Abstract
OBJECTIVES:
To determine the roles of body size and longitudinal body weight changes in the survival of incident peritoneal dialysis patients.
PATIENTS AND METHODS:
Patients (n = 1911) older than 18 years of age recruited from 114 dialysis centers (Dec/2004-Oct/2007) and participating in the Brazilian Peritoneal Dialysis Multicenter Cohort Study were included. Clinical and laboratory data were collected monthly (except if the patient received a transplant, recovered renal function, was transferred to hemodialysis, or died).
RESULTS:
Survival analyses were performed using Kaplan-Meier survival curves and Cox proportional hazards. Total follow-up was 34 months. The mean age was 59 years (54% female). The weight category percentages were as follows: underweight: 8%; normal: 51%; overweight: 29%; and obese 12%. The multivariate model showed a higher risk of death for a body mass index <18.5 kg/m2, a neutral risk between 25 and 29.9 kg/m2 and a protective effect for an index >30 kg/m2. Patients were divided into five categories according to quintiles of body weight changes during the first year of dialysis: <−3.1%, −3.1 to+0.12%, +0.12 to <+3.1% (reference category), +3.1 to +7.1% and >+7.1%. Patients in the lowest quintile had significantly higher mortality, whereas no negative impact was observed in the other quintiles.
CONCLUSION:
These findings suggest that overweight/obesity and a positive body weight variation during the first year of peritoneal dialysis therapy do not increase mortality in incident dialysis patients in Brazil.
Keywords: Overweight, Obesity, Incident, Peritoneal Dialysis, Survival, Cohort Study
INTRODUCTION
Over the last 20 years, the prevalence of overweight and obesity has increased dramatically in the Brazilian population, especially among those aged 20 years and over (1). Overweight and obesity are known risk factors for highly prevalent conditions such as type 2 diabetes, hypertension, cardiovascular diseases, and certain types of cancer (2). In patients with chronic kidney disease on hemodialysis (HD), a high body mass index (BMI) is associated with better survival, whereas a low BMI and weight loss are associated with increased mortality (3-7). The role of BMI in the survival of patients undergoing peritoneal dialysis (PD) has yet to be established; available studies have provided conflicting results (8-10). Although most physicians do not consider PD to be the treatment of choice for obese patients, the percentage of incident patients with high BMIs has risen progressively in some countries since 1980 (11,12,13).
McDonald et al. evaluated 9,440 PD patients in the ANZDATA registry and could not show a protective effect of BMI (11). However, in a prospective study with 45,982 PD patients, Snyder et al. reported an association between adjusted mortality and BMI changes over time, with better survival among overweight and obese patients until the third year of PD (14).
The conflicting results regarding the effect of body size on PD outcomes may relate in part to the inability of BMI to differentiate between muscle mass and fat tissue (9). Ram Kumar et al. evaluated the body size and body composition of 10,140 PD patients (15); the authors concluded that both factors influence survival in incident patients receiving PD and that those patients with higher BMIs associated with normal or high muscle mass exhibited the best survival. In a recently published paper, Pellicano et al. (16) followed up 60 incident HD (n = 39) and PD (n = 21) patients longitudinally for 12 months. Using gold standard methods, they found that dialysis modalities affected changes in total body fat; extended hours of home HD were associated with the smallest increase, and PD was associated with the greatest increase in total body fat, with a significant increase in the ratio of visceral to subcutaneous fat. Obese patients experienced greater preservation of total body protein compared with normal and overweight patients, suggesting that energy storage as fat mass is of value in the dialysis patient population.
The Brazilian Peritoneal Dialysis Multicenter Cohort Study (BRAZPD) is the first large observational PD cohort study performed in Brazil, and the percentage of obese PD patients (17) is similar to that of the general population (18).
The aim of this observational study is to determine the impact of both body size and longitudinal body weight changes on survival in a cohort of incident PD patients in the BRAZPD study.
PATIENTS AND METHODS
Setting and patients
Incident PD patients recruited from 114 dialysis centers treating more than 10 PD patients each and reporting monthly to BRAZPD were included in this study. All of the patients were 18 years or older, remained on PD for at least 90 days and provided complete information on body weight and height. This study was conducted in accordance with the Declaration of Helsinki, and all participants provided written informed consent before enrollment. Details of the study design and characteristics of the cohort are described elsewhere (17).
Of the 3,439 incident patients enrolled in the BRAZPD from December 2004 through October 2007, 1,911 were eligible for the study, and 1,528 patients were excluded (867 for not completing 90 days of therapy and 661 with more than 90 days of therapy but lacking either weight or height data). Out of the 1,528 excluded patients, 1,172 were still alive and on PD in October 2007, 210 died and 146 dropped out for reasons other than death as follows: 51 were transferred to HD, 13 received kidney transplantations, 4 recovered renal function, 13 were transferred to other clinics, 2 abandoned the study, 6 were lost for other reasons and 57 had no available data.
Data collection
Data were collected monthly from December 2004 through October 2007. Sociodemographic and clinical data were evaluated at baseline. Each patient's medical chart was thoroughly reviewed by nephrologists who extracted data pertaining to the underlying renal disease, history of cardiovascular disease and other comorbid conditions. The Davies comorbidity score was used to assess the severity of comorbid conditions (19). The data obtained from the patients' charts included the following: sociodemographic information, chronic kidney disease etiology, hypertension, and comorbidities. During the follow-up period, body weight (BW), height, and BMI were evaluated monthly. BMI, defined as weight in kilograms divided by the square of the height in meters, was classified according to the World Health Organization (WHO): underweight (<18.5 kg/m2), normal (18.5 to 24.9 kg/m2), overweight (25 to 29.9 kg/m2) and obese (>30 kg/m2). Body weight was measured monthly without PD fluid in the abdominal cavity. Laboratory measurements were taken monthly, including creatinine, urea, potassium, calcium, phosphate, alanine amino-transferase (ALT), glucose, hemoglobin, albumin, total cholesterol, and triglycerides, and were determined using routine methods.
