Abstract
OBJECTIVE:
The failure to wean from mechanical ventilation is related to worse outcomes after cardiac surgery. The aim of this study was to evaluate whether the serum level of B-type natriuretic peptide is a predictor of weaning failure from mechanical ventilation after cardiac surgery.
METHODS:
We conducted a prospective, observational cohort study of 101 patients who underwent on-pump coronary artery bypass grafting. B-type natriuretic peptide was measured postoperatively after intensive care unit admission and at the end of a 60-min spontaneous breathing test. The demographic data, hemodynamic and respiratory parameters, fluid balance, need for vasopressor or inotropic support, and length of the intensive care unit and hospital stays were recorded. Weaning failure was considered as either the inability to sustain spontaneous breathing after 60 min or the need for reintubation within 48 h.
RESULTS:
Of the 101 patients studied, 12 patients failed the weaning trial. There were no differences between the groups in the baseline or intraoperative characteristics, including left ventricular function, EuroSCORE and lengths of the cardiac procedure and cardiopulmonary bypass. The B-type natriuretic peptide levels were significantly higher at intensive care unit admission and at the end of the breathing test in the patients with weaning failure compared with the patients who were successfully weaned. In a multivariate model, a high B-type natriuretic peptide level at the end of a spontaneous breathing trial was the only independent predictor of weaning failure from mechanical ventilation.
CONCLUSIONS:
A high B-type natriuretic peptide level is a predictive factor for the failure to wean from mechanical ventilation after cardiac surgery. These findings suggest that optimizing ventricular function should be a goal during the perioperative period.
Keywords: B-Type Natriuretic Peptide, Cardiac Surgery, Mechanical Ventilation, Weaning Failure
INTRODUCTION
The failure to wean from mechanical ventilation after cardiac surgery is associated with worse outcomes, including increased length of hospital stays and higher costs (1-3). In a prospective study of 885 patients who underwent coronary artery bypass grafting (CABG), Wong et al. (4) identified increased age, female gender, the postoperative use of an intra-aortic balloon pump (IABP), the use of inotropes, bleeding, and atrial arrhythmia as risk factors for weaning failure and prolonged mechanical ventilation.
Perioperative ventricular dysfunction and cardiac failure are frequent causes of weaning failure from mechanical ventilation (4). Prolonged cardiopulmonary bypass (CPB), inadequate myocardial protection during surgery, perioperative myocardial ischemia and previous left ventricular dysfunction are associated with a higher incidence of perioperative heart failure (5,6). A diagnosis of low output syndrome after surgery is suggested by decreased central venous oxygen saturation (ScvO2), low urine output, low cardiac index, high cardiac filling pressure and elevated levels of B-type natriuretic peptide (BNP) (7-10).
BNP is produced by cardiac ventricular myocytes in response to volume or pressure overload. Active BNP and its inactive form, NT-proBNP, are derived from the cleavage of their precursor molecule, proBNP. BNP decreases systemic vascular resistance, improves myocardial relaxation, increases natriuresis and suppresses endothelin and the renin-angiotensin system (10). The BNP levels are increased in patients who have left ventricular dysfunction, right ventricular dysfunction or valvular dysfunction. Therefore, BNP is considered to be a quantitative biomarker of heart failure (10). BNP levels are also related to left ventricular dysfunction in the postoperative period, including after cardiac surgery (11-20). In a prospective study, Zapata et al. evaluated the value of the BNP level as a marker of failure to wean from mechanical ventilation in a mixed population of 100 medical and surgical patients (excluding cardiac surgery patients) who were treated with mechanical ventilation for over 48 h (21). BNP levels predicted failure to wean cases that were caused by ventricular dysfunction, and a ΔBNP of 48 ng/L identified heart failure as the cause of failed SBT, with 91.7% sensitivity and 88.5% specificity (21).
We hypothesized that BNP levels could help in the early identification of patients who cannot be weaned from mechanical ventilation due to postoperative ventricular dysfunction after cardiac surgery.
MATERIAL AND METHODS
The study was approved by the Ethics Committee, and written informed consent was obtained from all patients. We prospectively included all of the patients older than 18 years who underwent elective CABG surgery with CPB during a 1-year period from January 2009 and January 2010 at Heart Institute, University of Sao Paulo. The exclusion criteria were a history of pulmonary disease or chronic renal failure, the need for IABP and consent refusal.
