Preface
Allogeneic haematopoietic stem cell transplantation is used to treat a variety of disorders, but its efficacy is limited by the occurrence of graft-versus-host disease (GVHD). The past decade has brought impressive advances in our understanding of the role of both donor and host adaptive and innate immune stimulatory and immune suppressive factors that influence GVHD pathogenesis. New insights in basic immunology, preclinical models and clinical studies have led to novel prevention or treatment approaches. This review highlights recent advances in GVHD pathophysiology and its treatment with a focus on immune system manipulations that are amenable to clinical application.
Allogeneic haematopoietic stem cell transplantation (HSCT) is the only curative option for many haematological malignancies. However, the development of graft-versus-host disease (GVHD) limits allo-HSCT success. GVHD frequency depends on factors such as recipient age, conditioning regimen, haematopoietic graft source and GVHD prophylaxis. GVHD is fatal to ~15% of patients.1 Steroids are the first line treatment but patients with steroid-refractory acute GVHD (aGVHD) have a dismal outcome, with long-term mortality rates close to 90%.
cGVHD has been classically defined as GVHD occurring after the first 100 days post-HSCT. However, it is not the time of onset but its characteristic clinical presentation, resembling autoimmune vascular diseases, coupled with specific diagnostic criteria and when available tissue pathology that separates cGVHD from aGVHD. cGVHD occurs in 30–65% of allogeneic HSCT recipients with a 5-year mortality of 30–50% due predominantly to immune dysregulation and infections. aGVHD culminates in systemic inflammation and tissue destruction affecting multiple organs, particularly the gut, liver, lungs, bone marrow, thymus and skin. cGVHD, which can be highly debilitating in its extensive form, targets skin, subcutaneous connective tissues, oral mucosa, salivary and lacrimal glands, lungs, gut, liver and joints.
HSCT protocols include a conditioning regimen that is myelosuppressive, to deplete host stem cells and make space for donor stem cell engraftment, and immunosuppressive, to reduce host anti-donor graft rejection. Myelosuppression and immunosuppression enhances recipient inflammation and T cell:APC interactions precipitating GVHD. The more intense conditioning regimens result in greater tissue injury and inflammation that supports GVHD induction. Reducing conditioning regimen intensity or localizing the treatment (e.g total lymph node irradiation) has reduced GVHD incidence 2,3. Although non-myeloablative conditioning regimens and improved GVHD prophylaxis have decreased aGVHD incidence, there has been little impact on chronic GVHD (cGVHD)2,3.
This review provides an update of our current knowledge gleaned from the preclinical models and focuses on the most promising targets in each step of the multistep GVHD pathogenesis process, i.e. T-cell-APC interactions, T-cell activation pathways, pro-inflammatory cytokines, and T-cell trafficking and. Intimately tied with GVHD is also the process of graft-versus-tumor (GVT) effects (Box 1), which must be maintained in patients with malignancies.
BOX 1. GVHD versus GVT effects.
The beneficial effect of GVHD on the incidence of leukemia relapse and on overall survival of patients, known as a graft- versus-tumor effect (GVT) effect, has been known since early 80’s.172 The role of T cells became evident when T-cell depletion was found to eliminate GVHD but at the expense of an increased relapse rate. 173 With increasing popularity of non-myeloablative or reduced intensity allogeneic HSCT there is increased reliance on immune-mediated, GVT effects to control the underlying disease. The major GVT effectors are cytotoxic T cells that recognize allogeneic histocompatibility antigens and unique tumor antigens. In addition, NK and NK-like T cells can directly recognize HLA class I molecules and stress induced peptides. Current strategies to improve GVT effect targets common tumor escape mechanism such as the absence of tumor-specific molecules or presented peptides, downregulation or loss of HLA-class I molecules, lack of costimulatory molecules on target cells, functional defects in T cells or NK cells, soluble inhibitors of NK cell function, expression of death domain ligands such as Fas by tumor cells, or tumor resistance to apoptosis. The current and potential future approaches for the prevention or treatment of GVHD here can be divided into three broad categories; 1) Approaches that may negatively affect GVT such as systemic use of steroids, costimulation inhibition or the use of calcineurin inhibitors; 2) Strategies that can provide a relative preservation in GVT versus GVHD such as use of IL-11 or an anti-IL-21 antibody or cellular therapies with naïve or induced TRegs and 3) Treatments that may improve GVT effect such as those that are directly toxic to the tumor (proteosome inhibitors and anti-IL6 antibody) or cellular approaches (e.g NK cells; modified donor lymphocyte infusions). As an example of the latter, CD8-depleted donor lymphocytes have been used by several investigators, while more recently, a suicide gene was incorporated into donor T-cells as a safety switch to shut off GVHD if this occurs by inducing apoptosis in transgene expressing cells. 170
Overview of GVHD pathogenesis
GVHD was initially reported by Barnes and classically defined by Billingham as a syndrome in which donor immunocompetent cells recognize and attack host tissues in immunocompromised allogeneic recipients.4,5 Both aGVHD and cGVHD involve distinct pathological processes, highlighting the need for stage-specific treatment approaches. Whereas aGVHD has strong inflammatory components (Figure 1), cGVHD displays more autoimmune and fibrotic features (Figure 2). aGVHD is thought to be mainly a Th1/Th17-driven process whereas cGVHD was thought to be Th2-predominate; however this paradigm has been challenged in recent mouse and human studies and is not absolute.6–11 aGVHD involves alloreactive donor T-cell mediated cytotoxicity of recipient tissues, mediated by cell surface and secreted factors 12 The pivotal role of T cells in aGVHD has been evidenced by complete abrogation of GVHD when T cell depleted graft was transplanted, which remains to date the most effective approach to prevent aGVHD. Tissue damage leads to the recruitment of other effector cells (including NK cells and PMNs), further augmenting tissue injury and resulting in a self-perpetuating state, making it difficult to control GVHD once fully initiated.
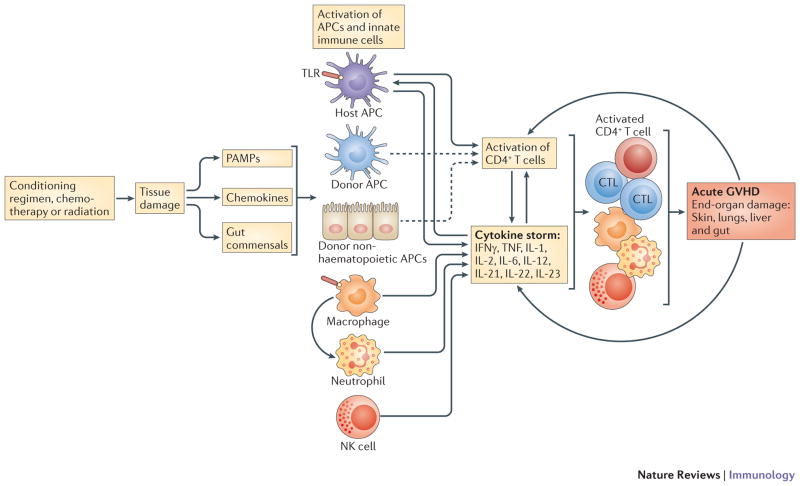
Figure 1. Overall aGVHD cascade.
Initiation and maintenance of aGVHD has been conceptualized into 4 phases with feedback loops that self-sustain the process. Although the effect of conditioning phase in aGVHD is not absolutely necessary, in many of the models it activates APCs, via tissue destruction, and improve APC capacity. It also, through release of gut bacteria, PAMPS and chemokines, can activate cellular components of innate immune system that can participate in direct tissue damage and contribute in cytokine storm. Host hematopoietic APCs have perhaps the most important role in initiation of GVHD, but this may depend on the model and the potential role of recipient APCs as well host non-hematopoietic APCs should not be ignored. Following antigen presentation, a strong cytokine response is initiated, promoting greater antigen presentation and recruitment of Teffs, and innate immune cells further contributing to the inflammatory cytokine milieu. Finally, the Teff cells, NK cells, macrophages and pro-inflammatory cytokines (e.g. TNF-α), will result in end organ damage, clinically recognized as aGVHD in the skin, lung, gut and liver. The resulting tissue damage, if not treated, will further escalate the disease, spiraling up the process to higher and more severe stages of GVHD pathology, which is extremely difficult to control.
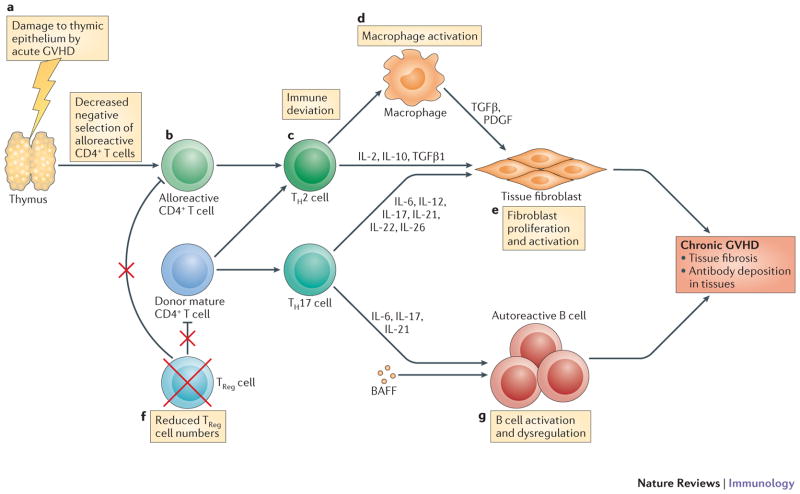
Figure 2. Critical Factors in development of cGVHD.
Pathophysiology of cGVHD. The pathophysiology of cGVHD mostly revolves on the polarization of Th cells to a Th2 cytokine phenotype but there are six hallmarks that are unique to this syndrome. These include damage to the thymus associated with the conditioning regimen and more importantly, occurrence of aGVHD earlier in the post-HSCT course resulting in decreased negative selection of alloreactive CD4+ T cells; Th2 cytokine pattern deviation resulting in release of fibrogenic cytokines such as IL-2, IL-10 and TGFβ; macrophage activation followed by tissue fibroblasts proliferations and activation through release of TGFβ and PDGF from macrophages; lower Treg levels and finally, dysregulation of B-cells leading to emergence of autoreactive B-cells and production of autoreactive antibodies. Its suggested that the latter maybe due to excessive presences of BAFF in the lymphoid microenvironment. All these will results in autoimmune-like systemic syndrome mostly associated with fibroproliferative changes that can occur in almost any organ in body but primarily affecting oral and ocular mucosal surfaces and the skin, lung, kidneys, liver and gut.
The primary preclinical model used for GVHD pathogenesis and prevention has been the mouse, although other animal models and particularly canine models have also provided us with significant insight into aGVHD prevention and therapy, especially using pharmacological agents. Owing to the availability of a plethora of reagents, knockout and transgenic strains, and transplantable tumor lines to assess potential roles in anti-tumor effects, mouse models have facilitated a thorough mechanistic dissection of GVHD immunological processes. Mouse aGVHD models usually involve the transplantation of bone marrow, as a source of haematopoietic stem cells, supplemented with varying numbers and different types of donor lymphocytes, into irradiated allogeneic recipients differing from donors in MHC class I and/or II molecules or multiple minor histocompatibility antigens. Current cGVHD models can be divided into sclerodermatous, autoantibody-mediated or lupus-like, and those associated with thymic dysfunction.13 Because none recapitulates all of the diverse characteristics of human cGVHD, there has been a relative paucity of drug candidates for clinical trials for the treatment of a growing population of patients, although newer models have been developed a wider breadth of target organs, including the lungs.14 Outside of species differences between mouse and man, there are other factors for consideration in GVHD studies. Mouse strain combination choice can have a marked impact on GVHD type, severity and pathophysiology.12 Differences in mouse vendor, age, sex, genetic drift, gut microbial flora and transplant protocols between laboratories each can have a marked impact on GVHD pathophysiology. Nonetheless, the mouse model has proven to be extremely useful for developing and testing of new treatment approaches.
