Abstract
Adoptive transfer of thymus-derived natural regulatory T-cells (nTregs) effectively suppresses disease in murine models of autoimmunity and graft-versus-host disease (GVHD). TGFβ induces Foxp3 expression and suppressive function in stimulated murine CD4+25- T cells, and these induced Treg (iTregs), like nTreg, suppress auto- and allo-reactivity in vivo. However, while TGFβ induces Foxp3 expression in stimulated human T-cells, the expanded cells lack suppressor cell function. Here we show that Rapamycin (Rapa) enhances TGFβ-dependent Foxp3 expression and induces a potent suppressor function in naïve (CD4+25-45RA+) T cells. Rapa/TGFβ iTregs are anergic, express CD25 at levels higher than expanded nTregs, and few cells secrete IL-2, IFNγ or IL-17 even after PMA and Ionomycin stimulation in vitro. Unlike other published methods of inducing Treg function, Rapa/TGFβ induces suppressive function even in the presence of memory CD4+ T-cells. A single apheresis unit of blood yields an average ~240×109 (range ~70–560×109) iTregs from CD4+25- T-cells in ≤ 2 weeks of culture. Most importantly, Rapa/TGFβ iTregs suppress disease in a xenogeneic model of GVHD. This study opens the door for iTreg cellular therapy for human diseases.
Keywords: GVHD, Treg, Foxp3, Rapamycin, TGFβ
Introduction
Adoptive transfer of natural regulatory T-cells (nTregs) that arise in the thymus or Tregulatory type I cells that arise in the periphery can ameliorate graft-versus-host disease (GVHD) and autoimmunity in mice(1–3). However, high nTreg doses (~1:1 with donor T-cells) are required to reproducibly suppress disease(4–6). Clinical application has been slowed greatly by the low frequency and proliferative potential of nTregs purified from peripheral blood (PB) or umbilical cord blood (UCB). Although 10-fold more nTregs can be isolated from PB than UCB, this source frequently contains CD25+ T-effector (Teff) or memory T-cells (Tmem) that preferentially expand over the anergic nTregs during culture, resulting in a product that has lost suppressor activity. We recently concluded a first-in-human phase I clinical trial assessing the safety and efficacy of ex vivo expanded UCB nTregs to suppress GVHD following UCB transplantation(7). While nTregs were well tolerated and median yield was 1.2×109 cells, further dose escalation studies were not possible due to inconsistently achieving target cell yields. While others have shown re-stimulation increases nTreg expansion to >1,000-fold, these studies required highly sort-purified cells(8, 9), which results in low initial and final yields not sufficiently robust to uniformly expand peripheral blood nTregs. Thus, other approaches are needed to achieve the ≥ 1:1 nTreg:PBMNC ratios that have been shown to be optimal in preventing GVHD in rodent to rodent and human to rodent PBMNC allografts.
In the periphery, a population of non-Treg CD4+ T-cells can be induced to acquire a Treg phenotype and function. Cellular therapy using induced Tregs (iTregs) has several potential advantages over UCB nTregs, including: ease of isolation, increased number of the starting population, greater proliferation potential, reduced production costs, and for treating autoimmunity, an autologous donor. Stimulation of murine CD4+ T-cells with anti-CD3/28 in the presence of TGFβ or all-trans-retinoic acid (ATRA) induces Foxp3 expression and potent suppressive function(10, 11). TGFβ or ATRA also induce Foxp3 expression in stimulated naïve human T-cells (CD4+25-45RA+), but while one study showed these cells to be suppressive(12), other studies observed modest or no suppression(13–15). Thus, additional strategies are needed to uniformly ensure potent suppressor cell function. Whereas the effects of these agents are synergistic, and naïve human T-cells incubated with TGFβ plus ATRA gain in vitro suppressive function(16), TGFβ/ATRA failed to induce Foxp3 expression and suppressive function in memory T-cells (Tmem:CD4+25-45RA-), which typically comprise ~50% of the CD4+ T-cell population. Moreover, Tmem cells co-cultured with naïve CD4+25-45RA+ T-cells inhibited FoxP3 induction through an IL-4 dependent mechanism(16).
Because recent experiments show Rapamycin (Rapa) enhances TGFβ-mediated Foxp3 expression and in vivo suppressive function in murine CD4 T-cells(17), we sought to determine whether Rapa could synergize with TGFβ and promote stable Foxp3 expression and suppressive function in human non-Treg (CD4+25-) cells. Here we show that activation of human naïve CD4+25-45RA+ T-cells in the presence of Rapa and TGFβ induces stable Foxp3 expression and a potent suppressive phenotype. In contrast to TGFβ/ATRA, Foxp3 expression and suppressive function could also be induced in cultures containing both naïve and memory CD4+ T-cells. Impressively, ~240×109 Rapa/TGFβ iTregs were produced from CD4+25- T-cells in a standard apheresis product after ≤2 weeks of culture. Importantly, Rapa/TGFβ iTreg were functional in vivo, and ameliorated disease in a xenogeneic GVHD model. This is the first demonstration that human iTregs are functionally suppressive in vivo. Since current good manufacturing practice (cGMP) reagents are available for all components of the isolation and expansion procedure, our data pave the way for using the clinical manufacture of iTreg as a cellular therapy to prevent GVHD, graft rejection and autoimmunity.
Materials and Methods
Cell purification and culture
CD4+ T-cells were enriched from non-mobilized PB apheresis products (Memorial Blood Center, St. Paul, MN) using MACS technology (Miltenyi Biotec, Auburn, CA) by depleting non-CD4 T-cells with cGMP-grade monoclonal antibody (mAb)-coated microbeads (cocktail of CD8, CD14, CD19) on an AutoMACS (Depletion 2.1). Unbound cells were washed and CD25++ nTregs were purified by positive selection using cGMP-grade anti-CD25 microbeads. Remaining CD25+ cells were depleted using an additional anti-CD25 step. Naïve or memory T-cells were isolated from CD8-/CD14-/CD19-/CD25- cells using anti-CD45RA microbeads. Bead incubations and washes were carried out as specified by the manufacturer (30’ at room temperature for cGMP-grade beads).
Purified cells were stimulated with a K562 cell line (KT) engineered to express CD86 and the high affinity Fc Receptor (CD64) (KT64/86) (2:1 Treg:KT)(18), irradiated with 10,000 cGray and incubated with anti-CD3 mAb (Orthoclone OKT3, Janssen-Cilag). Cells were cultured in X-Vivo-15 media (BioWhittaker) supplemented with 10% human AB serum (Valley Biomedical), GlutaMAX (Gibco) and N-acetylcysteine (USP). Recombinant IL-2 (300 IU/ml, Novartis) was added (day 2 for nTregs and day 0 for all others) and maintained throughout the culture. Where indicated, Rapa (Rapammune, Wyeth-Ayerst) at 109 nM was added on day 0 or 7, and with subsequent media supplementation. Where indicated, decitabine (5 µM, Berry&Associates, Inc., Ann Arbor, MI) was added to iTreg cultures for 12 hours on day 7. Cultures were maintained at 0.3 – 0.5×106 viable nucleated cells/ml every 2–3 days. Complete details for cell freezing and thawing, flow cytometry and intracellular cytokine staining can be found in Supporting Information.