The patients were anesthetized according to our standard institutional protocol for CABG surgery. Preoperative medication consisted of midazolam (0.1 to 0.2 mg/kg given orally 30 minutes before surgery). Anesthesia was induced with fentanyl (3-5 μg/kg), midazolam (0.05 mg/kg), etomidate (0.2- 0.3 mg/kg), and pancuronium bromide (0.1 mg/kg). Anesthesia was maintained with isoflurane in oxygen and fentanyl as needed. During CPB, additional doses of midazolam and pancuronium were administered as required to reach a bispectral index of approximately 40-60. After the tracheal intubation, all of the patients received invasive mechanical ventilation with intermittent positive pressure with a tidal volume of 8 mL/kg, positive end-expiratory pressure of 5 to 8 cm H2O, and fraction of inspired oxygen (FiO2) of 0.6 to 1 to maintain the arterial oxygen saturation above 95%. During surgery, the patients were monitored with a central venous line and indwelling radial artery catheter. Forty-four patients also had a pulmonary artery catheter inserted if the surgical and anesthesiology teams believed that it was warranted. In these patients, the cardiac index was obtained from a continuous cardiac output monitor, Vigilance II (Edwards Lifesciences, Irvine, CA 92614 USA).
Preoperative information, including demographic data, preoperative left ventricular ejection fraction (LVEF), and the European system for cardiac operative risk evaluation score (EuroSCORE) (22), were obtained for all patients. The intraoperative data, including the cardiac procedure and CPB durations and the use of inotropes or vasopressors, were also recorded. After ICU admission, the following hemodynamic parameters were obtained and recorded: heart rate, mean arterial pressure (MAP), central venous pressure (CVP), pulmonary arterial pressures, pulmonary capillary wedge pressure, cardiac output, cardiac index and systemic vascular resistance (SVR). We also recorded the mechanical ventilation parameters, including the ventilation mode, plateau pressure and positive end-expiratory pressure (PEEP), respiratory rate in controlled and spontaneous mode, fraction of inspired oxygen (FiO2), oxygen saturation by pulse oximetry (SpO2), tidal volume and minute volume, rapid shallow breathing index or respiratory rate/tidal volume ratio and static respiratory system compliance (23,24).
A blood gas analysis and measurement of hemoglobin levels were performed and recorded after ICU admission every 6 h during the first 24 h after cardiac surgery. The Triage® BNP Test (Biosite, San Diego, CA, USA) was used to determine the BNP levels in the plasma specimens through immunofluorescence, with EDTA as the anticoagulant. The BNP levels were measured immediately after ICU admission and at the end of the spontaneous breathing test (SBT). The fluid balance, need for vasopressor or inotropic support, and length of the ICU and hospital stays were also recorded.
Weaning protocol
After ICU admission, all of the patients were initially ventilated using the following parameters: synchronized intermittent mandatory ventilation (SIMV) using pressure-controlled ventilation with an I/E ratio of 1:2 and enough support pressure to give a tidal volume of approximately 8 mL/kg, 5 cm H2O PEEP, respiratory rate of 12 breaths/min, and FiO2 of 60% or greater if the SpO2 was less than 90%. The degree of support was reduced, if possible, by 2 to 4 cm H2O at least hourly. The first spontaneous breathing test was given if the patients were awake and hemodynamically stable, as defined by the absence of bleeding (chest tube drainage ≤100 ml per hour or ≤300 mL in one hour), ScvO2>65%, CI>2.2 L/min/m2 and MAP>65 mmHg with low-dose norepinephrine ≤0.2 μg/Kg/min) or no vasopressor agents. Additionally, SBT was started after correcting acid-base and electrolyte disorders. The SBT test lasted 60 min and was considered failed if the patient presented with one or more of the following signs at the end of the first SBT: respiratory rate <35 breaths/min; heart rate <140 beats/min; SpO2<90% or PaO2<60 mmHg; respiratory acidosis (pH<7.3 or PaCO2>50 mmHg); signs of respiratory distress, such as thoracoabdominal dyssynchrony, anxiety and diaphoresis, or reintubation within 48 h in patients who were successful in the first SBT.
Statistical analysis
We compared the baseline characteristics, follow-up measures, and clinical outcomes between the groups. Continuous variables were tested for normal distribution using the Kolmogorov-Smirnov test and compared using Student's t test or the Mann-Whitney U-test. The sample size was calculated based on a power of 80% and a 5% type-I error. Estimating an event incidence of 10%, 158 patients were needed to complete the study. An interim analysis was scheduled after 101 patients had been enrolled in the study.
