Summary
Cell-cell fusion in animal development and in pathophysiology involves expansion of nascent fusion pores formed by protein fusogens to yield an open lumen of cell-size diameter. Here we explored the enlargement of micron-scale pores in syncytium formation, which was initiated by a well-characterized fusogen baculovirus gp64. Radial expansion of a single or, more often, of multiple fusion pores proceeds without loss of membrane material in the tight contact zone. Pore growth requires cell metabolism and is accompanied by a local disassembly of the actin cortex under the pores. Effects of actin-modifying agents indicate that the actin cortex slows down pore expansion. We propose that the growth of the strongly bent fusion-pore rim is restricted by a dynamic resistance of the actin network and driven by membrane-bending proteins that are involved in the generation of highly curved intracellular membrane compartments.
Keywords: Cell fusion, Syncytium formation, Fusion-pore expansion, Actin cytoskeleton, Membrane-bending proteins, Baculovirus gp64
Introduction
Cell-cell fusion is a key event in fertilization and during differentiation of muscle, bone and trophoblast cells, and possibly also in stem-cell plasticity, carcinogenesis and tumor progression (Chen et al., 2007; Duelli and Lazebnik, 2007; Oren-Suissa and Podbilewicz, 2007; Shemer and Podbilewicz, 2003). Controlled fusion between biological membranes is also a crucial step in protein trafficking and exocytosis, and in viral infections (Chen et al., 2007; Jahn et al., 2003; Kielian and Rey, 2006; Shmulevitz and Duncan, 2000). Earlier work on fusion mechanisms has been focused on the earliest stages of the fusion pathways, which bring about the first measurable indications of fusion: lipid mixing and the opening of a narrow fusion pore – an aqueous connection between the membranes (Earp et al., 2005; Jahn et al., 2003; Kielian and Rey, 2006; Nolan et al., 2006; Sapir et al., 2008; Weissenhorn et al., 2007). At later stages of cell-cell fusion, these initial pores of a few nanometers in diameter expand to pores that are readily detectable by fluorescence microscopy (diameter >~0.2 μm) and finally yield an open lumen of cell-size diameter (~10–15 μm). Little is known about the properties of these larger pores and the mechanisms that underlie the enlargement of cytoplasmic bridges from early fusion pores to syncytia (Gattegno et al., 2007; Mohler et al., 1998; Podbilewicz and Chernomordik, 2005; Podbilewicz and White, 1994). For instance, we still do not know whether this enlargement proceeds spontaneously and, if not, whether it is driven by the cytoskeleton (Podbilewicz and White, 1994; Zheng and Chang, 1991), membrane tension (Knutton, 1980) or another, as-yet unidentified, cell machinery.
The role of the actin cytoskeleton in cell-cell fusion has drawn special attention in many recent studies. Syncytium formation, including cell fusion in development, involves major changes in cell shape and thus might be expected to involve, or even to be controlled by, dynamic rearrangements of the cytoskeleton. The shape of the plasma membrane is supported by the membrane-anchored actin cortex – a three-dimensional network of actin filaments – and, especially for adherent cells in culture, by rigid microfilamentous bundles or stress fibers. The effects of different modifications of the actin cytoskeleton on syncytium formation have been documented (Bitko et al., 2003; DeFife et al., 1999; Gallo et al., 2003; Gower et al., 2005; Kadiu et al., 2007; Kallewaard et al., 2005; Palovuori and Eskelinen, 2000; Pontow et al., 2004; Schowalter et al., 2006; Straube and Merdes, 2007; Sylwester et al., 1993; Yura et al., 2000). The importance of actin polymerization at the sites of fusion has been especially emphasized in a recent series of elegant and controversial studies on myoblast fusion in Drosophila (Berger et al., 2008; Kim et al., 2007; Massarwa et al., 2007; Richardson et al., 2007; Schafer et al., 2007). By contrast, in Caenorhabditis elegans, multiple mutants affecting actin and actin-associated proteins such as cadherins, catenins, β-spectrin, myosin and others do not affect the extent of cell fusion in the embryonic hypodermis (Costa et al., 1998; Ding et al., 2004; McKeown et al., 1998) (also B.P., unpublished results).
Exploration of the mechanisms of fusion-pore enlargement in syncytium formation is hindered by the fact that biologically relevant processes of cell-cell fusion involve complex multistep regulation and are driven by mostly unidentified protein fusogens (Kim et al., 2007; Oren-Suissa and Podbilewicz, 2007; Podbilewicz et al., 2006). In this work, we explored the expansion of the fusion pores in syncytium formation, which was initiated by a well-characterized fusion protein, baculovirus gp64 (Blissard and Wenz, 1992; Chernomordik et al., 1995; Leikina et al., 1992; Markovic et al., 1998; Plonsky et al., 1999; Plonsky and Zimmerberg, 1996). Baculovirus particles enter cells by endocytosis followed by fusion between the viral envelope and the endosomal membrane, which is triggered by acidification of the endosomal content. Studies of low-pH-triggered fusion between gp64-expressing Sf9 insect cells yielded detailed characterization of early fusion pores (Plonsky et al., 1999; Plonsky and Zimmerberg, 1996). Here, we have focused on the much bigger micron-sized pores detectable by fluorescence microscopy.
Low-pH-triggered conformational change in gp64 results in a fast opening of fusion pores and inactivation of the fusogen (Markovic et al., 1998; Plonsky et al., 1999; Plonsky and Zimmerberg, 1996). Thus, this system allows effective uncoupling of fusogen-specific early fusion stages from the pore-expansion stages. Note that the flat extended contacts between spherical Sf9 cells, where these pores form and expand, are topologically reminiscent of extended contacts between Drosophila myoblasts (Doberstein et al., 1997) and between C. elegans epithelial cells (Gattegno et al., 2007; Mohler et al., 1998; Podbilewicz and White, 1994; Shemer and Podbilewicz, 2003).
We report here that gp64-initiated syncytium formation progresses primarily through the opening of multiple rather than single fusion pores, followed by their expansion and eventual merger. The pores were roughly circular and expanded radially. Fusion-pore expansion within the zone of tight contact was accompanied by an increase in the cell-contact area, suggesting that fusion pores grow by displacement of membrane material towards the periphery of the contact zone. In contrast to the opening of a fusion pore driven by protein fusogens, pore expansion at the micron scale was a process that was dependent on cell metabolism. On the basis of the effects of actin-modifying agents and of the disassembly of the actin cortex under the pores, we conclude that fusion-pore expansion is not driven by the actin cytoskeleton but rather requires its local depolymerization. We propose that a dynamic resistance of the actin network slows down pore expansion; this expansion might be driven by membrane-bending proteins that are involved in the generation of highly curved membrane compartments.
Results
Experimental system
Round Sf9Op1D cells expressing gp64 establish large and flat contacts that are characterized by a relatively constant intermembrane distance of ~20 nm (data not shown) (Chernomordik et al., 1995), making these cells a convenient model in which to explore the development and evolution of fusion sites in the extended contact zone. Loosely attached to their substrate, Sf9Op1D cells have a relatively simple actin cytoskeleton, characteristic of spherical cells: a cortical shell with no elongated stress fibers. The fusion reaction between Sf9Op1D cells is triggered by a short application of low-pH medium, which activates gp64 (Blissard and Wenz, 1992; Leikina et al., 1992) (supplementary material Movie 1). As expected (Chernomordik et al., 1995), expression of fusogens in only one of a pair of fusing cells was sufficient for cell-cell fusion, and no syncytia were formed without low-pH application (not shown).
Characterization of the fusion pores
To visualize the three-dimensional morphological changes in the contact zone during the opening and expansion of the fusion pore(s), we labeled Sf9Op1D cells with fluorescent lipids (Vybrant DiI or FM4-64FX) and imaged the cells with three-dimensional time-lapse confocal microscopy as they underwent fusion. Before initiation of fusion, two Sf9Op1D cells were connected by a flat, discoidal contact zone that was about 10 μm in diameter; upon completion of the fusion, the two cells were connected by an open lumen of cell-size diameter (~20 μm). On the basis of 27 time-lapse recordings of cell-cell fusion, fusion pores (detected when larger than 0.2 μm) seem to appear with equal probability within the entire contact zone, both along its circumference and in the central part. These pores were roughly circular and expanded radially until they came into contact with one another (Fig. 1; supplementary material Movie 2). When two pores came into contact (were separated by less than 0.5 μm of membrane), they did not readily converge. Instead, expansion of the pores towards each other was blocked, whereas expansion in other directions continued. As a result, two adjacent pores became separated by a long string of membrane, which eventually broke, leaving vesicular material trapped within pore lumen. Thirteen of our recordings captured the entire process of cell-cell fusion from a flat contact zone to a fusion product in which the volumes of the fused cells are connected by an open lumen with the same diameter as the cell. The number of pores in these recordings ranged from 1 to 14 (4.8±3.9). The time of expansion ranged from 7.4 to 32.4 minutes (16.1±8.6). The first detectable pore was observed within 1 minute, and as late as 20 minutes, after low-pH application.
