Abstract
Purpose
To investigate changes in the area of the foveal avascular zone (FAZ) in patients with retinal vascular disease.
Patients and methods
This retrospective, consecutive study examined 53 eyes of 53 patients with macular edema due to branch retinal vein occlusion in 25 patients (47.2%) and nonproliferative diabetic retinopathy in 28 patients (52.8%). The macular edema was treated with an intravitreal injection of 0.05 mL equal to 1.25 mg bevacizumab. Before and 6–8 weeks after the injection, best corrected visual acuity, slit lamp biomicroscopy of the anterior segment and fundus, optical coherence tomography, and fluorescein angiography were conducted. The FAZ was manually circumscribed on early-phase angiography images and the area of the FAZ was measured.
Results
The preoperative overall mean FAZ area was 0.327 ± 0.126 mm2 (median 0.310 mm2). At the control consultation, the overall mean area was significantly larger (0.422 ± 0.259 mm2; median 0.380 mm2; P < 0.001). In the nonproliferative diabetic retinopathy subpopulation, the mean area was 0.361 ± 0.129 mm2 (median 0.330 mm2) before bevacizumab application and 0.434 mm2 at the follow-up visit (mean increase 0.071 mm2/19.7%). In the branch retinal vein occlusion group, the baseline FAZ area was 0.290 ± 0.115 mm2 and 0.407 ± 0.350 mm2 at follow-up (median 0.330 mm2; mean increase 0.117 mm2/40.3%). No cases of severe operation-associated complications were observed.
Conclusion
The results confirm the safety of intravitreal bevacizumab injection in patients with macular edema due to nonproliferative diabetic retinopathy and branch retinal vein occlusion. The enlargement of the FAZ could be equivalent to an increase in retinal ischemia. These results may be transient; a potential vascular risk, however, when applying antivascular endothelial growth factor therapy in eyes with preexistent vascular disease must be considered.
Keywords: foveal avascular zone, ischemia, diabetic retinopathy, branch retinal vein occlusion, anti-VEGF
Introduction
Management of macular edema following branch retinal vein occlusion (BRVO) and nonproliferative diabetic retinopathy (NPDR) is challenging. Except photocoagulation, which used to be the initial therapy of choice, the pharmacologic inhibition of vascular endothelial growth factor (VEGF) shows promise.1,2 In the meantime, ranibizumab has become the therapy of choice for BRVO with macular edema and a visual acuity of 20/40 and less.
VEGF is a multifunctional cytokine. It is not only crucial for cell proliferation, cell migration, proteolysis, cell survival, and maintenance of the choriocapillaris, it also facilitates survival and development of retinal neurons following ischemia and it is necessary for preservation of normal vessels.3–7 Its role in angiogenesis and organogenesis is vital; the loss of one single VEGF allele results in embryonic death.8 One of its major inducers is hypoxia.9 Therefore, it plays an important role in ocular diseases in which retinal malperfusion is developing, eg, diabetic macular disease or BRVO.10 Furthermore, VEGF stimulates endothelial mitogenesis, promotes endothelial survival, and controls vascular permeability. It is 50,000 times more potent in inducing vascular leakage than histamine, causing macular edema when released in the vitreous cavity of monkeys.11,12 Development of macular edema causes impairment of visual acuity in these pathological conditions.
There is great evidence from large prospective randomized trials for the efficacy of anti-VEGF therapy in diabetic macular edema (DME), macular edema following BRVO, and age-related macular degeneration.13–22 A novel agent, aflibercept (Eylea®, Regeneron Pharmaceuticals, Inc, Tarrytown, NY, USA), now augments the pharmacological anti-VEGF armamentarium.23 This soluble decoy receptor shows a higher affinity and faster association to VEGF-A than ranibizumab or bevacizumab.24
This study presents two groups of patients who were diagnosed with macular disease secondary to DME or BRVO and consequently treated with a single dose of intravitreal bevacizumab. Both groups demonstrated a significantly enlarged foveal avascular zone (FAZ) at the 6–8-week follow-up.
