Abstract
Purpose
To assess the impact of pain severity and time to diagnosis of depression on health care costs for primary care patients with pre-existing unexplained pain symptoms who subsequently received a diagnosis of depression.
Patients and methods
This retrospective cohort study analyzed 4000 adults with unexplained pain (defined as painful physical symptoms [PPS] without any probable organic cause) and a subsequent diagnosis of depression, identified from the UK General Practice Research Database using diagnostic codes. Patients were categorized into four groups based on pain severity (milder or more severe; based on number of pain-relief medications and use of opioids) and time to diagnosis of depression (≤1 year or>1 year from PPS index date). Annual health care costs were calculated (2009 values) and included general practitioner (GP) consultations, secondary care referrals, and prescriptions for pain-relief medications for the 12 months before depression diagnosis and in the subsequent 2 years. Multivariate models of cost included time period as a main independent variable, and adjusted for age, gender, and comorbidities.
Results
Total annual health care costs before and after depression diagnosis for the four patient groups were higher for the groups with more severe pain (£819–£988 versus £565–£628; P < 0.001 for all pairwise comparisons) and highest for the group with more severe pain and longer time to depression diagnosis in the subsequent 2 years (P < 0.05). Total GP costs were highest in the group with more severe pain and longer time to depression diagnosis both before and after depression diagnosis (P < 0.05). In the second year following depression diagnosis, this group also had the highest secondary care referral costs (P < 0.01). The highest drug costs were in the groups with more severe pain (P < 0.001), although costs within each group were similar before and after depression diagnosis.
Conclusion
Among patients with unexplained pain symptoms, significant pain in combination with longer time from pain symptoms to depression diagnosis contribute to higher costs for the UK health care system.
Keywords: depression, pain, cost, GPRD, UK
Introduction
Depression is one of the most prevalent, disabling, and costly mental disorders worldwide. According to recent estimates, the 12-month prevalence rate of depression in Europe is 6.9%1 and depression accounts for 33% of the total costs of all brain disorders.2 Similarly, each year in the UK, an estimated 6% of adults experience an episode of depression, and more than 15% of the population will experience an episode of depression during their lifetime.3 Depression is managed predominantly in primary care and accounts for 15% of all general practice consultations in the UK.3,4 In 2007, the estimated total cost of health care services for depression in England was £1.7 billion, which increased to £7.5 billion when indirect costs associated with lost productivity were included.5
While high quality care for depression in primary care settings is important to minimize its clinical and economic impact on patients and society as a whole, the identification and treatment of depression in primary care is often suboptimal and has been a target for improvement in recent years.3,4,6–9 A possible reason for poor detection and/or misdiagnosis of depression is that it often occurs together with pain, with reported co-occurrence rates of 30%–50%.10,11 Many patients with depression initially present with painful physical symptoms (PPS) (eg, headache, musculoskeletal pain), but as painful symptoms are not a prominent feature of the diagnostic criteria for depression (DSM-IV or ICD-10), this may negatively affect the recognition of depression in primary care.10,12 When the cause of pain is not known (often termed “unexplained pain”), the prevalence of unrecognized mood disorders has been reported to be as high as 80%.11,13 This may lead to a delay in the time to diagnosis and treatment of depression in primary care, although evidence of this from longitudinal studies is missing.13 More severe pain may cause greater delays in depression diagnosis, which can potentially lead to a more severe or chronic form of depression and poorer outcomes for the patient.10,14,15 This, in turn, is likely to have an impact on health care utilization and related costs. There is some evidence that concurrent pain and depression are associated with increased use of health care resources and costs compared with either condition alone.13,16–19 However, there is no information on the economic impact of a longer time to diagnosis of depression among primary care patients with pre-existing PPS.
This study used a retrospective cohort design to assess the impact of pain severity and time to diagnosis of depression on 12-month health care utilization and associated costs before and after diagnosis of depression in UK primary care patients with pre-existing unexplained pain. We also assessed if costs would increase differentially between the two consecutive 12-month time periods post-depression diagnosis.
Material and methods
Data source
Data for this study were obtained from the UK General Practice Research Database (GPRD), which is the world’s largest computerized database of anonymized electronic medical records of patients in primary care.20 The GPRD has records for over 13 million patients from 629 primary care practices throughout the UK, and the records for over 5 million currently registered patients meet the GPRD standards of acceptable quality for use in research, which is equivalent to approximately 8.5% of the UK population.21 The information collected on the GPRD includes demographics, medical diagnoses, clinical events, medication prescriptions, specialist referrals, hospital admission, and treatment outcomes. Recent systematic reviews have confirmed the validity of medical diagnoses and quality of information on the GPRD.20,22
Patients for this study were identified from the GPRD for the 5-year observation period of January 1, 2005 until December 31, 2009.
Study sample
The study population included all patients aged ≥ 18 years who were ever registered at one of the GP practices in the GPRD during the 5-year observation period and who had unexplained pain followed by a subsequent diagnosis of depression. Only patients with “acceptable quality” data as recorded in the GPRD21 were included in the study.
Firstly, we identified patients with pre-existing unexplained pain, which was defined as PPS without any probable organic cause, based on the Read code classification, which is a system of clinical classification frequently used in the UK National Health Service (NHS).23 We adapted the Read codes for PPS previously defined elsewhere.16 Patients with unexplained pain were required to have one or more PPS codes during the observation period, but no other diagnostic code (other than PPS or depression) recorded 90 days on either side of the PPS code. This was to ensure that the PPS diagnosis was not likely to be associated with or attributable to any other comorbid condition during this period. The index date of PPS was the first recorded date of PPS during the observation period. Secondly, we then identified those patients who had a subsequent diagnosis of depression, which was based on the Read codes for this condition as described in a previous study.24 The full list of Read codes used to identify patients with PPS and depression are available upon request.