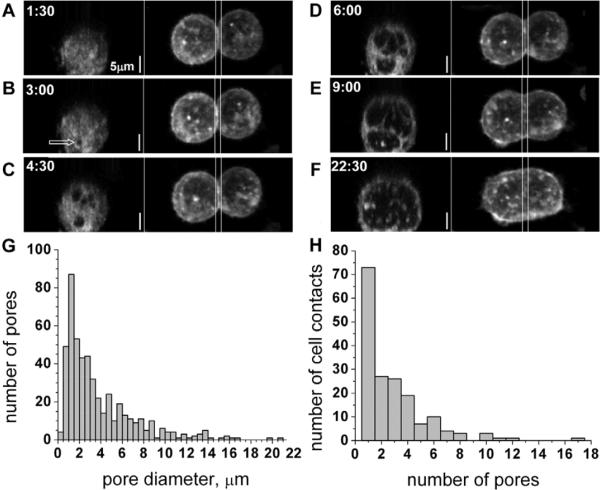
Fig. 1.
Fusion-pore opening and expansion in the cell-contact zone. (A–F) Time series of syncytia formation for cells that were labeled with FM4-64FX. For each time point, the top view (maximum intensity projection of the whole image stack along the z-axis) is shown on the right panel and the view of the contact zone as seen by looking from one fusing cell into another (maximum intensity projection along the x-axis of the region delimited by two vertical lines) is shown on the left panel. The arrow in B marks the location of the first pore that was observed in this series. (G,H) Pore-size (G) and pore-number per contact zone (H) distribution histograms based on analysis of 498 pores from 175 cell-cell contact zones. Because the average rates of fusion-pore expansion differed from day to day in more than ten experiments investigating the morphology of the fusion pores in fixed cells, these histograms include only the data collected in the same experiment. Cells were fixed 4 minutes after a 1-minute low-pH application. The height of each bar in G represents the number of pores with diameters in the interval between its lower and upper bounds on the x-axis.
To minimize the risk of photo damage, to increase the resolution of our images and to vastly increase the number of contact zones that were visualized, we performed experiments with fixed cells. Cells were induced to fuse then, at different time points, were labeled with fixable membrane dye FM4-64FX and, 1 minute later, treated with formaldehyde. The morphology of the fusion pores in fixed cells was similar to that observed in live-cell experiments. We collected hundreds of images of fusing contact zones; these images provided us with information on the distributions of pore sizes and the number of pores per contact zone (Fig. 1G,H).
In brief, late fusion stages progress through a radial expansion of a single pore or, more often, an opening and radial expansion of multiple pores, which involves interference between adjacent pores growing close to one another.
Fusion pores expand without loss of membrane material in the contact zone
Although, as mentioned above, interactions and merger of adjacent fusion pores often left some vesicles within the pore lumen, expansion of the pores was always manifested by local disappearance of the membrane material. Does this material vesiculate and move into fusing cells or is it laterally displaced to the periphery of the fusion site and remains within a contact plane? We measured membrane fluorescence around the initial contact zone up to a distance of 1.2 μm into each cell and found that it decreased as the fusion pore expanded (Fig. 2; supplementary material Fig. S1). At the same time, the diameter of the contact zone increased (Fig. 2B, insets). Moreover, the total intensity of membrane fluorescence within the whole contact plane did not decrease, indicating that there was no loss of cell-contact area until the very late stages of syncytium formation (Fig. 2; supplementary material Fig. S1). Although the kinetics of expansion of fusion pores differed between experiments, we observed similar qualitative behavior for all six cell pairs studied: an expansion of fusion pores that is not accompanied by a significant loss of total membrane area within the whole contact zone.
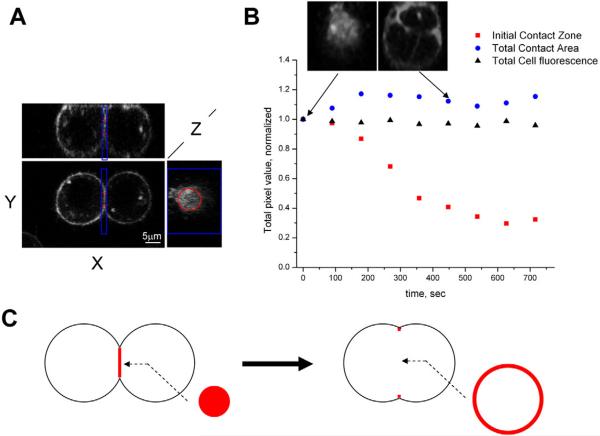
Fig. 2.
Fusion-pore expansion does not decrease the total area of the cell-contact zone. Fusion was triggered by a 1-minute low-pH pulse. (A) Orthogonal planes through a 3D-stack image of two fusing cells before the onset of syncytia formation (no pores detectable) are shown. The xy and xz planes are placed to cut through the middle of fusing cells, whereas the yz plane is placed to coincide with the contact zone between two cells. The red and blue lines outline 3D regions of interest (ROI) used for the analysis that is presented in B. Red lines outline a cylinder that encloses the initial contact zone and extends 1.2 μm into each cell. Blue lines outline a box that also extends 1.2 μm into each cell and is large enough to enclose the growing contact zone throughout the whole process of fusion-pore expansion. (B) Total membrane fluorescence within the initial contact zone [outlined by the red cylinder in A (red squares on the graph)] and within the total contact area [outlined by the blue box in A (blue circles on the graph)] were corrected for bleaching using the total cell fluorescence (black triangles). The data were normalized to the corresponding fluorescence intensity value at the initial time point using the equation Froi(t=T)Ftc(t=0)/[Froi(t=0)Ftc(t=T)], where Froi is an integrated pixel intensity of either initial-contact-zone or total-contact area, and Ftc is an integrated pixel intensity of the whole cell. Inset images show the contact zone at the time points that are indicated by the arrows. Cell membranes were labeled with FM4-64FX. The data are measurements from a single pair of fusing cells and represent one out of six experiments. (C) In this cartoon, fusion-pore growth is accompanied by an increase in the cell-contact diameter, whereas the total area of membranes in contact (shown in red) remains constant. As the contact zone expands, the total area of the initial contact zone (changes in the areas of the contact zone and fusion pore are drawn so as to be proportional to those of the fusing cells on A and B) is readily accommodated within a narrow rim (~5% of total-contact-zone diameter in thickness) along the edges of the expanded contact zone.
The lack of membrane material in the vicinity of fusion-pore lumen could be explained within the vesiculation model by a fast removal of the vesicles. However, this would not explain why the membrane area within the contact-zone plane remains essentially unchanged. Thus, taken together, our data substantiate the hypothesis that fusion pores mostly grow by displacement of membrane material towards the periphery of the contact zone, with conservation of the area of the tight cell-cell contact, as illustrated in Fig. 2C.
Fusion-pore expansion is not driven by membrane tension
It has been proposed that expansion of fusion pores during viral fusogen-initiated syncytium formation is driven by membrane tension that is generated by osmotic swelling of the fusing cells (Knutton, 1980). In this colloid-osmotic mechanism of tension development, small pores, formed by activated fusion proteins, in the membranes pass ions and thus dissipate transmembrane ion gradients but are not large enough to pass macromolecules. Water that enters the permeabilized cells to compensate the osmotic pressure of retained macromolecules swells the cells, building up the membrane tension. To test whether a colloid-osmotic mechanism is at work in gp64-mediated cell fusion, we induced fusion by applying low-pH medium containing propidium iodide (PI) (supplementary material Fig. S2). The lack of PI uptake by the fusing Sf9Op1D cells indicates that syncytium formation was not accompanied by membrane permeabilization and thus did not involve colloid-osmotic swelling.