Material and methods
In this retrospective consecutive study, more than 200 patients with NPDR or BRVO who visited the Department of Ophthalmology of Technical University of Munich (Munich, Germany) between June 2008 and December 2009 were screened, and 53 patients were included. These 53 individuals suffered from macular edema due to NPDR or BRVO and also had a well-circumscribed FAZ, both visible on fluorescein angiography. The study was approved by the local institutional review board (Ethics Commission, Faculty of Medicine, Technical University of Munich) and informed consent was obtained from every patient for the intravitreal injection. The subjects were also provided information about the off-label use of the drug.
The mean age of the patients was 67.4 years; 31 female (58.5%) and 22 male (41.5%) patients were included. The macular edema was caused by NPDR in 28 patients (52.8%) and by BRVO within the last 6 months in 25 patients (47.2%). The exclusion criteria were macular disease secondary to causes other than DME or BRVO, any ongoing ocular or periocular inflammation, any kind of ocular surgeries on the studied eye within the last 6 months, or any previous treatment of the macular edema (eg, any intravitreal injection for macular edema in the last 6 months or laser treatment at any time in the past for macular edema).
Examinations at baseline included best corrected visual acuity with a Snellen chart at a distance of 20 feet, slit lamp biomicroscopy of the anterior segment and fundus (Slit Lamp BM 900®; Haag-Streit AG, Koeniz, Switzerland), optical coherence tomography (Spectralis®; Heidelberg Engineering, Heidelberg, Germany), and fluorescein angiography (Heidelberg Retina Angiograph II; Heidelberg Engineering).
The intravitreal injection of 0.05 mL with 1.25 mg bevacizumab was performed in accordance with the guidelines for intravitreal injections published by the Macula Committee of the German Ophthalmological Society and the Professional Association of Ophthalmologists of Germany.25 Before injection administration, the eye was washed with povidone-iodine (5%) and the eyelashes and lid region were then wiped, also with povidone-iodine (5%). The drape (as used for intraocular intervention) was applied, a lid speculum was inserted. After dislocation of the conjunctivae, the injection was performed at a distance of 3.5 mm from the limbus. Consecutively, each patient received an injection of 1.25 mg bevacizumab (Avastin®; F Hoffmann-La Roche, Basel, Switzerland). Antibiotic eye drops were then applied. At day one and day three after the injection, a slit lamp examination was performed to rule out intraocular inflammation or elevated intraocular pressure.
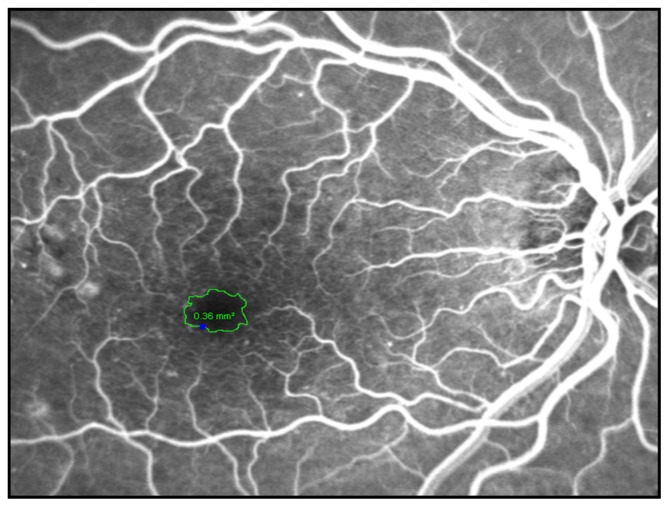
In order to visualize the FAZ, the angiography was performed with scanning laser technology, and only patients with good quality early-phase fluorescein angiograms without any bleeds or exudates hindering visualization of the macular region were included for evaluation. The FAZ was manually circumscribed by two experienced retina consultants in these early-phase fluorescein angiograms, once by each of them (Figure 1). Heidelberg Eye Explorer software (version 1.7.0.0; Heidelberg Engineering) yielded the area (in mm2). The mean value was used for further calculations and patients with differing values of more than 0.05 mm2 were excluded.
Figure 1.
The foveal avascular zone was manually circumscribed on an early-phase fluorescein angiogram and the software measured the area (in mm2).