For the present analyses, patients were classified into one of four groups based on the severity of their unexplained pain (milder versus more severe) and time to diagnosis of depression (shorter versus longer) following record of pain symptoms as follows: (1) milder pain and shorter time to depression diagnosis; (2) milder pain and longer time to depression diagnosis; (3) more severe pain and shorter time to depression diagnosis; and (4) more severe pain and longer time to depression diagnosis. As there is no record of pain severity available in the GPRD, we defined milder pain as less than three pain relief prescriptions (non-opioid analgesics, opioid analgesics, non-steroidal anti-inflammatory drugs [NSAIDs], and skeletal muscle relaxants) in the 180 days after the first pain code. More severe pain was defined as at least three pain relief prescriptions plus use of at least one opioid in the 180 days after the first pain code. Patients with at least three pain relief prescriptions and no opioid use were excluded from the analysis in order to have a clear dichotomy between mild and severe pain. The index date for depression was the first recorded date of a definite diagnostic code for depression after unexplained pain was recorded in the GPRD during the study period. Shorter time to depression diagnosis was defined as less than 1 year from the PPS index date, whereas longer time to depression diagnosis was defined as more than 1 year from the PPS index date. The 1-year cut-off was based on the median time to depression diagnosis within our data and on clinical advice, and allowed for groups of approximately equal size. Patients were required to remain in the GPRD for at least 2 years after the diagnosis of depression. The period after diagnosis of depression was examined as two separate but consecutive 12-month periods (POST1 and POST2) in order to examine potential differences in health care utilization and costs during these two time periods as a result of pain severity and time to depression diagnosis from pain symptoms.
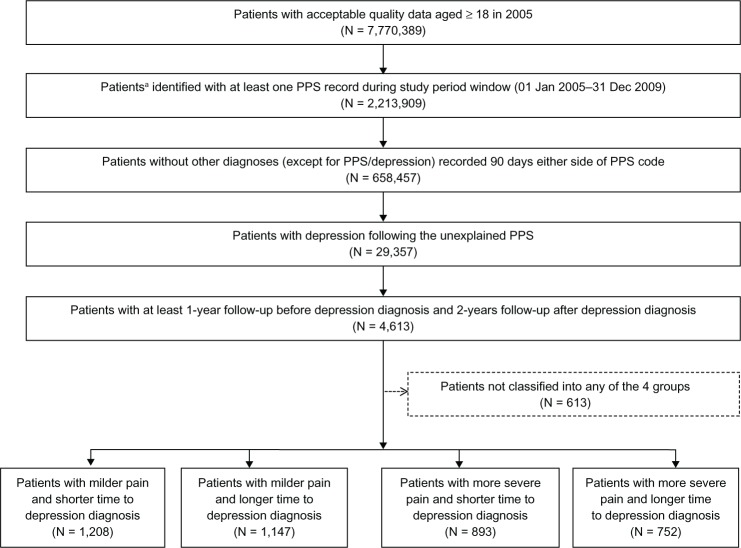
The flow chart of patient selection and classification into the four groups is presented in Figure 1.
Figure 1.
Flow diagram of patients and patient cohorts.
Note:a Patients ≥ 18 years old at the beginning of the study, continuously registered with their GP (ie, not transferred to or from another GP practice) and provided “acceptable quality” data. The 613 patients not classified into any of the 4 groups were those who were prescribed ≥3 pain relief prescriptions but had no use of opioids during 180 days after the first pain code, or had opioid use but <3 pain relief prescriptions.
Abbreviation: PPS, painful physical symptoms.
Data collection
Following selection of the study sample, data on patient age and gender were collected from the GPRD. In addition, information on comorbid conditions was collected and the Charlson Comorbidity Index (CCI) was calculated.25,26 The CCI is a summary measure of index, which represents the 1-year mortality for a patient, based on their history of a range of comorbid conditions. Khan et al27 previously defned 17 categories of comorbid conditions using Read codes for the calculation of CCI, and we adapted their codes and approach. We first identified any of the records related to the comorbid conditions in the 12-month period before diagnosis of depression (PRE), and then weighted them to produce a summary score.
Health care utilization and unit costs
Health care utilization (GP consultations, secondary care referrals, and pharmacotherapy) and the related costs (UK unit costs, 2009 values)28 were estimated for the 12-month period preceding depression diagnosis (PRE) and for the subsequent two 12-month periods after depression diagnosis (POST1 and POST2). The cost analysis was from the perspective of the UK NHS.
The costs associated with GP consultations were estimated from several major types of GP consultation, including clinical consultations, surgery visits, home visits, and telephone consultations. The list of GP consultations available in the GPRD data were classified into these four types of consultation, and the unit costs for each type of consultation were derived from the Unit Costs of Health and Social Care 2009 (Table 1).28
Table 1.
Unit costs (in pounds sterling) per episode for health care services provided
| Type of health care service provided | Unit cost/episode (2009 values) |
|---|---|
| GP consultation | |
| Clinical consultation | £52 |
| Surgery consultation | £35 |
| Home visit | £117 |
| Telephone consultation | £21 |
| Secondary care referrals | |
| Inpatient referral | £2,626 |
| Day care referral | £638 |
| Outpatient referral | £185 |
| A&E referral leading to hospitalization | £126 |
| A&E referral not leading to hospitalization | £93 |
In the present analyses, secondary care referrals consisted of inpatient referrals, day care referrals, outpatient referrals, as well as accident and emergency referrals that did and did not lead to hospital admission. The average costs per episode for each of these different types of secondary care referral were derived from the Unit Costs of Health and Social Care 2009 (Table 1).28
Information on prescriptions for medications for pain and depression were collected from the GPRD. This included non-opioid analgesics, opioid analgesics, NSAIDs, skeletal muscle relaxants, antidepressants, anxiolytics/hypnotics, and anticonvulsants. The average number of pain relief prescriptions per patient was determined for each 12-month period. The costs per day of therapy (DOT) of each medication were derived from the IMS PADDS® database.29
Statistical analyses
Descriptive statistics were used to summarize the demographics and pre-index clinical characteristics as well as the health care resource utilization for the 12-month period before depression diagnosis (PRE) for each of the four groups of patients. Comparisons between the four patient groups were made using Chi-square tests or Kruskal–Wallis tests as appropriate.