Even in the absence of any osmotic imbalances, plasma membranes might be subjected to a lateral tension, such as the apparent tension generated by membrane-cytoskeleton adhesion (Dai and Sheetz, 1999; Morris and Homann, 2001). To temporarily release any tension in the cell-membrane bilayer, we shrank the fusing cells in hypertonic medium. Immediately after a 1-minute application of acidic-pH buffer, Sf9Op1D cells were placed into either PBS or PBS supplemented with 250 mM stachyose. We found that an increase in the tonicity of the medium lowered the mean cell radius, which was measured with confocal microscopy, by ~20% (Fig. 3A) (Kwon and Handler, 1995). However, the final extents of syncytium formation that were observed in isotonic and hypertonic media were identical, indicating that fusion-pore expansion was unaffected by a temporary release in membrane tension induced by the cell shrinkage. This finding distinguishes our experimental system from osmotically regulated fusion between mating yeast cells (Philips and Herskowitz, 1997), in which an acute shift to hyperosmotic medium inhibits early stages of fusion-pore expansion and mating (Nolan et al., 2006).
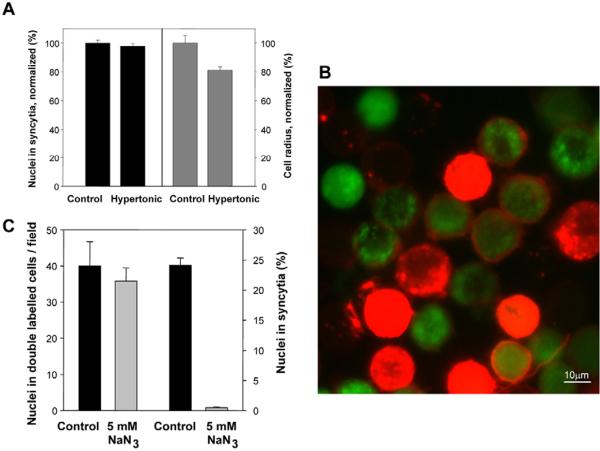
Fig. 3.
Syncytium formation is not driven by membrane tension and requires metabolic energy. (A) To explore the effects of a decrease in membrane tension on the extent of syncytium formation (black bars) and the mean radius of the cells (gray bars), immediately after a 1-minute application of acidic-pH buffer we placed Sf9Op1D cells into either PBS (control) or PBS supplemented with 250 mM stachyose (hypertonic medium). The syncytium-formation and cell-radii analyses were carried out 30 minutes after low-pH application for >1330 and >22 cells, respectively. The syncytium index and mean cell radius in hypertonic medium were normalized to those in the control experiments. (B,C) The metabolic inhibitor sodium azide (NaN3, 5 mM) did not prevent early fusion stages but blocked syncytium formation. (B) An overlay of a representative field of red and green fluorescence of the NaN3-treated cells fixed 1-hour after a 1-minute low-pH pulse. Membrane merger between cells that were pre-labeled with the red membrane dye Vybrant DiI and cells pre-labeled with content probe CellTracker Green yielded double-labeled cells. Unfused cells are seen as only red or only green ones. (C) Local fusion between cells that were pre-labeled with different probes in the presence of NaN3 and between controls was quantified as an average number of nuclei in double-labeled cells per field of view. Syncytium formation after a 1-minute low-pH pulse was quantified as the percentage of nuclei in syncytia. The total number of analyzed nuclei for each condition was >2500. A and C show means ± s.d. of ten replicates in a representative experiment, performed twice.
To summarize, the transition from local fusion to syncytia is not driven by membrane tension. Note that, although tension is not required for syncytia formation, application of tension by placing fusing cells into hypotonic medium promotes fusion both in our experimental system (data not shown) and in many other cell-fusion reactions (Chernomordik et al., 1998; Nolan et al., 2006; Podbilewicz et al., 2006; Wyke et al., 1980).
Syncytium formation requires metabolic activity of living cells
We co-plated cells that were labeled with either the red membrane dye Vybrant DiI or the cytosolic probe CellTracker Green, applied a low-pH pulse and detected fusion events by the appearance of cells that were co-labeled by membrane dye and content probes. Slowing down the metabolism either by lowering the cell-incubation temperature to 4°C (data not shown) or by pre-treating the cells with sodium azide (NaN3), a reversible inhibitor of mitochondrial respiration, had no effect on early fusion intermediates (Fig. 3B,C). NaN3 treatment had almost no effect on the number of double-labeled cells and, thus, did not affect early fusion stages, yielding fusion pores (Fig. 3C). By contrast, both NaN3 (Fig. 3C) and incubation at 4°C (not shown) completely blocked syncytium formation under conditions that yielded robust syncytium formation in NaN3-untreated cells at room temperature (supplementary material Fig. S3). This inhibition cannot be explained by a NaN3-induced down regulation of the cell-surface expression of gp64, because NaN3 also inhibited low-pH-induced syncytium formation, mediated by surface-bound baculovirus particles, between Sf9 cells (data not shown). Importantly, when the inhibitor was withdrawn (the temperature was raised to 22°C or NaN3 was washed out) to allow the cells to recover their normal metabolism, fusion ensued, confirming that the cells were still viable. For instance, the level of syncytia formation was restored to 85% of that observed in untreated controls when NaN3 was removed 30 minutes after a 1-minute low pH pulse. Because gp64 readily inactivates after a low-pH application, this result indicates that early fusion intermediates were already formed and that metabolic inhibitors blocked the fusion reaction downstream of the low-pH-dependent gp64-mediated fusion steps.
To summarize, cell metabolism plays a crucial role in the transition from low-pH-dependent early fusion intermediates to syncytium formation. This conclusion is consistent with several earlier studies on cell fusion induced by Semliki Forest virus (Kempf et al., 1987) and electroporation (Zheng and Chang, 1991).
Actin-cytoskeleton rearrangements during syncytium formation
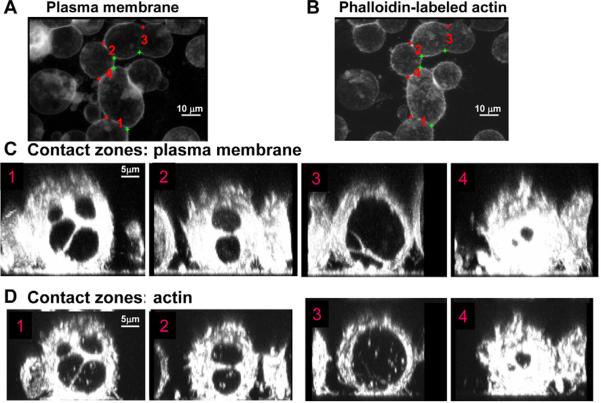
Many important properties of cell membranes are controlled by their interactions with the actin cytoskeleton and, especially, with the actin-based contractile cortex under the plasma membrane. A study of electroporation-induced fusion in mammalian cell culture has led to the suggestion that fusion pores are expanded by actin bundles that are assembled at the edges of the pores (Zheng and Chang, 1991). To visualize the structure of the actin network during Sf9Op1D syncytium formation, we labeled the actin filaments in permeabilized fusing cells using fluorescent phalloidin. We observed openings in the actin cortex that were colocalized with the lumen of the fusion pores in the membrane junction (Fig. 4). These observations imply, therefore, that development of gp64-induced fusion pores results in a disassembly of the actin cortex under the pores rather than in local accumulation of actin bundles, driving syncytium formation.
Fig. 4.
Fusion pores colocalize with openings in the actin cortex. Cells were labeled with FM4-64FX 9 minutes after a 2-minute low-pH pulse. 1 minute later, the cells were fixed and labeled with Alexa-Fluor-48-phalloidin. (A,B) xy-projections of representative contact zones (marked as 1–4) shown with membrane- and actin-labeling, respectively. (C,D) Maximum intensity projections of the image stacks of each of these zones along the z axis with membrane- (C) and, under it, actin- (D) labeling.
Effects of actin-modifying treatments on fusion-pore expansion
Is the actin cytoskeleton required for syncytium formation? To address this question, we used actin-modifying reagents. Latrunculin A (LatA; Fig. 5A), a drug that sequesters monomeric actin (Allingham et al., 2006) caused a fast (within 15 minutes; data not shown) dissociation of the actin cortex beneath Sf9Op1D cell membranes. In spite of a dramatic loss of actin-cortex labeling, LatA-treated cells, as the control cells, formed syncytia (marked by arrows in Fig. 5A). Moreover, LatA treatment resulted in a dramatic acceleration of pore expansion, which was detected as an increase in the total area of the open pore lumen observed 1 minute after low-pH application (Fig. 5B). LatA application also changed the shape of the fusion pores from almost circular to irregular (supplementary material Table S1).
Fig. 5.