Patients returned to the outpatient clinic for routine postinjection follow-up. Best corrected visual acuity, slit lamp biomicroscopy of the anterior segment and fundus, optical coherence tomography, and fluorescein angiography were conducted again at 6–8 weeks postinjection. The area of FAZ was assessed again in the above described manner. Statistical analysis was performed using SPSS version 13 (SPSS Inc, Chicago, IL, USA) with the Wilcoxon matched-pairs test and t-test.
Results
All patients tolerated the injection well. There were no cases of operation-associated complications such as retinal detachment, endophthalmitis, or persistent elevated intraocular pressure. Two patients reported ocular discomfort for a few days, and seven patients (13.2%) showed a mild hyposphagma at the injection site that disappeared without further treatment within 10 days. The mean FAZ was significantly larger at the control visit after 6–8 weeks compared to the previous values for all patients (Wilcoxon: P < 0.001). Preoperative parameters showed a mean area of 0.327 ± 0.126 mm2 (median 0.310 mm2). After the first visit, the mean area increased to 0.422 ± 0.259 mm2 (median 0.380 mm2) (Table 1; Figure 2).
Table 1.
Foveal avascular zone area measurements
| Foveal avascular zone (mm2) | DME | BRVO | Overall | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Pre OP | FU1 | Pre OP | FU1 | Pre OP | FU1 | |
| Mean | 0.36 | 0.43 | 0.29 | 0.41 | 0.33 | 0.42 |
| Median | 0.33 | 0.42 | 0.28 | 0.33 | 0.31 | 0.38 |
| First quartile | 0.26 | 0.34 | 0.21 | 0.26 | 0.24 | 0.30 |
| Third quartile | 0.46 | 0.51 | 0.36 | 0.41 | 0.41 | 0.47 |
| Minimum | 0.14 | 0.16 | 0.04 | 0.07 | 0.04 | 0.07 |
| Maximum | 0.63 | 0.71 | 0.62 | 1.96 | 0.63 | 1.96 |
| Standard deviation | 0.13 | 0.14 | 0.11 | 0.35 | 0.13 | 0.26 |
Abbreviations: BRVO, branch retinal vein occlusion; DME, diabetic macular edema; FU1, first follow-up visit; Pre OP, before injection of bevacizumab.
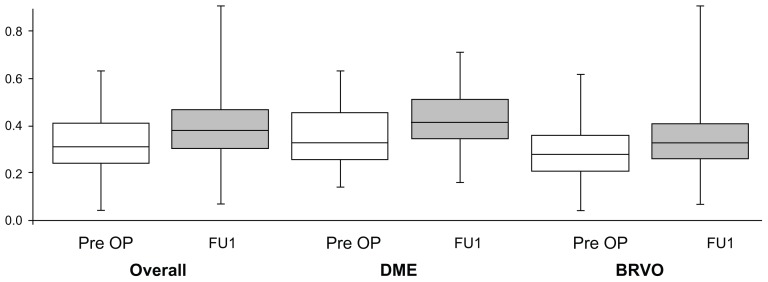
Figure 2.
Box plots summarize the increase in foveal avascular zone area (mm2) at FU1 compared to pre OP measurements for all patients (overall) and the patients with DME and BRVO.
Abbreviations: BRVO, branch retinal vein occlusion; DME, diabetic macular edema; FU1, first follow-up visit; Pre OP, before injection of bevacizumab.
An analysis to differentiate between the underlying diagnoses was also performed. In the NPDR group (n = 28), the baseline mean FAZ area was 0.361 ± 0.129 mm2 (median 0.330 mm2). At the follow-up, the mean area increased by 0.071 mm2 (or 19.7%) to 0.434 mm2 (Table 1; Figure 2).
The subjects with BRVO (n = 25) showed a slightly smaller FAZ area at baseline compared to the diabetic group (0.290 ± 0.115 mm2 versus 0.361 ± 0.129 mm2). At the follow-up, the mean area increased by 0.117 mm2 (or 40.3%) to 0.407 ± 0.350 mm2 (median 0.330 mm2; Table 1; Figure 2).