Suppression assays
In vitro suppressive capacity of expanded cultures was assessed with a 5-carboxyfluorescein diacetate succinimide ester (CFSE) inhibition assay(19). PB mononuclear cells (PBMNC) were purified, labeled with CFSE (InVitrogen), and stimulated with anti-CD3 mAb-coated beads (Dynal) ± cultured nTreg or iTreg (Treg:PBMNCs at ratios of 1:2–1:32), without adjustment for Foxp3 content in the product. On day 4, cells were stained with anti-CD8 mAb. Acquired data were analyzed using the proliferation platform in FlowJo, and suppression determined from the Division Index (Treestar, Ashland, OR). No attempt was made to HLA match PBMNC with Treg product.
In vivo xenogeneic GVHD model
T-, B- and NK-deficient Rag−/−/γc−/− or NOD/Scid/γc−/− mice (Jackson) were housed in a pathogen-free facility in micro-isolator cages and on day 0 human PBMNCs (30×106) were injected with or without expanded Tregs (30×106). Rag−/−/γc−/− mice also received clodronate containing liposomes (100µl/10g body weight) on day -1 and 400 cGy (137 cesium source) on day 0(16). Mice were assessed for clinical GVHD daily and weighed thrice weekly. To document PBMNC-associated T-cell expansion, animals were bled (10–40µl), and red blood cells lysed. Treg populations and PBMNC were enumerated by flow cytometry by staining with mAbs to human CD4, CD8, CD45 and acquired with a known number of counting beads (Sigma). Animal protocols were approved by IACUC at the University of Minnesota.
Statistical analysis
Data were analyzed by ANOVA or student’s t-test. Probability (P) values ≤0.05 were considered statistically significant.
Results
Rapa enhances TGFβ-mediated Foxp3 expression in stimulated non-Treg CD4+ T-cells and induces in vitro suppressive function
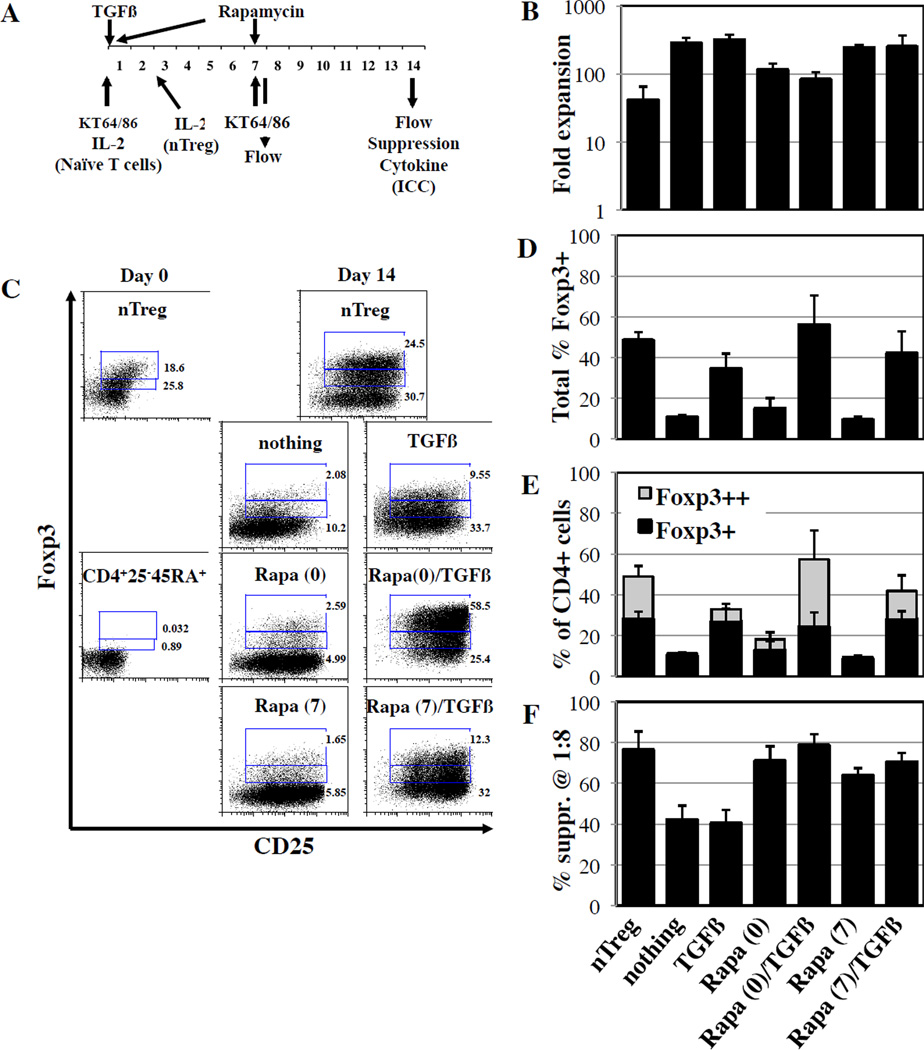
To determine whether Rapa could synergize with TGFβ to generate human iTregs in vitro, CD4+25-45RA+ T-cells were purified from PB using magnetic beads. For comparison, nTregs (CD4+25++) were MACS purified from the same samples (Figure S1A). Purified nTregs and naïve T-cells were stimulated with KT64/86 cells, a K562 cell-based artificial APC (aAPC) expressing CD86 and the high affinity Fc receptor, loaded with anti-CD3 mAb, and cultured with IL-2 (300U/ml). nTreg cultures were performed in the presence of Rapa, whereas naïve T-cells were re-stimulated on day 7 with aAPCs and cultured throughout the duration with nothing, Rapa, TGFβ, or Rapa/TGFβ (Figure 1A). CD4+25-45RA+ cells stimulated with nothing or TGFβ expanded 280±61- and 313±60-fold, respectively, by day 14 (Figure 1B). Rapa, known to inhibit T-cell and Treg proliferation, significantly decreased expansion when added to the culture either alone or with TGFβ (2.4- and 3.4-fold, respectively; p<0.05 for each). Nonetheless, overall CD4+25-45RA+ T-cell expansion with Rapa alone or with TGFβ (115±27 vs. 82±22-fold, p<0.05) was significantly higher than for nTregs (41±24-fold) and, combined with increased numbers after purification, indicates the yield of CD4+25-45RA+ iTreg is 2–5 fold higher than for nTreg.
Figure 1. Rapa enhances TGFβ induced Foxp3 expression and confers in vitro suppressive function.
nTreg (CD4+25++) and naïve T-cells (CD4+25-45RA-) were purified from peripheral blood using magnetic beads. A) Purified cells were stimulated on day 0 with a cell line expressing CD86 and CD64 (KT64/86) to which anti-CD3 mAb is bound. High dose IL-2 (300IU/ml) was added on day 0 for naïve T-cells and day 2 for nTreg. TGFβ (10ng/ml) was added to naïve T-cells on day 0, while Rapa (109nM) was added on either day 0 or day 7. Naïve T-cells were also re-stimulated on day 7. Cultures were phenotyped by flow cytometry on day 7 and 14, while suppressive function and cytokine secretion was tested on day 14. B) Fold expansion of total cell number over the 14 day assay. C) Representative example showing differential Foxp3 expression in nTreg and naïve T-cells cultured ±TGFβ and ±Rapa. D) Total % of Foxp3 expressing cells in the various cultures. E) Average % of CD4+ cells that are Foxp3+ and Foxp3++ in nTreg and naïve T-cells cultures expanded ±TGFβ and ±Rapamycin. F) Suppressive function of cultured cells was determined using a CFSE-based proliferation assay in which expanded nTreg or naïve T-cells were incubated with CFSE-loaded allogeneic PBMC at doses from 1:2 to 1:32 (expanded cell:PBMC) and stimulated with anti-CD3 beads for 4 days. Data shown are from the 1:8 ratio. n=3–5 experiments.