The patients were followed until they received kidney transplants, recovered renal function, were transferred to HD, died, or ended their participation in the study.
Statistics
The patients were divided into four groups according to BMI. The demographic, clinical, and laboratory data were evaluated for each group. Normally distributed variables were expressed as means±SD (unless noted otherwise), and non-normally distributed variables were expressed as medians and ranges. Differences between the BMI groups were examined using the Kruskal-Wallis analysis of variance (ANOVA), followed by a post hoc Dunn's test for non-parametric comparisons. A χ2 test was used for categorical variables.
Survival was analyzed with Kaplan-Meier survival curves and Cox proportional hazards models. The latter were used to examine survival differences after the analysis had been adjusted for potential confounding factors (age, gender, Davies score, and BMI) in incident patients. We used competing risk analysis to study the influence of BMI on survival in this cohort of incident PD patients. We also performed two sensitivity analyses. First, the main analysis with censoring of follow-up time for the patients (n = 1,615) who either reached the final follow-up time (n = 1,304) or ended PD therapy for any reason (drop out not related to death, n = 311). Second, we reclassified the patients who ended PD therapy for any reason. Patients who received a renal transplant, were transfer to HD or recovered renal function were excluded from the analysis. Survival was determined after a median follow-up of 13 months (range, 3 to 34 months). Moreover, survival for the entire follow-up period was analyzed according to the evolution of BW during the patients' first year of therapy (n = 1,738). Patients were divided into five quintiles (<−3.1%, −3.1 to +0.12%, +0.12 to <+3.1% [reference category], +3.1 to +7.1% and >+7.1%) according to the BW evolution expressed as a percentage normalized to the ideal body weight calculated from the Broca formula (ideal BW = (height in cm − 100) for males and ideal BW = (height in cm − 104) for females).
Restricted cubic splines were used to evaluate nonlinear relationships between BMI levels and mortality. This method has been suggested to offer adequately fit models and is a good compromise between flexibility and loss of precision caused by over-fitting a small sample. Statistical significance was set at p<0.05. Statistical analyses were performed using Stata software (Version 12.1; Stata Corp., College Station, TX, USA) and SAS statistical software (Version 9.2 SAS Institute Inc., Cary, NC, USA). As p-values were not adjusted for multiple testing, they should be considered descriptive.
RESULTS
This study included 1,911 incident patients treated by either Automated PD (APD) or Continuous Ambulatory Peritoneal Dialysis (CAPD), who started PD between December 2004 and October 2007 and were followed up until October 2007. According to the WHO classification, the BMI distribution of the cohort was as follows: underweight 8%; normal (51%); overweight (29%) and obese (12%).
The mean age of the patients was 59±16 years old, 54% were female and 62% were Caucasian. The most common chronic kidney disease etiology was diabetic nephropathy (38%). A Davies score greater than 2 was present in 15% of patients, and the most frequent comorbidities were hypertension (76%) and diabetes (39%). CAPD was the PD modality for 51% of the patients. The majority of patients (68%) starting PD were transferred from HD, and the median time on HD before switching to PD was 7.3 (range, 1.3-60.2) months. Patients were mainly referred to nephrologists from internists (31%), and 18% of patients were referred from an emergency unit. In this cohort, 58% of patients had not received pre-dialysis care, 67% were illiterate or had <4 years of schooling and 79% had a family income less than 5 times the national minimum wage (< USD$ 7.71/person/day) per month. Only glucose-based PD solutions were prescribed for all patients (Dianeal, Baxter Healthcare), and Homechoice™ (Baxter, Healthcare) was the cycler used for APD.
There were no significant differences in age, gender, socioeconomic status, and race distribution when comparing the 1,911 patients in this study and the 1,528 patients (867 for not completing 90 days of therapy and 661 with more than 90 days of therapy but lacking either weight or height data) excluded from the study. Moreover, there were no significant differences (p = 0.08) among the four BMI groups when analyzing the 867 patients excluded from the study for not having completed 90 days of therapy. In this group of excluded patients, 11.3% were underweight, 48.5% were normal, 27.2% were overweight, and 13% were obese. No significant differences in baseline characteristics were observed between the group of patients who had PD as their initial renal replacement therapy (RRT) (32%) and the group of patients who had HD as their first RRT (68%).
Table 1 shows some demographic characteristics and clinical and laboratory data for the incident PD patients according to BMI. Overweight patients were older (60±13 years old; p = 0.001), whereas obese patients were mostly female (64%; p = 0.001) and exhibited higher systolic (p = 0.001) and diastolic blood pressures (p = 0.003). The Davies score was higher than 2 in 18% of the overweight patients and 16% of the obese patients. Patients who were overweight or obese presented higher hemoglobin (p = 0.02), glucose (p = 0.001) and triglyceride (p = 0.007) levels than the other two groups (normal and underweight). There were no significant differences in the values of albumin, urea, potassium, calcium, phosphate, and cholesterol among the BMI groups. For creatinine, there was a trend towards a significant difference with higher levels in patients with higher BMIs.
Table 1.