Discussion
In February 2004, the US Food and Drug Administration approved bevacizumab as the first angiogenesis inhibitor for first-line treatment of cancer; and favorable results led to extended use in non-small-cell lung cancer, kidney cancer, and glioblastoma. As a nonselective inhibitor of all isoforms of the VEGF, this humanized antibody also suppresses vital physiological functions like its vasodilatory effect or the stimulated expression of plasminogen activators, resulting in arterial thromboembolic events.26–31 Ultrastructural analyses showed the reduction of choriocapillaris endothelial cell fenestrations and emerging thrombosis.32–34 Evidence for vasoconstriction of retinal vessels was published, altogether raising concerns about retinal vascular events.35–37
This retrospective study found a significant increase in the area of the FAZ 6–8 weeks after a single intravitreal injection of 1.25 mg bevacizumab. There were no significant complications after intravitreal bevacizumab, including endophthalmitis, retinal detachment, cataract formation, increased intraocular pressure, or retinal artery occlusion. The presented results are in accordance with previous observations.38 Several multicenter clinical trials also did not detect any elevated incidence of severe side effects in anti-VEGF therapy; and reports on increased intraocular pressure and ischemia are seldom, with no adverse event rate exceeding 0.21%.39–45 Mansour et al determined the overall risk of ocular vascular events following VEGF antagonist injection as 0.108% in a general population and 2.61% in diabetic patients, and refer to the patients’ perioperative stress and the natural history of their conditions.46
There are several publications that attempt to measure retinal ischemia on fluorescence angiography as the size of the FAZ. Neubauer et al used a high-resolution ultra wide-field scanning ophthalmoscopy system for counting retinal fields of nonperfusion in a group of 19 nonresponders to photocoagulation.47 The number of avascular fields dropped significantly, but the diameter of the FAZ remained quasi unchanged, as it did in a prospective, consecutive, noncomparative case series with 126 diabetic pretreated patients at a 12-month follow-up.14 Another group measured an area of capillary nonperfusion in the context of intravitreal bevacizumab for macular edema following BRVO; they found an unchanged mean area of nonperfusion.48 One reason for this could be the slightly different period of time between intervention and the second data acquisition. Nevertheless, the current study yielded different results.
A statistically significant enlargement of the FAZ 6 to 8 weeks after one intravitreal injection of 1.25 mg bevacizumab in patients with macular edema both secondary to NPDR and BRVO was found. This effect may be transient. Papadopoulou et al found a vasoconstrictor impact of ranibizumab on retinal arteries after application of ranibizumab, suggesting that closure of the smallest vessels approaching the FAZ could enlarge this area on fluorescein angiography.35 A recent report defined the FAZ as a factor of macular ischemia and found a negative effect of an enlarged FAZ on short-term visual outcomes after intravitreal bevacizumab for DME.49
Some limitations are inherent in the current study. First, the design was retrospective, the sample size was small (both groups totaled 53 eyes), and there was no control group. As the natural course of the disease also leads to increasing vascular stress and enlargement of the FAZ, it is not known without doubt that the results are secondary to the use of bevacizumab. Second, this one-time follow-up of the patients does not describe the development of the FAZ over time and the results may be transient, especially since the effects of intravitreal bevacizumab have been reported to vanish within weeks after a single application.50 Third, the period from BRVO or the onset of diabetes to the application of bevacizumab was not defined. It is likely that different durations of ischemia yield a varying response to anti-VEGF treatment.