Compared to other CD4+ T-cells, nTregs are known to be less susceptible to Rapa mediated mTOR inhibition, since Foxp3 induces the expression of pim2, a kinase that can substitute for mTOR and override the Rapa mTOR inhibitory effect(20, 21). Because TGFβ induces Foxp3 expression in CD4+25-45RA+ cells, we sought to determine whether delaying Rapa would increase iTreg expansion by decreasing the duration of naïve CD4+ T-cell exposure to Rapa. Cultures receiving Rapa on day 7 without TGFβ had increased expansion as compared to day 0 Rapa (Figure 1B). Adding Rapa on day 7 to naïve T-cells cultured with TGFβ increased expansion 3-fold as compared to adding Rapa and TGFβ on day 0 (p<0.05).
Expanded cells were stained for expression of CD4, CD25 and Foxp3. nTreg cultures maintained similar CD4 purity (>85% for both initial and final, with a range of 82–96% and 96–98%, respectively) and Foxp3 purity (41±5% of CD4+ cells were initially CD25+Foxp3+ compared to 48±4% final) (Figure 1C and D). After purification, CD4+25-45RA+ cells were >95% CD4+ and <1% CD25+Foxp3+. TCR stimulation alone, as previously reported(15), induces Foxp3 expression in a limited number of CD4+25- T-cells (11±1% CD25+Foxp3+), possibly in conjunction with endogenous TGFβ in the human serum containing culture media (Figure 1D). While Rapa alone did not significantly increase the frequency of CD25+Foxp3+ cells, expression was increased with TGFβ and further increased with Rapa plus TGFβ (34±8% and 56±15%, respectively; p = ≤0.01 vs. no addition and p≤0.05 vs. each other). As reported for human naïve CD4+ T-cells (CD4+25-45RA+) cultured TGFβ plus ATRA(16), two distinct levels of Foxp3 expression were apparent in TGFβ vs. Rapa/TGFβ cultures (Figure 1C). By gating on the major population of Foxp3++ cells in the Rapa/TGFβ cultures, we found that TGFβ treatment mainly drives low Foxp3 expression, whereas Rapa/TGFβ drives high FoxP3 expression (31±12% vs. 5±2% Foxp3++, p≤0.03; Figure 1E).
In vitro suppressive function was assessed using a CFSE-based proliferation assay(19). nTregs potently suppressed T-cell division 76±9% at an expanded cell:PBMNC ratio of 1:8 (Figure 1F). CD4+25-45RA+ T-cells expanded in the absence or presence of TGFβ alone had a lower degree of suppression (42±7% and 40±7%, respectively; p≤0.03 vs. nTregs for each), even though TGFβ cultures expressed Foxp3 at levels similar to nTregs. CD4+25-45RA+ T-cells expanded in Rapa alone had increased suppression (day 0 or 7 addition: 71±7% and 64±4%, respectively, p≤0.03 vs. no addition for each) and increased further in cultures receiving Rapa plus TGFβ on day 0 (79±6%, p≤0.05 vs. nTreg).
Naïve CD4+ T-cells treated with Rapa/TGFβ are anergic, express high levels of CD25, but not Latency Associated Peptide (LAP), and contain very few IL-2, IFNγ or IL-17 secreting cells
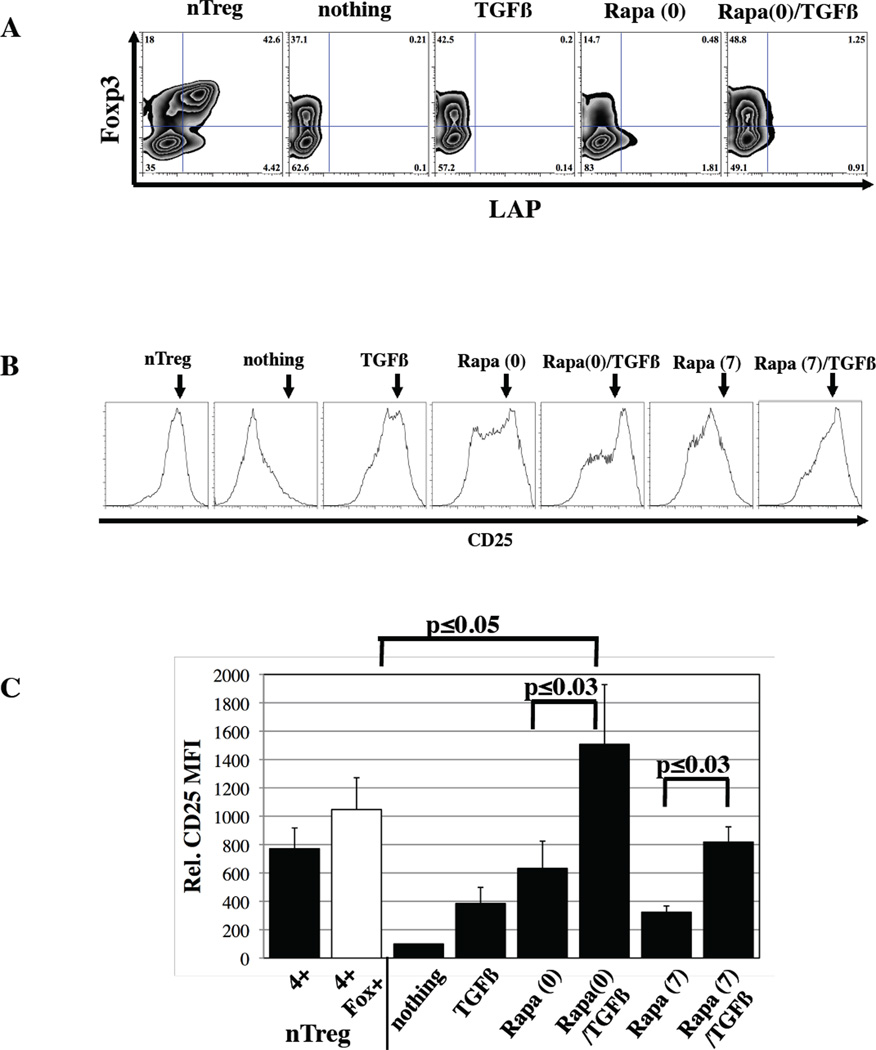
nTregs can be distinguished from Foxp3+ cells generated by stimulating CD4+25- T-cells in the presence of TGFβ by the expression of LAP, a precursor of TGFβ(9). Even though Rapa/TGFβ iTregs had high Foxp3 expression and were suppressive, LAP was not expressed, strengthening the argument that LAP is a marker of nTregs and not iTregs (Figure 2A).