Characteristics of incident patients with PD by underweight (<18.5 kg/m2), normal (18.5 to 24.9 kg/m2), overweight (25 to 30 kg/m2), and obese (>30 kg/m2).
| Under-weight N = 159 | Normal N = 985 | Over-weight N = 547 | Obese N = 220 | p-value | |
| Sex female (%) | 68% | 55% | 46% | 64% | 0.001 |
| Age (years) | 55±21 | 57±17 | 60±13 | 59±12 | 0.001 |
| Systolic BP (mm Hg) | 132±23.5 | 138.9±24 | 141.7±25.5 | 144.8±26.6 | 0.001 |
| Diastolic BP (mm Hg) | 81±14 | 83.2±14.2 | 85±14 | 85±14 | 0.003 |
| S-albumin (g/L) | 3.7±1.4 | 4.2±1.2 | 4.3±2.4 | 4.2±1.2 | NS |
| S-creatinine (mg/dL) | 6.8±3.1 | 7.5±4.3 | 7.7±4.6 | 8.2±4.8 | 0.07 |
| S-urea (mg/dL) | 109.6±47 | 116.3±48 | 117±48 | 115.7±44 | NS |
| Hemoglobin (g/L) | 10.4±2.3 | 10.4±2.9 | 10.8±2.6 | 10.5±2.1 | 0.02 |
| Phosphate (mg/dL) | 4.9±1.9 | 5.5±1.8 | 5.1±1.8 | 5.4±3.3 | NS |
| Calcium (mg/dL) | 8.5±2.2 | 8.4±3.5 | 8.3±2.4 | 8.38±2.4 | NS |
| Potassium (mEq/L) | 4.4±1.1 | 4.6±1 | 4.7±2.4 | 4.5±0.9 | NS |
| Glucose (mg/dL) | 106±67 | 115±76 | 122±78 | 127±75 | 0.001 |
| Triglycerides (mg/dL) | 143±86 | 172±101 | 209±166 | 223.2±97 | 0.007 |
| Tot cholesterol (mg/dL) | 190.8±55 | 190±63 | 184±61 | 199.9±62 | NS |
| Davies score: | 0.03 | ||||
| 0 | 27% | 22% | 16% | 16% | |
| 1-2 | 62% | 64% | 65% | 67% | |
| >2 | 11% | 14% | 18% | 16% |
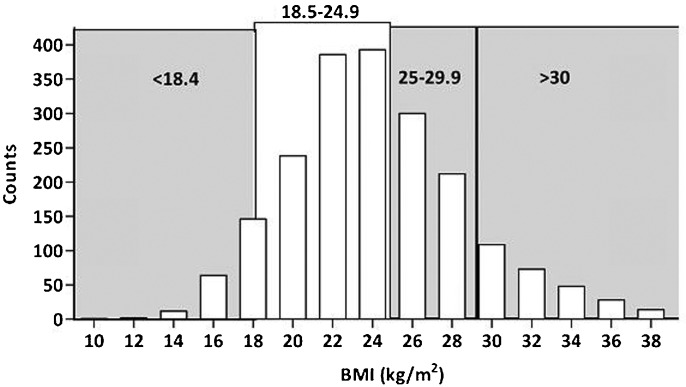
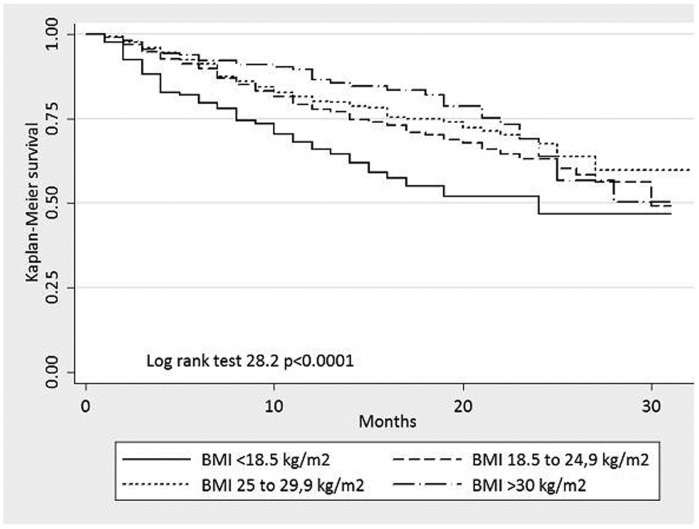
Figure 1 depicts the frequency distribution of BMI in the incident PD patients, with most patients being normal or overweight. Figure 2 shows an unadjusted Kaplan-Meier plot for incident patients according to BMI, with significant differences in the mortality curves among the different BMI groups (log rank χ2, p<0.0001). The majority (34%) of patients died due to cardio-vascular causes in all groups. The most common cause of dropout was not related to the PD technique in all groups (67.5%).
Figure 1.
Histogram of BMI (kg/m2) for all PD patients at baseline.
Figure 2.
Unadjusted Kaplan-Meier survival curves for incident PD patients and all-cause mortality, according to BMI levels.