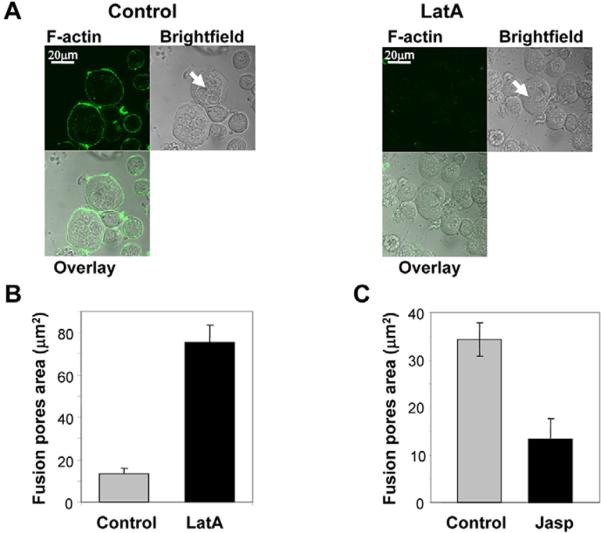
Actin-depolymerizing and -polymerizing reagents latrunculin A (LatA) and jasplakinolide (Jasp) promote and inhibit fusion-pore expansion, respectively. (A) Dissociation of the actin cortex by LatA. Sf9Op1D cells were incubated for 30 minutes with Grace medium with 2 μM LatA (`LatA') or without it (`Control') prior to a 5-minute low-pH pulse. In the former case, the cells were kept in the presence of LatA throughout the experiment. Fluorescence-microscopy and bright-field images of the cells, which were fixed and labeled with Alexa-Fluor-488-phalloidin 2 hours after low-pH application, are presented. Although LatA treatment resulted in a loss of actin-cortex labeling, LatA-treated cells, as did control cells, formed syncytia (marked by arrows). (B) LatA-treated cells have a larger average total area of fusion pores per contact zone than the control cells when fixed 1 minute after a 1-minute low-pH application. Each bar is based on analysis of 50 contact zones (152 and 127 pores for control and for LatA-treated cells, respectively). (C) Jasp slows down fusion-pore expansion. Cells treated with a 1-minute pulse of pH-4.9 medium followed by a 9-minute incubation in the presence of 0.5 μM Jasp and then fixed have a lower average total area of fusion pores per contact zone than the control Jasp-untreated cells. Results are based on the analysis of a total of 51 contact zones for control cells and 37 contact zones for Jasp-treated cells. B and C show the means ± s.e. based on the analysis of the data collected in the same experiment for the reagent-treated and untreated (control) FM4-64FX-labeled cells.
In contrast to LatA, jasplakinolide (Jasp), an actin-polymerizing and filament-stabilizing drug (Allingham et al., 2006) lowered the total area of the pores (Fig. 5C). The opening of initial fusion pores in gp64-mediated cell fusion after acidification is very fast [from 0.3 to 6 seconds (Plonsky et al., 1999)]. Thus, to focus on the Jasp effects on the expansion rather than on the opening of the pores, in these experiments we applied the reagent after a 1-minute pulse of low pH. To better highlight slowing down (Jasp) of pore growth relative to control conditions (no actin-modifying reagents), we assayed pore area 10 minutes after low-pH application, rather than 1 minute after acidification as in the case of LatA.
Analysis of the final extents of syncytium formation for the cells treated with either LatA or cytochalasin D (CD), a reagent that inhibits actin polymerization by blocking the barbed end of actin filaments (Wakatsuki et al., 2001), have further substantiated the conclusion that actin depolymerization does not block (LatA) and even promotes (CD) fusion-pore expansion (Fig. 6A). By contrast, the actin-filament-crosslinking reagents Jasp and phalloidin inhibited syncytium formation (Fig. 6A,B). To deliver a membrane-impermeable phalloidin into Sf9Op1D cells, we incubated the cells with phalloidin-loaded human RBC ghosts. In this case, low-pH-induced gp64-mediated fusion between Sf9Op1D cells and RBC ghosts delivered phalloidin into Sf9Op1D cells, lowering the observed extent of syncytium formation by Sf9Op1D cells.
Fig. 6.
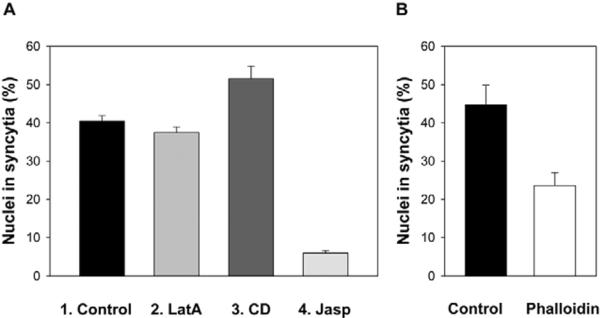
Effects of actin-modifying reagents on the final extents of syncytium formation. (A) Syncytium formation for Sf9Op1D cells treated with the cell-permeable actin-depolymerizing reagents LatA (2 μM, bar 2) and CD (0.1 μM, bar 3), and actin-polymerizing reagent Jasp (0.5 μM, bar 4), and for the untreated cells (bar 1). LatA was applied 30 minutes before a fusion-triggering low-pH pulse. CD and Jasp were applied immediately after the pulse (see Materials and Methods). (B) The membrane-impermeable actin-polymerizing reagent phalloidin that was delivered into Sf9Op1D cells by their fusion with phalloidin-loaded erythrocyte ghosts inhibited syncytium formation. In the control experiments we used unloaded erythrocyte ghosts. (A,B) Fusion was triggered by a 1-minute application of pH 4.9 medium. Syncytium-formation extents were scored 30 minutes later and presented as means+s.e. (n≥3).
To summarize, the effects of actin-modifying agents confirmed an important role of the actin cortex in cell-cell fusion. Polymerization and depolymerization of actin filaments respectively inhibited and promoted pore expansion and syncytium formation, indicating that the actin cytoskeleton restricts rather than drives the expansion of fusion pores.
Discussion
Earlier work on membrane-fusion mechanisms has focused on the identification and structural characterization of the protein fusogens and on early fusion intermediates, such as hemifusion and a nascent fusion pore. These early fusion stages driven by fusogens are independent of cell physiology and have been observed in ATP-depleted cells (Fig. 3) (Kempf et al., 1987; Zheng and Chang, 1991). Although the mechanisms of fusion initiation are essential for understanding any fusion reaction, cell-cell fusion in development, in viral infections and possibly in tumor progression proceeds beyond small initial fusion pores to cell-sized cytoplasmic connections. In this work, we addressed these late cell-cell fusion stages, using, as a model system, syncytium formation mediated by baculovirus gp64. Similarly to yeast vacuoles (Wang et al., 2002), and to developmental fusion-committed cells such as myoblasts (Doberstein et al., 1997) and C. elegans epithelial cells (Shemer and Podbilewicz, 2003), gp64-expressing Sf9Op1D cells establish extended tight contacts. In gp64-induced syncytia, as in fusion between myoblasts and between C. elegans epithelial cells, fusion pores develop over the entire contact zone (reviewed by Podbilewicz and Chernomordik, 2005). By contrast, in the case of yeast vacuoles, fusion pore(s) develop along the edge of the contact zone (Wang et al., 2002) and, in the case of cell-cell fusions in the tail tip and the embryonic hypodermal cells of C. elegans, fusion pores appear only at or near adherens junctions (Mohler et al., 1998; Nguyen et al., 1999).
The number of the fusion pores developing in the contact zone might be different in diverse developmental fusion reactions. Elegant transmission electron microscopy (TEM) data indicate that cell fusion in the hypodermis of wild-type C. elegans embryos proceeds by expansion of single expanding pores (Mohler et al., 1998). However, this model is based on a limited number of sectioned embryos and has not yet been supported by other ultrastructural studies on different fusion reactions (Doberstein et al., 1997; Gattegno et al., 2007; Nguyen et al., 1999; Shemer et al., 2004). More-recent studies on adult hypodermis and pharyngeal muscles of eff-1 temperature-sensitive mutants of C. elegans showed multiple pores that expand to 50–100 nm but fail to complete the merger of the extended tight contacts (Gattegno et al., 2007; Shemer et al., 2004). Syncytium formation that is initiated by gp64 uses both of the discussed pathways: an expansion of a single fusion pore and, more often, an expansion and interactions of multiple pores. The numbers of activated fusogens, the waiting time for fusion-pore opening and, thus, probably the numbers of fusion pores per cell contact zone might be varied by altering the pH of the medium, used to trigger fusion, or the duration of the low-pH application. Thus, gp64-initiated cell fusion in the future might be used to explore both single-pore and multiple-pore pathways.