Figure 2. Naïve (CD4+25-45RA+) cultures treated with Rapa plus TGFβ do not express LAP, but express high levels of CD25.
In vitro expanded nTreg and naïve T-cells treated with TGFβ and/or Rapa were stained with antibodies to LAP and CD25 to determine potential mechanisms used for suppression. A) Representative example of LAP expression in the various cultures. Representative example (B) and summary (C) of relative CD25 expression (naïve T-cells expanded without TGFβ or Rapa was set at 100). Staining for CD25 was performed on day 14 whereas staining for LAP, which is only transiently expressed, was performed on day 7. Data are representative of at least five independent experiments.
As demonstrated in Figure 2B, a wide range of CD25 expression was observed on expanded cells. CD4+25-45RA+ T-cells receiving only TCR stimulation and CD86 co-stimulation expressed low levels of CD25 (relative expression arbitrarily set at 100), which was increased 4±1 fold with TGFβ (p<0.02), and 6±2 or 3±0.4-fold with Rapa addition on day 0 or 7, respectively (Figure 2C, p<0.01 for each). As with Foxp3 expression, the effects of Rapa and TGFβ were synergistic, and increased CD25 expression another 2.5-fold over Rapa alone, whether added on day 0 or 7 (p≤0.03 for each). CD25 expression in naive T-cells expanded in Rapa and TGFβ from day 0 was even higher than in nTreg cultures (1507±422 vs. 771±146, respectively, p≤0.05). High expression of CD25 on Rapa/TGFβ iTreg could mediate suppression via IL-2 consumption or, alternatively, enhance survival; which we have shown increases suppressive function(19).
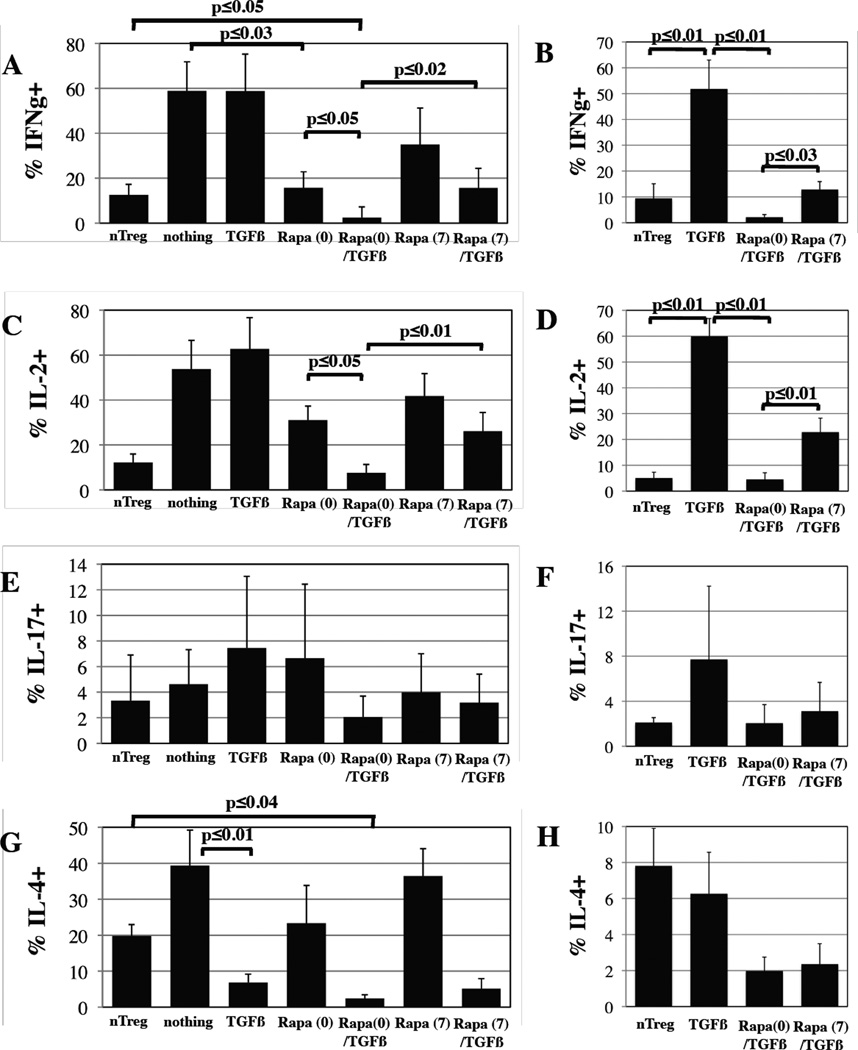
Repetitive stimulation causes T-cells to differentiate into Teffs capable of secreting cytokines, which could exacerbate GVHD. Some reports also indicate that iTregs are not stable and can convert to Teffs(23, 24). Therefore, we re-stimulated the various cultures with PMA and Ionomycin and performed intracellular cytokine staining. All conditions were co-stained for Foxp3 to differentiate secretion by Tregs vs. non-Treg cells. Only ~10% of total cells in PB nTreg cultures secrete IFNγ or IL-2, compared to >50% for control CD4+25-45RA+ cells expanded without treatment or with TGFβ alone (Figure 3A and C). Similarly, <10% of Foxp3+ cells in PB nTreg cultures secreted IFNγ or IL-2, while >50% of Foxp3+ cells in TGFβ cultures were secretors (Figure 3B and D). Adding Rapa on day 0 reduced the % of total cells secreting IFNγ or IL-2 to 16±6 and 31±6, respectively (p<0.03 and 0.07, respectively). Combining Rapa and TGFβ further reduced the % of IFNγ or IL-2 secreting cells to 2.5±1.2% and 7.6±3.7%, respectively (p<0.05 for each), resulting in even fewer IFNγ secreting cells than nTreg cultures (3±1% vs. 13±5%, respectively, p<0.05). While adding Rapa on day 7 to TGFβ cultures significantly reduced contamination with IFNg or IL-2 secreting cells, is was significantly less effective than day 0 addition (p<0.02 and 0.01 for IFNγ and IL-2, respectively). The % of cells secreting IL-17 (total or Foxp3+) in all cultures was very low (<8%), and no significant differences were observed (Figure 3E and F). nTreg cultures displayed a Th2 phenotype and contained 20% IL-4 secreting cells, although less than 8% of Foxp3+ cells secreted this cytokine (Figure 3G and H). Unlike it’s effects on IFNγ and IL-2 and consistent with studies showing Rapa permits Th2 cell expansion(25), Rapa only weakly inhibited IL-4 secretion in stimulated CD4+25-45RA+ T-cells, and the suppressive nature of IL-4 may contribute to the partial suppressive phenotype observed for CD4+25-45RA+ T-cells expanded in Rapa only (Figure 1F). Conversely, TGFβ, whether added alone or in combination with Rapa on day 0 or 7, significantly reduced the % of cells (total or Foxp3+) secreting IL-4 (p≤0.01 for each). Similar to what was observed for IFNγ, Rapa (0)/TGFβ cultures contained significantly fewer IL-4 secreting cells (total % or gated on Foxp3+) than nTreg cultures (p<0.04 for each). The overall % of cells secreting IL-10 in all cultures was <2%, and no significant differences were observed (data not shown). Taken together, these results show that even though nTreg and Rapa/TGFβ iTreg cultures contain Foxp3- cells, few are capable of secreting inflammatory cytokines (IFNγ, IL-2, -17) that may intensify disease.