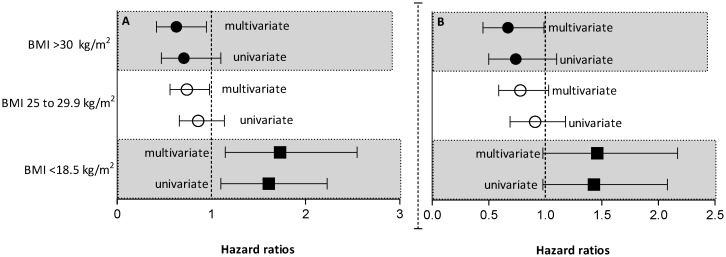
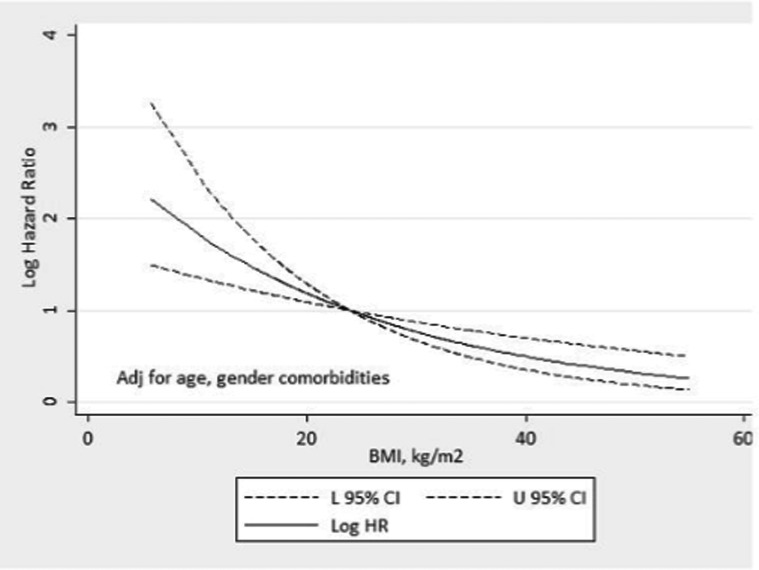
Figure 3A (univariate and multivariate analysis; conventional Cox method), Figure 3B (univariate and multivariate analysis; competing Cox method), and Figure 4 (Spline curve) show the Cox proportional hazard for the incident patients, according to BMI. In a conventional Cox multivariate analysis (Figure 3A), both BMI 25-29.9 kg/m2 (HR = 0.74, CI: 0.56 to 0.98, p<0.05) and BMI>30 kg/m2 (HR = 0.63, CI: 0.42 to 0.95, p<0.05) reflected protection from mortality risk, whereas BMI<18.5 kg/m2 reflected a higher mortality risk (HR = 1.72, CI: 1.15 to 2.55, p<0.001). Figure 3B indicates that in the multivariate Cox analysis with competing risks, BMI<18.5 kg/m2 (HR = 1.46 (0.98-2.1), p = 0.05) and BMI 25-29.9 kg/m2 (HR = 0.78 (0.59-1.04), p = 0.08) were not significant, whereas BMI>30 kg/m2 reflected protection from mortality risk (HR = 0.67 (0.45-0.99), p = 0.04). Figure 4 shows the Spline Cox proportional hazard based on baseline BMI; the model is adjusted for age and gender. The curve depicts the relationship between log Hazard Ratios and BMI levels in incident PD patients, demonstrating that low BMI is associated with higher mortality and that high BMI is associated with lower mortality.
Figure 3.
A) Univariate and multivariate Cox regressions for predictors of all-cause mortality in incident patients. B) Univariate and multivariate Cox regressions for predictors of all-cause mortality in incident patients with competing risk analysis. Hazard Ratios (HR) for all-cause mortality [and 95% confidence intervals (CI)] for patients with BMI <18.5 kg/m2, BMI 25 to 29.9 kg/m2 and BMI >30 kg/m2. Patients with BMIs ranging from 18.5 to 24.9 kg/m2 were considered the reference. p-value, significance level <0.05. The models are adjusted for potential confounders, i.e., baseline comorbidities (cardiovascular disease (CVD) and diabetes mellitus (DM), automated peritoneal dialysis or continuous ambulatory peritoneal dialysis, renal replacement therapy, and calendar year (CY) (2005, 2006 and 2007).
Figure 4.
In the left Y-axis, restricted spline curve showing the age, gender and comorbidities-adjusted Hazard ratios and 95% confidence intervals (CI) (dashed lines) for all-cause mortality associated with BMI in 1,911 incident PD patients. The model is plotted as restricted cubic splines with four knots. Log HR, Log transformed Hazard ratio; 95% CI, lower and upper 95% confidence intervals, respectively. P for linearity = 0.01.
Table 2A depicts the Cox proportional hazard for the evolution of body weight during the first year of PD in incident patients. The patients were divided into five categories according to the quintile distribution: <−3.1%, −3.1 to +0.12%, +0.12 to <+3.1% (reference category), +3.1 to +7.1% and >+7.1%. There was significantly higher mortality (HR = 2.10, p<0.001) in the lowest quintile (-3.1%), whereas no impact was observed in the 3.1 to +0.12% quintile when compared to the reference category. In patients who gained +3.1 to +7.1% or >+7.1%, no impact was observed when compared to the reference weight. The patients who gained weight (>+7.1%) had an HR = 0.81 with a narrow CI 0.54-1.24 in relation to the reference weight. In a multivariate Cox proportional hazard analysis (Table 2B), the lowest quintile (-3.1%) was associated with higher mortality (HR = 1.94, p<0.0001) when compared to the reference weight, whereas no impact was observed in the other quintiles.
Table 2.
Univariate (A) and multivariate (B) Cox regressions for predictors of all causes of mortality in according to the evolution of body weight during the first year of PD treatment.
| A- Univariate analysis – all causes of mortality. | ||||
| Variable | Hazard Ratio | 95% CI | χ2 | p-value |
| <−3.1%, vs 0.12 to <3.1% | 2.10 | 1.47-3.02 | 4.06 | <0.001 |
| −3.1 to <0.12% vs 0.12 to <3.1% | 1.18 | 0.81-1.77 | 0.84 | 0.41 |
| +3.1 to <+7.1% vs 0.12 to <3.1% | 1.08 | 0.73- 1.61 | 0.41 | 0.68 |
| >+7.1%. vs 0 to 0.12 to <3.1% | 0.85 | 0.56-1.29 | 0.74 | 0.46 |
| B- Multivariate -all cause of mortality adjusted for age, gender, CVD, DM, APD/CAPD, RRT and calendar year | ||||
| <−3.1%, vs 0.12 to <3.1% | 1.94 | 1.35 - 2.8 | 3.59 | <0.001 |
| −3.1 to <0.12% vs 0.12 to <3.1% | 1.29 | 0.86 - 1.95 | 1.24 | 0.21 |
| +3.1 to <+7.1% vs 0.12 to <3.1% | 1.10 | 0.74 - 1.63 | 0.47 | 0.63 |
| >+7.1% vs 0 to 0.12 to <3.1% | 0.81 | 0.54 - 1.24 | 0.94 | 0.34 |
Indicated are Hazard Ratios (HRs) for all-cause mortality [and 95% confidence intervals (CI)] for patients with weight evolution <−3.1%, −3.1 to <0.12%, +3.1 to <+7.1% and >+7.1%. The patients with weight evolution between 0.12 to <3.1% were considered as reference. A p-value with significance level <0.05. The models adjust for potential confounders i.e. baseline comorbidities (cardiovascular disease (CVD), and diabetes mellitus (DM), automated peritoneal dialysis or continuous ambulatory peritoneal dialysis, renal replacement therapy, calendar year (CY) (2005, 2006, 2007).