The results are expressed as means with 95% confidence intervals (CIs) or medians with interquartile ranges (IQRs). A multiple logistic regression analysis was performed to assess the predictive factors for weaning failure from mechanical ventilation, and the significance level was set at p<0.10 in the univariate model (i.e., the patient age, body mass index [calculated as weight in kilograms divided by height in meters squared], comorbidity, left ventricular ejection fraction, EuroSCORE, surgery type, CPB duration, initial and final hemoglobin concentrations, lactate concentration, and ScvO2 and BNP levels). We built a multivariate Cox proportional hazard model in the overall population with weaning failure as the dependent factor using the variables above.
To determine the best cut-off for BNP, we calculated the area under the receiver operating characteristic curve (AUC) and compared the BNP levels in the failed weaning group with those in the successful weaning group.
A two-sided p-value<0.05 was considered statistically significant. The statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL).
RESULTS
An interim analysis was performed when 101 patients had been included, and because a significant p-value was found, we interrupted the data collection. Of the 101 patients studied, 12 failed the weaning trial. Of those 12 patients, five required reintubation, and seven were weaned from mechanical ventilation after they failed to wean in the first SBT. There were no differences between the groups in their baseline or intraoperative characteristics, including the left ventricular function, EuroSCORE, and the durations of the cardiac procedure and CPB bypass (Table 1). The patients who failed weaning had longer ICU (9 (4-14) vs. 3 (2-5), p = 0.024) and hospital (15 (13-20) vs. 8 (7-13), p = 0.047) lengths of stay compared with successfully weaned patients.
Table 1.
Baseline, intraoperative characteristics and clinical outcomes of patients.
| Variable | Total (101) | Successful weaning (n = 89) | Failure to wean (n = 12) | p-value |
| Sex*) | ||||
| Male | 76 (75.2%) | 69 (77.5%) | 7 (58.3%) | 0.165 |
| Female | 25 (24.8%) | 20 (22.5%) | 5 (41.7%) | |
| Age (years)** | 63 (61-65) | 63 (61-65) | 63 (55-72) | 0.289 |
| LVEF (%)*** | 54 (40-64) | 54 (40-64) | 56 (40-68) | 0.560 |
| EuroSCORE*** | 5 (3-7) | 4 (3-7) | 6 (4-8) | 0.246 |
| Duration of procedure (min)*** | 270 (240-330) | 270 (240-330) | 283 (240-383) | 0.281 |
| Duration of CBP (min)*** | 95 (80-110) | 94 (80-108) | 111 (91-120) | 0.433 |
| Duration of mechanical ventilation (min) *** | 420 (360-613) | 420 (360-625) | 488 (390-559) | 0.868 |
| ICU length of stay (days) *** | 3 (2-5) | 3 (2-5) | 9 (4-14) | 0.024 |
| Hospital length of stay (days) *** | 9 (7-14) | 8 (7-13) | 15 (13-20) | 0.047 |
| Hospital Mortality | 5 (5%) | 2 (2,2%) | 3 (25%) | 0.011 |
*Chi-square test, **mean (95% confidence Interval), t-test, ***median (interquartile range), Mann-Whitney test. EuroSCORE: European System for Cardiac Operative Risk Evaluation; LVEF: left ventricular ejection fraction; CPB: cardiopulmonary bypass.
The patients who failed to wean from mechanical ventilation had higher CVP values at the end of the SBT than the patients who weaned successfully (11 (9-15) vs. 9 (5-10) mmHg, p = 0.023); they also had lower ScvO2 values (62 [58-65] vs. 69% [68-71], p = 0.002) at this time point and required higher doses of dobutamine (15 (11-18) vs. 12 (9-16) μg/Kg/min, p = 0.044) (Table 2). There were no differences between the groups in the other hemodynamic variables or in the cumulative fluid balance.
Table 2.
Hemodynamic, respiratory and gas exchange variables.