Figure 3. CD4+25-45RA+ cells expanded in the presence of Rapa and TGFβ were hypoproliferative and contain very few IL-2, IFNγ or IL-17 secreting cells.
nTreg and naïve T-cells expanded ±TGFβ and ±Rapa for 14 days were re-stimulated for 4 hours with PMA and Ionomycin in the presence of Brefeldin followed by intracellular staining for Foxp3 and either IFNγ (A, B), IL-2 (C, D), IL-17 (E, F) or IL-4 (G, H) to quantitate the percentage of effector cells present and determine their phenotype (i.e. Th1 or Th2). Data presented are either the % total cytokine+ cells (A, C, E, G) or % of Foxp3+ cells that are cytokine+ (B, D, F, H). Data represent the average ± SEM for 3–5 independent experiments.
Rapa/TGFβ induces equivalent Foxp3 expression and suppressive function in unfractionated (CD4+25-) and naive (CD4+25-45RA+) T-cells
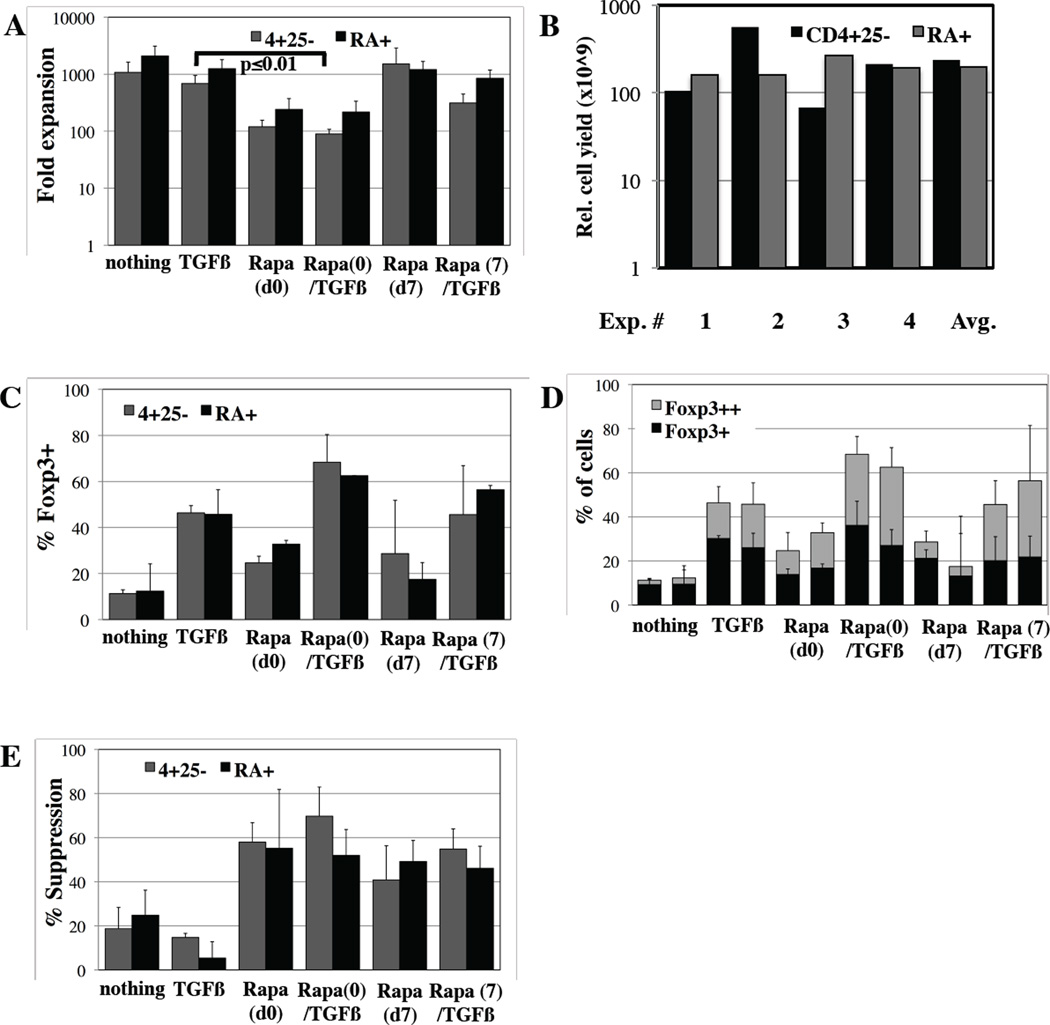
Our initial studies focused on naïve cells because previous studies with TGFβ alone or in combination with either ATRA or an aryl hydrocarbon receptor agonist found Foxp3 expression could be not be induced on Tmem, and Tmem also inhibited Foxp3 induction in naïve T-cells(10, 16, 26). Because a cGMP anti-CD45RA reagent is not currently available to the broad community for isolation of naïve T-cells and only ~50% CD4+25- cells in PB are CD45RA+, we investigated whether Rapa/TGFβ treatment could induce Foxp3 expression and suppressive function on unfractionated CD4+25- T-cells. Unfractionated (CD4+25-) or CD4+25-45RA+ T-cells were purified and stimulated in the presence of nothing, TGFβ, Rapa or Rapa/TGFβ and cell number determined after 14 days of culture. Expansion of CD4+25- T-cells was ~2-fold lower than naïve T-cells for each condition tested, although this was not significant. Like naïve T-cells, CD4+25- T-cell cultures supplemented with Rapa/TGFβ on day 0 had the lowest overall expansion (>8-fold decrease over controls) (Figure 4A, p<0.01). Based on four independent experiments, 240×109 (range ~70–560×109) Rapa/TGFβ iTregs could be generated from CD4+25- T-cells using a single non-mobilized apheresis product containing ~10×109 total cells (Figure 4B).
Figure 4. Rapa/TGFβ induces equivalent Foxp3 expression and suppressive function in unfractionated (CD4+25-) and naive (CD4+25-45RA+) T-cells.
Total CD4+25- and naïve (CD4+25-45RA+) T-cells were purified from PB using magnetic beads and expanded in vitro ±TGFβ and ±Rapa for 14 days. A) Fold expansion of total cells for cultures started with CD4+25- (gray bar) or naïve (black bar) T-cells expanded ±TGFβ and ±Rapa on day 0 or 7. B) Total calculated cell yield for CD4+25- and CD4+25-45RA+ Rapa(d0)/TGFβ iTreg cultures after 14 days, based on 4 independent experiments starting with an average apheresis unit of 10×109 cells. C) Total % of Foxp3 expressing cells in the various cultures. D) Average % of CD4+ cells that are Foxp3+ and Foxp3++ in unfractionated or naïve T-cell cultures expanded ±TGFβ and ±Rapamycin. E) Suppressive function of cultures assessed by CFSE-based proliferation assay at a 1:8 ratio of expanded cells per CFSE-loaded PBMC. n=3 independent experiments.