There were no differences in the frequencies of categories of BW evolution during the first year of therapy among the baseline BMI groups in 1,738 incident PD patients. BW gain during the first year was similarly distributed among the four groups (underweight, 42%; normal, 42%; overweight, 38%; and obese, 37%). Thirty-four percent of patients were more than 65 years of age. The younger and older (>65 years of age) patients presented similar underweight (7.8% vs. 8.3%), overweight (27.5% vs. 31.4%), and obesity (12% vs. 11.3%) distributions (p = 0.31).
DISCUSSION
In this large observational study of incident PD patients performed in Brazil, we demonstrated that a higher BMI (overweight and obese) is not associated with higher mortality when compared to normal (neutral risk) or underweight (higher risk of death). In a multivariate Cox proportional hazard analysis, the evolution of BW in the lowest quintile was associated with a higher mortality when compared to the reference, whereas no negative impact was observed in the other quintiles.
In the last 20 years, the number of people with obesity has increased dramatically in Brazil (www.ibge.gov.br, accessed in November 2007) (18). The BRAZPD is the first large observational PD study performed in this country, and the percentage of PD patients with obesity is similar to that of the general population (18).
In the general population, obesity is considered unhealthy; obesity is associated with a higher risk of cardiovascular disease (20), diabetes (21), cancer (22), kidney disease (23,24), and other comorbidities, and it correlates with mortality. It is important to note that while obesity has been shown to be a risk factor for mortality in the general population with more than 10 years of follow-up (25), CVD mortality in the dialysis patient population, even after stratification, is 10 to 20 times higher than in the general population (26). Consequently, the follow-up time in studies evaluating dialysis patients and the general population should be different (27). However, obesity may be protective against mortality under several conditions, including in elderly and HD patients and in cases of chronic obstructive pulmonary disease, acquired immunodeficiency syndrome and rheumatoid arthritis (3,28). Obesity has also been reported to have a protective effect for patients with congestive heart failure and geriatric patients, i.e., populations with extraordinarily high mortality rates (28).
Exploring the causes and consequences of this epidemiological observation for obesity in PD patients may enhance our insights into similar paradoxes observed for other conventional risk factors, such as blood pressure, serum cholesterol and homocysteine (23,29-33),. It is important to note that in this study adverse clinical characteristics are readily observed in the epidemiological profile of the overweight and obese PD patients, i.e., higher age, increased hypertension, diabetes and higher Davies comorbidity scores. However, in this study, obesity is still not associated with higher mortality risk. Similar results have been observed by Chazot et al. (3) in a cohort of 5,592 HD patients; despite increased comorbidities, overweight and obese HD patients carried a significantly lower mortality risk than those HD patients with normal and lower BMI ranges.
Agarwal et al. (34) evaluated the relationship between blood pressure (BP) control and BMI in HD patients. They observed that a lower BMI was associated with worse BP control and higher mortality during patients' first two years on dialysis, justifying this higher mortality to the patients with greater severity. However, no time-dependent analysis of BP, body composition or inflammation was evaluated (35). These findings that higher mortality is associated with lower BMI are in agreement with our results. However, in our study, the overweight/obese patients did not have higher mortality, even though they presented with higher Davies comorbidity scores than the lower BMI group.
De Mutsert et al. (36) evaluated incident PD patients selected from the NECOSAD study and followed them for five years; they found that obese incident PD patients did not have worse survival compared with normal BMI incident PD patients. In the same study, PD patients with a low BMI had a two-fold increased mortality risk. Our results also demonstrate higher mortality in patients either presenting lower BMI (<18.5 kg/m2) or a longitudinal weight loss of >-3.1 kg during the first year of therapy.
A recent study involving the NECOSAD database examined the differences between the impact of BMI on mortality in HD and PD patients, stratified by age (>65 years and <65 years). They found that in patients younger than 65 years of age, obesity was associated with higher mortality (37). This study presents relevant differences in relation to our study: first, the percentage of older patients was higher (47.3%) than in our study (34%); second, BMI was evaluated only at baseline, and there was no information about the variation of the BMI over time. Pellicano et al. (16), in a longitudinal study evaluating BMI, stratified the patients by age and did not observe differences between age groups. In our study, the prevalences of malnutrition and overweight/obesity in patients younger and older than 65 years were the same; like the majority of the studies, we adjusted for age, and no impact was observed.
In this study, mortality curves adjusted for age, gender, and Davies score indicated that obesity does not increase mortality for incident PD patients (HR = 0.67, CI: 0.47 to 0.95, p = 0.02). Pellicano et al. have hypothesized that a higher BMI is protective because of increased energy storage as total body fat, which could lead to relative preservation of lean body mass (16). In their study, obese patients experienced greater preservation of total body protein when compared with normal and overweight patients. Recently, Kalantar-Zadeh et al. (7) raised the question, “what is better: fat or muscle?”, and concluded that body fat is a protective factor for patients on hemodialysis.
In our study, BW gain during the first year was similarly distributed among the four groups. However, a BW loss >3.1% (first quintile) during the first year of treatment was associated with significantly increased mortality when compared to the reference, whereas no impact was observed in the other quintiles. Our findings are in agreement with the study by Kalantar-Zadeh et al. (32), which examined a cohort of 54,535 MHD patients in the United States over two years. The authors found that for each BW loss category below -1%, there was a significant increase in patient death. Obesity, including morbid obesity, was associated with better survival and reduced cardiovascular death, even after accounting for changes in BMI and laboratory values. Over time, progressively worsening weight loss was associated with poor survival, whereas weight gain showed a tendency toward decreased cardiovascular death.