Several electron-microscopy studies on different developmental cell fusion reactions reported vesicles in the lumen of the growing pores, suggesting that pore growth proceeds by vesiculation (Doberstein et al., 1997; Mohler et al., 1998). In our system, interaction between multiple growing pores within the same contact zone in many cases left vesicles in the pore lumen. However, observed conservation of the contact-zone area suggests that most of the membrane material from expanding pores is laterally displaced within the contact zone rather than removed by vesiculation. Nonetheless, additional experimental approaches and experiments on biologically relevant cell-cell fusion reactions are needed to unambiguously establish the validity and generality of the hypothesis that the expansion of fusion pores mostly proceeds by lateral membrane displacement.
In contrast to a fast opening of fusion pores driven by protein fusogens, the extension of initial pores to sizes detectable with light microscopy and their further growth at the micron scale require metabolic activity of the cells. Thus, pore evolution at the micron scale is controlled by the cell machinery. The relative simplicity of our experimental systems allowed us to explore the contributions of the cytoskeleton and to address the intriguing question of what drives fusion-pore extension in syncytium formation.
Syncytium formation and the cytoskeleton
The actin cytoskeleton plays an important and variable role in both intracellular and intercellular fusion. The actin cortex blocks access of exocytotic granules to the plasma membrane (Ehre et al., 2005; Miyake et al., 2001). By contrast, the actin cytoskeleton facilitates the delivery of granules to their site of fusion and drives the expansion of exocytotic fusion pores (Larina et al., 2007; Muallem et al., 1995; Valentijn et al., 1999). Although actin cytoskeleton has also been implicated in syncytium formation, it remains unclear whether actin structures promote fusion-pore extension (Massarwa et al., 2007; Zheng and Chang, 1991), restrict it (Chernomordik and Sowers, 1991; Schowalter et al., 2006) or influence only the stages of cell-cell fusion that precede the opening of fusion pores (Kim et al., 2007).
Our analysis of the actin structures during the fusion expansion stage of gp64-initiated syncytium formation uncovered a local disruption of the actin cortex beneath the pore. These `pores' in the cortex resemble local and transient disruptions in the actin meshwork that are detected under microneedle-puncture-induced pores in the plasma membrane (Miyake et al., 2001). In the latter case, a local actin depolymerization was explained by an increase in Ca2+ concentration in the vicinity of the membrane pore, which connects the intracellular volume with the Ca2+-rich extracellular medium. In the case of fusion pores connecting two volumes with similarly low concentrations of Ca2+, such an interpretation is unlikely. One may hypothesize that fusion pores yield openings in a tangled and cross-linked network of actin filaments by local disruption of dynamic interactions between actin filaments and plasma membrane and across the cortex.
Regardless of the specific mechanism, dissociation of the actin cytoskeleton at the fusion site appears to be a prerequisite for pore growth. Indeed, we found reagents that promote actin polymerization and stabilize actin filaments to inhibit fusion-pore expansion, and reagents that depolymerize actin filaments to promote pore expansion and/or syncytium formation. Our data indicate that, in our experimental system, any direct or indirect promoting effect of the actin skeleton on pore formation (if it exists) is overshadowed by the restricting effect. The conclusion that the actin cytoskeleton guides and restricts fusion-pore expansion rather than drives it is consistent with a recent finding that myoblast fusion is preceded by dissolution of an actin focus at the site of fusion (Richardson et al., 2007).
Mechanism of fusion-pore expansion
Evolution of a fusion pore after its formation depends on the properties of the inter-membrane contact. In the simplest case (no attractive interactions or links between the membranes in the contact zone), expansion of a fusion pore should be driven by unbending of the pore wall, which is accompanied by an increase in the inter-membrane distance (Chizmadzhev et al., 1995). However, this is not the case in the system of two bound cells forming syncytium. The membranes of two Sf9Op1D cells establish a very tight contact. The area of close contact did not decrease in the course of pore growth, indicating that the enlargement of fusion pores formed within the contact zone moves apart, rather than disrupts, the inter-membrane links.
Expansion of large fusion pores with a strongly bent rim requires a persistent energy input (Chernomordik and Kozlov, 2003) and hence must be driven by physical forces. There are three possible sources of such forces:
-
(1)
Pore growth can be driven by membrane lateral tension pulling on the pore rim to enlarge the pore area (Zimmerberg et al., 1980).
-
(2)
The force expanding the fusion pore between two cells can be generated by the actin structures. For instance, polymerizing actin propagating into the pore lumen should exert a force pushing the pore rim that is analogous to the force acting on the leading edge of the lamellipodium of a moving cell (Kaksonen et al., 2006).
-
(3)
And, finally, the force driving the pore expansion can come from the factors relaxing the bending energy of the pore rim and making the pore energetically favorable. This process might be described in terms of a negative line tension of the pore edge (Chernomordik and Kozlov, 2003). These factors will lead to a tendency of the pore to increase its circumference.
The first two reasons for pore expansion can be ruled out on the basis of our experimental results. Neither release in membrane tension induced by the cell shrinkage nor depolymerization of the actin cortex by LatA slowed down fusion-pore growth. Hence, the driving force of pore growth must come from the factors generating the negative line tension of the pore rim. The shape of the fusion-pore rim is similar to a half cylinder with a radius of about 20 nm (Fig. 7A). We hypothesize that the pore expansion is based on the pool of membrane-bending proteins existing within the cell and involved in the cell-controlled generation of highly curved membrane compartments such as endocytic vesicles and tubular, spherical and pleomorphic intracellular transport intermediates, all characterized by curvature radii of a few tens of nanometers (Antonny, 2006; Itoh and De Camilli, 2006; McMahon and Gallop, 2005; Zimmerberg and Kozlov, 2006). The expanding list of protein families currently implicated in shaping plasma membranes into such kinds of structures includes the small G-proteins that contain amphipathic helices, and the proteins containing the N-BAR domain, the F-BAR domain and the EH (epsin homology) domain (Antonny, 2006; Itoh and De Camilli, 2006; McMahon and Gallop, 2005; Zimmerberg and Kozlov, 2006).
Fig. 7.
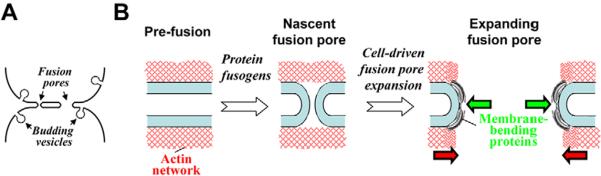
Proposed mechanism of the fusion-pore expansion in syncytium formation. (A) Membrane curvature of an expanding fusion pore is similar to that in budding vesicles. (B) Proposed pathway of syncytium formation. Red networks under the contacting membranes in the pre-fusion state depict actin structures. Protein fusogens, such as baculovirus gp64, form nascent fusion pores. The subsequent expansion of these pores to yield syncytium is controlled by cell machinery. A dynamic resistance of the actin network disrupted by a pore slows down pore expansion, which is driven by membrane-bending proteins (banana-like shapes). These proteins, which are involved in the generation of highly curved intracellular membrane compartments, accumulate at the strongly bent rim of the fusion pores, lower the line tension of the pores and, thus, drive their expansion.
On the basis of a similarity of membrane bending in the fusion-pore rim and highly curved membrane compartments such as budding endocytic vesicles (Fig. 7A), we propose the following scenario of fusion-pore growth (Fig. 7B). The membrane-bending proteins are capable of a concerted grouping on a fragment of membrane surface and of shaping it into a bent form with a few tens of nanometers radius of curvature. This event is kinetically controlled by the generation of an initial curved membrane spot serving as a nucleation site for protein enrichment and for growth of the curved membrane structure. Nucleation of the curved membrane compartments is produced by specialized proteins, such as clathrin-adaptor protein complexes. We suggest that the rim of a nascent fusion pore that is formed as the result of action of protein fusogens also nucleates localized binding of the membrane-bending proteins followed by rim growth and, hence, enlargement of the pore. This implies that the pore rim with bound membrane-bending proteins is energetically favorable and hence is characterized by a negative line tension. We do not expect the binding of the protein to the fusion-pore edge to significantly affect the amount of the available membrane-bending proteins. The edge area is probably much smaller than the total area of endocytotic vesicles, which bud every minute [the pore edge area ~0.2 μm2 for a 2 μm pore vs the area of budding vesicles, which might be as high as 10 μm2 per minute, assuming as a rough estimate that the vesicles represent ~1–2% of the plasma-membrane area (Griffiths et al., 1989)].