Role of innate immune response
With our improved understanding of the induction of innate and adaptive immune responses by microbial products, the gut microbiome impact on GVHD is receiving increased attention. Current data points to the innate immune response as being responsible for initiating or amplifying aGVHD. Molecules, such as bacterial lipopolysaccharide (LPS), released from the injured gut during conditioning, activate innate immune receptors including Toll-like receptors, and cause a cytokine storm favoring aGVHD.15 Mutations in TLR4 (an LPS receptor) have been shown in mice and patients to reduce GVHD risk16 and bacterial DNA mediated TLR9 ligation can enhance aGVHD induction.17,18 In clinical practice, polymorphisms of the genes encoding the nucleotide-binding domain, leucine-rich-repeat-containing family receptors (NLRs), such as NOD2 and TLR4 are associated with a higher GVHD incidence and can explain the seemingly unpredictable nature of GVHD. NOD2 and the TLR5 ligand flagellin have an inhibitory effect on GVHD by suppressing APC function and favoring immune suppressive Regulatory T cells (TRegs) generation.19,20 One of the most direct lines of TLR effects on GVHD was derived by applying a TLR7 activator to the skin before inducing GVHD, which resulted in massive T-cell infiltrates and GVHD pathology only at the site of pretreated skin.21 A role for TLR9 and its downstream signaling pathway, MyD88 was observed in an intestinal GVHD model.18 Together, these data suggest that MyD88 inhibitors such as ST2825 may be useful in reducing the innate and adaptive immune responses triggered by TLR activation.22 Increased numbers of enterobacteria, enterococci and Bacteroides/Prevotella species have been seen in murine models of colitis and ileitis and are suspected to modulate innate immune responses via TLRs.23,24 Changing the gut flora to a less GVHD favourable flora can potentially prevent GVHD, evidenced by decreased severity of GVHD and improved survival of animals upon administration of probiotic bacteria.25
Other molecules, known as damage-associated molecular patterns (DAMPs), released following conditioning regimen-induced tissue damage, may have a role in GVHD induction. For example, ATP released by dying cells in the gut of mice and the peritoneal fluid of GVHD patients binds to its receptor P2X7R on host APCs and activates the inflammasome, leading to co-stimulatory molecules upregulation on APCs26 Pharmacological blockade of P2X7R decreased aGVHD incidence and increased TRegnumber. P2X7R polymorphisms are associated with survival differences in allogeneic HSCT patients, supporting the possibility that blockade of P2X7R signaling may be a useful strategy to prevent or treat GVHD.27
It is important to mention that despite the prominent role of innate immune system in pathogenesis of GVHD, in the absence of appropriate TLR signaling, T cells can still be activated and GVHD can still occur.28
Role of APCs
There has been tremendous progress recently on discerning the role of APCs, their subsets and importance of their origin (donor or host) in GVHD and GVT responses [for GVT, see Box 1]. The presentation of minor histocompatibility antigens by MHC class I molecules on recipient haematopoietic APCs is important, although not required, for CD8+ T cell-dependent aGVHD. Donor APCs can augment this response29,30. The previously thought obligatory nature of MHC class II-bearing host haematopoietic APCs on the induction of CD4+ T cell-dependent aGVHD has been called into question31–34. Recent studies have shown that professional haematopoietic host APCs within lymphoid organs may have only a limited capacity to induce GVHD, and host DCs may not be required.35–37 Parenchymal tissue cells can acquire APC functions and have been shown to promote marked alloreactive donor T-cell expansion within the gastrointestinal tract. In the absence of functional host haematopoietic APCs, the presentation of minor histocompatibility antigens by donor haematopoietic or host non-haematopoietic APCs is sufficient for GVHD induction 35,38. These data indicate that experimental aGVHD can be induced by non-haematopoietic recipient APCs, although it is difficult to gauge the impact of these alternative pathways in humans. Because donor and host haematopoietic APCs and host non-haematopoietic APCs each can contribute to GVHD, approaches that selectively deplete a single type of APC, rather than globally impair APC function, may prove inefficient for aGVHD prevention or in the case of haematopoietic host APCs may be even deleterious. Therefore, while a long-term strategy to prevent GVHD may focus on antigen presentation on APCs, there is no clear approach that can accomplish this goal at the present time.
Targeting B-cells
In mice, B-cell depletion results in a decreased incidence of aGVHD perhaps by their effect on host APCs.39 Paradoxically, B-cells can also play a protective role in GVHD by controling naïve T-cell differentiation into Teffector cells (Teffs) and inhibiting the proliferation of alloantigen-specific Teffs, via the IL-10 secretion and alloantigen-specific Treg expansion.40 In clinical practice, incorporating an anti-CD20 monoclonal antibody, rituximab, into conditioning regimen resulted in reduced incidence and severity of aGVHD. An association between elevated donor graft B-cells with both aGVHD and cGVHD has been demonstrated41 although post-HSCT rituximab did not reduce GVHD incidence.42 The role of B-cells in cGVHD is more straightforward and promising with both preclinical and clinical evidence pointing to the importance of B-cell dysregulation in cGVHD pathogenesis and treatment.43 Targeting germinal center formation (using lymphotoxin-beta receptor blocking agents)14 and the IL-17-BAFF axis,44 a cause of B-cell dysregulation, are particularly interesting for future cGVHD clinical intervention(s) that are worthy of investigation at the present time.
Targeting T-cell responses
Both donor CD4+ and CD8+ T-cells have crucial roles in GVHD pathogenesis. Thus, arguably the most effective approaches for GVHD prevention and therapy will focus on depletion, tolerization or functional incapacitation of donor T cells. It is important to note this is a result of naive T-cell responses. Central and memory T-cells do not appear to induce GVHD although they mediate GVT responses45. Clinical trials using memory T-cell transfer are underway at several institutions and may provide a readily exportable and effective approach to GVHD prevention. Donor T-helper cells are particularly important in GVHD initiation since they can reciprocally differentiate into Th1, Th2, and Th17 cells that mediate organ-specific GVHD.
TH1 and TH2 cell responses
Th1 cells and pro-inflammatory molecules such as IL-1, IL-6, TNF-α, IL-12 and nitric oxide have been shown to be etiological factors in GVHD induction.46,47 This inflammatory cascade results in a systemic syndrome with variable presentations of weight loss, diarrhea, skin changes; and increased mortality. Although the Th1 cell-associated cytokines IFNγ, IL-2 and TNF-α have been implicated in aGVHD pathophysiology48, some studies have shown opposite effects. IFNγ may regulate immune suppression as well as support cellular cytotoxicity.49 The aGVHD impact of IFNγ may depend on the timing of its production, as IFNγ can have immunosuppressive effects when present immediately after HSCT but can exacerbate disease via its inflammatory properties at later stages.50 In rodents, TNF-α neutralization has been associated with variable benefits in reducing aGVHD and a phase II randomized study in steroid refractory GVHD demonstrated a relatively low response rate compared to other second-line anti-GVHD medications.51
Th2-type cytokines, such as IL-4, can reduce aGVHD, but, similar to IFNγ, its effects may depend on timing.52,53 Mouse donor T-cells lacking the ability to secrete all four classical Th2-type cytokines (IL-4, IL-5, IL-9 and IL-13) showed enhanced T-cell proliferation and more GVHD.54 However, in studies involving the transfer of donor Th1-deficient and Th2-deficient T cells (using STAT4−/ −, STAT6−/ − transgenic mice, respectively), both subsets contributed to aGVHD, albeit the pattern of tissue injury that developed was distinct.9 The lack of conclusive and reproducible evidence supporting roles for Th1 or Th2 cells in GVHD suggests that other subsets are involved.
Due to the paradoxical and variable effects of TH1 and TH2 cytokine targeting, such approaches alone will not likely be sufficient to prevent aGVHD but may serve as adjunct strategies to reduce the tissue injury of GVHD.
TH17 cell responses
TH17 cell subsets, characterized by production of IL-17A, IL-17F, IL-21 and IL-22, have been shown to have a direct role in GVHD pathobiology. Initial studies reported that a lack of donor Th17 cells augmented Th1 cell differentiation and exacerbated aGVHD.55 Other studies have shown that the absence of donor IL-17 production markedly impaired CD4+ T-cell-mediated aGVHD, albeit not when both CD4+ and CD8+ T-cells mediated GVHD.56 Adoptive transfer of in vitro differentiated Th17 cells resulted in lethal aGVHD57 whereas disruption of Th17 cell by deleting the Th17-specific transcription factor RORγt did not affect GVHD58, demonstrating that Th17 cells were sufficient but not necessary to induce GVHD. In patients with active GVHD, IL-17 cells can be found in gut but not skin biopsy samples.6 Thus, IL-17 may yet prove to be a viable target for neutralization in patients with gut GVHD.
The Th17-type cytokine IL-21 is another potential neutralization target given its role in promoting the activation, differentiation, maturation or expansion of NK cells, B-cells, T-cells and APCs. It exerts anti-tumor effects59 and can facilitate autoimmunity.60 IL-21 increases Th17 cell activity not only by directly augmenting Th17 cell responses61 but also by inhibiting TRegs.62,63 Inhibiting IL-21/IL-21R signaling in vivo reduced aGVHD activity in the gut, associated with decreased Th1 cells and increased TRegs in gut mucosa.64 A similar effect was observed using a neutralizing antibody specific for human IL-21 in a human-into-mouse xenogeneic model of gut GVHD.65 The GVT effect was mostly preserved in the absence of IL-21 signaling, although such an effect may not be seen in all tumor models or patients.66,67 Nonetheless, based upon the available preclinical data, IL-21 neutralization is a particularly attractive approach to preventing and treating aGVHD and perhaps cGVHD in the clinic.
An alternative approach to manipulating the Th17 cell response is to target the cytokines involved in the induction of Th17 cells such as IL-6, which together with TGFβ, promotes naïve T-cell differentiation into Th17 cells, whereas in its absence TRegs are induced.68,69 Accordingly, high serum IL-6 levels can be predictive of severe aGVHD70 and IL6 gene polymorphisms are associated with aGVHD and cGVHD in patients.71,72 Infusion of an anti-IL-6R-specific monoclonal antibody in an aGVHD model led to TReg expansion and a reduction in GVHD pathological damage, particularly in the gut.73 IL-6 inhibition has been recently applied clinically although only modest protection was observed in preliminary studies.69,70 IL-6 neutralization may result in direct antitumor responses, particularly in multiple myeloma wherein IL-6 supports plasma cell growth.71 IL-6 inhibition may have more pronounced effects in cGVHD, since a direct relationship between IL-6 polymorphism and cGVHD has been demonstrated67 and IL-6 induced Th17 effects on B-cell dysregulation is a cGVHD hallmark.
For their survival and proliferation, Th17 cells also require IL-23, a member of IL-12 family with shared subunits and similar Stat4 downstream signaling pathway. In mice, infusion of IL-23-deficient splenocytes or use of an anti-IL-23 (p19) antibody, decreased GVHD-associated morbidity, while preserving a GVT effect suggesting IL-23 as an interesting therapeutic target for GVHD control that warrants consideration for clinical testing.74 Neutralizing IL-12 can be an effective means of preventing aGVHD.75 However, administration of high IL-12 doses early but not later after HSCT protects mice from aGVHD76 via an IFNγ-dependent mechanism.77 Preliminary results suggest that targeting the IL12/IL23 axis has activity in treatment refractory aGVHD in patients.78
Targeting co-stimulatory and co-inhibitory pathways
Co-stimulatory molecules and their importance in transplant biology have been known for the last two decades. 79 They are necessary to induce T-cell proliferation, cytokine secretion and effector function after TCR activation in response to antigen. The most extensively studied pathways involve the CD28:B7 molecules and CD40:CD40L molecules. T-cell activity is counter-regulated by co-inhibitory molecules, such as PD1 and PDL1.
