, Berger J, et al. Combined intrinsic and extrinsic influences pattern cranial neural crest migration and pharyngeal arch morphogenesis in axolotl. Dev Biol. 2004b;266:252–269. doi: 10.1016/j.ydbio.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of the skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- De Jongh HJ. Functional morphology of the jaw apparatus of larval and metamorphosing Rana temporaria L. Netherlands J Zool. 1968;18:1–103. [Google Scholar]
- Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381–385. doi: 10.1126/science.1075703. [DOI] [PubMed] [Google Scholar]
- Depew MJ, Simpson CA, Morasso M, et al. Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J Anat. 2005;207:501–561. doi: 10.1111/j.1469-7580.2005.00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottori M, Gross MK, Labosky P, et al. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development. 2001;128:4127–4138. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- Engleka KA, Wu M, Zhang M, et al. Menin is required in cranial neural crest for palatogenesis and perinatal viability. Dev Biol. 2007;311:524–537. doi: 10.1016/j.ydbio.2007.08.057. [DOI] [PubMed] [Google Scholar]
- Epperlein H, Meulemans D, Bronner-Fraser M, et al. Analysis of cranial neural crest migratory pathways in axolotl using cell markers and transplantation. Development. 2000;127:2751–2761. doi: 10.1242/dev.127.12.2751. [DOI] [PubMed] [Google Scholar]
- Ericsson R, Cerny R, Falck P, et al. The role of cranial neural crest cells in visceral arch muscle positioning and morphogenesis in the Mexican axolotl, Ambystoma mexicanum. Dev Dyn. 2004;231:237–247. doi: 10.1002/dvdy.20127. [DOI] [PubMed] [Google Scholar]
- Ericsson R, Olsson L. Patterns of spatial and temporal visceral arch muscle development in the Mexican axolotl (Ambystoma mexicanum. J Morphol. 2004;261:131–140. doi: 10.1002/jmor.10151. [DOI] [PubMed] [Google Scholar]
- Francis-West P, Ladher R, Barlow A, et al. Signalling interactions during facial development. Mech Dev. 1998;75:3–28. doi: 10.1016/s0925-4773(98)00082-3. [DOI] [PubMed] [Google Scholar]
- Freund R, Dorfler D, Popp W, et al. The metameric pattern of the head mesoderm – does it exist? Anat Embryol (Berl) 1996;193:73–80. doi: 10.1007/BF00186835. [DOI] [PubMed] [Google Scholar]
- Gegenbaur C. Die Metamerie des Kopfes und die Wirbeltheorie des Kopfskelettes. Morphol Jahrbuch. 1888;13:1–144. [Google Scholar]
- Gomez-Skarmeta JL, de la Calle-Mustienes E, Modolell J, et al. Xenopus brain factor-2 controls mesoderm, forebrain and neural crest development. Mech Dev. 1999;80:15–27. doi: 10.1016/s0925-4773(98)00190-7. [DOI] [PubMed] [Google Scholar]
- Goodrich ES. Studies on the Structure and Development of Vertebrates. London: Macmillan; 1930. [Google Scholar]
- Gross JB, Hanken J. Use of fluorescent dextran conjugates as a long-term marker of osteogenic neural crest in frogs. Dev Dyn. 2004;230:100–106. doi: 10.1002/dvdy.20036. [DOI] [PubMed] [Google Scholar]
- Gross JB, Hanken J. Cranial neural crest contributes to the bony skull vault in adult Xenopus laevis: insights from cell labeling studies. J Exp Zool B Mol Dev Evol. 2005;304:169–176. doi: 10.1002/jez.b.21028. [DOI] [PubMed] [Google Scholar]
- Gross JB, Hanken J. Review of fate-mapping studies of osteogenic cranial neural crest in vertebrates. Dev Biol. 2008a;317:389–400. doi: 10.1016/j.ydbio.2008.02.046. [DOI] [PubMed] [Google Scholar]
- Gross JB, Hanken J. Segmentation of the vertebrate skull: neural crest derivation of adult cartilages in the clawed frog, Xenopus laevis. Int Comp Biol. 2008b;48:681–696. doi: 10.1093/icb/icn077. [DOI] [PubMed] [Google Scholar]