Multivariate analyses were used to estimate the total costs and the costs of each component (GP consultations, secondary care referrals, and pharmacotherapy) in the 12 months before depression diagnosis (PRE) and in the two 12-month periods after depression diagnosis (POST1 and POST2). The models included time period as a main independent variable, adjusting for the following covariates: age, gender, and CCI summary score. All multivariate analyses were carried out using generalized estimating equation models with a loglink function to correct for the skewness of cost data and also take into account correlation within the same subjects across the time periods using AR1 correlation structure. Standard errors of the costs were estimated using the non-parametric bootstrap method. Bootstrap t-tests were used for pairwise cost comparisons (within-group and between-group comparisons).
Data extraction and management were carried out using SAS 9.2 (SAS Institute Inc, Cary, NC), and all costing analyses were conducted using STATA SE 10 (StataCorp L P, College Station, TX).
Results
The mean age of the total study sample of 4000 adults was 48.9 years and 68.2% were female. Table 2 summarizes the characteristics of the total sample and the four patient groups and shows that the demographics (gender, age) differed significantly between the four patient groups. In particular, the two groups of patients with more severe pain were older than the two groups with milder pain and there were fewer females in the more severe group with longer time to depression diagnosis. The mean CCI score for the total sample was 0.12 (standard deviation 0.45) and differed significantly between the four patient groups (P = 0.015), with the highest score in the group with more severe pain and longer time to depression diagnosis (mean CCI = 0.21). For the 17 comorbid conditions included in the CCI, the percentages of patients with these comorbidities at baseline were very low, ranging from 0% for AIDS, metastatic tumor, and moderate liver disease to 3.3% for diabetes in the total sample. There were significant differences between the four patient groups in the frequencies of the following comorbidities: cancer, chronic pulmonary disease, diabetes, renal disease, and rheumatologic disease (P < 0.05, Chi-square test) (data available upon request).
Table 2.
Patient characteristics at baseline (PRE, before diagnosis of depression) for the overall sample and for the four patient groups
| Overall sample (n = 4,000) | Milder pain and shorter time to depression diagnosis (n = 1,208) | Milder pain and longer time to depression diagnosis (n = 1,147) | More severe pain and shorter time to depression diagnosis (n = 893) | More severe pain and longer time to depression diagnosis (n = 752) | P valuea | |
|---|---|---|---|---|---|---|
| Females, % | 68.2 | 69.8 | 68.1 | 69.5 | 64.0 | 0.039 |
| Age, mean (SD) | 48.9(15.8) | 45.1 (14.7) | 45.5(15.3) | 53.9(15.9) | 54.3(15.1) | <0.00l |
| Age group, n (%) | <0.00l | |||||
| 18–34 years | 749(18.7) | 297 (24.6) | 288(25.1) | 88 (9.9) | 76(10.1) | |
| 35–49 years | 1,435(35.9) | 484(40.1) | 436 (38.0) | 295 (33.0) | 220 (29.3) | |
| 50–64 years | 1,191 (29.8) | 319(26.4) | 299(26.1) | 297 (33.3) | 276 (36.7) | |
| 65+ years | 625(15.6) | 108(8.9) | 124(10.8) | 213 (23.9) | 180(23.9) | |
| CCI, mean (SD) | 0.12(0.5) | 0.06 (0.3) | 0.11 (0.5) | 0.12(0.4) | 0.21 (0.6) | 0.015 |
Notes:
Chi-square test for female and Kruskal–Wallis test for age and CCI. Age = 2005 – year of birth. 17 comorbidities were included in the CCI summary score.
Abbreviations: CCI, Charlson Comorbidity Index; PRE, the 12-month period before diagnosis of depression; SD, standard deviation.
Table 3 provides a descriptive summary of the 12-month resource use before depression diagnosis (PRE) in the four groups of patients. There were significant differences between groups for each of the four types of GP consultation (P < 0.001), with more GP consultations in the two groups with more severe pain. The frequencies of secondary care referrals in the 12 months before depression diagnosis was similar across groups, with the exception of a higher number of accident and emergency visits leading to hospital admission in the group with more severe pain and a longer time to depression diagnosis (P = 0.034). The average number of pain relief prescriptions in the 12-month period before depression diagnosis differed significantly between the four patient groups for each type of medication, and was higher in the two groups with more severe pain, consistent with the definition used (Table 3).
Table 3.