Initial studies focused on the in vivo blockade of interactions between CD28 or CTLA4 and their B7 ligands, CD80 or CD86, using CTLA4-immunoglobulin fusion protein or B7-specific antibodies.80,81 Limitations of CD28/B7 pathway blockade is the potential adverse effects on TReg survival that would impede aGVHD inhibition as well as co-blockade of the CD28 stimulatory as well as the CTLA4 inhibitory pathways., the latter increasing aGVHD. An ongoing trial to prevent aGVHD is being conducted with CTLA4-immunoglobulin and future approaches will likely explore a potentially superior approach using a mutated CTLA4-immunoglobulin fusion protein that preferentially inhibits CD28/B7 and not CTLA4/B7 interactions. Finally, while studies in rodents and humans demonstrated that ex vivo blockade of the B7 pathway can induce tolerance and prevent aGVHD,82,83 although this approach is cumbersome.
Blockade of CD40 ligand:CD40 interactions was efficacious in reducing GVHD and, unlike CD28/B7 blockade, augments nTReg function in mice.80,83 Despite its promise, clinical applications of an anti-CD40 ligand antibody were limited due to toxicity associated with endothelial and platelet binding.84 As such, non-mitogenic anti-CD40 antibodies are being developed and based upon rodent data may prove to be useful adjuvants to achieve tolerization and aGVHD prevention post-HSCT.
Other co-stimulatory pathways that successfully reduced aGVHD have included the TNF-α family members OX40, 4-1BB, CD30, BTLA, LIGHT and HVEM, as well as the B7 superfamily members ICOS and VSTM3. Blocking reagents are not yet available for clinical GVHD applications and therefore will not be further discussed.85
The T cell co-inhibitory molecule PD1 binds to PD-L1 (primarily expressed by DCs and GVHD parenchymal cells) and PDL2 (primarily expressed by resting monocytes and GVHD-target parenchymal cells). PD1-expressing T-cells have been shown at the site of GVHD and PD1 blockade results in an IFNγ-dependent increase in aGVHD severity.86 Delayed PDL1 blockade at later time-points after HSCT improved GVT effects without exacerbating GVHD in some models, suggesting a venue for safer application.87–89. CTLA4- and PD1-specific blocking antibodies are being tested in the clinic to improve GVT responses after HSCT, although there are not yet reagents that selectively signal via these inhibitory pathways that may be useful in GVHD prevention or therapy.89,90
Targeting T-cell signaling pathways
There are currently several pharmacological agents that are in routine use in allogeneic recipients that target donor T-cell activation. These include calcineurin inhibitors (cyclosporine A, FK506), anti-metabolites (mycophenolate) or lymphocyte-depleting agents (steroids, anti-thymocyte globulin, CD52-specific antibody, or early post-transplant cyclophosphamide, which induces selectively apoptosis of alloactivated T cells).91 Each of these reagents has proven useful for either GVHD prevention or therapy, although none are entirely efficacious in either venue.
Targeting kinases
Protein kinase C (PKC) is crucial for T cell activation and survival. PKCθ has been shown to be required for alloreactivity and GVHD induction but not for a GVT or viral challenge (Figure 3).92 The PKCθ inhibitor AEB071 decreases IL-2 and IFNγ production by T-cells and extends rat heart and kidney allograft survive in primates. Although PKCθ inhibition decreases Teff responses, TReg potency is increased because PKCθ reduces TReg suppressive capacity when present at the immunological synapse.93,94 Blocking this dual role of PKC-θ in controlling T-cell function is ideal for inhibiting GVHD.
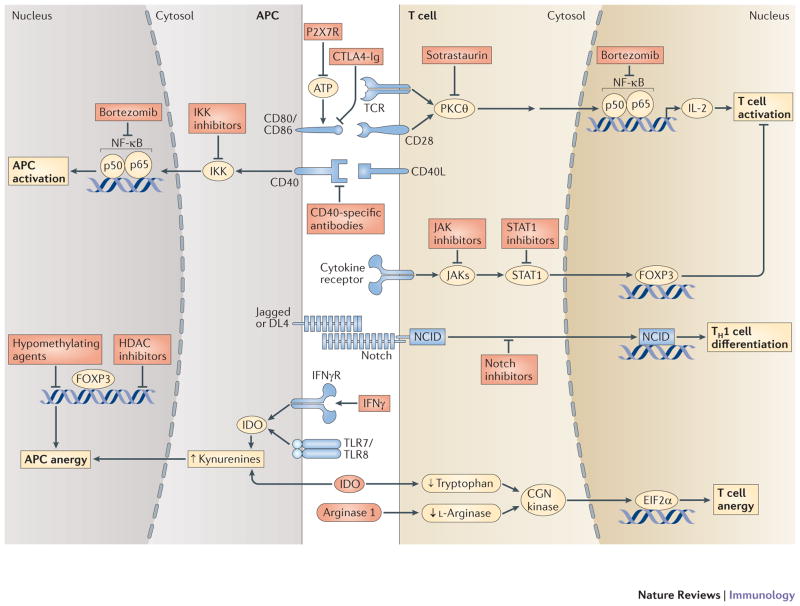
Figure 3. Some of the common pathways in T-cells-APC interactions targeted by therapeutic intervention using antibodies or small molecules.
The diagram represents naïve T-cell and APC interactions with some of their interactions and the downstream pathways resulting in augmentation of T cell activity and antigen presentation or resulting in anergy and tolerization. Some of the agents that have been used to inhibit these pathways are depicted.
Because of strong role for pro-inflammatory cytokines in aGVHD, inhibition of cytokine-induced signal transduction is an appealing approach for GVHD treatment. Janus kinases (JAKs) are cytoplasmic protein tyrosine kinases that initiate cytokine-triggered signaling events by activating STAT protein cytoplasmic latent forms.95 In preclinical models, a small molecule JAK3 inhibitor has shown high promise in reducing lethality from GVHD without impeding the GVT effect.96,97 JAK2 inhibition has been shown to have in vitro tolerogenic effects through its effect on DC/allostimulated T-cells while preserving nominal antigen responses.98 Small molecule JAK2 or JAK3 inhibitors may prove to be useful in inducing donor anti-host tolerance.
Other tyrosine kinase inhibitors such as imatinib (commonly used in chronic myelogenous leukaemia) has been shown to have a significant anti-GVHD effects, especially in cGVHD patients.99,100 Although inmatinib’s exact GVHD mechanism seems independent of a PDGF inhibitory function, imatinib represents an attractive approach for suppressing cGVHD and preserving GVT responses.
Proteasome inhibitors
Proteasome inhibitors, such as bortezomib, have been recently used in clinical trials based on the prevention or treatment of murine GVHD and associated inhibitory effects on cytokine signaling and NF-κB activation (Figure 4). Bortezomib, even at very low doses, can specifically deplete alloreactive T-cells, allowing TReg survival and attenuating IL-6-mediated cell growth.101 Bortezomib can inhibit APCs by targeting TLR4-mediated activation.102 In mice, as well as some limited clinical studies,103 early post-HSCT bortezomib administration protects against aGVHD without impairing engraftment. There is a differential effect of proteasome inhibition with bortezomib on GVHD that is critically dependent on the timing of bortezomib administration with delayed administration causing a TNF dependent gut GVHD exacerbation although this may also due to species-specific sensitivity differences.104 Because it can preserve or even augment GVT responses by sensitizing tumour cells to cytolytic effector mechanisms, bortezomib and possibly other proteasome inhibitors are attractive therapeutic agents and likely will be tested in a number of clinical HSCT settings for its potent GVHD prevention and GVT effects.
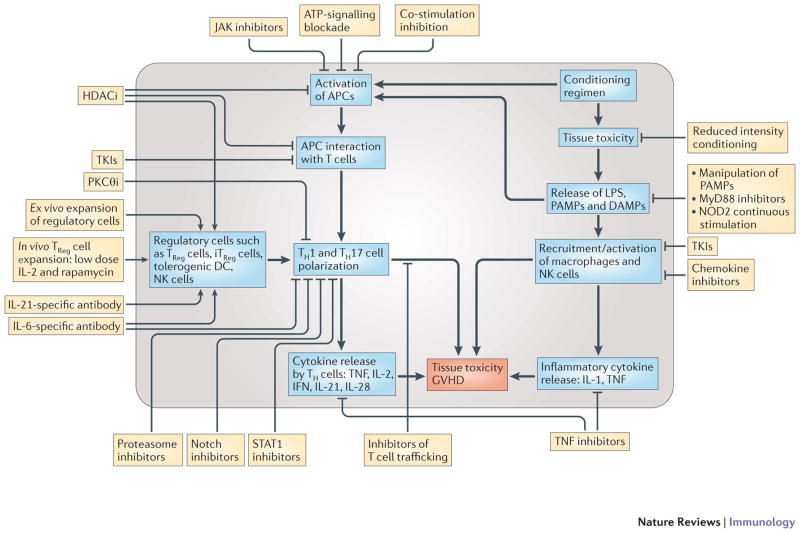
Figure 4. General Overview of Promising Anti-GVHD Therapies.
The pathways inside the circle shows the most important pathophysiological pathways in the generation of aGVHD. The boxed outside the circle are some of the promising categories of therapeutic interventions and their most relevant immunological targets in GVHD. Plus signs represent the stimulatory effects and the minus signs represent the inhibitory effects of the therapeutics on a pathway.
Targeting T-cell homing
Modulating the trafficking patterns of alloreactive T-cells had been identified as an efficacious means of ameliorating experimental GVHD disease.105 Inhibition of T-cell homing into tissues harboring active inflammation can be accomplished by interrupting one of four key stages: tethering and rolling on the endothelium, chemokine ligand-receptor interactions, adhesion to the endothelium and migration in response to sphingosine-1 phosphate (S1P). In this section we review some of the components of T-cell homing with more immediate potential applications on management of GVHD.
Selectins and their ligands
P-selectin, one of a family of three glycosylated lectins (E-, L- and P-selectin), is constitutively expressed on vascular endothelium of the skin and bone marrow, and inducibly expressed by other endothelial cells during inflammation. P-selectin is critical for endothelium tethering and rolling. mRNA encoding P-selectin glycoprotein ligand 1 (PSGL1) is upregulated during GVHD.106 P-selectin-deficient recipients exhibit decreased GVHD of the skin, liver and small bowel, associated with diminished infiltration of alloactivated T-cells into the Peyer’s patches and small bowel, and increased donor T-cells in the spleen and secondary lymphoid organs.107 Blockade of selectin/ligand interactions can be used for homing inhibition of alloreactive T-cells, although concerns have been raised about the deleterious effects on wound healing and protection against infection.
Chemokine ligand–receptor interactions
Distinct chemokine ligand-receptor interactions mediate Teff homing to different tissues. CCR9 expression by alloreactive T-cells facilitates their recruitment into the gut and skin. CCR4 and CCR10 are important for skin homing, and CXCR3 has been demonstrated to attract Th1 cells to sites of tissue injury. Recipients of a CCR2-deficient CD8+ T-cell transplant developed less damage in the gut and liver108, while the GVT effect was preserved. In mice, CXCR3 inhibition reduced the severity of GVHD.109 Steroid therapy may affect chemokine levels such as CXCL 9–11 and CCL2–3, and, due to differential expression, the recruitment of T-cells to gut but not liver.110 Although there is no direct evidence from animal studies on the role of CCR9 in GVHD, CCR9 polymorphisms have been linked to GVHD severity in humans,111 making it a potential target for future clinical trials. However, one limitation of the use of chemokine receptor antagonists, such as CCR5, is the possible interference with TReg recruitment to GVHD target organs105.