Similar to naïve T-cells, CD4+25- T-cells receiving only TCR and co-stimulatory signals were ~15% Foxp3+ (Figure 4C). As before, the effects of Rapa and TGFβ were synergistic, and >60% of expanded CD4+25- T-cells expressed Foxp3 (p<0.03), with >30% being Foxp3++ cells (Figure 4C and D). Adding Rapa to TGFβ cultures on day 7 was less effective in supporting the generation of FoxP3+ cells than day 0 addition. The % of cells expressing Foxp3 and the proportion of Foxp3++ cells in each condition was similar when total CD4+25- T-cells were used. CD4+25- T-cells expanded with TGFβ and Rapa (day 0 or 7) were as potent suppressors as iTreg cultures from naïve T-cells (Figure 4E). Taken together, these data show that CD45RA positive selection is not needed to generate iTregs in the presence of Rapa and TGFβ.
Rapa/TGFβ human iTregs suppress a xenogeneic GVHD lethality in vivo
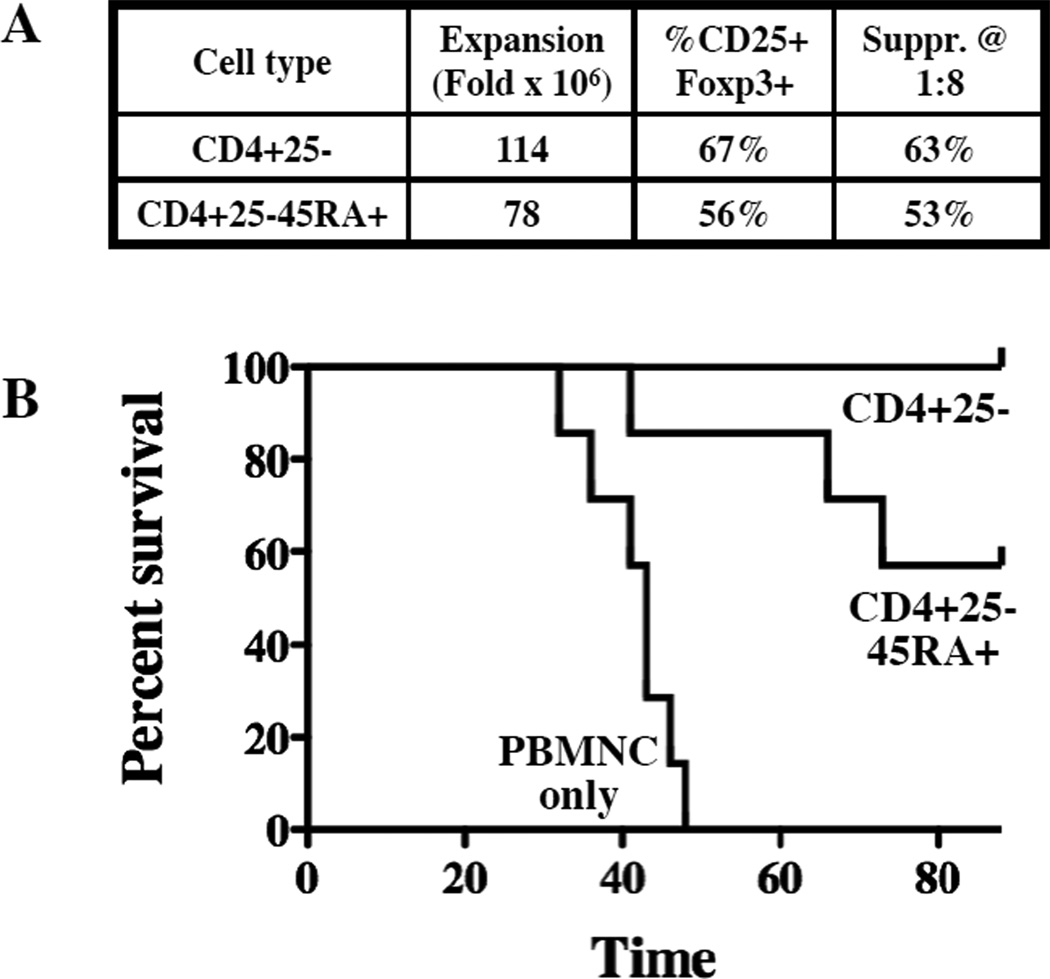
Rapa/TGFβ iTreg display stable Foxp3 expression and suppressive function in vitro. However, recent studies show TGFβ-induced Foxp3 expression is not stable in vivo, and iTreg can de-differentiate and cause disease(24). We have previously used xenogeneic models of human GVHD to show that ex vivo expanded nTregs are safe and ameliorate disease(19), whereas naïve T-cells (CD4+25-45RA+) expanded in the presence or absence of TGFβ appear to exacerbate disease (manuscript submitted). This model was also used to test the safety and efficacy of Treg induced from CD4+25- or CD4+25-45RA+ T-cells with Rapa/TGFβ. As shown in Figure 5A, the CD4+25- and CD4+25-45RA+ iTreg used in these studies were >55% CD25+Foxp3+ and had >50% suppression of T-cell responses even at a 1:8 ratio of iTreg:PBMNC. While recipients of PBMNCs only (30×106) uniformly succumbed to GVHD (median survival of 43 days), both groups of mice treated with iTreg (1:1 iTreg to PBMNC) had significantly prolonged survival, with 7/7 and 4/7 mice treated with CD4+25- or CD4+25-45RA+ iTreg, respectively, surviving long-term (Figure 5B; n = 6–7 per group; p<0.002).
Figure 5. Rapamycin/TGFβ induced Treg are suppressive in vivo.
In vitro expanded CD4+25- or CD4+25-45RA+ Rapa/TGFβ iTreg (30×106 cells) were co-transferred with allogeneic PBMCs (30×106 cells) into NOD/Scid/γc−/− mice to assess the ability to ameliorate xenogeneic GVHD. A) Characteristics of the Rapa/TGFβ iTreg cultures that were used. B) Kaplan-Meier survival curves for mice receiving PBMCs only or PBMCs plus adoptive transfer of Rapa/TGFβ Treg induced from CD4+25- or CD4+25-45RA+ T-cells.