Conclusion
The results show that intravitreal injection of anti-VEGF drugs may induce an increase in the area of the FAZ, a morphological retinal change visualized by fluorescein angiography. In the light of retinal ischemia, this may cause further problems. Altogether, the findings emphasize only one single aspect of intravitreal anti-VEGF treatment in patients with preexistent vascular disease; they underline the necessity to consider the potential vascular risk when applying anti-VEGF therapy in eyes with preexistent vascular disease, which is common in patients with DME or BRVO. A standardized method to assess the FAZ and further investigations are necessary to describe the relationship between intravitreal bevacizumab, microvascular changes in the FAZ, and long-term visual acuity results.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;98(Suppl 5):766–785. [PubMed] [Google Scholar]
- 2.The Branch Vein Occlusion Study Group. Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984;98(3):271–282. doi: 10.1016/0002-9394(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 3.Thakker GD, Hajjar DP, Muller WA, Rosengart TK. The role of phosphatidylinositol 3-kinase in vascular endothelial growth factor signaling. J Biol Chem. 1999;274(15):10002–10007. doi: 10.1074/jbc.274.15.10002. [DOI] [PubMed] [Google Scholar]
- 4.Tilton RG, Chang KC, LeJeune WS, Stephan CC, Brock TA, Williamson JR. Role for nitric oxide in the hyperpermeability and hemodynamic changes induced by intravenous VEGF. Invest Ophthalmol Vis Sci. 1999;40(3):689–696. [PubMed] [Google Scholar]
- 5.Bernatchez PN, Rollin S, Soker S, Sirois MG. Relative effects of VEGF-A and VEGF-C on endothelial cell proliferation, migration and PAF synthesis: role of neuropilin-1. J Cell Biochem. 2002;85(3):629–639. doi: 10.1002/jcb.10155. [DOI] [PubMed] [Google Scholar]
- 6.Marneros AG, Fan J, Yokoyama Y, et al. Vascular endothelial growth factor expression in the retinal pigment epithelium is essential for choriocapillaris development and visual function. Am J Pathol. 2005;167(5):1451–1459. doi: 10.1016/S0002-9440(10)61231-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saint-Geniez M, Maldonado AE, D’Amore PA. VEGF expression and receptor activation in the choroid during development and in the adult. Invest Ophthalmol Vis Sci. 2006;47(7):3135–3142. doi: 10.1167/iovs.05-1229. [DOI] [PubMed] [Google Scholar]
- 8.Haigh JJ. Role of VEGF in organogenesis. Organogenesis. 2008;4(4):247–256. doi: 10.4161/org.4.4.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 10.Funatsu H, Yamashita H, Noma H, Mimura T, Yamashita T, Hori S. Increased levels of vascular endothelial growth factor and interleukin-6 in the aqueous humor of diabetics with macular edema. Am J Ophthalmol. 2002;133(1):70–77. doi: 10.1016/s0002-9394(01)01269-7. [DOI] [PubMed] [Google Scholar]
- 11.Ozaki H, Hayashi H, Vinores SA, Moromizato Y, Campochiaro PA, Oshima K. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood–retinal barrier in rabbits and primates. Exp Eye Res. 1997;64(4):505–517. doi: 10.1006/exer.1996.0239. [DOI] [PubMed] [Google Scholar]
- 12.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146(5):1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 13.Chang TS, Bressler NM, Fine JT, Dolan CM, Ward J, Klesert TR. Improved vision-related function after ranibizumab treatment of neovascular age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 2007;125(11):1460–1469. doi: 10.1001/archopht.125.11.1460. [DOI] [PubMed] [Google Scholar]
- 14.Kook D, Wolf A, Kreutzer T, et al. Long-term effect of intravitreal bevacizumab (Avastin) in patients with chronic diffuse diabetic macular edema. Retina. 2008;28(8):1053–1060. doi: 10.1097/IAE.0b013e318176de48. [DOI] [PubMed] [Google Scholar]
- 15.Prager F, Michels S, Kriechbaum K, et al. Intravitreal bevacizumab (Avastin) for macular oedema secondary to retinal vein occlusion: 12-month results of a prospective clinical trial. Br J Ophthalmol. 2009;93(4):452–456. doi: 10.1136/bjo.2008.141085. [DOI] [PubMed] [Google Scholar]