High-affinity integrins. There has only recently been a greater appreciation of the significance of high affinity integrins in inflammatory conditions including GVHD.112 For example, natalizumab, a humanized monoclonal antibody against α4-integrin has been approved for patients with multiple sclerosis but resulted in a progressive demyelinating disease (PML). The beta7 subunit of this integrin has been shown to play a pivotal role in homing of alloreactive cells specifically into the gut and its inhibition can significantly moderate the effects of gut aGVHD, sparing GVT effect while avoiding its negative effect on PML.113 It is this level of tissue specificity of integrins that makes them interesting targets for future GVHD therapy. It is possible that clinical trials in GVHD using natalizumab will not be accompanied by PML due to lack of the underlying CNS inflammation that occurs with multiple sclerosis.
Sphingosine 1-phosphate receptors
FTY720 is a high-affinity agonist for 4 of 5 known S1P receptors, crucial for cell survival, cytoskeletal rearrangements, cell motility and cell migration. FTY720 induces receptor internalization, rendering the cells unresponsive to serum lipid S1P FTY720 exerts its immunomodulatory effects primarily by sequestering lymphocytes within secondary lymphoid organs, preventing circulation to peripheral inflammatory sites. FTY720 decreases aGVHD mortality without loss of GVT effects,114,115 although the mechanism is unclear. In one study, FTY720 differentially modified Teff migration to lymphoid organs but it did not retain Teffs in lymph nodes nor did it prevent early Teff migration into GVHD tissues.116 Rather, FTY720 reduced splenic DCs and donor T-cell responder frequency early post-HSCT. Because FTY720 is an approved agent for multiple sclerosis, FTY720 may be tested for GVHD prevention or therapy, although results in solid organ transplantation did not show therapeutic benefit.
TRegs and tolerigenic DCs
Natural TRegs (nTRegs)
nTRegs, defined by CD4, CD25 and the transcription factor forkhead box P3 (FOXP3) expression, suppress autoreactive lymphocytes, and controls innate and adaptive immune responses.117–119 TReg impairment is associated with loss of tolerance, autoimmunity and cGVHD120 (Figure 5). In preclinical models, nTReg adoptive transfer was highly effective at suppressing aGVHD121–123 and improving immune recovery.124,125 Surprisingly, GVT responses were preserved, likely due to retention of cytolytic T cell function or differences in TReg versus Teff homing patterns.126 Two TReg phase I clinical aGVHD prevention trials have been reported. TRegs expanded from umbilical cord blood significantly reduced aGVHD compared to historical controls,126,127 although the TReg:Teff ratios achieved were suboptimal based upon rodent GVHD models. Improvements in ex vivo nTreg cell production should permit the expansion of large numbers of otherwise hypoproliferative nTregs.128 In another study, freshly isolated TRegs from haploidentical donors virtually eliminated aGVHD when given with Teffs at numbers that typically cause aGVHD.124 New methodologies to generate antigen-specific nTRegs are likely to be tested in future trials as a means of restricting nTReg-mediated suppression to aGVHD while sparing GVT responses. Given the remarkable efficacy of nTReg in preclinical studies and encouraging preliminary data in human clinical trials, nTReg infusion could provide a degree of specificity of aGVHD prevention not possible with T cell depletion or globally immune suppressive drugs.
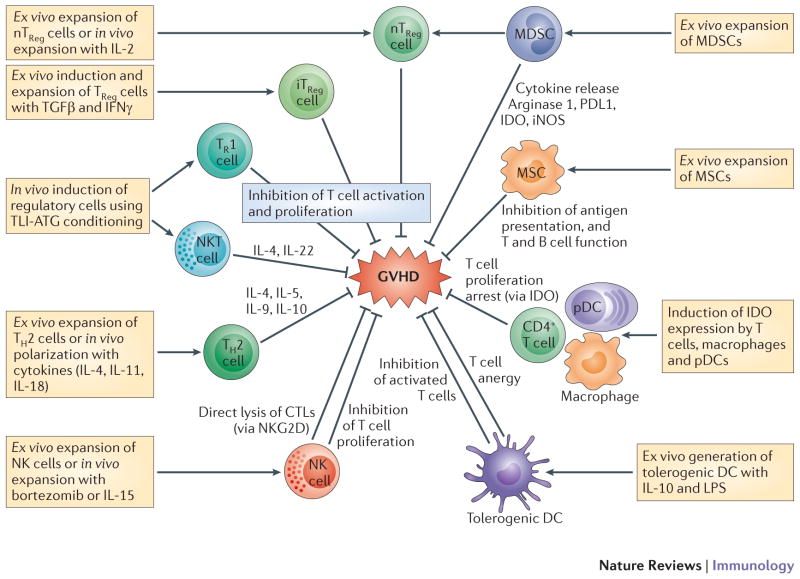
Figure 5. Potential targets for cellular immunotherapies in GVHD.
Tregs are either formed naturally by thymic differentiation (nTRegs) or are induced in the periphery from naive T-cells. Induced Tregs (iTRegs) can be divided into IL4, IL-10 and TGFβ-producing Th3 cells (CD4+CD25+FOXP3+) and CD25- but CD4+FOXP3+ iTregs that also produce IL-10 and TGFβ. FOXp3- Tr1 cells produce IL-10 and have shown potent suppressive effects on GVHD in the context of total lymphoid irradiation and anti-thymocyte globulin (TLI-ATG) conditioning regimen which also induces the generation of IL4-producing NKT cells. Ex vivo expanded Th2 polarized cell are already in clinical trials for the treatment of aGVHD. NK cells trials are also underway using NK cell infusion or activations of NK cells in vivo to delete alloreactive T cells. Substantial data on suppressive effects of IDO+ T cells, macrophages and DCs, make them prime candidates for future clinical trials. Third party infusion of mesenchymal stem cells (MSCs) for the treatment of GVHD, has created mixed results. Transfer of donor-derived Tregs expanded ex vivo has been more promising. Infusion of ex vivo expanded Myeloid derived stem cells (MDSCs) in pre-clinical models using G-& GM-CSF +/− IL-13 has shown to be feasible with anti-GVHD effect. Injection of pegylated-arginase may have the same benefit and is more practical therapeutically.
The cytokine IL-2 has a critical role in the proliferation of TRegs and Teffs. Its effect on TReg proliferation may be one reason for lack of robust response to IL-2 inhibitors observed in a Phase II randomized aGVHD therapy trial.36 cGVHD was ameliorated in some patients by low dose of IL-2, associated with increased TRegs. Although it remains to be determined if TRegs were essential103, the potential TReg contribution is in agreement with mouse studies demonstrating that IL-2 given with the molecular target of rapamycin inhibitor sirolimus resulted in aGVHD protection due to TReg expansion.129 While IL-2 was originally thought to be deleterious in GVHD due to its ability to promote Teff function, low dose IL-2 effects on TReg proliferation may prove dominant and hence beneficial. Thus, IL-2 alone or coupled with nTReg infusion may further harness the power of TRegs for GVHD prevention and therapy.
Inducible TRegs (iTRegs)
Investigators have shown that rodent antigen-specific iTRegs, generated from CD4+25- T-cells in the presence of TGFβ or induced in vivo by tolerogenic DCs, as well as human polyclonally expanded iTRegs, generated using TGFβ and sirolimus, could reduce GVHD in rodent models.130–132 Owing to the higher frequency of CD4+CD25- T-cells than CD4+CD25+ nTRegs and the greater expansion efficiency of iTRegs than nTRegs, a clinical iTReg aGVHD prevention trial for aGVHD is expected to begin soon.
Tolerogenic DCs
APCs do not always promote immune responses and can be tolerogenic and inhibit GVHD in mice.131,133 Regulatory DCs are obtained by exposing bone marrow-derived cells to GM-CSF, IL-10, TGFβ and then LPS or isolated from a mixed lymphocyte reaction response to which TGFβ and retinoic acid are added. Tolerogenic DC infusion rescued animals from lethal aGVHD, associated with iTreg generation.133 Correlative studies in patients have indicated an association between reduced cGVHD and high donor graft DC content.134
Additional immunomodulatory cellular therapies
NK cells
Donor-type NK cells have also been shown to have a role in the inhibition of aGVHD. Preclinical studies have shown that donor NK cells can suppress aGVHD and promote GVT responses.135 Subsequent studies have shown that donor T-cells exhibited less proliferation, lower CD25 expression and decreased IFNγ production in the presence of donor NK cells and cytokine-induced killer cells, a mixture of NK cells and highly activated CD8+ T-cells that mediate MHC-unrestricted cytotoxicity.135 Third-party NK cells are being designed to investigate the clinical potential of such an approach.136 Such an effect has been indirectly shown in clinical studies in which infused NK cell dose at HSCT directly related with GVHD occurrence and severity.137 The demonstration on the importance of killer cell immunoglobulin receptor (KIR) family signaling on NK cell subset activity and outcome after allogeneic HSCT for acute myeloid leukemia (AML)138 spurred great interest in optimizing NK cell use not only to suppress GVHD but promote GVT. Haploidentical NK cell transfer can increase GVT responses in AML patients.139 Means to promote this effect via concurrent administration of NK cell stimulating cytokines, such as IL-2 or IL-15, as well as understanding the potential inhibitory effects of HSCT immunosuppressive regimens on NK cell activity/recovery are being examined. A subpopulation of NK cells that co-express T cell markers (NKT cells) have also been shown to control mouse GVHD in an IFN-γ and IL-4 dependent manner.53 A similar GVHD reducing effect has been proposed after certain conditioning regimen such as total lymphoid irradiation and anti-thymoglobulin infusion in non-myeloablative clinical trials. 140 Alternatively, in vivo activation of NK T cells, and particularly their invariant form of them, with glycosphingolipid such as α-galactosylceramide (α-GalCer), has been shown to inhibit GVHD in murine models. A liposomal form of this compound is currently been tested in clinical trials. Such an approach however, has recently been challenged as early administration of synthetic form of α-GalCer, KRN7000, in mice resulted in hyperacute GVHD. 141 Thus, the long-term utility of NKT cell based therapeutic approaches remains to be determined.
MDSCs
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous cell population of myeloid origin. MDSCs consist of progenitors and immature macrophages, granulocytes and DCs, defined as CD11b+Gr1+ in mice and in humans, Lin-HLA-DR-CD33+ or CD11b+CD14-CD33+, although they have also been defined within a CD15+ peripheral blood population.142,143 MDSCs can be expanded in vitro and can suppress T-cell function by inducing enzymes that regulate essential amino acid metabolism [namely arginase or indoleamine 2,3 dioxygenase (IDO), see below], by releasing soluble mediators (such as IL-10, reactive oxygen species or iNOS), by direct cell-to-cell contact, and by inducing TReg expansion144, although their effect on TRegs is variable.140,141 Thus, MDSCs share features with alternatively activated M2 macrophages. Preclinical studies have shown that MDSCs can suppress aGVHD.145,146 In one study,144,177 in vivo arginine depletion by MDSCs could be accomplished by use of a drug, pegylated arginase-I, suggesting a new pharmacological approach to aGVHD prevention.