In general, a pore with a vanishing or negative line tension tends to increase its perimeter at any given area of the pore lumen, and hence the shape of the pore should not be circular. At the same time, the pores we observed had nearly circular shapes until they collided with each other. This pattern can be explained by a dynamic resistance of the actin cortex to fusion-pore expansion. A growing pore deforms the cortex by removing membrane-anchored actin from underneath the pore, and the energy of these deformations must be minimal for the case of circular pores generating a symmetric distribution of cortex strains. In agreement with this interpretation, dissociation of the actin cortex by LatA results in a faster expansion of fusion pores and uneven rather than circular pore edges.
Conclusions
The expansion of the early fusion pores to a cell-size lumen in syncytium formation has been thought (but never experimentally established) to either proceed spontaneously as a way of releasing membrane deformations in the pores, or be driven by actin cytoskeleton. Our data argue against both of these hypotheses. We found that fusion-pore expansion is not a spontaneous process but rather an active one, dependent on cell metabolism. Furthermore, actin structures did not drive but rather slowed down syncytium formation. Controlled expansion of fusion pores is also an important stage of exocytosis. Interestingly, in analogy to our findings for syncytium formation, the actin cortex restricts expansion of a fusion pore connecting exocytosing granules and plasma membrane, and LatA-induced disruption of the cortex promotes pore expansion and the rapid collapse of granules into the plasma membrane (Sokac et al., 2003).
gp64 proteins, similar to many viral fusogens, rapidly inactivate after low-pH application (Markovic et al., 1998) and are probably inactivated by the time the pores become visible in light microscopy. Thus, although syncytium formation is initiated by an action of protein fusogens that generate nascent fusion pores and drive their initial expansion, the subsequent cell-physiology-dependent stages of cell-cell fusion might be mostly independent of the specific `pioneer' fusogens. Indeed, we recently found that syncytium formation that is initiated by influenza hemagglutinin also proceeds in cells with depolymerized actin and is blocked by ATP depletion (Richard et al., 2008). Thus, although the current study explored a relatively simple cell-fusion reaction among cells not normally destined to fuse, we expect that the mechanisms of syncytium formation described here are of general significance, and contribute to the more complex cell-fusion reactions that occur during animal development and in pathological processes.
Materials and Methods
Cells and virus
Sf9 cells (derived from pupal ovarian tissue of the Fall armyworm, Spodoptera frugiperda) and Sf9Op1D cells, i.e. stably transfected Sf9 cells expressing a fusion protein of baculovirus OpMNPV gp64 (Plonsky et al., 1999), generously provided by Gary Blissard (Cornell University, Ithaca, NY), were cultured at 27°C as the exponentially growing subconfluent monolayers in 75-cm2 flasks in Grace's Insect Cell Culture Medium (Invitrogen) supplemented with 10% FBS and gentamicin (Invitrogen). In some experiments, instead of fusion between Sf9Op1D cells we studied fusion between Sf9 cells that were pre-incubated with baculovirus AcNPV particles (Invitrogen; multiplicity of infection 100). In some experiments, we used human erythrocyte ghosts freshly isolated from whole blood.
Cell-labeling protocols
Plasma membrane was labeled with either Vybrant DiI or FM4-64FX (Invitrogen). In the experiments using confocal microscopy, we routinely used FM4-64FX, a dye designed for labeling membranes in cell-fixation protocols. For non-fixed cells, any change in the medium would remove the dye and, thus, in all experiments involving changes in the medium we used Vybrant DiI. For Vybrant-DiI labeling, attached cells were incubated at room temperature with 5 μM Vybrant DiI in Grace's medium for 5 minutes, then washed twice with growth medium. FM4-64FX labeling was done according to the manufacturer's protocol, using glucose to adjust Hank's Balanced Salt Solution (HBSS) tonicity to that of Grace's media. Actin cytoskeleton was labeled with Alexa-Fluor-488–phalloidin (Invitrogen) according to the manufacturer's protocol. In experiments involving both plasma-membrane and actin labeling, slides were marked with a permanent marker before cells were added. We labeled and imaged plasma membrane first, and then actin; using the marks as reference points, we located and imaged the same cells again.
To detect and quantify local fusion events, we divided cells into two subpopulations and labeled one subpopulation of the cells in suspension with fluorescent lipid Vybrant DiI (Invitrogen) and another with cytoplasm probe CellTracker Green CMFDA (5-chloromethylfluorescein diacetate) (Invitrogen) according to manufacturer protocols.
Fluorescence and confocal microscopy, and image analysis
We performed fluorescence and confocal microscopy using an Olympus IX70 inverted microscope and a Zeiss LSM 510 system, respectively. We carried out image processing and visualization of confocal images with an in-house software written in Matlab with the Image Processing Toolbox. Time-series: 4D images were registered in the axial (z) direction by automatic detection of the maximum intensity change in adjacent z-slices. 4D images were registered in the x-y direction by user selection of two points parallel to the contact zone on four or five xy-projections along the time series; x-y registration reference points were linearly interpolated between user selections. 4D images were linearly interpolated in the z-direction to achieve a 1:1:1 aspect ratio. A 3×3 median filter was used to reduce noise. To analyze fixed cells with confocal microscopy, we cropped cell-contact areas using user-selected coordinates. 3D images were linearly interpolated in the z-direction to achieve a 1:1:1 aspect ratio. To analyze the sizes of the pores, we manually outlined them and measured their area. Pore diameter, estimated as a diameter of the circle with the same area, was necessarily quantized by our imaging system because we can acquire only a limited number of pixels per image. To evaluate the effects of hypertonic medium on apparent cell sizes, we measured the cross sectional area of the cells using confocal microscopy. The mean cell radius was calculated from the mean cross-sectional area of 22 cells for each condition.
Fusion and permeability assays
gp64-mediated fusion was triggered by a short-term application of Grace's medium titrated with citrate to pH 4.9, followed by restoration of the normal medium pH of 6.4. Because fusion rates varied from day to day, apparently because of variation in the level of gp64 expression, the number of attached cells and temperature, we routinely started the experiments by choosing the duration of the pH 4.9 pulse between 1 and 10 minutes (1 minute if not stated otherwise) to get the final extents of syncytium formation in the control experiments (untreated cells) to ~40%.
We counted the percentage of cells in syncytia (the ratio of nuclei within syncytia to the total number of cell nuclei in the same field) 20–30 minutes after low-pH application, using light microscopy for the fixed cells with Hoechst-33342 (Invitrogen)-labeled nuclei (supplementary material Fig. S3). We assayed the final extents of local fusion using fluorescence-microscopy analysis of the redistribution of membrane and cytoplasm probes between co-plated labeled and unlabeled cells or between co-plated differently labeled cells. To visualize cells with permeabilized plasma membrane (including dead cells) as cells accumulating the membrane-impermeable DNA probe PI (MW 668), we incubated the cells in PBS supplemented with 10 μM PI (Invitrogen) and imaged them 10 minutes later.
Cell treatments
Cell metabolism was inhibited by incubation with 5 mM NaN3 (Sigma-Aldrich) in Grace's medium for 1 hour prior to fusion. The actin cytoskeleton was disrupted by incubation with 2 μM LatA (Invitrogen) in Grace's medium for 30 minutes prior to low-pH application. Jasp (Invitrogen) and CD (Sigma-Aldrich) were applied at 0.5 and 0.1 μM, respectively, immediately after the end of the low-pH application. After a 30-minute incubation, still in the presence of the actin-modifying reagents, the cells were fixed with 3.7% formaldehyde (Sigma) in either Grace's medium or HBSS, labeled with Hoechst 33342 (Invitrogen) and scored for syncytia.
In the experiments investigating the effects of Alexa-Fluor-488–phalloidin on syncytium formation, we first loaded the reagent into human erythrocyte ghosts that were formed by mild hypotonic lysis (Melikyan et al., 1995) and were resealed after a 1-hour incubation on ice in the presence of 10 μM Alexa-Fluor-488–phalloidin. After washings (Melikyan et al., 1995), a small number of the ghosts were incubated with Sf9Op1D cells for 30 minutes. Fusion was triggered by a 1-minute application of pH 4.9 medium. Phalloidin was delivered into Sf9Op1D cells by their fusion with erythrocyte ghosts and influenced fusion between Sf9Op1D cells. Syncytium formation was scored 20 minutes later.
We are indebted to Gary Blissard for the generous gift of Sf9Op1D cells and Myoungsoon S. Hong for her kind help with electron-microscopy experiments. We thank Alexander Bershadsky, Jean-Philippe Richard and Joshua Zimmerberg for very helpful discussions. This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (L.V.C.).