- 16.Kondo M, Kondo N, Ito Y, et al. Intravitreal injection of bevacizumab for macular edema secondary to branch retinal vein occlusion: results after 12 months and multiple regression analysis. Retina. 2009;29(9):1242–1248. doi: 10.1097/IAE.0b013e3181aa8e20. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Gregori NZ, Gaitan J, Rosenfeld PJ, et al. Long-term safety and efficacy of intravitreal bevacizumab (Avastin) for the management of central retinal vein occlusion. Retina. 2008;28(9):1325–1337. doi: 10.1097/IAE.0b013e318188501f. [DOI] [PubMed] [Google Scholar]
- 20.Gregori NZ, Rattan GH, Rosenfeld PJ, et al. Safety and efficacy of intravitreal bevacizumab (Avastin) for the management of branch and hemiretinal vein occlusion. Retina. 2009;29(7):913–925. doi: 10.1097/IAE.0b013e3181aa8dfe. [DOI] [PubMed] [Google Scholar]
- 21.Campochiaro PA, Brown DM, Awh CC, et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology. 2011;118(10):2041–2049. doi: 10.1016/j.ophtha.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 22.Heier JS, Campochiaro PA, Yau L, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012;119(4):802–809. doi: 10.1016/j.ophtha.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Boyer D, Heier J, Brown DM, et al. Vascular endothelial growth factor Trap-Eye for macular edema secondary to central retinal vein occlusion: six-month results of the phase 3 COPERNICUS study. Ophthalmology. 2012;119(5):1024–1032. doi: 10.1016/j.ophtha.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos N, Martin J, Ruan Q, et al. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15(2):171–185. doi: 10.1007/s10456-011-9249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaissle GB, Szurman P, Bartz-Schmidt KU. Recommendation for the implementation of intravitreal injections – statement of the German Retina Society, the German Society of Ophthalmology (DOG) and the German Professional Association of Ophthalmologists (BVA) Klin Monbl Augenheilkd. 2005;222(5):390–395. doi: 10.1055/s-2005-858231. German. [DOI] [PubMed] [Google Scholar]
- 26.Scappaticci FA, Skillings JR, Holden SN, et al. Arterial thromboembolic events in patients with metastatic carcinoma treated with chemotherapy and bevacizumab. J Natl Cancer Inst. 2007;99(16):1232–1239. doi: 10.1093/jnci/djm086. [DOI] [PubMed] [Google Scholar]
- 27.Cannistra SA, Matulonis UA, Penson RT, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25(33):5180–5186. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 28.Eremina V, Jefferson JA, Kowalewska J, et al. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358(11):1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hurwitz HI, Saltz LB, Van Cutsem E, et al. Venous thromboembolic events with chemotherapy plus bevacizumab: a pooled analysis of patients in randomized phase II and III studies. J Clin Oncol. 2011;29(13):1757–1764. doi: 10.1200/JCO.2010.32.3220. [DOI] [PubMed] [Google Scholar]
- 30.Schutz FA, Je Y, Azzi GR, Nguyen PL, Choueiri TK. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22(6):1404–1412. doi: 10.1093/annonc/mdq587. [DOI] [PubMed] [Google Scholar]
- 31.Prager GW, Breuss JM, Steurer S, Mihaly J, Binder BR. Vascular endothelial growth factor (VEGF) induces rapid prourokinase (pro-uPA) activation on the surface of endothelial cells. Blood. 2004;103(3):955–962. doi: 10.1182/blood-2003-07-2214. [DOI] [PubMed] [Google Scholar]
- 32.Peters S, Heiduschka P, Julien S, Ziemssen F, Fietz H, Bartz-Schmidt KU. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol. 2007;143(6):995–1002. doi: 10.1016/j.ajo.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Meyer T, Robles-Carrillo L, Robson T, et al. Bevacizumab immune complexes activate platelets and induce thrombosis in FCGR2A transgenic mice. J Thromb Haemost. 2009;7(1):171–181. doi: 10.1111/j.1538-7836.2008.03212.x. [DOI] [PubMed] [Google Scholar]