Resource use per patient in the 12-month period before depression diagnosis (PRE) for the four groups of patients
| Type of resource use | Milder pain and shorter time to depression diagnosis (n = 1,208) | Milder pain and longer time to depression diagnosis (n = 1,147) | More severe pain and shorter time to depression diagnosis (n = 893) | More severe pain and longer time to depression diagnosis (n = 752) | P value |
|---|---|---|---|---|---|
| GP consultations, mean (SD) | |||||
| Clinical consultation | 0.9(1.9) | 1.0 (2.6) | I.I (2.2) | 1.4(3.1) | <0.00l |
| Surgery consultation | 8.8 (5.8) | 9.8 (7.4) | 11.3 (8.3) | 13.3 (10.0) | <0.00l |
| Home visit | 0.1 (0.6) | 0.1 (0.7) | 0.3 (I.I) | 0.3 (I.I) | <0.00l |
| Telephone consultation | 0.5(1.3) | 0.6(1.4) | 1.0(2.2) | I.I (3.0) | <0.00l |
| Secondary care referrals, % yes | |||||
| Inpatient admission | 0.6 | 0.4 | 0.7 | 1.1 | 0.406 |
| Day care | 0 | 0.2 | 0 | 0.3 | 0.186 |
| Outpatient visit | 24.3 | 23.9 | 25.9 | 26.2 | 0.568 |
| A&E visit linked to admission | 0 | 0 | 0 | 0.3 | 0.034 |
| A&E visit not linked to admission | 0.7 | 0.6 | 0.5 | 1.2 | 0.303 |
| All types of A&E visits | 0.7 | 0.6 | 0.5 | 1.5 | 0.084 |
| Pain relief prescriptions, mean (SD) | |||||
| Non-opioid analgesic | 0.2 (0.7) | 0.2 (0.8) | 1.2(3.5) | 1.3 (3.3) | <0.00l |
| Non-opioid combinations | 0.1 (0.4) | 0.1 (0.4) | 0.2(1.2) | 0.3 (1.7) | <0.00l |
| Opioid | 0.3 (1.0) | 0.4(1.7) | 4.1 (6.6) | 4.6 (8.6) | <0.00l |
| Opioid combinations | 0.8(1.3) | 0.8 (2.0) | 4.6 (5.4) | 4.7 (6.0) | <0.00l |
| NSAID | 1.3(1.5) | 1.2(2.1) | 4.2 (4.9) | 4.5 (6.0) | <0.00l |
| Skeletal muscle relaxant | 0.0 (0.3) | 0.0 (0.4) | 0.4(1.9) | 0.6 (2.3) | <0.00l |
| Anxiolytic | 0.8 (2.5) | 0.9 (3.0) | 2.7 (6.3) | 3.0 (8.0) | <0.00l |
| Anticonvulsant | 0.2(1.7) | 0.2(1.2) | 0.7 (3.2) | I.I (3.8) | <0.00l |
| TCA | 0.6 (2.0) | 0.6(2.1) | 1.7(4.1) | 1.7(4.3) | <0.00l |
| SSRI | 2.3 (3.3) | 1.8 (2.9) | 3.2 (4.3) | 2.4 (3.7) | <0.00l |
| SNRI | 0.4(2.1) | 0.2(1.4) | 0.8 (3.0) | 0.4 (2.3) | <0.00l |
| Other antidepressants | 0.2(1.1) | 0.1 (1.2) | 0.2(1.4) | 0.3 (1.7) | 0.002 |
| MAOI | 0 | 0 | 0 | 0 |
Notes: Bold indicates statistical significance (P < 0.05) for between-group comparisons using the Kruskal-Wallis test for GP consultations and pain-relief prescriptions and the Chi-square test for secondary care referrals. Non-opioid combinations include those prescriptions that had more than one active ingredient which were all non-opioid. Opioid combinations included those prescriptions with more than one active ingredient that included at least one ingredient of opioid.
Abbreviations: A&E, accident and emergency; GP, general practitioner; MAOI, monoamine oxidase inhibitor; NSAID, non-steroidal anti-inflammatory drug; PRE, the 12-month period before diagnosis of depression; SD, standard deviation; SNRI, serotonin norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
Estimates of the 12-month total health care costs (adjusted) for each of the four groups of patients before (PRE) and after depression diagnosis (POST1 and POST2) are shown in Table 4. The total costs were higher for the two groups with more severe pain compared with the two groups with milder pain (P < 0.001 for all pairwise comparisons). The pattern remained largely similar even when the total costs were estimated without drug costs. Patients with more severe pain and longer time to depression diagnosis had the highest annual total costs both before (£925 at PRE) and after diagnosis of depression (£933 at POST1 and £988 at POST2). When the two groups with more severe pain were compared those with a longer time to depression diagnosis had higher 12-month total costs after depression diagnosis (POST1 andPOST2) compared with those who had a shorter time to depression diagnosis (P < 0.05). Likewise, pairwise comparisons between the two groups with milder pain showed that those with a longer time to depression diagnosis had higher total costs at POST1 than those with a shorter time to depression diagnosis (P < 0.05); cost comparisons at the other time points were not significant.
Table 4.
Estimated 12-month total costs per patienta before (PRE) and in the first 2 years after (POST1, POST2) diagnosis of depression for the four patient groups
| Milder pain and shorter time to depression diagnosis | Milder pain and longer time to depression diagnosis | More severe pain and shorter time to depression diagnosis | More severe pain and longer time to depression diagnosis | |
|---|---|---|---|---|
| Mean (SE) 12-month costs in pounds sterling (£) | ||||
| PRE | 582 (19) | 572 (18) | 844 (33)*,† | 925 (43)*,† |
| POST1 | 565 (17) | 628 (21)- | 820 (32)*,† | 933 (43)*,†,§ |
| POST2 | 599 (20) | 614 (19) | 819 (33)*,† | 988 (47)*,†,§ |
Notes:
Adjusted costs from GEE model with log link function (AR1 correlation): group and time variable included, adjusting for age, gender, and CCi. Total cost estimate based on total cost incurred by each patient. Bootstrapped t-tests for pairwise comparisons:
P < 0.001 versus milder pain and shorter time to depression diagnosis;
P < 0.001 versus milder pain and longer time to depression diagnosis;
P < 0.05 versus more severe pain and shorter time to depression diagnosis; ~P < 0.05 versus milder pain and shorter time to depression diagnosis.
Abbreviations: CCI, Charlson Comorbidity Index; GEE, generalized estimating equation; POST1, the first 12-month period following diagnosis of depression; POST2, the 12-month period after POST1; PRE, the 12-month period before diagnosis of depression; SE, bootstrapped standard errors (1,000 iterations).
The mean 12-month cost estimates of the various cost components (GP consultations, secondary care referrals, and pharmacotherapy) for the four groups of patients before and after depression diagnosis are presented in Figure 2. Total GP consultation costs (Figure 2A) were the largest cost component and were highest both before and after depression diagnosis in the group with more severe pain and longer time to depression diagnosis (P < 0.001 versus the other three groups). Secondary care referral costs (Figure 2B) were highest in the second year after depression diagnosis in the group with more severe pain and longer time to depression diagnosis (P < 0.05 versus the other three groups at POST2). Mean total drug costs per year (Figure 2C) were higher in the group with more severe pain and longer time to depression diagnosis compared with the two groups with milder pain (P < 0.001 for all pairwise comparisons), but were similar to the group with more severe pain and shorter time to depression diagnosis. Annual total drug costs were similar before and in the two years after depression diagnosis in all four patient groups.