| Variable | Successful weaning (n = 89) | Failure to wean (n = 12) | p-value |
| Hemodynamic | |||
| HR-1 (beats/min)*) | 96 (92-100) | 93 (80-105) | 0.435 |
| HR-2 (beats/min)*) | 103 (101-106) | 96 (89-103) | 0.087 |
| MAP-1 (mmHg)*) | 92 (89-96) | 93 (83-102) | 0.983 |
| MAP-2 (mmHg)*) | 89 (87-92) | 85 (78-93) | 0.239 |
| PAOP-1 (mmHg)*)$ | 14 (13-15) | 14 (10-17) | 0.855 |
| PAOP-2 (mmHg)*)$ | 10 (9-12) | 12 (9-15) | 0.440 |
| CVP-1 (mmHg)*) | 10 (9-11) | 10 (8-12) | 0.883 |
| CVP-2 (mmHg)** | 9 (5-10) | 11 (9-15) | 0.023 |
| PCP-1 (%)*)$ | 14 (13-15) | 14 (10-17) | 0.855 |
| PCP-2 (%)*)$ | 10 (9-12) | 12 (9-15) | 0.440 |
| ScvO2-1 (%)** | 77 (70-81) | 77 (69-77) | 0.781 |
| ScvO2-2 (%)*) | 69 (68-71) | 62 (58-65) | 0.002 |
| CI-1 (L/min/m2)$*) | 3.17 (2.83-3.50) | 2.75 (2.31-3.20) | 0.361 |
| CI-2 (L/min/m2)$*) | 3.33 (3.08-3.58) | 2.76 (2.21-3.30) | 0.068 |
| Lactate-1 (mmol/L)** | 4.0 (2.9-6.1) | 2.3 (1.3-5.0) | 0.313 |
| Lactate-2 (mmol/L)** | 2.9 (1.8-4.6) | 1.9 (1.4-3.6) | 0.296 |
| Hb-1 (g/dL)** | 10.8 (9.8-11.8) | 9.8 (9.2-11.7) | 0.154 |
| Hb-2 (g/dL)** | 10.6 (9.6-11.8) | 10 (8.9-10.2) | 0.084 |
| Dobutamine-1** | 10 (8-17) | 14 (9-18) | 0.519 |
| Dobutamine-2** | 12 (9-16) | 15 (11-18) | 0.044 |
| Respiratory | |||
| PaO2-1 (mmHg)*) | 145 (136-154) | 142 (113-171) | 0.754 |
| PaO2-2 (mmHg)** | 107 (90-143) | 117 (88-152) | 0.550 |
| PaCO2-1 (mmHg)** | 40 (36-44) | 39 (36-45) | 0.806 |
| PaCO2-2 (mmHg)** | 37 (33-40) | 37 (34-41) | 0.303 |
| pH-1** | 7.3 (7.3-7.4) | 7.4 (7.3-7.4) | 0.313 |
| pH-2** | 7.4 (7.3-7.4) | 7.4 (7.3-7.4) | 0.232 |
| Tobin-2** | 36 (26-45) | 45 (29-83) | 0.098 |
| SC-1 (mL/cmH2O)** | 38 (32-49) | 39 (33-51) | 0.889 |
| Natriopeptides (ng/L) | |||
| BNP-1** | 73 (28-127) | 214 (65-487) | 0.020 |
| BNP-2** | 140 (80-226) | 416 (311-561) | <0.001 |
*mean (95% Confidence Interval), t-test, **median (Interquartile Range), Mann-Whitney test. $evaluated in the 44 patients who had a pulmonary artery catheter inserted – 36 in the successful weaning group and 8 in the failure to wean group. 1- at intensive care unit admission; 2- at the end of the spontaneous breathing test; HR: heart rate; MAP: mean arterial pressure; PAOP: pulmonary artery occlusion pressure; CVP: central venous pressure; ScvO2: central venous oxygen saturation; Hb: hemoglobin concentration; SC = Static compliance; CI: cardiac index.
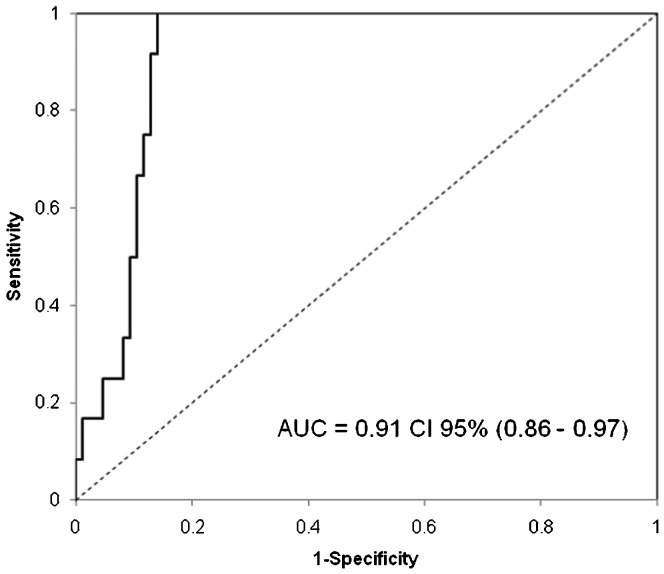
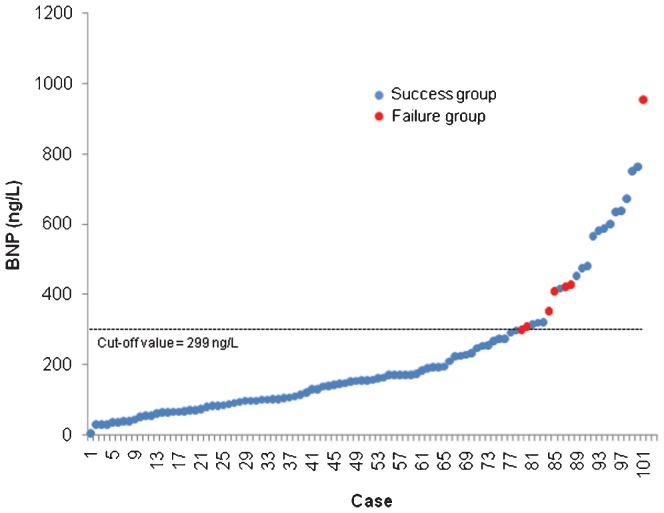
The BNP levels were significantly higher in the patients who failed to wean compared with those who weaned successfully, both at ICU admission (214 ng/mL [65-487] vs. 73 [28-127], p = 0.02) and after the SBT (416 ng/mL [311-561] vs. 140 [80-226], p<0.001) (Table 2). In the multivariate model, only BNP at the end of the SBT (BNP-2) was predictive of weaning failure from mechanical ventilation (odds ratio [OR], 1.006 per ng/mL [95% CI, 1.003-1.009]; p<0.001). A BNP concentration of 299 ng/L at the end of the SBT identified weaning failure with 92% sensitivity and 88% specificity, with an AUC of 0.91 (CI 95% [0.86 - 0.97], p<0.001) (Figure 1). Figure 2) shows the BNP concentrations for the individual patients.