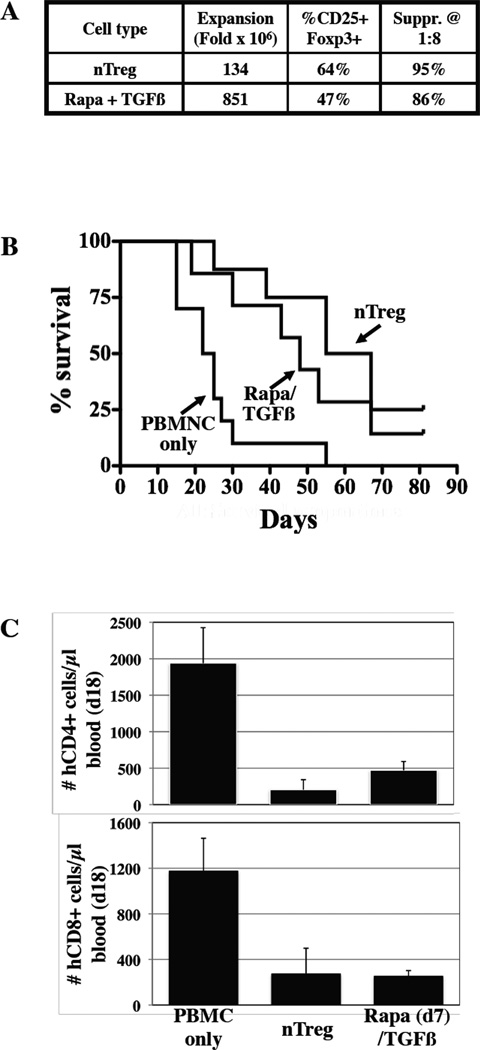
We next sought to compare the in vivo potency of nTreg and Rapa/TGFβ iTreg. To maximize cell recovery relevant to clinical application, Rapa(d7)/TGFβ iTreg were used and, although the frequency of CD4+25+FoxP3+ cells for the expanded nTregs was somewhat higher than for Rapa/TGFβ iTregs (64% vs. 47%, respectively), suppression at a 1:8 ratio of Treg:PBMNC was similar (95% vs. 86%, respectively).
To compare the in vivo safety and potency of nTregs and Rapa/TGFβ iTregs, expanded cells were co-transferred with allogeneic PBMNCs (30×106) into mice at a suboptimal Treg:PBMNC ratio (1:3 vs. more optimal ratio of 1:1) to help stratify potential differences in outcome. nTregs and Rapa/TGFβ iTregs each significantly decreased GVHD-induced mortality, and with similar potency (Figure 5B; p<0.01 and 0.05, respectively vs. PBMNCs alone; p=0.98 for nTregs vs. iTregs). nTregs and Rapa (d7)/TGFβ iTregs were similarly effective at decreasing the number of circulating human T-cells expressing CD4 (p<0.002 and 0.008, respectively) or CD8 (p<0.02 and 0.005, respectively) on day 18 (Figure 5C). Both Treg populations resulted in a similar reduction in human T-cell expansion from PBMNCs on day 18 and comparable long-term (day 82) survival.
Discussion
Here we show that Rapa enhances TGFβ-induced Foxp3 expression in human naïve (CD4+25-45RA+) T-cells and, more importantly, resulted in potent in vitro and in vivo suppressive function. Unlike previous studies, Rapa plus TGFβ also induced Foxp3 expression and suppressive function in CD4+25- cultures which contained naïve and memory T-cells. In as short as 2 weeks of culture, an average of 240×109 or 200×109 Rapa(d0)/TGFβ iTregs could be generated from CD4+25- or CD4+25-45RA+ T-cells, respectively, in a single apheresis unit, which would provide a high ratio of iTregs to Teffs at the time of initial transplant for recipients of UCB, bone marrow or PB hematopoietic stem/progenitor cell grafts. Alternatively, iTreg yield from a buffy coat may be sufficient, which would be favorable over donating an apheresis product 14 days prior to the stem/progenitor cells. The availability of the needed cGMP reagents makes this a practical approach for multiple iTreg infusions for GVHD prevention or therapy as well as opens the possibility for generating a master cell bank for frozen iTregs that could be used as partially HLA class II matched products. Cultured iTreg maintained Foxp3 expression and in vitro suppressive function after cryopreservation and thawing (data not shown).
A concern in the field has been whether nTregs and especially iTregs would be unstable and reprogrammed to become Teffs. We do not have evidence for this occurrence in our xenogeneic GVHD model since both nTregs and iTregs comparably suppressed GVHD lethality after a long-term observation period (82 days) even at suboptimal Treg:PBMNC ratios. Both Treg populations comparably suppressed human CD4+ and CD8+ T-cells derived from PBMNCs to low levels on day 18 post-transplant, indicating that neither Treg population was substantially contributing to the peripheral CD4+ T-cell pool. Although it is possible that iTregs experienced reprogramming(27–29), an initial experiment in which PBMNC and Treg can be distinguished based upon HLA-A2 allotype demonstrated that, despite being easily detectable in blood on day 6 (~100 cells/µl) neither nTreg or iTreg were found in the blood or spleen of animals examined at death, even as early as day 18 (data not shown). These data agree with our published data that nTregs typically persist in the circulation only 1–2 weeks in vivo(19) and are not found in GVHD target organs, although persistence in this xenogeneic system may not be predicative for persistence in humans. However, while nTregs and iTregs were comparable in significantly reducing GVHD-associated weight loss in the first 1 month post-transplant, nTregs were superior at later times post-transplant (data not shown). One potential explanation for the superior weight curves with nTregs vs. iTregs is the relative higher frequency of IL-4 secreting cells in Treg cultures, since increased IL-4 production has been shown to ameliorate disease in a murine model of GVHD(30).
Multiple types of murine and human iTregs have been defined, although significant differences exist between species. For example, while TGFβ or ATRA individually induce Foxp3 expression and suppressive function in murine T-cells(9, 10), human T-cells do not acquire suppressive function until ATRA (which enhances TGFβ function by increasing SMAD3 expression(11)) is combined with TGFβ(16). Rapa, in addition to inhibiting mTOR, enhances TGFβ signaling by inhibiting Smad7, a negative regulator that targets TGFβ receptors for degradation(31, 32). In contrast, IFNγ suppresses TGFβ signaling(33) and peripheral induction of Tregs in mice(34) by inducing Smad7. We show >50% of naïve human CD4+25- T-cells expanded in TGFβ secrete IFNγ (Figure 3A). Therefore, Rapa is likely able to induce Treg from cultures containing naïve and memory cells because, unlike ATRA or aryl hydrocarbon receptor ligands, it inhibits both IFNγ secretion and Smad7 downregulation of TGFβ signaling. In addition to inducing Treg in cultures containing a mixture of naïve and memory T-cells, Rapa/TGFβ induced Foxp3 expression and suppressive function in purified CD4+25-45RA- (data not shown). Similarly, Zhang, et. al. found Rapa enhanced TGFβ induction murine Treg and could improve allograft survival in a mouse islet transplant model(17). Stable Foxp3 expression in nTreg is controlled through the Treg specific demethylated region (TSDR) in the Foxp3 gene(35). To date, no method to induce Treg in murine or human cells has resulted in TSDR methylation, and we found no evidence for Rapa/TGFβ iTreg induced from either naïve or unfractionated CD4+25- T-cells (Figure S2). However, the enhanced TGFβ signaling found in these cells might drive stable expression through another mechanism. For example, naïve CD4+25- T-cells treated with TGFβ and aryl hydrocarbon receptor agonists induce Smad1 expression, which then binds the Foxp3 enhancer and regulates Foxp3 expression(36). Decitabine, a DNA methyltransferase inhibitor previously shown to increase Foxp3 gene methylation and expression, did not enhance Foxp3 expression or suppressive function in Rapa/TGFβ iTreg, providing further evidence that Foxp3 methylation status does not control Treg induction (Figure S3).