Figure 2.
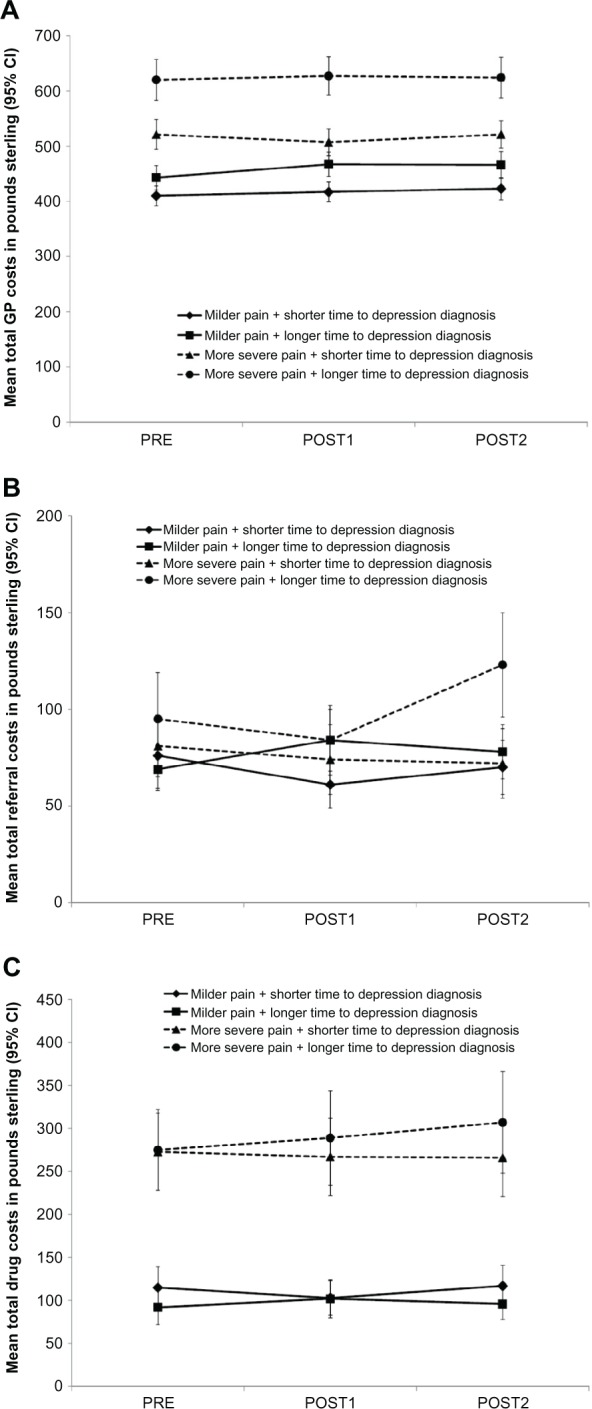
Estimated 12-month costs per patient before (PRE) and after (POST1, POST2) diagnosis of depression for the four patient groups for the following cost components: (A) total GP costs, (B) total referral costs, and (C) total drug costs.
Note: The sum of these cost component estimates is not exactly the same as the total cost estimates presented in Table 4 but is consistent with the total cost estimates.
Abbreviations: CI, confidence interval; GP, general practitioner; POST1, the first 12-month period following diagnosis of depression; POST2, the 12-month period after POST1; PRE, the 12-month period before diagnosis of depression.
There was no clear pattern of results when assessing 12-month costs over the PRE, POST1, and POST2 periods within each group. Neither patients with more severe pain nor those with a longer time to depression diagnosis demonstrated consistent increased costs at POST1.
Discussion
In this study, we identified primary care patients in the UK GPRD who had unexplained pain and a subsequent diagnosis of depression and estimated their annual health care utilization costs in the year before depression diagnosis and in the first 2 years after depression diagnosis. Our findings show that unexplained pain and depression impose a significant economic burden on the UK health care system.
Patients with more severe pain (based on prescription of at least three pain relief medications plus opioid use) and a longer time to depression diagnosis after identification of pain symptoms had higher total costs than those in the other three groups. More severe pain was the main factor driving costs; the two groups with more severe pain had higher annual costs in the year before and in each of the 2 years after depression diagnosis than the two groups with milder pain, after adjusting for age, sex, and CCI scores. Furthermore, comparisons between the two groups of patients with more severe pain showed that those with a longer time to depression diagnosis had higher annual total costs in the 2 years after depression diagnosis than those with a shorter time to depression diagnosis. However, a longer time to depression diagnosis was not associated with higher total costs among patients with milder pain except in the first year after depression diagnosis.
Several previous studies have shown that comorbid pain and depression is associated with increased use of health care services.13,16–19 In a GPRD study of adults with depression, 66% of patients also had codes for PPS, and patients with depression and concurrent pain had higher health care resource use (GP consultations, secondary care referrals, and drug use) than those with no pain.16
No previous studies have examined the impact of pain and depression on health care costs in the same way as our analysis. All patients in our study had a diagnosis of depression subsequent to a diagnosis of pain and we did not examine the health care costs associated with either condition alone. In a previous population-based study in Sweden, the average total health care costs (primary health care costs, hospital costs, and drug costs) per patient were higher for those with a diagnosis of both depression and back pain (SEK 46,909), compared with patients with a depression diagnosis (SEK 36,904) or a diagnosis of back pain (SEK 26,152).30 Comorbidity of depression and pain had a negative interaction effect on health care costs, such that the costs were less than additive.30 This negative interaction effect was largely related to lower hospital care costs, while there was actually an increase in the number of general practitioner visits among patients with both conditions.30
Primary care consultations were the largest cost component in our study and were highest in the group with more severe pain and a longer time to depression diagnosis. This group also had higher costs due to pain relief prescriptions and to secondary care referrals in year two after depression diagnosis. Our findings suggest that there may be potential cost savings for the UK NHS with earlier diagnosis of depression in patients presenting with more severe unexplained pain.