Figure 1.
Area under receiving operating characteristic curve for BNP-2 (at the end of spontaneous breathing test) to predict weaning failure.
Figure 2.
Individual values of BNP concentration. A cut-off of 299 ng/L at the end of the SBT predicted failure to wean from mechanical ventilation after cardiac surgery with 92% sensitivity and 87% specificity. Red dots represent patients with weaning failure.
Clinical outcomes
A total of 716 patients were assessed for eligibility during the study period. In total, 101 patients were enrolled, and the mortality rate was 5%. The patients who failed to wean from mechanical ventilation after the first SBT had higher ICU mortality rates than the patients who did not fail (3 [25%] vs. 2 [2.2%], p<0.0011). Five patients in the failure group were reintubated within 48 h after the SBT because of congestive heart failure. Two of the reintubated patients died from cardiogenic shock. Seven patients had failure to wean in the first SBT but were not reintubated in the first 48 h. One of these patients died from pneumonia, septic shock and multiple organ failure.
DISCUSSION
Our study shows that a high level of BNP is an independent risk factor for the failure to wean from mechanical ventilation after cardiac surgery. In clinical practice, BNP levels are widely used to diagnose and stratify risk in heart failure patients and as a predictor of outcomes, including re-hospitalization and death (10,25). An elevated BNP level is considered to be a biomarker of ventricular dysfunction and can identify early decompensated heart failure after cardiac surgery (11-14,16-20,25,26). Elevated postoperative BNP levels have also been associated with prolonged hospital stays and mortality in patients undergoing cardiac surgery (11,17,18).
Underlying left ventricular dysfunction is an important cause of weaning failure in critically ill patients, particularly during the postoperative period after cardiac surgery, and can be the result of perioperative myocardial ischemia, prolonged CPB, or inadequate myocardial protection (27-30). This condition may be difficult to recognize within the first 24 h after cardiac surgery. Previous studies have reported that early indirect parameters of cardiac function, such as capnometric recirculation gas tonometry and gastric intramucosal pH, were altered in patients who presented with failure to wean from mechanical ventilation. These findings can be explained by the likelihood that the increased respiratory workload during spontaneous test breathing can induce an intestinal mucosa hypoperfusion because of the adrenergic response and effort required to increase the blood flow to the respiratory muscles (31-33). Similarly, the BNP levels during the spontaneous breathing test might help physicians identify earlier those patients with postoperative heart failure and support decisions to discontinue mechanical ventilation after cardiac surgery, potentially decreasing the risk of weaning failure (6,18,21).