MSCs
Bone marrow-derived mesenchymal stem cells (MSCs) are a group of heterogeneous plastic-adherent cells with the capacity to differentiate in vitro into osteoblasts, adipocytes and chrondroblasts. MSCs have a wide range of immunosuppressive and immunomodulating effects on innate and adaptive immune cells147. MSCs may have a protective effect against GVHD148 although this effect has not been seen consistently in murine models.149 Results of clinical trials are also confusing with earlier trials showing significant benefits whereas two recent phase III trials at least with one source of MSCs did not show any benefit.150,151 Differences in manufacturing, definition of MSCs, expression of homing receptors and type of GVHD injury may all contribute to the difficulty in comparing results between laboratories and clinical outcomes.
Targeting intracellular pathways for immune regulation
IDO is the first and rate-limiting enzyme of the catabolism of the essential amino acid, tryptophan. By tryptophan depletion and/or tryptophan catabolic (termed kynurenines) accumulation, T-cell proliferation is arrested and T-cells die. IDO is produced by some alternatively activated macrophages and other immunoregulatory cells (also used as an immune subversion strategy by many tumors). More importantly, tryptophan starvation and presence of kynurenines can induce the conversion of naïve T-cells into TRegs.152 GVHD induces IDO expression in the gut but the timing is too late to avoid disease.153 Both APCs and epithelial cells contribute to GVHD inhibition by IDO in rodents. In HSCT patients, IDO positivity was seen not only on CD16+ macrophages and DCs, but more significantly in a subset of CD4+ T cell correlating with aGVHD severity.154,155 Therapeutically, IDO can be induced in mice by the time of HSCT in an IFNγ-dependent mechanism by TLR7 agonist or kynurenine administration, indicating new therapeutic aGVHD approach.156 However, GVT effects need to be assessed as does long-term tolerance induction in the absence of continuous tryptophan starvation or kynurenine administration.
Notch signaling controls cell fate and tissue homeostasis. Notch1–4 receptors interact with Jagged and δ-like family ligands; upon γ-scretase proteolytic cleavage, Notch receptors are translocated to the nucleus to exert their biological effects. Notch regulates Th1, Th2, Th17, TRegs as well as Teffs. Recent studies have indicated that aGVHD can be inhibited Notch inactivation in donor CD4 T cells which preserving GVT157 Because several pharmacologic methods are entering human clinical trials, inactivating the Notch axis may prove to be an effective strategy to inhibit aGVHD and based upon highly encouraging preclinical data, further development of these reagents toward the clinic is indicated.
Hypomethylating agents such as azacytidine can prevent aGVHD in mice without interfering with GVT effects158 and to increase TRegs in patients.159 In preclinical models of aGVHD and cGVHD patients treated with extracorporal phototherapy using a rhodamine-derived photosensitizing agent, alloreactive T-cells are depleted and TRegs preserved.160,161 Inhibition of histone methylation using 3-deazaneplanocin A has shown to arrests ongoing aGVHD in mice by activating proapoptotic gene Bim, resulting in selective apoptosis of alloantigen-activated Teffs and importantly preserving GVT effects.162 The clinical testing of hypomethylating agents specifically for GVHD prevention deserves strong consideration for future trials.
Histone deacetylase (HDAC) inhibitors, currently in clinical trials for GVHD, modify histones and chromatin and have shown an aGVHD protective effect163,164, associated with suppression of host APCs and enhancement of IDO and Tregs,.164 Additionally, HDAC inhibitors may impact T-cell activation by inhibiting STAT3 phosphorylation.165,166 Use of a small molecule inhibitor of STAT1, a natural inhibitor of FoxP3, can also be exploited as a means to iTReg conversion and nTReg expansion, although such reagents are not currently being tested for such purpose in the clinic.167,168
Finally, statins have been shown to reduce GVHD in mruine models of GVHD by their immunomodulatory effects on APC and T cells perhaps through interruption of l-mevalonate and its downstream isoprenoid metabolites.169 Retrospective analysis of recipients who were taking statins however, has shown that such a benefit may occur at the cost of increased cancer relapse due to the non-specific effects of statins on GVT effect.170
Conclusions and future directions
Despite improvements in our understanding of transplant immunology and clinical and supportive care, both aGVHD and cGVHD remain a clinical challenge and a major cause of morbidity and mortality for HSCT recipients. Systemic corticosteroid therapy despite its significant shortcoming still remains the standard primary therapy for GVHD while clearly better therapies are needed, particularly in cases of steroid-refractory cGVHD. Although the mouse is an imperfect model for the human disease, such models are highly useful for testing reagents for GVHD prevention and GVT retention. Nonetheless, improvement of the preclinical models, particularly for cGVHD are still needed, as regimens and patient populations receiving allogeneic HSCT are constantly changing. There has been much recent progress with regard to understanding the mechanisms underlying GVHD, particularly with regard to the role of the APC. Most of the currently used anti-GVHD approaches are broad spectrum approaches that target T cells and are therefore likely have a potential significant negative impact on GVT as well as immune reconstitution. Alternatively, more selective approaches that specifically influence alloreactive T-cell activation, survival or function should avoid some of the adverse side effects of globally immune-suppressive therapy. Targeting of cytokines, leukocyte homing and migration patterns, in vivo augmentation or induction of TRegs, tolerance-inducing strategies and cellular therapies derived from preclinical GVHD studies are attractive approaches for improving our GVHD armamentarium. Novel and unique approaches, such as inhibition of neovascularizartion, an early even in the inflammatory phase of aGVHD, may prove to be even more promising.171
The potential deleterious effects of some of the anti-GVHD on engraftment, immune reconstitution and anti-tumor effects approaches will affect their clinical applicability. The suppression of GVHD without impairing GVT remains the “Holy Grail” of allogeneic HSCT. While no one preclinical approach has proven to be uniformly efficacious in preventing GVHD and retaining GVT, recent clinical studies derived from murine GVHD models including nTReg infusion, memory T-cell isolation, suicide gene transfer, TLI-ATG, bortezomib, HDAC inhibitors, or anti-IL21 antibodies are particularly noteworthy. It also is clear that as more is learned with regard to the science of immunology that revisiting of prior reagents and applications in GVHD may arise as evidenced by the recent use of IL2 to induce TRegs in cGVHD. Additionally, by capitalizing on reagents already in the clinic for other purposes (e.g. autoimmune diseases; oncology; solid organ transplants), those interested in preclinical modeling and clinical applications should have a rich source of new strategies to prevent or treat GVHD in the future.
Table 1.
Newer approaches for treatment of GVHD that have been or are currently in clinical trials
| Category | Targets | agents | Phase of trials | Comments |
|---|---|---|---|---|
| Small Molecules | PKC inhibitors | AEB071 | Phase II, | T cell anergy, solid organ Tx |
| CCR5 inhibitors | Maraviroc | Phase I/II | ||
| mTor inhibitors | Sirolimus Everolimus |
Phase II & III Phase I |
Th1↓, Treg↔ | |
| Hypomethylating agents | 5-Azacytidine | Phase I/II | APC↓, T cell anergy | |
| HDAC inhibitors |
LBH589 Vorinostat |
Phase I Phase II |
APC↓, T cell anergy | |
| Tyrosine Kinase Inhbitors |
Imatinib Nilotinib |
Phase II Phase II |
PDGF↓ | |
| Antibodies and fusion proteins | Anti-T cells | Thymoglobulin | Phase II, III | General T cell suppression |
| TNF-α inhbitors | Infliximab Etanercept |
|||
| Anti-CD25 or IL-2 receptor | Denileukin diftitox Basiliximab Daclizumab |
Pahse II | Deletion of activated T cells | |
| Anti-CD3 antibody | Visilizumab | Phase I/II, | T cell stimulation ↓ | |
| Anti-CD2 antibody | Siplizumab (MEDI-507) | Phase I | T cell activation↓ | |
| Anti-IL6 receptor antibodies | Tocilizumab | Phase I | Th17↓, Treg↑ | |
| Anti-CD20 antibody | Rituximab | Phase II | B cell inhibition, APCs↓ | |
| Anti-CD52 antibody | Alemtuzumab | Phase I–IV | T & B cell suppression | |
| Anti-CD147 antibody | ABX-CBL | Phase II/III | Activated T cells↓ | |
| LFA3-Ig fusion protein | Alefacept | Phase I–III | inhibiting LFA-3/CD2 interaction | |
| Anti-CD7 antibody | Anti-CD-7 RI | Phase I/II | Mature T cells↓ | |
| Effect on Microbiome | Anti-bacterial, | Rifaximin | Phase I | Gut decontamination |
| Probiotic Use | Lactobacillus | Phase I | Changing gut microbiome | |
| Cellular Therapies | TRegs | Ex vivo or in vivo TRegs |
Phase I–II | Inhibition T cell activation |
| Mesenchymal Stem Cells | Prochymal, etc | Phase I–III | immunomodulating T & B cells, NK cells, and APCs | |
| NK cells | Different populations of NK cells | Phase I | Alloreactive T cells↓, modulating Tregs | |
| DC | HDC Vax-001 | Phase I | Induce tolerance | |
| Donor Th2 cells | IL4 & IFn producing Th2 cells | Phase I | Teff↓ | |
| Donor T-cells with caspase-9 suicide gene | AP1903 | Phase I | If GVHD occurs, AP1903 delete of alloreactive T cells. | |
| CD45RA+ naive T-cells | Depletion before HSCT | Phase II | Decrease GVHD while preserve GVT | |
| Cytokine | KGF | rHuKGF (palifermin) | Phase I–II | Protection of thymic environment. |
| IL-2 | Low dose IL-2 | Phase II | in vivo Treg↑ | |
| Chemo-agent | T cells specific | Pentostatin | Phase II | Deletion of T &B cells |
| B & T cell specific | cyclophosphamide | Phase II | Deletion of proliferating T celsl | |
| Miscellaneous | NK T cell receptors | RGI-2001 | Phase I–II | Activate type II NKT cells |
| Protesome inhitors | bortezomib | Phase I–II | Teff↓, Treg↑ | |
| photopheresis | Extracorporal photopheresis | Phase II | Teff↓, Treg↑ | |
| Statins | Atorvastatin | Phase II | Th1↓, Th2↑, Treg↑ |
Acknowledgments
The authors thank the current and past members of our laboratories and our colleagues who have collaborated with us for their scientific contributions and Angela Baptiste for expert technical assistance in preparation of the manuscript.