- Gross JB, Hanken J, Oglesby E, et al. Use of a ROSA26:GFP transgenic line for long-term Xenopus fate-mapping studies. J Anat. 2006;209:401–413. doi: 10.1111/j.1469-7580.2006.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall EK. Experimental modifications of muscle development in Amblystoma punctatum. J Exp Zool. 1950;113:355–377. [Google Scholar]
- Hall BK. The Neural Crest and Neural Crest Cells in Vertebrate Development and Evolution. New York: Springer; 2009. [Google Scholar]
- Hall BK, Hörstadius S. The Neural Crest. Oxford: Oxford University Press; 1988. [Google Scholar]
- Hanken J, Gross JB. Evolution of cranial development and the role of neural crest: insights from amphibians. J Anat. 2005;207:437–446. doi: 10.1111/j.1469-7580.2005.00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanken J, Hall BK. The Skull, Vol. I–III. Chicago: University of Chicago Press; 1993. [Google Scholar]
- Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10:233–240. doi: 10.1038/nrg2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong CS, Saint-Jeannet JP. Sox proteins and neural crest development. Semin Cell Dev Biol. 2005;16:694–703. doi: 10.1016/j.semcdb.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Huang R, Christ B. Origin of the epaxial and hypaxial myotome in avian embryos. Anat Embryol (Berl) 2000;202:369–374. doi: 10.1007/s004290000130. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Ordahl CP, et al. The fate of the first avian somite. Anat Embryol (Berl) 1997;195:435–449. doi: 10.1007/s004290050063. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Izpisua-Belmonte JC, et al. Origin and development of the avian tongue muscles. Anat Embryol (Berl) 1999;200:137–152. doi: 10.1007/s004290050268. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Patel K, et al. Contribution of single somites to the skeleton and muscles of the occipital and cervical regions in avian embryos. Anat Embryol (Berl) 2000;202:375–383. doi: 10.1007/s004290000131. [DOI] [PubMed] [Google Scholar]
- Jacobson AG. Somitomeres: mesodermal segments of vertebrate embryos. Development. 1988;104:209–220. doi: 10.1242/dev.104.Supplement.209. [DOI] [PubMed] [Google Scholar]
- Jacobson AG, Meier SP. Morphogeneis of the head of a newt: mesodermal segments, neuromeres, and distribution of neural crest. Dev Biol. 1984;106:181–193. doi: 10.1016/0012-1606(84)90074-5. [DOI] [PubMed] [Google Scholar]
- Jouve C, Iimura T, Pourquie O. Onset of the segmentation clock in the chick embryo: evidence for oscillations in the somite precursors in the primitive streak. Development. 2002;129:1107–1117. doi: 10.1242/dev.129.5.1107. [DOI] [PubMed] [Google Scholar]
- Kaestner KH, Knochel W, Martinez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14:142–146. [PubMed] [Google Scholar]
- Kleinteich T, Haas A. Cranial musculature in the larva of the caecilian, Ichthyophis kohtaoensis (Lissamphibia: Gymnophiona) J Morphol. 2007;268:74–88. doi: 10.1002/jmor.10503. [DOI] [PubMed] [Google Scholar]
- Kleinteich T, Haas A. The hyal and ventral branchial muscles in caecilian and salamander larvae: homologies and evolution. J Morphol. 2011;272:598–613. doi: 10.1002/jmor.10940. [DOI] [PubMed] [Google Scholar]
- Köntges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Kuratani S. Craniofacial development and the evolution of the vertebrates: the old problems on a new background. Zool Sci. 2005;22:1–19. doi: 10.2108/zsj.22.1. [DOI] [PubMed] [Google Scholar]
- LeDouarin NM, Kalcheim C. The Neural Crest. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- LeLièvre C, LeDouarin NM. Mesenchymal derivatives of the neural crest: analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- Mayr E. Animal Species and Evolution. Cambridge: Harvard University Press; 1963. [Google Scholar]