In summary, we have shown that that Rapa enhances TGFβ-dependent Foxp3 expression and generates iTregs with potent in vivo suppressor function. Our protocol generates an average of 240×109 iTregs from CD4+25- or 200×109 iTregs from CD4+25-RA+ T-cells capable of suppressing third party responses in vivo. Because all the necessary clinical reagents are available and iTreg were cultured on a clinically relevant scale (≥500×106 cells), these data set the stage for clinical trials testing the feasibility and safety of iTreg cellular therapy for human diseases.
Supplementary Material
Figure 6. Rapa(d7)/TGFβ induced Treg are as potent as nTreg at suppressing GVHD in vivo.
In vitro expanded nTreg and Rapa(d7)/TGFβ iTreg (30×106 cells) were co-transferred with allogeneic PBMCs (30×106 cells) into Rag−/−,γc−/− mice to assess the relative potency for preventing xenogeneic GVHD. A) Characteristics of the nTreg and iTreg cultures that were used. B) Kaplan-Meier survival curves for mice receiving PBMC only or PBMC plus adoptive transfer of nTreg of iTreg. C) Animals were bled on day 18, and the degree of human T-cell expansion (CD4+ or CD8+) was determined using counting beads. n=10, 8, and 7 mice for PBMC, nTreg and iTreg, respectively.
Acknowledgements
This work was supported in part by research grants from the Children’s Cancer Research Fund and Blood and Marrow Transplant Research Fund (KLH), Leukemia and Lymphoma Translational Research Grant R6029-07 (BRB), R37 HL56067 (BRB), P01 AI056299 (BRB), NCI P01 CA067493 (BRB, JEW, JSM) and NHLBI N01HB037164 (JEW, JSM); and support from the JDRF Collaborative Centers for Cell Therapy and the JDRF Center on Cord Blood Therapies for Type 1 Diabetes (JLR, CHJ).
Footnotes
Disclosure Statement
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Bluestone JA, Thomson AW, Shevach EM, Weiner HL. What does the future hold for cell-based tolerogenic therapy? Nat Rev Immunol. 2007;7(8):650–654. doi: 10.1038/nri2137. [DOI] [PubMed] [Google Scholar]
- 2.Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7(8):585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 3.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30(5):656–665. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluestone JA, Tang Q, Sedwick CE. T regulatory cells in autoimmune diabetes: past challenges, future prospects. J Clin Immunol. 2008;28(6):677–684. doi: 10.1007/s10875-008-9242-z. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196(3):389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99(10):3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 7.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2010 doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58(3):652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran DQ, Andersson J, Hardwick D, Bebris L, Illei GG, Shevach EM. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113(21):5125–5133. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29(9):429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, et al. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181(4):2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A metabolites induce gut-homing FoxP3+ regulatory T cells. J Immunol. 2007;179(6):3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 13.Golovina TN, Mikheeva T, Brusko TM, Blazar BR, Bluestone JA, Riley JL. Retinoic acid and rapamycin differentially affect and synergistically promote the ex vivo expansion of natural human T regulatory cells. PLoS One. 2011;6(1):e15868. doi: 10.1371/journal.pone.0015868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322(5907):1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110(8):2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Huizinga TW, Toes RE. De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J Immunol. 2009;183(6):4119–4126. doi: 10.4049/jimmunol.0901065. [DOI] [PubMed] [Google Scholar]
- 17.Zhang W, Zhang D, Shen M, Liu Y, Tian Y, Thomson AW, et al. Combined administration of a mutant TGF-beta1/Fc and rapamycin promotes induction of regulatory T cells and islet allograft tolerance. J Immunol. 2010;185(8):4750–4759. doi: 10.4049/jimmunol.1000769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suhoski MM, Golovina TN, Aqui NA, Tai VC, Varela-Rohena A, Milone MC, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007;15(5):981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hippen KL, Harker-Murray P, Porter SB, Merkel SC, Londer A, Taylor DK, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112(7):2847–2857. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox CJ, Hammerman PS, Thompson CB. The Pim kinases control rapamycin-resistant T cell survival and activation. J Exp Med. 2005;201(2):259–266. doi: 10.1084/jem.20042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Basu S, Golovina T, Mikheeva T, June CH, Riley JL. Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. Journal of Immunology. 2008;180(9):5794–5798. doi: 10.4049/jimmunol.180.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi J, Ritchey J, Prior JL, Holt M, Shannon WD, Deych E, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116(1):129–139. doi: 10.1182/blood-2009-12-257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koenecke C, Czeloth N, Bubke A, Schmitz S, Kissenpfennig A, Malissen B, et al. Alloantigen-specific de novo-induced Foxp3+ Treg revert in vivo and do not protect from experimental GVHD. Eur J Immunol. 2009;39(11):3091–3096. doi: 10.1002/eji.200939432. [DOI] [PubMed] [Google Scholar]
- 24.Radhakrishnan S, Cabrera R, Schenk EL, Nava-Parada P, Bell MP, Van Keulen VP, et al. Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J Immunol. 2008;181(5):3137–3147. doi: 10.4049/jimmunol.181.5.3137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Foley JE, Jung U, Miera A, Borenstein T, Mariotti J, Eckhaus M, et al. Ex vivo rapamycin generates donor Th2 cells that potently inhibit graft-versus-host disease and graft-versus-tumor effects via an IL-4-dependent mechanism. J Immunol. 2005;175(9):5732–5743. doi: 10.4049/jimmunol.175.9.5732. [DOI] [PubMed] [Google Scholar]
- 26.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, et al. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11(9):854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma MD, Hou DY, Baban B, Koni PA, He Y, Chandler PR, et al. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33(6):942–954. doi: 10.1016/j.immuni.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams LM, Rudensky AY. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nature immunology. 2007;8(3):277–284. doi: 10.1038/ni1437. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nature immunology. 2009;10(9):1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foley JE, Mariotti J, Ryan K, Eckhaus M, Fowler DH. Th2 cell therapy of established acute graft-versus-host disease requires IL-4 and IL-10 and is abrogated by IL-2 or host-type antigen-presenting cells. Biol Blood Marrow Transplant. 2008;14(9):959–972. doi: 10.1016/j.bbmt.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamaguchi T, Kurisaki A, Yamakawa N, Minakuchi K, Sugino H. FKBP12 functions as an adaptor of the Smad7-Smurf1 complex on activin type I receptor. J Mol Endocrinol. 2006;36(3):569–579. doi: 10.1677/jme.1.01966. [DOI] [PubMed] [Google Scholar]
- 32.Aghdasi B, Ye K, Resnick A, Huang A, Ha HC, Guo X, et al. FKBP12, the 12-kDa FK506-binding protein, is a physiologic regulator of the cell cycle. Proc Natl Acad Sci U S A. 2001;98(5):2425–2430. doi: 10.1073/pnas.041614198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulloa L, Doody J, Massague J. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-gamma/STAT pathway. Nature. 1999;397(6721):710–713. doi: 10.1038/17826. [DOI] [PubMed] [Google Scholar]
- 34.Caretto D, Katzman SD, Villarino AV, Gallo E, Abbas AK. Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells. J Immunol. 2010;184(1):30–34. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9(2):83–89. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 36.Gandhi R, Kumar D, Burns EJ, Nadeau M, Dake B, Laroni A, et al. Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3(+) regulatory T cells. Nat Immunol. 2010;11(9):846–853. doi: 10.1038/ni.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.