GLOSSARY
- Conditioning regimen
Conditioning regimens, also referred to as preparative regimens, are combinations of chemotherapy, radiation therapy and/or immunosuppressive medications designed not only to destroy residual malignant cells, but also to provide space for donor stem cell engraftment and to provide immunosuppression to prevent rejecting the donor’s stem cells
- Myelosuppressive
Refers to conditioning regimens that inhibit bone marrow activity, resulting in marked decrease in production of blood cells and platelets
- Non-myeloablative
Refers to conditioning regimens that do not inhibit bone marrow activity, resulting in no or minimal decrease in production of blood cells and platelets yet still help with engraftment and prevent rejection of donor cells
- Minor histocompatibility antigens
Minor histocompatibility antigens are due to normal proteins that are in themselves polymorphic in a given population. Even when a transplant donor and recipient are identical with respect to their major histocompatibility complex genes, the amino acid differences in minor proteins can cause the grafted tissue to be slowly rejected
- Genetic drift
Genetic drift is the process of change in the genetic composition of a population due to chance or random events rather than by natural selection, resulting in changes in allele frequencies over time
- Toll-like receptors
(TLRs). A family of membrane-spanning proteins that recognize pathogen-associated molecular patterns (which are shared by various microorganisms), as well as damaged host cell components. TLRs signal to the host that a microbial pathogen is present or that tissue damage has occurred. They are characterized by an ectodomain that has varying numbers of leucine-rich repeat motifs and a cytoplasmic Toll/IL-1 receptor (TIR) domain that recruits adaptors, such as the myeloid differentiation primary response protein 88 (MYD88) and TIR domain-containing adaptor protein inducing IFNβ (TRIF; also known as TICAM1)
- Cytokine storm
Hyperrelease of inflammatory mediators in a relatively short period of time in response to stimulation of T cells, NK cells, monocytes and macrophages by pathogens, tissue injury from conditioning regimen or other immune insults triggering Graft versus host disease. Typically, cytokine storm consists of one or more positive feedback loops between cytokines and immune cells spiraling up the reaction
- Nucleotide-binding domain, leucine-rich-repeat-containing family receptors
(NLRs). The human NLR family comprises 22 members. They share a domain organization that usually includes an amino-terminal caspase recruitment domain (CARD) or pyrin domain (PYD), followed by an intermediary nucleotide-binding oligomerization domain (NOD) and carboxy-terminal leucine-rich repeat motifs. NLRs are thought to survey the host cytosol and intracellular compartments for pathogen- and damage-associated molecular patterns to activate signalling pathways that contribute to the host innate immune response
- P2X7R
P2X purinoceptor 7 (P2X7R) is an ATP-gated cation channel expressed in hematopoietic cells that participates in both cell proliferation and apoptosis. Expression and function of the P2X7R have been associated with the clinical course of patients affected by chronic lymphocytic leukemia (CLL). It is encoded by the P2RX7 gene. The product of this gene belongs to the family of purinoceptors for ATP. It is responsible for ATP-dependent lysis of macrophages through the formation of membrane pores permeable to large molecules
- Inflammasome
A large multiprotein complex formed by a NOD- and LRR-containing (NLR) protein, the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC; also known as PYCARD) and pro-caspase 1. The assembly of the inflammasome leads to the activation of caspase 1, which cleaves pro-interleukin-1 β (pro-IL-1β) and pro-IL-18 to generate the active pro-inflammatory cytokines
- Th1 cells
(T helper 1 cells). TH1 cells secrete interferon-γ and tumour necrosis factor to promote cell-mediated immunity by supporting the classical activation of macrophages and the proliferation of cytotoxic CD8+ T cells
- Janus kinases
(JAK–STAT pathway). An evolutionarily conserved signalling pathway that is associated with type I and type II cytokines. Receptor ligation by these cytokines leads to a series of events that includes the recruitment and activation of JAKs and the phosphorylation of various STATs, which in turn translocate to the nucleus where they transactivate various genes involved in cell differentiation, survival, apoptosis and proliferation
- Tolerogenic DCs
A subpopulation of dendritic cells capable of inducing antigen specific both peripheral and centeral tolerance by indction of antigen specific TReg cells, T cell anergy and antigen- specific cytotoxic T cell deletion
- Mixed lymphocyte reaction
A tissue-culture technique for testing T cell reactivity and APC activity. A population of T cells is cultured with MHC-mismatched APCs, and proliferation of the T cells is determined by measuring the incorporation of 3H-thymidine into the DNA of dividing cells
- Azacytidine
A pyrimidine nucleoside analogue of cytidine that inhibits DNA methyltransferase, thereby blocking DNA methylation. Hypomethylation of DNA by azacitidine may activate an array of genes such as tumor suppressor genes silenced by hypermethylation, resulting for example in an antitumor effect. This agent is also incorporated into RNA, thereby disrupting normal RNA function and impairing tRNA cytosine-5-methyltransferase activity
- Indoleamine 2, 3 dioxygenase
(IDO). An intracellular haem-containing enzyme that catalyses the oxidative catabolism of tryptophan. Insufficient availability of tryptophan can lead to T cell apoptosis and anergy
- Alternatively activated macrophages
(M2 macrophage). A macrophage stimulated by IL-4 or IL-13 that expresses arginase 1, the mannose receptor CD206 and IL-4 receptor-α. There may be pathogen-associated molecular patterns expressed by helminths that can also drive the alternative activation of macrophages
- Mesenchymal stem cells (MSCs)
Mesenchymal stem cells, are marrow derived multipotent stem cells that can differentiate into a variety of cell types of mesenchymal origin including: osteoblasts, chondrocytes, and adipocytes. Terms of MSCs and and Marrow Stromal Cell have been used interchangeably. MSCs have been shown to have immunomodulatory and immunosuppressive effect
References
- 1.Pasquini MC, Wang Z, Horowitz MM, Gale RP. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin Transpl. 2010:87–105. [PubMed] [Google Scholar]
- 2.Alyea EP, et al. Impact of conditioning regimen intensity on outcome of allogeneic hematopoietic cell transplantation for advanced acute myelogenous leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2006;12:1047–1055. doi: 10.1016/j.bbmt.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Scott BL, et al. Myeloablative vs nonmyeloablative allogeneic transplantation for patients with myelodysplastic syndrome or acute myelogenous leukemia with multilineage dysplasia: a retrospective analysis. Leukemia. 2006;20:128–135. doi: 10.1038/sj.leu.2404010. [DOI] [PubMed] [Google Scholar]
- 4.Barnes DW, Loutit JF, Micklem HS. “Secondary disease” of radiation chimeras: a syndrome due to lymphoid aplasia. Ann N Y Acad Sci. 1962;99:374–385. doi: 10.1111/j.1749-6632.1962.tb45321.x. [DOI] [PubMed] [Google Scholar]
- 5.Billingham RE. The biology of graft-versus-host reactions. Harvey Lect. 1966;62:21–78. [PubMed] [Google Scholar]
- 6.Broady R, et al. Cutaneous GVHD is associated with the expansion of tissue-localized Th1 and not Th17 cells. Blood. 2010;116:5748–5751. doi: 10.1182/blood-2010-07-295436. [DOI] [PubMed] [Google Scholar]
- 7.Imanguli MM, et al. Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood. 2009;113:3620–3630. doi: 10.1182/blood-2008-07-168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy WJ, et al. Differential effects of the absence of interferon-gamma and IL-4 in acute graft-versus-host disease after allogeneic bone marrow transplantation in mice. J Clin Invest. 1998;102:1742–1748. doi: 10.1172/JCI3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nikolic B, Lee S, Bronson RT, Grusby MJ, Sykes M. Th1 and Th2 mediate acute graft-versus-host disease, each with distinct end-organ targets. J Clin Invest. 2000;105:1289–1298. doi: 10.1172/JCI7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratajczak P, et al. Th17/Treg ratio in human graft-versus-host disease. Blood. 2010;116:1165–1171. doi: 10.1182/blood-2009-12-255810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakoda Y, et al. Donor-derived thymic-dependent T cells cause chronic graft-versus-host disease. Blood. 2007;109:1756–1764. doi: 10.1182/blood-2006-08-042853. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan M, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood. 2012;119:1570–1580. doi: 10.1182/blood-2011-07-364414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–2759. [PubMed] [Google Scholar]
- 16.Imado T, et al. The protective role of host Toll-like receptor-4 in acute graft-versus-host disease. Transplantation. 2010;90:1063–1070. doi: 10.1097/TP.0b013e3181f86947. [DOI] [PubMed] [Google Scholar]
- 17.Calcaterra C, et al. Critical role of TLR9 in acute graft-versus-host disease. J Immunol. 2008;181:6132–6139. doi: 10.4049/jimmunol.181.9.6132. [DOI] [PubMed] [Google Scholar]
- 18.Heimesaat MM, et al. MyD88/TLR9 mediated immunopathology and gut microbiota dynamics in a novel murine model of intestinal graft-versus-host disease. Gut. 2010;59:1079–1087. doi: 10.1136/gut.2009.197434. [DOI] [PubMed] [Google Scholar]
- 19.Hossain MS, et al. Flagellin, a TLR5 agonist, reduces graft-versus-host disease in allogeneic hematopoietic stem cell transplantation recipients while enhancing antiviral immunity. J Immunol. 2011;187:5130–5140. doi: 10.4049/jimmunol.1101334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Penack O, Holler E, van den Brink MR. Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood. 2010;115:1865–1872. doi: 10.1182/blood-2009-09-242784. [DOI] [PubMed] [Google Scholar]
- 21.Chakraverty R, et al. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J Exp Med. 2006;203:2021–2031. doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loiarro M, et al. Pivotal Advance: Inhibition of MyD88 dimerization and recruitment of IRAK1 and IRAK4 by a novel peptidomimetic compound. J Leukoc Biol. 2007;82:801–810. doi: 10.1189/jlb.1206746. [DOI] [PubMed] [Google Scholar]
- 23.Heimesaat MM, et al. Gram-negative bacteria aggravate murine small intestinal Th1-type immunopathology following oral infection with Toxoplasma gondii. J Immunol. 2006;177:8785–8795. doi: 10.4049/jimmunol.177.12.8785. [DOI] [PubMed] [Google Scholar]
- 24.Lupp C, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe. 2007;2:204. doi: 10.1016/j.chom.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Gerbitz A, et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 2004;103:4365–4367. doi: 10.1182/blood-2003-11-3769. [DOI] [PubMed] [Google Scholar]
- 26.Wilhelm K, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010;16:1434–1438. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 27.Lee KH, et al. P2X7 receptor polymorphism and clinical outcomes in HLA-matched sibling allogeneic hematopoietic stem cell transplantation. Haematologica. 2007;92:651–657. doi: 10.3324/haematol.10810. [DOI] [PubMed] [Google Scholar]
- 28.Li H, et al. Graft-versus-host disease is independent of innate signaling pathways triggered by pathogens in host hematopoietic cells. J Immunol. 2011;186:230–241. doi: 10.4049/jimmunol.1002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matte CC, et al. Donor APCs are required for maximal GVHD but not for GVL. Nat Med. 2004;10:987–992. doi: 10.1038/nm1089. [DOI] [PubMed] [Google Scholar]
- 30.Shlomchik WD, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 31.Anderson BE, et al. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. [DOI] [PubMed] [Google Scholar]
- 32.Duffner UA, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 33.Markey KA, et al. Conventional dendritic cells are the critical donor APC presenting alloantigen after experimental bone marrow transplantation. Blood. 2009;113:5644–5649. doi: 10.1182/blood-2008-12-191833. [DOI] [PubMed] [Google Scholar]
- 34.Teshima T, et al. Acute graft-versus-host disease does not require alloantigen expression on host epithelium. Nat Med. 2002;8:575–581. doi: 10.1038/nm0602-575. [DOI] [PubMed] [Google Scholar]
- 35.Koyama M, et al. Recipient nonhematopoietic antigen-presenting cells are sufficient to induce lethal acute graft-versus-host disease. Nat Med. 2012;18:135–142. doi: 10.1038/nm.2597. [DOI] [PubMed] [Google Scholar]
- 36.Toubai T, et al. Induction of acute GVHD by sex-mismatched H-Y antigens in the absence of functional radio-sensitive host hematopoietic-derived antigen presenting cells. Blood. 2011 doi: 10.1182/blood-2011-10-384057. [DOI] [PMC free article] [PubMed]