PPS and depression appear to be closely linked, but the relationship is complex and not yet fully understood: depression may be both a cause and a consequence of PPS.10,11,31 Moreover, when pain and depression are both present, they impact on each other,11,32 which may refect common underlying neurobiological mechanisms.10,33 However, it has been estimated that about half of all patients with depression may go undiagnosed because they present with somatic or physical symptoms.31 Primary care physicians often fail to look for symptoms of depression in patients presenting with pain despite reports that about 50%–80% of patients with depression initially have somatic symptoms including PPS,34,35 and that there is an increased risk of depression in patients presenting with pain.36 Particular attention should be given to unexplained pain as it is associated with increased depression comorbidity and poorer outcomes.33 A recent study in primary care found that 80% of patients presenting with unexplained chronic pain had an undiagnosed depression or anxiety disorder.13 Although medication for pain may be initiated, delayed recognition of depression and initiation of antidepressant treatment may negatively affect the course and severity of illness and result in poorer treatment outcomes. These results highlight the need for education on the role of pain and unexplained painful symptoms in depression for primary care providers.
Our study is the first to examine the economic impact of pain severity and time to depression diagnosis in patients presenting initially with pain. Previous studies have found that moderate to severe pain is common in patients with depression.31,37 More severe pain has been associated with more severe depression,38 a worse course of depression,10,39 a lower likelihood of or longer time to response or remission,15,40,41 a poorer health-related quality of life,14 and increased health care utilization.10 Recent data extracted from the GPRD has shown greater health service use and costs in patients with non-remission of depression compared with those who achieve remission (defined as successful cessation of antidepressant treatment for at least 6 months).42
We included a large sample of patients from the GPRD, which is representative of the UK population, and for which data are recorded during the consultation. Also, we included only data from general practices that were considered to be of a suitable standard by the GPRD. Moreover, when estimating costs, we controlled for patient demographics and comorbidities. This is important because recent data from the GPRD had shown that the incidence of depression varies according to age and sex.43 Chronic medical illnesses (eg, osteoarthritis, rheumatoid arthritis, diabetes, coronary artery disease, congestive heart failure, asthma, and COPD) are common comorbidities in patients with depression and pain.44,45 However, the CCI score for the primary care patients with pain and depression in the present study was very low, indicating that they have a low risk of mortality as a result of their comorbid diseases.27 The low prevalence of comorbidities at baseline resulted from the exclusion of patients with any diagnoses (other than depression) recorded 90 days on either side of the frst pain code. Because we selected patients without any comorbid diagnosis to ensure that the unexplained pain was not due to any other medical conditions, the costs in our study may be underestimated. It is likely that patients with depression and pain with comorbid conditions would have higher medical costs.
There are some limitations that need to be considered when interpreting the results. First, selection of patients was based on diagnostic Read codes and we do not know whether the episode of depression identified by the diagnostic code was related to the pain identified by the pain code. Previous research has indicated that the relationship between pain and depression is bidirectional,11 but our study can provide no information on cause and effect relationships. Second, the dates of diagnosis of pain and depression were based on the first recorded diagnostic codes for PPS and depression during the 5-year study period. Although the validity of the medical diagnoses in the GPRD has been confirmed,20,22 we did find considerable antidepressant use in the 12 months prior to depression diagnosis which tends to blur the diagnostic picture. This may account for the smaller than expected differences within groups over the time periods (especially PRE vs POST1). However, the statistically significant difference in health care costs between the groups at each time period remains a robust fnding. Moreover, a register-based study in Sweden found that almost 30% of patients with a depression diagnosis were treated with antidepressants during the year before diagnosis.46 There may be considerable variation among GPs in the specific diagnostic codes used for recording depression and in the date of entry of a diagnostic code. Thus, we used a large number of diagnostic Read codes for depression, covering a wide range of depression disorders. This lack of standardization of diagnostic codes can cause problems of interpretation between comparison groups, but the sample size within each group is sufficiently large to be valid. We were restricted to capturing the record of depression diagnosis rather than when the depression episode occurred in relation to the pain symptoms. In addition, severity of depression was not reliably available in the GPRD and, therefore, the cut-off for differentiating between a longer and shorter time to depression diagnosis was chosen as 1 year. Another cut-off time period may have produced different results. The 1-year cut-off was not based on any standardized reference as information on average time from presentation of PPS to depression diagnosis is not available. The cut-off time was based on the median time to depression diagnosis within our data and on clinical opinion that the cut-off should be long enough for PPS unrelated to depression to resolve spontaneously, such that we would expect unrelated PPS to resolve within less than 1 year, whereas PPS lasting for more than 1 year would be a more chronic condition and have implications for NHS health care costs. Additionally, using the 1-year cut-off allowed groups of approximately equal sizes. Third, pain severity was classified indirectly, based on the number of pain relief prescriptions, and did not take into account the use of over-the-counter (OTC) drugs. Therefore, patients who exclusively used OTC pain relief were excluded from the study; such patients are likely to have milder pain. Patients in the milder pain group may have used more than three pain relief medications due to the possible use of OTC drugs in addition to prescribed pain medication. Patients with more severe pain are less likely to use OTC drugs and we consider that patients with at least three pain relief prescriptions can be regarded as having more severe pain. Excluding those with OTC drug use/milder pain from the mild pain groups has potentially increased mean resource use, strengthening the between-group differences identified. Moreover, patients in the more severe pain group were required to be prescribed at least one opioid analgesic. It is important to note that the data refects the prescription of medication and not the dispensing or taking of the medication. Fourth, it is possible that those with unexplained pain may have received a diagnosis outside of the 90 days on either side of the pain symptom record to account for their pain. This is a more conservative approach than has been used previously (30 days on either side of the pain symptom)16 to limit misclassification and results in a relatively small sample size which does not refect the prevalence of PPS in patients receiving a depression diagnosis. By eliminating patients with comorbidities in this way in order to have confidence that the recorded pain was not related to an explainable cause, we may have eliminated patients with more severe or more chronic depression, thus underestimating costs. More accurate data collection would be achieved if there was a diagnostic code that is uniformly used to record unexplained pain. Finally, we analyzed only direct medical costs for the UK NHS and did not consider other costs (eg, costs of OTC drugs), or indirect costs, such as costs due to lost productivity, which are a major component of the total cost of depression.5
Conclusion
Our results show that UK primary care patients with more severe pre-existing unexplained pain and a subsequent diagnosis of depression have greater health care service costs, especially drug costs, than patients with milder pain. Pain (particularly more severe pain) in combination with longer time from pain symptoms to depression diagnosis contribute to higher overall costs for the UK health care system compared to a shorter time to depression diagnosis. Our findings suggest that earlier diagnosis of depression in primary care patients presenting with unexplained pain may lead to health care cost savings.