- Meier SP, Packard DSJ. Morphogenesis of the cranial segments and distribution of neural crest in embryos of the snapping turtle, Chelydra serpentina. Dev Biol. 1984;102:309–323. doi: 10.1016/0012-1606(84)90196-9. [DOI] [PubMed] [Google Scholar]
- Moczek AP. On the origins of novelty and diversity in development and evolution: a case study on beetle horns. Cold Spring Harb Symp Quant Biol. 2009;74:289–296. doi: 10.1101/sqb.2009.74.010. [DOI] [PubMed] [Google Scholar]
- Müller GB, Wagner GP. Novelty in evolution: restructuring the concept. Ann Rev Ecol Syst. 1991;22:229–256. [Google Scholar]
- Noden DM. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am J Anat. 1983a;168:257–276. doi: 10.1002/aja.1001680302. [DOI] [PubMed] [Google Scholar]
- Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983b;96:144–165. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- Noden DM. Origins and patterning of craniofacial mesenchymal tissues. J Craniofac Genet Dev Biol Suppl. 1986a;2:15–31. [PubMed] [Google Scholar]
- Noden DM. Patterning of avian craniofacial muscles. Dev Biol. 1986b;116:347–356. doi: 10.1016/0012-1606(86)90138-7. [DOI] [PubMed] [Google Scholar]
- Noden DM, Francis-West P. The differentiation and morphogenesis of craniofacial muscles. Dev Dyn. 2006;235:1194–1218. doi: 10.1002/dvdy.20697. [DOI] [PubMed] [Google Scholar]
- Noden DM, Marcucio R, Borycki AG, et al. Differentiation of avian craniofacial muscles: I. Patterns of early regulatory gene expression and myosin heavy chain synthesis. Dev Dyn. 1999;216:96–112. doi: 10.1002/(SICI)1097-0177(199910)216:2<96::AID-DVDY2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Olsson L, Hanken J. Cranial neural-crest migration and chondrogenic fate in the Oriental fire-bellied toad Bombina orientalis: defining the ancestral pattern of head development in anuran amphibians. J Morphol. 1996;229:105–120. doi: 10.1002/(SICI)1097-4687(199607)229:1<105::AID-JMOR7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Olsson L, Falck P, Lopez K, et al. Cranial neural crest cells contribute to connective tissue in cranial muscles in the anuran amphibian, Bombina orientalis. Dev Biol. 2001;237:354–367. doi: 10.1006/dbio.2001.0377. [DOI] [PubMed] [Google Scholar]
- Pati D, Keller C, Groudine M, et al. Reconstitution of a MEC1-independent checkpoint in yeast by expression of a novel human fork head cDNA. Mol Cell Biol. 1997;17:3037–3046. doi: 10.1128/mcb.17.6.3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatt J. Morphogenesis of the cranial muscles of Amblystoma punctatum. J Morphol. 1938;63:531–587. [Google Scholar]
- Piekarski N. A long-term fate map of the anterior somites in the Mexican axolotl (Ambystoma mexicanum. Jena: Friedrich-Schiller-Universität; 2009. Unpublished PhD thesis. [Google Scholar]
- Piekarski N, Olsson L. Muscular derivatives of the cranialmost somites revealed by long-term fate mapping in the Mexican axolotl (Ambystoma mexicanum. Evol Dev. 2007;9:566–578. doi: 10.1111/j.1525-142X.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- Piekarski N, Olsson L. A somitic contribution to the pectoral girdle in the axolotl revealed by long-term fate mapping. Evol Dev. 2011;13:47–57. doi: 10.1111/j.1525-142X.2010.00455.x. [DOI] [PubMed] [Google Scholar]
- Pigliucci M. What, if anything, is an evolutionary novelty? Phil Sci. 2008;75:887–898. [Google Scholar]
- Pillai R, Coverdale LE, Dubey G, et al. Histone deacetylase 1 (HDAC-1) required for the normal formation of craniofacial cartilage and pectoral fins of the zebrafish. Dev Dyn. 2004;231:647–654. doi: 10.1002/dvdy.20168. [DOI] [PubMed] [Google Scholar]
- Richman JM, Lee SH. About face: signals and genes controlling jaw patterning and identity in vertebrates. BioEssays. 2003;25:554–568. doi: 10.1002/bies.10288. [DOI] [PubMed] [Google Scholar]