- 37.Wang X, et al. Mechanisms of antigen presentation to T cells in murine graft-versus-host disease: cross-presentation and the appearance of cross-presentation. Blood. 2011;118:6426–6437. doi: 10.1182/blood-2011-06-358747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, et al. Mechanisms of antigen presentation to T cells in murine graft-versus-host disease: cross-presentation and the appearance of cross-presentation. Blood. 118:6426–6437. doi: 10.1182/blood-2011-06-358747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schultz KR, Paquet J, Bader S, HayGlass KT. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transplant. 1995;16:289–295. [PubMed] [Google Scholar]
- 40.Knoechel B, Lohr J, Kahn E, Abbas AK. The link between lymphocyte deficiency and autoimmunity: roles of endogenous T and B lymphocytes in tolerance. J Immunol. 2005;175:21–26. doi: 10.4049/jimmunol.175.1.21. [DOI] [PubMed] [Google Scholar]
- 41.Shimabukuro-Vornhagen A, Hallek MJ, Storb RF, von Bergwelt-Baildon MS. The role of B cells in the pathogenesis of graft-versus-host disease. Blood. 2009;114:4919–4927. doi: 10.1182/blood-2008-10-161638. [DOI] [PubMed] [Google Scholar]
- 42.Glass B, Hasenkamp J, Görlitz A, Dreger P, Schubert J, Wulf G, Nickelsen M, Truemper LH, Schmitz N. Rituximab for Graft-Versus-Host-Disease-Prophylaxis after Allogeneic Stem Cell Transplantation Given as Treatment of High Risk Relapse of Aggressive Lymphoma: Results of a Randomized Phase II Study. 1974;11 [Google Scholar]
- 43.Kharfan-Dabaja MA, Cutler CS. Rituximab for prevention and treatment of graft-versus-host disease. Int J Hematol. 2011;93:578–585. doi: 10.1007/s12185-011-0855-2. [DOI] [PubMed] [Google Scholar]
- 44.Doreau A, et al. Interleukin 17 acts in synergy with B cell-activating factor to influence B cell biology and the pathophysiology of systemic lupus erythematosus. Nat Immunol. 2009;10:778–785. doi: 10.1038/ni.1741. [DOI] [PubMed] [Google Scholar]
- 45.Zheng H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111:2476–2484. doi: 10.1182/blood-2007-08-109678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooke KR, et al. Tumor necrosis factor- alpha production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J Clin Invest. 1998;102:1882–1891. doi: 10.1172/JCI4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J Exp Med. 1992;175:405–413. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reddy P. Pathophysiology of acute graft-versus-host disease. Hematol Oncol. 2003;21:149–161. doi: 10.1002/hon.716. [DOI] [PubMed] [Google Scholar]
- 49.Lu Y, Waller EK. Dichotomous role of interferon-gamma in allogeneic bone marrow transplant. Biol Blood Marrow Transplant. 2009;15:1347–1353. doi: 10.1016/j.bbmt.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brok HP, Vossen JM, Heidt PJ. IFN-gamma-mediated prevention of graft-versus-host disease: pharmacodynamic studies and influence on proliferative capacity of chimeric spleen cells. Bone Marrow Transplant. 1998;22:1005–1010. doi: 10.1038/sj.bmt.1701478. [DOI] [PubMed] [Google Scholar]
- 51.Alousi AM, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114:511–517. doi: 10.1182/blood-2009-03-212290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fowler DH, Gress RE. Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma. Leuk Lymphoma. 2000;38:221–234. doi: 10.3109/10428190009087014. [DOI] [PubMed] [Google Scholar]
- 53.Leveson-Gower DB, et al. Low doses of natural killer T cells provide protection from acute graft-versus-host disease via an IL-4-dependent mechanism. Blood. 2011;117:3220–3229. doi: 10.1182/blood-2010-08-303008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tawara I, et al. Combined Th2 cytokine deficiency in donor T cells aggravates experimental acute graft-vs-host disease. Exp Hematol. 2008;36:988–996. doi: 10.1016/j.exphem.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yi T, et al. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood. 2008;112:2101–2110. doi: 10.1182/blood-2007-12-126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kappel LW, et al. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113:945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carlson MJ, et al. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113:1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iclozan C, et al. T helper17 cells are sufficient but not necessary to induce acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:170–178. doi: 10.1016/j.bbmt.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis ID, et al. Interleukin-21 signaling: functions in cancer and autoimmunity. Clin Cancer Res. 2007;13:6926–6932. doi: 10.1158/1078-0432.CCR-07-1238. [DOI] [PubMed] [Google Scholar]
- 60.Spolski R, Kashyap M, Robinson C, Yu Z, Leonard WJ. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc Natl Acad Sci U S A. 2008;105:14028–14033. doi: 10.1073/pnas.0804358105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peluso I, et al. IL-21 counteracts the regulatory T cell-mediated suppression of human CD4+ T lymphocytes. J Immunol. 2007;178:732–739. doi: 10.4049/jimmunol.178.2.732. [DOI] [PubMed] [Google Scholar]
- 62.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 63.Fantini MC, et al. IL-21 regulates experimental colitis by modulating the balance between Treg and Th17 cells. Eur J Immunol. 2007;37:3155–3163. doi: 10.1002/eji.200737766. [DOI] [PubMed] [Google Scholar]
- 64.Bucher C, et al. IL-21 blockade reduces graft-versus-host disease mortality by supporting inducible T regulatory cell generation. Blood. 2009;114:5375–5384. doi: 10.1182/blood-2009-05-221135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hippen KL, et al. Blocking IL-21 signaling ameliorates xenogeneic GVHD induced by human lymphocytes. Blood. 2012;119:619–628. doi: 10.1182/blood-2011-07-368027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanash AM, et al. Abrogation of donor T-cell IL-21 signaling leads to tissue-specific modulation of immunity and separation of GVHD from GVL. Blood. 2011;118:446–455. doi: 10.1182/blood-2010-07-294785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meguro A, et al. Lack of IL-21 signal attenuates graft-versus-leukemia effect in the absence of CD8 T-cells. Bone Marrow Transplant. 2011;46:1557–1565. doi: 10.1038/bmt.2010.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 69.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Symington FW, et al. The relationship of serum IL-6 levels to acute graft-versus-host disease and hepatorenal disease after human bone marrow transplantation. Transplantation. 1992;54:457–462. doi: 10.1097/00007890-199209000-00014. [DOI] [PubMed] [Google Scholar]
- 71.Ambruzova Z, et al. Association of IL6 and CCL2 gene polymorphisms with the outcome of allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2009;44:227–235. doi: 10.1038/bmt.2009.16. [DOI] [PubMed] [Google Scholar]
- 72.Cavet J, et al. Interferon-gamma and interleukin-6 gene polymorphisms associate with graft-versus-host disease in HLA-matched sibling bone marrow transplantation. Blood. 2001;98:1594–1600. doi: 10.1182/blood.v98.5.1594. [DOI] [PubMed] [Google Scholar]
- 73.Chen X, et al. Blockade of interleukin-6 signaling augments regulatory T-cell reconstitution and attenuates the severity of graft-versus-host disease. Blood. 2009;114:891–900. doi: 10.1182/blood-2009-01-197178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Das R, et al. Blockade of interleukin-23 signaling results in targeted protection of the colon and allows for separation of graft-versus-host and graft-versus-leukemia responses. Blood. 2010;115:5249–5258. doi: 10.1182/blood-2009-11-255422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williamson E, Garside P, Bradley JA, More IA, Mowat AM. Neutralizing IL-12 during induction of murine acute graft-versus-host disease polarizes the cytokine profile toward a Th2-type alloimmune response and confers long term protection from disease. J Immunol. 1997;159:1208–1215. [PubMed] [Google Scholar]
- 76.Sykes M, et al. Dose and timing of interleukin (IL)-12 and timing and type of total-body irradiation: effects on graft-vs-host disease inhibition and toxicity of exogenous IL-12 in murine bone marrow transplant recipients. Biol Blood Marrow Transplant. 1999;5:277–284. doi: 10.1016/s1083-8791(99)70002-9. [DOI] [PubMed] [Google Scholar]
- 77.Yang YG, Dey BR, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102:2126–2135. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pidala J, Perez L, Beato F, Anasetti C. Ustekinumab demonstrates activity in glucocorticoid-refractory acute GVHD. Bone Marrow Transplant. 2011 doi: 10.1038/bmt.2011.172. [DOI] [PubMed] [Google Scholar]
- 79.Briones J, Novelli S, Sierra J. T-cell costimulatory molecules in acute-graft-versus host disease: therapeutic implications. Bone Marrow Res. 2011;2011:976793. doi: 10.1155/2011/976793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blazar BR, et al. CD28/B7 interactions are required for sustaining the graft-versus-leukemia effect of delayed post-bone marrow transplantation splenocyte infusion in murine recipients of myeloid or lymphoid leukemia cells. J Immunol. 1997;159:3460–3473. [PubMed] [Google Scholar]
- 81.Saito K, et al. Involvement of CD40 ligand-CD40 and CTLA4-B7 pathways in murine acute graft-versus-host disease induced by allogeneic T cells lacking CD28. J Immunol. 1998;160:4225–4231. [PubMed] [Google Scholar]
- 82.Guinan EC, et al. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340:1704–1714. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 83.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. J Exp Med. 2001;193:1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sidiropoulos PI, Boumpas DT. Lessons learned from anti-CD40L treatment in systemic lupus erythematosus patients. Lupus. 2004;13:391–397. doi: 10.1191/0961203304lu1032oa. [DOI] [PubMed] [Google Scholar]
- 85.Socie G, Blazar BR. Acute graft-versus-host disease: from the bench to the bedside. Blood. 2009;114:4327–4336. doi: 10.1182/blood-2009-06-204669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Blazar BR, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 87.Asakura S, et al. Alloantigen expression on non-hematopoietic cells reduces graft-versus-leukemia effects in mice. J Clin Invest. 2010;120:2370–2378. doi: 10.1172/JCI39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Flutter B, et al. Nonhematopoietic antigen blocks memory programming of alloreactive CD8+ T cells and drives their eventual exhaustion in mouse models of bone marrow transplantation. J Clin Invest. 2010;120:3855–3868. doi: 10.1172/JCI41446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koestner W, et al. PD-L1 blockade effectively restores strong graft-versus-leukemia effects without graft-versus-host disease after delayed adoptive transfer of T-cell receptor gene-engineered allogeneic CD8+ T cells. Blood. 2011;117:1030–1041. doi: 10.1182/blood-2010-04-283119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Sharpe AH, Vallera DA. Opposing roles of CD28:B7 and CTLA-4:B7 pathways in regulating in vivo alloresponses in murine recipients of MHC disparate T cells. J Immunol. 1999;162:6368–6377. [PubMed] [Google Scholar]
- 91.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 2010;47:65–77. doi: 10.1007/s12026-009-8139-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valenzuela JO, et al. PKCtheta is required for alloreactivity and GVHD but not for immune responses toward leukemia and infection in mice. J Clin Invest. 2009;119:3774–3786. doi: 10.1172/JCI39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zanin-Zhorov A, et al. Protein kinase C-theta mediates negative feedback on regulatory T cell function. Science. 2010;328:372–376. doi: 10.1126/science.1186068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zanin-Zhorov A, Dustin ML, Blazar BR. PKC-theta function at the immunological synapse: prospects for therapeutic targeting. Trends Immunol. 2011;32:358–363. doi: 10.1016/j.it.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 96.Cetkovic-Cvrlje M, et al. Treatment of post-bone marrow transplant acute graft-versus-host disease with a rationally designed JAK3 inhibitor. Leuk Lymphoma. 2002;43:1447–1453. doi: 10.1080/1042819022386581. [DOI] [PubMed] [Google Scholar]
- 97.Cetkovic-Cvrlje M, Roers BA, Waurzyniak B, Liu XP, Uckun FM. Targeting Janus kinase 3 to attenuate the severity of acute graft-versus-host disease across the major histocompatibility barrier in mice. Blood. 2001;98:1607–1613. doi: 10.1182/blood.v98.5.1607. [DOI] [PubMed] [Google Scholar]
- 98.Betts BC, et al. Janus kinase-2 inhibition induces durable tolerance to alloantigen by human dendritic cell-stimulated T cells yet preserves immunity to recall antigen. Blood. 2011;118:5330–5339. doi: 10.1182/blood-2011-06-363408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Olivieri A, et al. Imatinib for refractory chronic graft-versus-host disease with fibrotic features. Blood. 2009;114:709–718. doi: 10.1182/blood-2009-02-204156. [DOI] [PubMed] [Google Scholar]
- 100.Stadler M, et al. Limited efficacy of imatinib in severe pulmonary chronic graft-versus-host disease. Blood. 2009;114:3718–3719. doi: 10.1182/blood-2009-07-231159. author reply 3719–3720. [DOI] [PubMed] [Google Scholar]