- 34.Vidinova C, Vidinov N. The effect of bevacizumab on the ultrastructure of choroidal neovascular membranes in patients with age-related macular degeneration (AMD) Klin Monbl Augenheilkd. 2009;226(6):491–495. doi: 10.1055/s-0028-1109429. German. [DOI] [PubMed] [Google Scholar]
- 35.Papadopoulou DN, Mendrinos E, Mangioris G, Donati G, Pournaras CJ. Intravitreal ranibizumab may induce retinal arteriolar vasoconstriction in patients with neovascular age-related macular degeneration. Ophthalmology. 2009;116(9):1755–1761. doi: 10.1016/j.ophtha.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Sacu S, Pemp B, Weigert G, et al. Response of retinal vessels and retrobulbar hemodynamics to intravitreal anti-VEGF treatment in eyes with branch retinal vein occlusion. Invest Ophthalmol Vis Sci. 2011;52(6):3046–3050. doi: 10.1167/iovs.10-5842. [DOI] [PubMed] [Google Scholar]
- 37.Soliman W, Vinten M, Sander B, et al. Optical coherence tomography and vessel diameter changes after intravitreal bevacizumab in diabetic macular oedema. Acta Ophthalmol. 2008;86(4):365–371. doi: 10.1111/j.1600-0420.2007.01057.x. [DOI] [PubMed] [Google Scholar]
- 38.Feucht N, Matthias H, Lohmann CP, Maier M. Pegaptanib sodium treatment in neovascular age-related macular degeneration: clinical experience in Germany. Clin Ophthalmol. 2008;2(2):253–259. doi: 10.2147/opth.s2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung AE, Rosenfeld PJ, Reichel E. The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90(11):1344–1349. doi: 10.1136/bjo.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenfeld PJ, Rich RM, Lalwani GA. Ranibizumab: phase III clinical trial results. Ophthalmol Clin North Am. 2006;19(3):361–372. doi: 10.1016/j.ohc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 42.van der Reis MI, La Heij EC, De Jong-Hesse Y, Ringens PJ, Hendrikse F, Schouten JS. A systematic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina. 2011;31(8):1449–1469. doi: 10.1097/IAE.0b013e3182278ab4. [DOI] [PubMed] [Google Scholar]
- 43.Yokoyama K, Choshi T, Kimoto K, Shinoda K, Nakatsuka K. Retinal circulatory disturbances following intracameral injection of bevacizumab for neovascular glaucoma. Acta Ophthalmol. 2008;86(8):927–928. doi: 10.1111/j.1755-3768.2008.01187.x. [DOI] [PubMed] [Google Scholar]
- 44.Kim KS, Chang HR, Song S. Ischaemic change after intravitreal bevacizumab (Avastin) injection for macular oedema secondary to non-ischaemic central retinal vein occlusion. Acta Ophthalmol. 2008;86(8):925–927. doi: 10.1111/j.1755-3768.2008.01175.x. [DOI] [PubMed] [Google Scholar]
- 45.Huang ZL, Lin KH, Lee YC, Sheu MM, Tsai RK. Acute vision loss after intravitreal injection of bevacizumab (Avastin) associated with ocular ischemic syndrome. Ophthalmologica. 2010;224(2):86–89. doi: 10.1159/000235726. [DOI] [PubMed] [Google Scholar]
- 46.Mansour AM, Shahin M, Kofoed PK, Parodi MB, Shami M, Schwartz SG. Insight into 144 patients with ocular vascular events during VEGF antagonist injections. Clin Ophthalmol. 2012;6:343–363. doi: 10.2147/OPTH.S29075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neubauer AS, Kook D, Haritoglou C, et al. Bevacizumab and retinal ischemia. Ophthalmology. 2007;114(11):2096. doi: 10.1016/j.ophtha.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 48.Terui T, Kondo M, Sugita T, et al. Changes in areas of capillary nonperfusion after intravitreal injection of bevacizumab in eyes with branch retinal vein occlusion. Retina. 2011;31(6):1068–1074. doi: 10.1097/IAE.0b013e31820c83c2. [DOI] [PubMed] [Google Scholar]
- 49.Chung EJ, Roh MI, Kwon OW, Koh HJ. Effects of macular ischemia on the outcome of intravitreal bevacizumab therapy for diabetic macular edema. Retina. 2008;28(7):957–963. doi: 10.1097/IAE.0b013e3181754209. [DOI] [PubMed] [Google Scholar]
- 50.Mirshahi A, Roohipoor R, Lashay A, Mohammadi SF, Abdoallahi A, Faghihi H. Bevacizumab-augmented retinal laser photocoagulation in proliferative diabetic retinopathy: a randomized double-masked clinical trial. Eur J Ophthalmol. 2008;18(2):263–269. doi: 10.1177/112067210801800215. [DOI] [PubMed] [Google Scholar]