- Rinon A, Lazar S, Marshall H, et al. Cranial neural crest cells regulate head muscle patterning and differentiation during vertebrate embryogenesis. Development. 2007;134:3065–3075. doi: 10.1242/dev.002501. [DOI] [PubMed] [Google Scholar]
- Samaan G, Yugo D, Rajagopalan S, et al. Foxn3 is essential for craniofacial development in mice and a putative candidate involved in human congenital craniofacial defects. Biochem Biophys Res Commun. 2011;400:60–65. doi: 10.1016/j.bbrc.2010.07.142. [DOI] [PubMed] [Google Scholar]
- Schilling TF. Genetic analysis of craniofacial development in the vertebrate embryo. BioEssays. 1997;19:459–468. doi: 10.1002/bies.950190605. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Kimmel CB. Musculoskeletal patterning in the pharyngeal segments of the zebrafish embryo. Development. 1997;124:2945–2960. doi: 10.1242/dev.124.15.2945. [DOI] [PubMed] [Google Scholar]
- Schlade-Bartusiak K, Macintyre G, Zunich J, et al. A child with deletion (14)(q24.3q32.13) and auditory neuropathy. Am J Med Genet A. 2008;146A:117–123. doi: 10.1002/ajmg.a.32064. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Schuff M, Olsson L. A role for FoxN3 in the development of cranial cartilages and muscles in Xenopus laevis (Amphibia: Anura: Pipidae) with special emphasis on the novel rostral cartilages. J Anat. 2011;218:226–242. doi: 10.1111/j.1469-7580.2010.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff M, Rossner A, Wacker SA, et al. FoxN3 is required for craniofacial and eye development of Xenopus laevis. Dev Dyn. 2007;236:226–239. doi: 10.1002/dvdy.21007. [DOI] [PubMed] [Google Scholar]
- Scott KL, Plon SE. CHES1/FOXN3 interacts with Ski-interacting protein and acts as a transcriptional repressor. Gene. 2005;359:119–126. doi: 10.1016/j.gene.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Spokony RF, Aoki Y, Saint-Germain N, et al. The transcription factor Sox9 is required for cranial neural crest development in Xenopus. Development. 2002;129:421–432. doi: 10.1242/dev.129.2.421. [DOI] [PubMed] [Google Scholar]
- Stone LS. Further experiments on the transplantation of neural crest (mesectoderm) in amphibians. Proc Soc Exp Biol Med. 1927;24:945–948. [Google Scholar]
- Stone LS. Experiments showing the role of migrating neural crest (mesectoderm) in the formation of head skeleton and loose connective tissue in Rana palustris. Roux’s Arch Entw Mech Org. 1929;118:40–77. doi: 10.1007/BF02108871. [DOI] [PubMed] [Google Scholar]
- Svensson ME, Haas A. Evolutionary innovation in the vertebrate jaw: a derived morphology in anuran tadpoles and its possible developmental origin. BioEssays. 2005;27:526–532. doi: 10.1002/bies.20224. [DOI] [PubMed] [Google Scholar]
- Taneyhill LA. To adhere or not to adhere: the role of Cadherins in neural crest development. Cell Adh Migr. 2008;2:223–230. doi: 10.4161/cam.2.4.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokita M, Schneider RA. Developmental origins of species-specific muscle pattern. Dev Biol. 2009;331:311–325. doi: 10.1016/j.ydbio.2009.05.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GP, Lynch VJ. Evolutionary novelties. Curr Biol. 2009;20:R48–R52. doi: 10.1016/j.cub.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Wasik BR, Rose DJ, Moczek AP. Beetle horns are regulated by the Hox gene, Sex combs reduced, in a species- and sex-specific manner. Evol Dev. 2010;12:353–362. doi: 10.1111/j.1525-142X.2010.00422.x. [DOI] [PubMed] [Google Scholar]
- Ziermann JM, Olsson L. Patterns of spatial and temporal cranial muscle development in the African clawed frog, Xenopus laevis (Anura: Pipidae) J Morphol. 2007;268:791–804. doi: 10.1002/jmor.10552. [DOI] [PubMed] [Google Scholar]