Chazot et al. (3) have also demonstrated that BW variation during the first year of HD treatment is associated with patient survival, reinforcing the importance of nutrition in this setting. Our results, based on a large cohort of incident PD patients, corroborate the findings reported by Chazot et al. (3) and give support to the recently published results by Pellicano et al. (16) with both incident PD and HD patients. Our incident PD patients who gained weight did not present increased mortality when compared to the reference. However, a BW loss >3.1% (first quintile) during the first year of treatment was associated with significantly increased mortality, whereas the second quintile (−3.1 to 0.12%) had no significant reduction in survival.
As a matter of a fact, there are more review articles than original papers on the BMI and mortality theme, especially in the PD field. Moreover, these articles report on populations from North America, Europe, Asia and Australia-New Zealand but not on Latin American populations. Brazil was settled by the Portuguese, and the Brazilian ethnicity is primarily mixed. Therefore, some of the positive aspects of this paper are its originality, as it is characterized by Brazilian incident PD patients and a longitudinal approach to body changes.
As far as we know, this is the second study in PD patients evaluating changes in BMI over time. Although this is an observational study, the large number of patients and statistically significant findings indicate that a BMI>30 kg/m2 does not increase the mortality risk for incident patients on PD. Despite the negative selection (only 32% of patients actually chose PD as an RRT modality), as most patients were either transferred from HD or started PD as late referrals and showed a great deal of poor social indicators, the clinical outcomes of the BRAZPD cohort (38) are not different from those reported elsewhere (39,40). However, this observational study does not provide an explanation for the effect of BMI, and future prospective interventional studies may lead to a clarification of the “obesity paradox”, adding substantially to the strength of our findings.
Our study has some limitations. First, we relied on registry data, and the limitations of this study model are well known. Second, we lack data on residual renal function and D/P creatinine as potential modifiers of outcomes. Third, the fact that BMI does not measure body composition is an inherent limitation. Finally, the short median follow-up time and lack of adjustment for smoking are additional study limitations. It is important to note that studies evaluating body weight longitudinally in dialysis patients have reported body weight changes up to 12 months, as we did in our study. Our total follow-up time was 34 months.
It is of utmost importance for us to stress that this study does not suggest that dialysis patients should become obese. Our findings reflect routine dialysis practice where overweight/obese incident PD patients seem to have a protective survival advantage. Therefore, there is a compelling need to design studies to understand the possible mechanisms that lead to a protective effect of higher BMI in dialysis patients.
The study designed, performed and published by Pellicano et al. (16) with a small number of patients serves as timely support to our novel finding, as they suggest that energy storage as total body fat is of value in the dialysis population. A novel observation from the results of the large cohort (BRAZPD) with incident PD patients in this study is that positive BW variation during the first year of therapy does not have a significant impact on mortality, allowing us to suggest that both overweight/obesity and BW gain during the first year of therapy do not increase mortality in incident PD patients in Brazil.
The following centers participated in the Brazilian Peritoneal Dialysis multicentric study and contributed to the preparation of this paper: Ameneg, Associação Hospital Bauru, Biocor Hospital Doenças Cardiológicas, Casa de Saúde e Mat. Nossa Sra. Perpétuo do Socorro, CDR Curitiba, CDR Goiania, CDR Imperatriz, CDR São José Pinhais, CDTR - Centro Diálise Transplante Renal, Centro Nefrologia Teresópolis, Centro Nefrológico Minas Gerais, Centro Tratamento Doenças Renais Joinville, Centro Tratamento Renal Zona Sul, Clinef Rio de Janeiro, Clinepa Clínica de Nefrologia Da Paraiba, Clines, Clinese, Clínica do Rim do Carpina, Clínica Evangélico S/C Ltda., Clínica Nefrologia de Franca, Clínica Nefrologia Santa Rita, Clínica Nefrológica São Gonçalo, Clínica Paulista Nefrologia, Clínica Renal Manaus, Clínica Senhor do Bonfim, Clínica Senhor do Bonfim Ltda. Filial, Clínica Tratamento Renal, Cuiaba - Cenec, Clire Clínica Doenças Renais, FAMESP Botucatu, Unicamp - Universidade Estadual de Campinas, Hospital Clínicas FMRPUSP, Fundação Civil Casa Mis Franca, Fundação Instituto Mineiro Est. Pesq. Nefrol, Gamen Rio de Janeiro, GDF Hospital de Base, Histocom Sociedade Civil Ltda, Hospital Universidade Prof. Edgard Santos, Hospital Beneficencia Portuguesa Pernambuco, Hospital Cidade Passo Fundo, Hospital Clínica Universidade Federal Goiás, Hospital e Maternidade Angelina Caron, Hospital Evangélico Vila Velha ES, Hospital Geral Bonsucesso, Hospital Geral de Goiania, Hospital Infantil Joana de Gusmão, Hospital São João Deus, Hospital São Jorge, Hospital São Jose do Avai, Hospital São Vicente de Paula - João Pessoa, Hospital São Vicente De Paulo, Hospital Servidor do Estado Ipase, Hospital Universidade Presidente Dutra MA, Hospital Universitário Antônio Pedro, Hospital Vita Volta Redonda S/A, IAMSPE São Paulo, IMIP, Instituto Capixaba Doenças Renais, Instituto Capixaba Doenças Renais Cariacica, Instituto Capixaba Doenças Renais Serra, Instituto do Rim de Fortaleza, Instituto do Rim de Marilia, Instituto do Rim do Parana S/C Ltda., Instituto do Rim Santo Antônio da Platina, Instituto Hemodiálise de Sorocaba, Instituto Medicina Nuclear Endocrina, Instituto Nefrologia de Mogi das Cruzes, Instituto Nefrologia de Suzano, Instituto Nefrologia Souza e Costa, Instituto Urologia e Nefrol Barra Mansa, Instituto Urologia e Nefrol São José do Rio Preto, MEDSERVSP, Nefrocentro, Nefroclínica Caxias do Sul, Nefroclínica Foz do Iguaçu, Nefroclínica Uberlandia, Nefron Clínica Natal, Nefron Contagem, Nephron Pelotas, Nephron São Paulo, Núcleo Nefrologia Belo Horizonte, Pro Nephron, Prorim Campos dos Goitacazes, PUC Porto Alegre, Renalcare Serviços Médicos Ltda, Renalcor Angra dos Reis, Renalcor Rio de Janeiro, Renalvida, Rien Rio de Janeiro, Santa Casa de Adamantina, Santa Casa de Jaú - UNEFRO, Santa Casa de Marília, Santa Casa de Ourinhos, Santa Casa de Santo Amaro, Santa Casa de São José dos Campos, Santa Casa de Votuporanga, Serviço de Nefrologia de Ribeirão Preto, UERJ - Hospital das Clínicas da Universidade Estadual do Rio de Janeiro, Uni Rim João Pessoa, Unidade Nefrologia Assis, Unirim Unidade de Doenças Renais, UNIRIM Unidade Renal do Portão, UNTR Unidade Nefrologia Transplante.