All of the patients with weaning failure in the current study had higher BNP levels both at their ICU admissions and at the end of the SBT compared with the patients who had successful weaning. The highest BNP levels were found at the end of the SBT, suggesting that postoperative left ventricular dysfunction was involved in the weaning failure. In addition, these patients had higher CVP and lower ScvO2 levels and needed higher doses of dobutamine compared with the patients who were successfully weaned. Paulus et al. (34) reported that in patients with left ventricular dysfunction who had presented a failure in the first or additional spontaneous breathing tests after cardiac surgery, the use of enoximone, a phosphodiesterase III inhibitor, was associated with an improvement in hemodynamic parameters and successful withdrawal of mechanical ventilation. Although these hemodynamic parameters were not predictors of weaning failure in our study, they reinforce our hypothesis that underlying heart failure caused weaning failure in this subgroup of patients.
A previous study showed that increased age, female gender, the postoperative use of an intra-aortic balloon pump (IABP), inotropes, bleeding, and atrial arrhythmia were risk factors of delayed extubation after cardiac surgery (4). Our data did not show any differences in gender. However, our findings suggested that poor ventricular function, expressed through high levels of BNP, is a predictor of weaning failure after cardiac surgery.
In our study, the prevalence of weaning failure was 12%. This failure rate is higher than that reported in other studies (1), most likely because our study was performed in a cardiac surgery referral center and included severely ill patients, as shown by the high EuroSCOREs (22). The patients who had weaning failure in our study may have developed myocardial dysfunction secondary to CPB. A CPB duration of more than 120 minutes has been considered a predictive factor for weaning failure in patients after cardiac surgery in previous studies (35), but there was no significant difference in the duration of CPB between our groups. Moreover, none of the patients with weaning failure met the perioperative myocardial infarction criteria. Nevertheless, the etiology of myocardial dysfunction after CPB is multifactorial and can occur even without prolonged CPB or perioperative infarction (6). Using transesophageal echocardiography, Bernard et al. described a 30% rate of diastolic dysfunction after cardiac surgery with CPB (27).
If BNP levels are predictors of weaning failure from mechanical ventilation in patients after cardiac surgery, as suggested by our results, and ventricular dysfunction is one cause of weaning failure, then optimizing ventricular function may result in better outcomes after cardiac surgery. A bundle of interventions could be used to optimize ventricular function during the perioperative period in such patients, including effective myocardial protection during CPB and the judicious administration of inotropic agents and vasodilators to obtain adequate cardiac indices and ScvO2 levels (6). The careful evaluation of fluid status is also needed to prevent elevated filling pressures and pulmonary congestion.
Our study has several limitations. First, it was an observational and single-center study with a small number of patients and relatively few failure events. Second, BNP levels can be altered in various conditions in patients undergoing cardiac surgery, and we are unable to identify the exact cause of the myocardial dysfunction that caused the weaning failure in our patients. Third, there are many other conditions in critically ill patients that may be associated with increased BNP levels. Therefore, these investigations should be extended to a larger patient population in a multicenter setting.
In conclusion, our study demonstrated that a high BNP level is a predictor of weaning failure from mechanical ventilation in patients after cardiac surgery. Therefore, measuring BNP levels may help to guide and evaluate the effects of therapeutic strategies, such as optimizing ventricular function during cardiac surgery, prior to weaning from mechanical ventilation.
This report describes human research. IRB contact information: CAPPesq - Comissão de Ética para Análise de Projetos de Pesquisa (cappesq@hcnet.usp.br), do Hospital das Clinicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo/SP, Brazil.
Footnotes
No potential conflict of interest was reported.
ACNOWLEDGMENTS
This study was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP), Brazil – Number 08553750.