Acknowledgments
The study was sponsored by Eli Lilly and Company Limited. The authors thank Deirdre Elmhirst, PhD, for her help in the editorial development of the manuscript.
Footnotes
Disclosures
Catherine Reed, Diego Novick, Alan Lenox-Smith, and Michael Happich are employees of Eli Lilly and Company Limited. Jihyung Hong is a consultant for Eli Lilly and Company Limited. The authors have no other conflicts to disclose.
References
- 1.Wittchen HU, Jacobi F, Rehm J, et al. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21(9):655–679. doi: 10.1016/j.euroneuro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Sobocki P, Jönsson B, Angst J, Rehnberg C. Cost of depression in Europe. J Ment Health Policy Econ. 2006;9(2):87–98. [PubMed] [Google Scholar]
- 3.Vedavanam S, Steel N, Broadbent J, Maisay S, Howe A. Recorded quality of care for depression in general practice: an observational study. Br J Gen Pract. 2009;59(559):e32–e37. doi: 10.3399/bjgp09X395085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scott J, Thorne A, Horn P. Quality improvement report: Effect of a multifaceted approach to detecting and managing depression in primary care. BMJ. 2002;325(7370):951–954. doi: 10.1136/bmj.325.7370.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCrone P, Dhanasiri S, Patel A, Knapp M, Lawton-Smith S. Paying the Price. The cost of mental health care in England to 2026. London: The King’s Fund; 2008. [March 26, 2012]. Available from: http://www.kingsfund.org.uk/sites/files/kf/Paying-the-Price-the-cost-of-mental-health-care-England-2026-McCrone-Dhanasiri-Patel-Knapp-Lawton-Smith-Kings-Fund-May-2008_0.pdf. [Google Scholar]
- 6.Gilbody SM, Whitty PM, Grimshaw JM, Thomas RE. Improving the detection and management of depression in primary care. Qual Saf Health Care. 2003;12(2):149–155. doi: 10.1136/qhc.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell AJ, Vaze A, Rao S. Clinical diagnosis of depression in primary care: a meta-analysis. Lancet. 2009;374(9690):609–619. doi: 10.1016/S0140-6736(09)60879-5. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell AJ, Rao S, Vaze A. International comparison of clinicians’ ability to identify depression in primary care: meta-analysis and meta-regression of predictors. Br J Gen Pract. 2010;61(583):e72–e80. doi: 10.3399/bjgp11X556227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendrick T, Peveler R. Guidelines for the management of depression: NICE work? . Br J Psychiatry. 2010;197(5):345–347. doi: 10.1192/bjp.bp.109.074575. [DOI] [PubMed] [Google Scholar]
- 10.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163(20):2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 11.Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12(9):964–973. doi: 10.1016/j.jpain.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohayon MM, Schatzberg AF. Chronic pain and major depressive disorder in the general population. J Psychiatr Res. 2010;44(7):454–461. doi: 10.1016/j.jpsychires.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Agüera L, Failde I, Cervilla JA, Díaz-Fernández P, Mico JA. Medically unexplained pain complaints are associated with underlying unrecognized mood disorders in primary care. BMC Family Pract. 2010;11:17. doi: 10.1186/1471-2296-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bair MJ, Robinson RL, Eckert GJ, Stang PE, Croghan TW, Kroenke K. Impact of pain on depression treatment response in primary care. Psychosom Med. 2004;66(1):17–22. doi: 10.1097/01.psy.0000106883.94059.c5. [DOI] [PubMed] [Google Scholar]
- 15.Karp JF, Scott J, Houck P, Reynolds CF, 3rd, Kupfer DJ, Frank E. Pain predicts longer time to remission during treatment of recurrent depression. J Clin Psychiatry. 2005;66(5):591–597. doi: 10.4088/jcp.v66n0508. [DOI] [PubMed] [Google Scholar]
- 16.Watson L, Baird J, Hösel V, Peveler R. The effect of concurrent pain on the management of patients with depression: an analysis of NHS health care resource utilization using the GPRD database. Int J Clin Pract. 2009;63(5):698–706. doi: 10.1111/j.1742-1241.2009.02017.x. [DOI] [PubMed] [Google Scholar]
- 17.Bao Y, Sturm R, Croghan TW. A national study of the effect of chronic pain on the use of health care by depressed persons. Psychiatr Serv. 2003;54(5):693–697. doi: 10.1176/appi.ps.54.5.693. [DOI] [PubMed] [Google Scholar]
- 18.Gameroff MJ, Olfson M. Major depressive disorder, somatic pain, and health care costs in an urban primary care practice. J Clin Psychiatry. 2006;67(8):1232–1239. doi: 10.4088/jcp.v67n0809. [DOI] [PubMed] [Google Scholar]
- 19.Arnow BA, Blasey CM, Lee J, et al. Relationships among depression, chronic pain, chronic disabling pain, and medical costs. Psychiatr Serv. 2009;60(3):344–350. doi: 10.1176/ps.2009.60.3.344. [DOI] [PubMed] [Google Scholar]
- 20.Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69(1):4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.General Practice Research Database [database on the Internet] London: The Clinical Practice Research Datalink Group; 2012. [March 21, 2012]. Available from: http://www.cprd.com/intro.asp. [Google Scholar]
- 22.Khan NF, Harrison SE, Rose PW. Validity of diagnostic coding within the General Practice Research Database: a systematic review. Br J Gen Pract. 2010;60(572):e128–e136. doi: 10.3399/bjgp10X483562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NHS Connecting for Health TRUD Service. Clinical Terminology Browser v1.04 [webpage on the Internet] London: Department of Health; 2012. [July 31, 2010]. Available from http://www.uktcregistration.nss.cfh.nhs.uk/trud3/user/guest/group/0/pack/9;jsessionid=46E878BE5BB11B5696FC82E6F16A6975. [Google Scholar]