Footnotes
No potential conflict of interest was reported.
ACKNOWLEDGMENTS
We would like to thank all of the dialysis centers for participating in the BRAZPD and the Brazilian Baxter Renal team for their important contributions to this project. This study was funded by Baxter Laboratories, Brazil.
REFERENCES
- 1.Sichieri R, do Nascimento S, Coutinho W. The burden of hospitalization due to overweight and obesity in Brazil. Cad Saude Publica. 2007;23(7):1721–7. doi: 10.1590/s0102-311x2007000700025. [DOI] [PubMed] [Google Scholar]
- 2.Abbott KC, Oliver DK, Hurst FP, Das NP, Gao SW, Perkins RM. Body mass index and peritoneal dialysis: "exceptions to the exception" in reverse epidemiology. Semin Dial. 2007;20(6):561–5. doi: 10.1111/j.1525-139X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Chazot C, Gassia JP, Di Benedetto A, Cesare S, Ponce P, Marcelli D. Is there any survival advantage of obesity in Southern European haemodialysis patients. Nephrol Dial Transplant. 2009;24(9):2871–6. doi: 10.1093/ndt/gfp168. [DOI] [PubMed] [Google Scholar]
- 4.Beddhu S. The body mass index paradox and an obesity, inflammation, and atherosclerosis syndrome in chronic kidney disease. Semin Dial. 2004;17(3):229–32. doi: 10.1111/j.0894-0959.2004.17311.x. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K. Causes and consequences of the reverse epidemiology of body mass index in dialysis patients. J Ren Nutr. 2005;15(1):142–7. doi: 10.1053/j.jrn.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 6.Kalantar-Zadeh K, Abbott KC, Salahudeen AK, Kilpatrick RD, Horwich TB. Survival advantages of obesity in dialysis patients. Am J Clin Nutr. 2005;81(3):543–54. doi: 10.1093/ajcn/81.3.543. [DOI] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, Kopple JD. Obesity paradox in patients on maintenance dialysis. Contrib Nephrol. 2006;151:57–69. doi: 10.1159/000095319. [DOI] [PubMed] [Google Scholar]
- 8.Pliakogiannis T, Trpeski L, Taskapan H, Shah H, Ahmad M, Fenton S, et al. Reverse epidemiology in peritoneal dialysis patients: the Canadian experience and review of the literature. Int Urol Nephrol. 2007;39(1):281–8. doi: 10.1007/s11255-006-9142-1. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DW. What is the optimal fat mass in peritoneal dialysis patients. Perit Dial Int. 2007;27 Suppl 2:S250–4. [PubMed] [Google Scholar]
- 10.de Araujo Antunes A, Vannini FD, Martin LC, Balbi AL, Ponce D, Nunes HR, et al. Inflammation and overweight in peritoneal dialysis: is there an association. Renal Fail. 2009;31(7):549–54. doi: 10.1080/08860220903050397. [DOI] [PubMed] [Google Scholar]
- 11.McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J Am Soc Nephrol. 2003;14(11):2894–901. doi: 10.1097/01.asn.0000091587.55159.5f. [DOI] [PubMed] [Google Scholar]
- 12.McDonald SP, Collins JF, Rumpsfeld M, Johnson DW. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int. 2004;24(4):340–6. [PubMed] [Google Scholar]
- 13.Bernardo AP, Fonseca I, Rodrigues A, Carvalho MJ, Cabrita A. Overweight rather than malnutrition is widely prevalent in peritoneal dialysis patients. Adv Perit Dial. 2009;25:119–24. [PubMed] [Google Scholar]
- 14.Snyder JJ, Foley RN, Gilbertson DT, Vonesh EF, Collins AJ. Body size and outcomes on peritoneal dialysis in the United States. Kidney Int. 2003;64(5):1838–44. doi: 10.1046/j.1523-1755.2003.00287.x. [DOI] [PubMed] [Google Scholar]
- 15.Ramkumar N, Pappas LM, Beddhu S. Effect of body size and body composition on survival in peritoneal dialysis patients. Perit Dial Int. 2005;25(5):461–9. [PubMed] [Google Scholar]
- 16.Pellicano R, Strauss BJ, Polkinghome KR, Kerr PG. Longitudinal Body Composition Changes Due to Dialysis. Clin J Am Soc Nephro. 2011;6(7):1668–75. doi: 10.2215/CJN.06790810. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes N, Bastos MG, Cassi HV, Machado NL, Ribeiro JA, Martins G, et al. The Brazilian Peritoneal Dialysis Multicenter Study (BRAZPD) : characterization of the cohort. Kidney Int Suppl. 2008;108:S145–51. doi: 10.1038/sj.ki.5002616. [DOI] [PubMed] [Google Scholar]
- 18.IBGE. Brazilian Institute of Geography and Statistical - IBGE. Brazil: Brazilian Institute of Geography and Statistical - IBGE; 2007 [cited 2008 april 2008]
- 19.Davies SJ, Phillips L, Naish PF, Russell GI. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002;17(6):1085–92. doi: 10.1093/ndt/17.6.1085. [DOI] [PubMed] [Google Scholar]
- 20.Vongpatanasin W. Cardiovascular morbidity and mortality in high-risk populations: epidemiology and opportunities for risk reduction. J Clin Hypertens (Greenwich) 2007;9(11 Suppl 4):11–5. doi: 10.1111/j.1524-6175.2007.07722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21(12):1443–55. doi: 10.1101/gad.1550907. [DOI] [PubMed] [Google Scholar]