REFERENCES
- 1.Shirzad M, Karimi A, Ahmadi SH, Marzban M, Tazik M, Aramin H. Predictors and early outcome of prolonged mechanical ventilation in contemporary heart valve surgery. Monaldi Arch Chest Dis. 2010;74(1):22–7. doi: 10.4081/monaldi.2010.276. [DOI] [PubMed] [Google Scholar]
- 2.Trouillet JL, Combes A, Vaissier E, Luyt CE, Ouattara A, Pavie A, et al. Prolonged mechanical ventilation after cardiac surgery: outcome and predictors. J Thorac Cardiovasc Surg. 2009;138(4):948–53. doi: 10.1016/j.jtcvs.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 3.Ji Q, Chi L, Mei Y, Wang X, Feng J, Cai J, et al. Risk factors for late extubation after coronary artery bypass grafting. Heart Lung. 2010;39(4):275–82. doi: 10.1016/j.hrtlng.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Wong DT, Cheng DC, Kustra R, Tibshirani R, Karski J, Carroll-Munro J, et al. Risk factors of delayed extubation, prolonged length of stay in the intensive care unit, and mortality in patients undergoing coronary artery bypass graft with fast-track cardiac anesthesia: a new cardiac risk score. Anesthesiology. 1999;91(4):936–44. doi: 10.1097/00000542-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Royster RL, Butterworth JF, 4th, Prough DS, Johnston WE, Thomas JL, Hogan PE, et al. Preoperative and intraoperative predictors of inotropic support and long-term outcome in patients having coronary artery bypass grafting. Anesth Analg. 1991;72(6):729–36. doi: 10.1213/00000539-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Mebazaa A, Pitsis AA, Rudiger A, Toller W, Longrois D, Ricksten SE, et al. Clinical review: practical recommendations on the management of perioperative heart failure in cardiac surgery. Crit Care. 2010;14(2):201. doi: 10.1186/cc8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Circulation. 2007;116(17):1971–96. doi: 10.1161/CIRCULATIONAHA.107.185700. [DOI] [PubMed] [Google Scholar]
- 8.Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole-Wilson PA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29(19):2388–442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 9.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 10.Maisel A, Mueller C, Adams K, Jr, Anker SD, Aspromonte N, Cleland JG, et al. State of the art: using natriuretic peptide levels in clinical practice. Eur J Heart Fail. 2008;10(9):824–39. doi: 10.1016/j.ejheart.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Fox AA, Shernan SK, Collard CD, Liu KY, Aranki SF, DeSantis SM, et al. Preoperative B-type natriuretic peptide is as independent predictor of ventricular dysfunction and mortality after primary coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2008;136(2):452–61. doi: 10.1016/j.jtcvs.2007.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berendes E, Schmidt C, Van Aken H, Hartlage MG, Rothenburger M, Wirtz S, et al. A-type and B-type natriuretic peptides in cardiac surgical procedures. Anesth Analg. 2004;98(1):11–9. doi: 10.1213/01.ANE.0000093249.35075.F1. [DOI] [PubMed] [Google Scholar]
- 13.Fox AA, Muehlschlegel JD, Body SC, Shernan SK, Liu KY, Perry TE, et al. Comparison of the utility of preoperative versus postoperative B-type natriuretic peptide for predicting hospital length of stay and mortality after primary coronary artery bypass grafting. Anesthesiology. 2010;112(4):842–51. doi: 10.1097/ALN.0b013e3181d23168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fellahi JL, Daccache G, Rubes D, Massetti M, Gérard JL, Hanouz JL. Does preoperative B-type natriuretic peptide better predict adverse outcome and prolonged length of stay than the standard European System for Cardiac Operative Risk Evaluation after cardiac surgery. J Cardiothorac Vasc Anesth. 2011;25(2):256–62. doi: 10.1053/j.jvca.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Cuthbertson BH, Amiri AR, Croal BL, Rajagopalan S, Brittenden J, Hillis GS. Utility of B-type natriuretic peptide in predicting medium-term mortality in patients undergoing major non-cardiac surgery. Am J Cardiol. 2007;100(8):1310–3. doi: 10.1016/j.amjcard.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 16.Cuthbertson BH, Croal BL, Rae D, Gibson PH, McNeilly JD, Jeffrey RR, et al. N-terminal pro-B-type natriuretic peptide levels and early outcome after cardiac surgery: a prospective cohort study. Br J Anaesth. 2009;103(5):647–53. doi: 10.1093/bja/aep234. [DOI] [PubMed] [Google Scholar]
- 17.Hutfless R, Kazanegra R, Madani M, Bhalla MA, Tulua-Tata A, Chen A, et al. Utility of B-type natriuretic peptide in predicting postoperative complications and outcomes in patients undergoing heart surgery. J Am Coll Cardiol. 2004;43(10):1873–9. doi: 10.1016/j.jacc.2003.12.048. [DOI] [PubMed] [Google Scholar]