- 101.Sun K, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci U S A. 2004;101:8120–8125. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yannaki E, et al. The proteasome inhibitor bortezomib drastically affects inflammation and bone disease in adjuvant-induced arthritis in rats. Arthritis Rheum. 2010;62:3277–3288. doi: 10.1002/art.27690. [DOI] [PubMed] [Google Scholar]
- 103.Koreth J, et al. Bortezomib, tacrolimus, and methotrexate for prophylaxis of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation from HLA-mismatched unrelated donors. Blood. 2009;114:3956–3959. doi: 10.1182/blood-2009-07-231092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sun K, et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005;106:3293–3299. doi: 10.1182/blood-2004-11-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wysocki CA, et al. Critical role for CCR5 in the function of donor CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood. 2005;106:3300–3307. doi: 10.1182/blood-2005-04-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou L, Askew D, Wu C, Gilliam AC. Cutaneous gene expression by DNA microarray in murine sclerodermatous graft-versus-host disease, a model for human scleroderma. J Invest Dermatol. 2007;127:281–292. doi: 10.1038/sj.jid.5700517. [DOI] [PubMed] [Google Scholar]
- 107.Lu SX, et al. Absence of P-selectin in recipients of allogeneic bone marrow transplantation ameliorates experimental graft-versus-host disease. J Immunol. 2010;185:1912–1919. doi: 10.4049/jimmunol.0903148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Terwey TH, et al. CCR2 is required for CD8-induced graft-versus-host disease. Blood. 2005;106:3322–3330. doi: 10.1182/blood-2005-05-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He S, et al. A new approach to the blocking of alloreactive T cell-mediated graft-versus-host disease by in vivo administration of anti-CXCR3 neutralizing antibody. J Immunol. 2008;181:7581–7592. doi: 10.4049/jimmunol.181.11.7581. [DOI] [PubMed] [Google Scholar]
- 110.Bouazzaoui A, et al. Steroid treatment alters adhesion molecule and chemokine expression in experimental acute graft-vs.-host disease of the intestinal tract. Exp Hematol. 2011;39:238–249. e231. doi: 10.1016/j.exphem.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 111.Inamoto Y, et al. Donor single nucleotide polymorphism in the CCR9 gene affects the incidence of skin GVHD. Bone Marrow Transplant. 2010;45:363–369. doi: 10.1038/bmt.2009.131. [DOI] [PubMed] [Google Scholar]
- 112.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Waldman E, et al. Absence of beta7 integrin results in less graft-versus-host disease because of decreased homing of alloreactive T cells to intestine. Blood. 2006;107:1703–1711. doi: 10.1182/blood-2005-08-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hashimoto D, et al. FTY720 enhances the activation-induced apoptosis of donor T cells and modulates graft-versus-host disease. Eur J Immunol. 2007;37:271–281. doi: 10.1002/eji.200636123. [DOI] [PubMed] [Google Scholar]
- 115.Kim YM, Sachs T, Asavaroengchai W, Bronson R, Sykes M. Graft-versus-host disease can be separated from graft-versus-lymphoma effects by control of lymphocyte trafficking with FTY720. J Clin Invest. 2003;111:659–669. doi: 10.1172/JCI16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Taylor PA, et al. Insights into the mechanism of FTY720 and compatibility with regulatory T cells for the inhibition of graft-versus-host disease (GVHD) Blood. 2007;110:3480–3488. doi: 10.1182/blood-2007-05-087940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Janssens W, et al. CD4+CD25+ T cells lyse antigen-presenting B cells by Fas-Fas ligand interaction in an epitope-specific manner. J Immunol. 2003;171:4604–4612. doi: 10.4049/jimmunol.171.9.4604. [DOI] [PubMed] [Google Scholar]
- 118.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 119.Serra P, et al. CD40 ligation releases immature dendritic cells from the control of regulatory CD4+CD25+ T cells. Immunity. 2003;19:877–889. doi: 10.1016/s1074-7613(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 120.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849–859. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cohen JL, Trenado A, Vasey D, Klatzmann D, Salomon BL. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med. 2002;196:401–406. doi: 10.1084/jem.20020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 124.Gaidot A, et al. Immune reconstitution is preserved in hematopoietic stem cell transplantation coadministered with regulatory T cells for GVHD prevention. Blood. 2011;117:2975–2983. doi: 10.1182/blood-2010-08-299974. [DOI] [PubMed] [Google Scholar]
- 125.Trenado A, et al. Recipient-type specific CD4+CD25+ regulatory T cells favor immune reconstitution and control graft-versus-host disease while maintaining graft-versus-leukemia. J Clin Invest. 2003;112:1688–1696. doi: 10.1172/JCI17702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Edinger M, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 127.Brunstein CG, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hippen KL, et al. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med. 2011;3:83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shin HJ, et al. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood. 2011;118:2342–2350. doi: 10.1182/blood-2010-10-313684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hippen KL, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant. 2011;11:1148–1157. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM. Dendritic cells induce antigen-specific regulatory T cells that prevent graft versus host disease and persist in mice. J Exp Med. 2011;208:2489–2496. doi: 10.1084/jem.20110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Semple K, Yu Y, Wang D, Anasetti C, Yu XZ. Efficient and selective prevention of GVHD by antigen-specific induced Tregs via linked-suppression in mice. Biol Blood Marrow Transplant. 2011;17:309–318. doi: 10.1016/j.bbmt.2010.12.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sato K, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367–379. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 134.Waller EK, et al. Larger numbers of CD4(bright) dendritic cells in donor bone marrow are associated with increased relapse after allogeneic bone marrow transplantation. Blood. 2001;97:2948–2956. doi: 10.1182/blood.v97.10.2948. [DOI] [PubMed] [Google Scholar]
- 135.Asai O, et al. Suppression of graft-versus-host disease and amplification of graft-versus-tumor effects by activated natural killer cells after allogeneic bone marrow transplantation. J Clin Invest. 1998;101:1835–1842. doi: 10.1172/JCI1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Olson JA, et al. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293–4301. doi: 10.1182/blood-2009-05-222190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tanaka M, et al. The impact of the dose of natural killer cells in the graft on severe acute graft-versus-host disease after unrelated bone marrow transplantation. Leuk Res. 2011 doi: 10.1016/j.leukres.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 138.Ruggeri L, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 139.Miller JS, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kohrt HE, et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood. 2009;114:1099–1109. doi: 10.1182/blood-2009-03-211441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kuns RD, et al. Invariant natural killer T cell-natural killer cell interactions dictate transplantation outcome after alpha-galactosylceramide administration. Blood. 2009;113:5999–6010. doi: 10.1182/blood-2008-10-183335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–2284. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peranzoni E, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22:238–244. doi: 10.1016/j.coi.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 144.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Highfill SL, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by interleukin-13. Blood. 2010;116:5738–5747. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhou Z, et al. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells. 2010;28:620–632. doi: 10.1002/stem.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 148.Polchert D, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–1755. doi: 10.1002/eji.200738129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sudres M, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761–7767. doi: 10.4049/jimmunol.176.12.7761. [DOI] [PubMed] [Google Scholar]
- 150.Tolar J, Le Blanc K, Keating A, Blazar BR. Concise review: hitting the right spot with mesenchymal stromal cells. Stem Cells. 2010;28:1446–1455. doi: 10.1002/stem.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kebriaei P, Robinson S. Treatment of graft-versus-host-disease with mesenchymal stromal cells. Cytotherapy. 2011;13:262–268. doi: 10.3109/14653249.2010.549688. [DOI] [PubMed] [Google Scholar]
- 152.Fallarino F, Grohmann U. Using an ancient tool for igniting and propagating immune tolerance: IDO as an inducer and amplifier of regulatory T cell functions. Curr Med Chem. 2011;18:2215–2221. doi: 10.2174/092986711795656027. [DOI] [PubMed] [Google Scholar]
- 153.Jasperson LK, et al. Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood. 2008;111:3257–3265. doi: 10.1182/blood-2007-06-096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ratajczak P, et al. IDO in human gut graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18:150–155. doi: 10.1016/j.bbmt.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Furuzawa-Carballeda J, et al. High levels of IDO-expressing CD16+ peripheral cells, and Tregs in graft biopsies from kidney transplant recipients under belatacept treatment. Transplant Proc. 2010;42:3489–3496. doi: 10.1016/j.transproceed.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 156.Jasperson LK, et al. Inducing the tryptophan catabolic pathway, indoleamine 2,3-dioxygenase (IDO), for suppression of graft-versus-host disease (GVHD) lethality. Blood. 2009;114:5062–5070. doi: 10.1182/blood-2009-06-227587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang Y, et al. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood. 2011;117:299–308. doi: 10.1182/blood-2010-03-271940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Choi J, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116:129–139. doi: 10.1182/blood-2009-12-257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Goodyear OC, et al. Azacitidine augments expansion of regulatory T cells after allogeneic stem cell transplantation in patients with acute myeloid leukemia. Blood. 2012 doi: 10.1182/blood-2011-09-377044. [DOI] [PubMed] [Google Scholar]
- 160.Bastien JP, et al. Photodepletion differentially affects CD4+ Tregs versus CD4+ effector T cells from patients with chronic graft-versus-host disease. Blood. 2010;116:4859–4869. doi: 10.1182/blood-2010-03-273193. [DOI] [PubMed] [Google Scholar]
- 161.Gatza E, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.He S, et al. Inhibition of histone methylation arrests ongoing graft-versus-host disease in mice by selectively inducing apoptosis of alloreactive effector T cells. Blood. 2012;119:1274–1282. doi: 10.1182/blood-2011-06-364422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Choi S, Reddy P. HDAC inhibition and graft versus host disease. Mol Med. 2011;17:404–416. doi: 10.2119/molmed.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reddy P, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci U S A. 2004;101:3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lu SX, et al. STAT-3 and ERK 1/2 phosphorylation are critical for T-cell alloactivation and graft-versus-host disease. Blood. 2008;112:5254–5258. doi: 10.1182/blood-2008-03-147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Radojcic V, et al. STAT3 signaling in CD4+ T cells is critical for the pathogenesis of chronic sclerodermatous graft-versus-host disease in a murine model. J Immunol. 2010;184:764–774. doi: 10.4049/jimmunol.0903006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ma H, et al. Absence of Stat1 in donor CD4 T cells promotes the expansion of Tregs and reduces graft-versus-host disease in mice. J Clin Invest. 2011;121:2554–2569. doi: 10.1172/JCI43706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Neufert C, et al. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 169.Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 170.Rotta M, et al. Impact of recipient statin treatment on graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1463–1466. doi: 10.1016/j.bbmt.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Penack O, Socie G, van den Brink MR. The importance of neovascularization and its inhibition for allogeneic hematopoietic stem cell transplantation. Blood. 2011;117:4181–4189. doi: 10.1182/blood-2010-10-312934. [DOI] [PubMed] [Google Scholar]
- 172.Weiden PL, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 173.Apperley JF, et al. Bone marrow transplantation for chronic myeloid leukaemia in first chronic phase: importance of a graft-versus-leukaemia effect. Br J Haematol. 1988;69:239–245. doi: 10.1111/j.1365-2141.1988.tb07628.x. [DOI] [PubMed] [Google Scholar]