- 24.Martinez C, Rietbrock S, Wise L, et al. Antidepressant treatment and the risk of fatal and non-fatal self harm in first episode depression: nested case-control study. BMJ. 2005;330(7488):389–393. doi: 10.1136/bmj.330.7488.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 26.Charlson M, Szatrowski T P, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 27.Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract. 2010;11:1. doi: 10.1186/1471-2296-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis L. Unit Costs of Health and Social Care 2009. Canterbury: PSSRU University of Canterbury; 2009. [November 11, 2012]. Available from http://www.pssru.ac.uk/archive/pdf/uc/uc2009/uc2009.pdf. [Google Scholar]
- 29.IMS Health Inc. PADDS. 2009. [November 11, 2012]. Available at http://www.imspadds.com/midas/
- 30.Carstensen J, Andersson D, André M, Engström S, Magnussen H, Borgquist LA. How does comorbidity infuence health care costs? A population-based cross-sectional study of depression, back pain and osteoarthritis. BMJ Open. 2012;2(2):e000809. doi: 10.1136/bmjopen-2011-000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Cebrian A, Gandhi P, Demyttenaere K, Peveler R. The association of depression and painful physical symptoms – a review of the European literature. Eur Psychiatry. 2006;21(6):379–388. doi: 10.1016/j.eurpsy.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Linton SJ, Bergbom S. Understanding the link between depression and pain. Scand J Pain. 2011;2(2):47–54. doi: 10.1016/j.sjpain.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Beesdo K, Jacobi F, Hoyer J, Low NC, Höfer M, Wittchen HU. Pain associated with specifc anxiety and depressive disorders in a nationally representative population sample. Soc Psychiatry Psychiatr Epidemiol. 2010;45(1):89–104. doi: 10.1007/s00127-009-0045-1. [DOI] [PubMed] [Google Scholar]
- 34.Kirmayer LJ, Robbins JM, Dworkind M, Yaffe MJ. Somatization and the recognition of depression and anxiety in primary care. Am J Psychiatry. 1993;150(5):734–741. doi: 10.1176/ajp.150.5.734. [DOI] [PubMed] [Google Scholar]
- 35.Simon GE, VonKorff M, Piccinelli M, Fullerton C, Ormel J. An international study of the relation between somatic symptoms and depression. N Engl J Med. 1999;341(18):1329–1335. doi: 10.1056/NEJM199910283411801. [DOI] [PubMed] [Google Scholar]
- 36.Arola HM, Nicholls E, Mallen C, Thomas E. Self-reported pain interference and symptoms of anxiety and depression in community-dwelling older adults: can a temporal relationship be determined? Eur J Pain. 2010;14(9):966–971. doi: 10.1016/j.ejpain.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Demyttenaere K, Reed C, Quail D, et al. Presence and predictors of pain in depression: results from the FINDER study. J Affect Disord. 2010;125(1–3):53–60. doi: 10.1016/j.jad.2010.02.106. [DOI] [PubMed] [Google Scholar]
- 38.Vaccarino AL, Sills TL, Evans KR, Kalali AH. Multiple pain complaints in patients with major depressive disorder. Psychsom Med. 2009;71(2):159–162. doi: 10.1097/PSY.0b013e3181906572. [DOI] [PubMed] [Google Scholar]
- 39.Gerrits MM, Vogelzangs N, van Oppen P, van Marwijk HW, van der Horst H, Penninx BW. Impact of pain on the course of depressive and anxiety disorders. Pain. 2012;152(2):429–436. doi: 10.1016/j.pain.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Demyttenaere K, Verhaeghen A, Dantchev N, et al. “Caseness” for depression and anxiety in a depressed outpatient population: symptomatic outcome as a function of baseline diagnostic categories. Prim Care Companion J Clin Psychiatry. 2009;11(6):307–315. doi: 10.4088/PCC.08m00748blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leuchter AF, Husain MM, Cook IA, et al. Painful physical symptoms and treatment outcome in major depressive disorder: a STAR*D (Sequenced Treatment Alternatives to Relieve Depression) report. Psychol Med. 2010;40(2):239–251. doi: 10.1017/S0033291709006035. [DOI] [PubMed] [Google Scholar]
- 42.Byford S, Barrett B, Despiégel N, Wade A. Impact of treatment success on health service use and cost in depression: longitudinal database analysis. Pharmacoeconomics. 2011;29(2):157–170. doi: 10.2165/11537360-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.Moore M, Yuen HM, Dunn N, Mullee A, Maskell J, Kendrick T. Explaining the rise in antidepressant prescribing: a descriptive study using the general practice research database. BMJ. 2009;339:b3999. doi: 10.1136/bmj.b3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin-Merino E, Ruigómez A, Johansson S, Wallander MA, García-Rodriguez LA. Study of a cohort of patients newly diagnosed with depression in general practice: prevalence, incidence, comorbidity and treatment patterns. Prim Care Companion J Clin Psychiatry. 2010;12((1)) doi: 10.4088/PCC.08m00764blu. PCC.08m00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry. 2007;29(2):147–155. doi: 10.1016/j.genhosppsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 46.Andersson D, Magnusson H, Carstensen J, Borgquist L. Co-morbidity and healthcare utilisation five years prior to diagnosis for depression. A register-based study in a Swedish population. BMC Public Health. 2011;11:552. doi: 10.1186/1471-2458-11-552. [DOI] [PMC free article] [PubMed] [Google Scholar]