Supplementary Material
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/121/21/3619/DC1
References
- Allingham JS, Klenchin VA, Rayment I. Actin-targeting natural products: structures, properties and mechanisms of action. Cell Mol. Life Sci. 2006;63:2119–2134. doi: 10.1007/s00018-006-6157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B. Membrane deformation by protein coats. Curr. Opin. Cell Biol. 2006;18:386–394. doi: 10.1016/j.ceb.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Berger S, Schafer G, Kesper DA, Holz A, Eriksson T, Palmer RH, Beck L, Klambt C, Renkawitz-Pohl R, Onel SF. WASP and SCAR have distinct roles in activating the Arp2/3 complex during myoblast fusion. J. Cell Sci. 2008;121:1303–1313. doi: 10.1242/jcs.022269. [DOI] [PubMed] [Google Scholar]
- Bitko V, Oldenburg A, Garmon NE, Barik S. Profilin is required for viral morphogenesis, syncytium formation, and cell-specific stress fiber induction by respiratory syncytial virus. BMC Microbiol. 2003;3:9. doi: 10.1186/1471-2180-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blissard GW, Wenz JR. Baculovirus gp64 envelope glycoprotein is sufficient to mediate pH-dependent membrane fusion. J. Virol. 1992;66:6829–6835. doi: 10.1128/jvi.66.11.6829-6835.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen EH, Grote E, Mohler W, Vignery A. Cell-cell fusion. FEBS Lett. 2007;581:2181–2193. doi: 10.1016/j.febslet.2007.03.033. [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Sowers AE. Evidence that the spectrin network and a nonosmotic force control the fusion product morphology in electrofused erythrocyte ghosts. Biophys. J. 1991;60:1026–1037. doi: 10.1016/S0006-3495(91)82140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu. Rev. Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- Chernomordik L, Leikina E, Zimmerberg J. Control of baculovirus gp64-induced syncytia formation by membrane lipid composition. J. Virol. 1995;69:3049–3058. doi: 10.1128/jvi.69.5.3049-3058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Frolov VA, Leikina E, Bronk P, Zimmerberg J. The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation. J. Cell Biol. 1998;140:1369–1382. doi: 10.1083/jcb.140.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizmadzhev YA, Cohen FS, Shcherbakov A, Zimmerberg J. Membrane mechanics can account for fusion pore dilation in stages. Biophys. J. 1995;69:2489–2500. doi: 10.1016/S0006-3495(95)80119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J. Cell Biol. 1998;141:297–308. doi: 10.1083/jcb.141.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J, Sheetz MP. Membrane tether formation from blebbing cells. Biophys. J. 1999;77:3363–3370. doi: 10.1016/S0006-3495(99)77168-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFife KM, Jenney CR, Colton E, Anderson JM. Disruption of filamentous actin inhibits human macrophage fusion. FASEB J. 1999;13:823–832. doi: 10.1096/fasebj.13.8.823. [DOI] [PubMed] [Google Scholar]
- Ding M, Woo WM, Chisholm AD. The cytoskeleton and epidermal morphogenesis in C. elegans. Exp. Cell Res. 2004;301:84–90. doi: 10.1016/j.yexcr.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Doberstein SK, Fetter RD, Mehta AY, Goodman CS. Genetic analysis of myoblast fusion: blown fuse is required for progression beyond the prefusion complex. J. Cell Biol. 1997;136:1249–1261. doi: 10.1083/jcb.136.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duelli D, Lazebnik Y. Cell-to-cell fusion as a link between viruses and cancer. Nat. Rev. Cancer. 2007;7:968–976. doi: 10.1038/nrc2272. [DOI] [PubMed] [Google Scholar]
- Earp LJ, Delos SE, Park HE, White JM. The many mechanisms of viral membrane fusion proteins. Curr. Top. Microbiol. Immunol. 2005;285:25–66. doi: 10.1007/3-540-26764-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehre C, Rossi AH, Abdullah LH, De Pestel K, Hill S, Olsen JC, Davis CW. Barrier role of actin filaments in regulated mucin secretion from airway goblet cells. Am. J. Physiol. Cell Physiol. 2005;288:C46–C56. doi: 10.1152/ajpcell.00397.2004. [DOI] [PubMed] [Google Scholar]
- Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, Puri A, Durell S, Blumenthal R. The HIV Env-mediated fusion reaction. Biochim. Biophys. Acta. 2003;1614:36–50. doi: 10.1016/s0005-2736(03)00161-5. [DOI] [PubMed] [Google Scholar]
- Gattegno T, Mittal A, Valansi C, Nguyen KC, Hall DH, Chernomordik LV, Podbilewicz B. Genetic control of fusion pore expansion in the epidermis of Caenorhabditis elegans. Mol. Biol. Cell. 2007;18:1153–1166. doi: 10.1091/mbc.E06-09-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower TL, Pastey MK, Peeples ME, Collins PL, McCurdy LH, Hart TK, Guth A, Johnson TR, Graham BS. RhoA signaling is required for respiratory syncytial virus-induced syncytium formation and filamentous virion morphology. J. Virol. 2005;79:5326–5336. doi: 10.1128/JVI.79.9.5326-5336.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Back R, Marsh M. A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J. Cell Biol. 1989;109:2703–2720. doi: 10.1083/jcb.109.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, De Camilli P. BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim. Biophys. Acta. 2006;1761:897–912. doi: 10.1016/j.bbalip.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Jahn R, Lang T, Sudhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Kadiu I, Ricardo-Dukelow M, Ciborowski P, Gendelman HE. Cytoskeletal protein transformation in HIV-1-infected macrophage giant cells. J. Immunol. 2007;178:6404–6415. doi: 10.4049/jimmunol.178.10.6404. [DOI] [PubMed] [Google Scholar]
- Kaksonen M, Toret CP, Drubin DG. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell. Biol. 2006;7:404–414. doi: 10.1038/nrm1940. [DOI] [PubMed] [Google Scholar]
- Kallewaard NL, Bowen AL, Crowe JE., Jr Cooperativity of actin and microtubule elements during replication of respiratory syncytial virus. Virology. 2005;331:73–81. doi: 10.1016/j.virol.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Kempf C, Kohler U, Michel MR, Koblet H. Semliki Forest virus-induced polykaryocyte formation is an ATP-dependent event. Arch. Virol. 1987;95:111–122. doi: 10.1007/BF01311338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M, Rey FA. Virus membrane-fusion proteins: more than one way to make a hairpin. Nat. Rev. Microbiol. 2006;4:67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Shilagardi K, Zhang S, Hong SN, Sens KL, Bo J, Gonzalez GA, Chen EH. A critical function for the actin cytoskeleton in targeted exocytosis of prefusion vesicles during myoblast fusion. Dev. Cell. 2007;12:571–586. doi: 10.1016/j.devcel.2007.02.019. [DOI] [PubMed] [Google Scholar]
- Knutton S. Studies of membrane fusion. VI. Mechanism of the membrane fusion and cell swelling stages of Sendai virus-mediated cell fusion. J. Cell Sci. 1980;43:103–118. doi: 10.1242/jcs.43.1.103. [DOI] [PubMed] [Google Scholar]
- Kwon HM, Handler JS. Cell volume regulated transporters of compatible osmolytes. Curr. Opin. Cell Biol. 1995;7:465–471. doi: 10.1016/0955-0674(95)80002-6. [DOI] [PubMed] [Google Scholar]
- Larina O, Bhat P, Pickett JA, Launikonis BS, Shah A, Kruger WA, Edwardson JM, Thorn P. Dynamic regulation of the large exocytotic fusion pore in pancreatic acinar cells. Mol. Biol. Cell. 2007;18:3502–3511. doi: 10.1091/mbc.E07-01-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikina E, Onaran HO, Zimmerberg J. Acidic pH induces fusion of cells infected with baculovirus to form syncytia. FEBS Lett. 1992);304:221–224. doi: 10.1016/0014-5793(92)80623-O. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic I, Pulyaeva H, Sokoloff A, Chernomordik LV. Membrane fusion mediated by baculovirus gp64 involves assembly of stable gp64 trimers into multiprotein aggregates. J. Cell Biol. 1998;143:1155–1166. doi: 10.1083/jcb.143.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarwa R, Carmon S, Shilo BZ, Schejter ED. WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev. Cell. 2007;12:557–569. doi: 10.1016/j.devcel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- McKeown C, Praitis V, Austin J. sma-1 encodes a betaH-spectrin homolog required for Caenorhabditis elegans morphogenesis. Development. 1998;125:2087–2098. doi: 10.1242/dev.125.11.2087. [DOI] [PubMed] [Google Scholar]