- 22.Hagymasi K, Tulassay Z. [Role of obesity in colorectal carcinogenesis.] Orv Hetil. 2007;148(51):2411–6. doi: 10.1556/OH.2007.28244. Az elhizas szerepe a vastagbeldaganatok kialakulasaban. [DOI] [PubMed] [Google Scholar]
- 23.Axelsson J. Obesity in chronic kidney disease: good or bad. Blood Purif. 2008;26(1):23–9. doi: 10.1159/000110559. [DOI] [PubMed] [Google Scholar]
- 24.Kramer H, Luke A. Obesity and kidney disease: a big dilemma. Curr Opin Nephrol Hypertens. 2007;16(3):237–41. doi: 10.1097/MNH.0b013e32803578e4. [DOI] [PubMed] [Google Scholar]
- 25.Dekker FW, de Mutsert R, van Dijk PC, Zoccali C, Jager KJ. Survival analysis: time-dependent effects and time-varying risk factors. Kidney Int. 2008;74(8):994–7. doi: 10.1038/ki.2008.328. [DOI] [PubMed] [Google Scholar]
- 26.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(5 Suppl 3):S112–9. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 27.de Mutsert R, Snijder MB, van der Sman-de Beer F, Seidell JC, Boeschoten EW, Krediet RT, et al. Association between body mass index and mortality is similar in the hemodialysis population and the general population at high age and equal duration of follow-up. J Am Soc Nephrol. 2007;18(3):967–74. doi: 10.1681/ASN.2006091050. [DOI] [PubMed] [Google Scholar]
- 28.Horwich TB, Fonarow GC. Reverse epidemiology beyond dialysis patients: chronic heart failure, geriatrics, rheumatoid arthritis, COPD and AIDS. Semin Dial. 2007;20(6):549–53. doi: 10.1111/j.1525-139X.2007.00346.x. [DOI] [PubMed] [Google Scholar]
- 29.Horwich TB, Fonarow GC. The impact of obesity on survival in patients with heart failure. Heart Fail Monit. 2002;3(1):8–14. [PubMed] [Google Scholar]
- 30.Schrier RW, Abraham WT. Hormones and hemodynamics in heart failure. N Engl J Med. 1999;341(8):577–85. doi: 10.1056/NEJM199908193410806. [DOI] [PubMed] [Google Scholar]
- 31.Stenvinkel P, Marchlewska A, Pecoits-Filho R, Heimburger O, Zhang Z, Hoff C, et al. Adiponectin in renal disease: relationship to phenotype and genetic variation in the gene encoding adiponectin. Kidney Int. 2004;65(1):274–81. doi: 10.1111/j.1523-1755.2004.00370.x. [DOI] [PubMed] [Google Scholar]
- 32.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, Wu DY. Reverse epidemiology: a spurious hypothesis or a hardcore reality. Blood Purif. 2005;23(1):57–63. doi: 10.1159/000082012. [DOI] [PubMed] [Google Scholar]
- 33.Duran M, Unal A, Inanc MT, Esin F, Yilmaz Y, Ornek E. Effect of maintenance hemodialysis on diastolic left ventricular function in end-stage renal disease. Clinics. 2010;65(10):979–84. doi: 10.1590/S1807-593220100010000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agarwal R. Body mass index-mortality paradox in hemodialysis: can it be explained by blood pressure. Hypertension. 2011;58(6):1014–20. doi: 10.1161/HYPERTENSIONAHA.111.180091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weir MR. Body mass index-mortality paradox in hemodialysis patients: blood pressure, blood volume, and nutritional status. Hypertension. 2011;58(6):989–90. doi: 10.1161/HYPERTENSIONAHA.111.181818. [DOI] [PubMed] [Google Scholar]
- 36.de Mutsert R, Grootendorst DC, Boeschoten EW, Dekker FW, Krediet RT. Is obesity associated with a survival advantage in patients starting peritoneal dialysis. Contrib Nephrol. 2009;163:124–31. doi: 10.1159/000223790. [DOI] [PubMed] [Google Scholar]
- 37.Hoogeveen EK, Halbesma N, Rothman KJ, Stijnen T, van Dijk S, Dekker FW, et al. Obesity and Mortality Risk among Younger Dialysis Patients. Clin J Am Soc Nephrol. 2012;7(2):280–8. doi: 10.2215/CJN.05700611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Andrade Bastos K, Qureshi AR, Lopes AA, Fernandes N, Barbosa LM, Pecoits-Filho R, et al. Family income and survival in Brazilian Peritoneal Dialysis Multicenter Study Patients (BRAZPD): time to revisit a myth. Clin J Am Soc Nephrol. 2011;6(7):1676–83. doi: 10.2215/CJN.09041010. [DOI] [PubMed] [Google Scholar]
- 39.J Am Soc Nephrol. 1996;7(2):198–207. doi: 10.1681/ASN.V72198. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada-USA (CANUSA) Peritoneal Dialysis Study Group. [DOI] [PubMed] [Google Scholar]
- 40.Brown E, Davies S, Rutherford P, Meeus F, Borras M, Riegel W, et al. Survival of functionally anuric patients on automated peritoneal dialysis: the European APD Outcome Study. J Am Soc Nephrol. 2003;14(11):2948–57. doi: 10.1097/01.asn.0000092146.67909.e2. [DOI] [PubMed] [Google Scholar]