- 18.Nozohoor S, Nilsson J, Algotsson L, Sjögren J. Postoperative increase in B-type natriuretic peptide levels predicts adverse outcome after cardiac surgery. J Cardiothorac Vasc Anesth. 2011;25(3):469–75. doi: 10.1053/j.jvca.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Jeong DS, Kim KH, Kim CY, Kim JS. Relationship between plasma B-type natriuretic peptide and ventricular function in adult cardiac surgery patients. J Int Med Res. 2008;36(1):31–9. doi: 10.1177/147323000803600105. [DOI] [PubMed] [Google Scholar]
- 20.Schachner T, Wiedemann D, Fetz H, Laufer G, Kocher A, Bonaros N. Influence of preoperative serum N-terminal pro-brain type natriuretic peptide on the postoperative outcome and survival rates of coronary artery bypass patients. Clinics. 2010;65(12):1239–45. doi: 10.1590/S1807-59322010001200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zapata L, Vera P, Roglan A, Gich I, Ordonez-Llanos J, Betbesé AJ. B-type natriuretic peptides for prediction and diagnosis of weaning failure from cardiac origin. Intensive Care Med. 2011;37(3):477–85. doi: 10.1007/s00134-010-2101-4. [DOI] [PubMed] [Google Scholar]
- 22.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16(1):9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 23.Yang KL, Tobin MJ. A prospective study of indexes predicting the outcome of trials of weaning from mechanical ventilation. N Engl J Med. 1991;324(21):1445–50. doi: 10.1056/NEJM199105233242101. [DOI] [PubMed] [Google Scholar]
- 24.Rossi A, Gottfried SB, Zocchi L, Higgs BD, Lennox S, Calverley PM, Begin P, et al. Measurement of static compliance of the total respiratory system in patients with acute respiratory failure during mechanical ventilation. The effect of intrinsic positive end-expiratory pressure. Am Rev Respir Dis. 1985;131(5):672–7. doi: 10.1164/arrd.1985.131.5.672. [DOI] [PubMed] [Google Scholar]
- 25.Jankowski M. B-type natriuretic peptide for diagnosis and therapy. Recent Pat Cardiovasc Drug Discov. 2008;3(2):77–83. doi: 10.2174/157489008784705395. [DOI] [PubMed] [Google Scholar]
- 26.Eliasdottir SB, Klemenzson G, Torfason B, Valsson F. Brain natriuretic peptide is a good predictor for outcome in cardiac surgery. Acta Anaesthesiol Scand. 2008;52(2):182–7. doi: 10.1111/j.1399-6576.2007.01451.x. [DOI] [PubMed] [Google Scholar]
- 27.Bernard F, Denault A, Babin D, Goyer C, Couture P, Couturier A, Buithieu J. Diastolic dysfunction is predictive of difficult weaning from cardiopulmonary bypass. Anesth Analg. 2001;92(2):291–8. doi: 10.1097/00000539-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Demoule A, Lefort Y, Lopes ME, Lemaire F. Successful weaning from mechanical ventilation after coronary angioplasty. Br J Anaesth. 2004;93(2):295–7. doi: 10.1093/bja/aeh185. [DOI] [PubMed] [Google Scholar]
- 29.Rao V, Ivanov J, Weisel RD, Ikonomidis JS, Christakis GT, David TE. Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg. 1996;112(1):38–51. doi: 10.1016/s0022-5223(96)70176-9. [DOI] [PubMed] [Google Scholar]
- 30.Nozawa E, Azeka E, Ignêz Z M, Feltrim Z, Auler Júnior JO. Factors associated with failure of weaning from long-term mechanical ventilation after cardiac surgery. Int Heart J. 2005;46(5):819–31. doi: 10.1536/ihj.46.819. [DOI] [PubMed] [Google Scholar]
- 31.Maldonado A, Bauer TT, Ferrer M, Hernandez C, Arancibia F, Rodriguez-Roisin R, et al. Capnometric recirculation gas tonometry and weaning from mechanical ventilation. Am J Respir Crit Care Med. 2000;161(1):171–6. doi: 10.1164/ajrccm.161.1.9904080. [DOI] [PubMed] [Google Scholar]
- 32.Bouachour G, Guiraud MP, Gouello JP, Roy PM, Alquier P. Gastric intramucosal pH: an indicator of weaning outcome from mechanical ventilation in COPD patients. Eur Respir J. 1996;9(9):1868–73. doi: 10.1183/09031936.96.09091868. [DOI] [PubMed] [Google Scholar]
- 33.Mohsenifar Z, Hay A, Hay J, Lewis MI, Koerner SK. Gastric intramural pH as a predictor of success or failure in weaning patients from mechanical ventilation. Ann Intern Med. 1993;119(8):794–8. doi: 10.7326/0003-4819-119-8-199310150-00004. [DOI] [PubMed] [Google Scholar]
- 34.Paulus S, Lehot JJ, Bastien O, Piriou V, George M, Estanove S. Enoximone and acute left ventricular failure during weaning from mechanical ventilation after cardiac surgery. Crit Care Med. 1994;22(1):74–80. doi: 10.1097/00003246-199401000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Natarajan K, Patil S, Lesley N, Ninan B. Predictors of prolonged mechanical ventilation after on-pump coronary artery bypass grafting. Ann Card Anaesth. 2006;9(1):31–6. [PubMed] [Google Scholar]