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438:590–596. doi: 10.1038/nature04396. [DOI] [PubMed] [Google Scholar]
- Melikyan GB, White JM, Cohen FS. GPI-anchored influenza hemagglutinin induces hemifusion to both red blood cell and planar bilayer membranes. J. Cell Biol. 1995;131:679–691. doi: 10.1083/jcb.131.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K, McNeil PL, Suzuki K, Tsunoda R, Sugai N. An actin barrier to resealing. J. Cell Sci. 2001;114:3487–3494. doi: 10.1242/jcs.114.19.3487. [DOI] [PubMed] [Google Scholar]
- Mohler WA, Simske JS, Williams-Masson EM, Hardin JD, White JG. Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr. Biol. 1998;8:1087–1090. doi: 10.1016/s0960-9822(98)70447-6. [DOI] [PubMed] [Google Scholar]
- Morris CE, Homann U. Cell surface area regulation and membrane tension. J. Membr. Biol. 2001;179:79–102. doi: 10.1007/s002320010040. [DOI] [PubMed] [Google Scholar]
- Muallem S, Kwiatkowska K, Xu X, Yin HL. Actin filament disassembly is a sufficient final trigger for exocytosis in nonexcitable cells. J. Cell Biol. 1995;128:589–598. doi: 10.1083/jcb.128.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CQ, Hall DH, Yang Y, Fitch DH. Morphogenesis of the Caenorhabditis elegans male tail tip. Dev. Biol. 1999;207:86–106. doi: 10.1006/dbio.1998.9173. [DOI] [PubMed] [Google Scholar]
- Nolan S, Cowan AE, Koppel DE, Jin H, Grote E. FUS1 regulates the opening and expansion of fusion pores between mating yeast. Mol. Biol. Cell. 2006;17:2439–2450. doi: 10.1091/mbc.E05-11-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren-Suissa M, Podbilewicz B. Cell fusion during development. Trends Cell Biol. 2007;17:537–546. doi: 10.1016/j.tcb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Palovuori R, Eskelinen S. Role of vinculin in the maintenance of cell-cell contacts in kidney epithelial MDBK cells. Eur. J. Cell Biol. 2000;79:961–974. doi: 10.1078/0171-9335-00120. [DOI] [PubMed] [Google Scholar]
- Philips J, Herskowitz I. Osmotic balance regulates cell fusion during mating in Saccharomyces cerevisiae. J. Cell Biol. 1997;138:961–974. doi: 10.1083/jcb.138.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plonsky I, Zimmerberg J. The initial fusion pore induced by baculovirus GP64 is large and forms quickly. J. Cell Biol. 1996;135:1831–1839. doi: 10.1083/jcb.135.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plonsky I, Cho MS, Oomens AG, Blissard G, Zimmerberg J. An analysis of the role of the target membrane on the Gp64-induced fusion pore. Virology. 1999;253:65–76. doi: 10.1006/viro.1998.9493. [DOI] [PubMed] [Google Scholar]
- Podbilewicz B, White JG. Cell fusions in the developing epithelial of C. elegans. Dev. Biol. 1994;161:408–424. doi: 10.1006/dbio.1994.1041. [DOI] [PubMed] [Google Scholar]
- Podbilewicz B, Chernomordik LV. Cell fusion in development and disease. In: Tamm LK, editor. Protein-lipids Interactions. Wiley VCH; New York: 2005. pp. 223–246. [Google Scholar]
- Podbilewicz B, Leikina E, Sapir A, Valansi C, Suissa M, Shemer G, Chernomordik LV. The C. elegans developmental fusogen EFF-1 mediates homotypic fusion in heterologous cells and in vivo. Dev. Cell. 2006;11:471–481. doi: 10.1016/j.devcel.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Pontow SE, Heyden NV, Wei S, Ratner L. Actin cytoskeletal reorganizations and coreceptor-mediated activation of rac during human immunodeficiency virus-induced cell fusion. J. Virol. 2004;78:7138–7147. doi: 10.1128/JVI.78.13.7138-7147.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard J.-Ph., Leikina E, Chernomordik LV. Cytoskeleton reorganization in influenza hemagglutinin – initiated syncytium formation. Biochim. Biophys. Acta. 2008 doi: 10.1016/j.bbamem.2008.09.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BE, Beckett K, Nowak SJ, Baylies MK. SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development. 2007;134:4357–4367. doi: 10.1242/dev.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapir A, Avinoam O, Podbilewicz B, Chernomordik LV. Viral and developmental cell fusion mechanisms: conservation and divergence. Dev. Cell. 2008;14:11–21. doi: 10.1016/j.devcel.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer G, Weber S, Holz A, Bogdan S, Schumacher S, Muller A, Renkawitz-Pohl R, Onel SF. The Wiskott-Aldrich syndrome protein (WASP) is essential for myoblast fusion in Drosophila. Dev. Biol. 2007;304:664–674. doi: 10.1016/j.ydbio.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Schowalter RM, Wurth MA, Aguilar HC, Lee B, Moncman CL, McCann RO, Dutch RE. Rho GTPase activity modulates paramyxovirus fusion protein-mediated cell-cell fusion. Virology. 2006;350:323–334. doi: 10.1016/j.virol.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Shemer G, Podbilewicz B. The story of cell fusion: big lessons from little worms. BioEssays. 2003;25:672–682. doi: 10.1002/bies.10301. [DOI] [PubMed] [Google Scholar]
- Shemer G, Suissa M, Kolotuev I, Nguyen KC, Hall DH, Podbilewicz B. EFF-1 is sufficient to initiate and execute tissue-specific cell fusion in C. elegans. Curr. Biol. 2004;14:1587–1591. doi: 10.1016/j.cub.2004.07.059. [DOI] [PubMed] [Google Scholar]
- Shmulevitz M, Duncan R. A new class of fusion-associated small transmembrane (FAST) proteins encoded by the non-enveloped fusogenic reoviruses. EMBO J. 2000;19:902–912. doi: 10.1093/emboj/19.5.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokac AM, Co C, Taunton J, Bement W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat. Cell Biol. 2003;5:727–732. doi: 10.1038/ncb1025. [DOI] [PubMed] [Google Scholar]
- Straube A, Merdes A. EB3 regulates microtubule dynamics at the cell cortex and is required for myoblast elongation and fusion. Curr. Biol. 2007;17:1318–1325. doi: 10.1016/j.cub.2007.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylwester A, Wessels D, Anderson SA, Warren RQ, Shutt DC, Kennedy RC, Soll DR. HIV-induced syncytia of a T cell line form single giant pseudopods and are motile. J. Cell Sci. 1993;106:941–953. doi: 10.1242/jcs.106.3.941. [DOI] [PubMed] [Google Scholar]
- Valentijn K, Valentijn JA, Jamieson JD. Role of actin in regulated exocytosis and compensatory membrane retrieval: insights from an old acquaintance. Biochem. Biophys. Res. Commun. 1999;266:652–661. doi: 10.1006/bbrc.1999.1883. [DOI] [PubMed] [Google Scholar]
- Wakatsuki T, Schwab B, Thompson NC, Elson EL. Effects of cytochalasin D and latrunculin B on mechanical properties of cells. J. Cell Sci. 2001;114:1025–1036. doi: 10.1242/jcs.114.5.1025. [DOI] [PubMed] [Google Scholar]
- Wang L, Seeley ES, Wickner W, Merz AJ. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell. 2002;108:357–369. doi: 10.1016/s0092-8674(02)00632-3. [DOI] [PubMed] [Google Scholar]
- Weissenhorn W, Hinz A, Gaudin Y. Virus membrane fusion. FEBS Lett. 2007;581:2150–2155. doi: 10.1016/j.febslet.2007.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke AM, Impraim CC, Knutton S, Pasternak CA. Components involved in virally mediated membrane fusion and permeability changes. Biochem. J. 1980;190:625–638. doi: 10.1042/bj1900625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura Y, Kusaka J, Bando T, Yamamoto S, Yoshida H, Sato M. Enhancement of herpes simplex virus-induced polykaryocyte formation by 12-O-tetradecanoyl phorbol 13-acetate: association with the reorganization of actin filaments and cell motility. Intervirology. 2000;43:129–138. doi: 10.1159/000025038. [DOI] [PubMed] [Google Scholar]
- Zheng QA, Chang DC. Reorganization of cytoplasmic structures during cell fusion. J. Cell Sci. 1991;100:431–442. doi: 10.1242/jcs.100.3.431. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell. Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- Zimmerberg J, Cohen FS, Finkelstein A. Micromolar Ca2+ stimulates fusion of lipid vesicles with planar bilayers containing a calcium-binding protein. Science. 1980;210:906–908. doi: 10.1126/science.7434004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.