Abstract
In the current era of effective anti-retroviral therapy, immuno-compromised patients with HIV-1 infection do live long enough to suffer diseases caused by many opportunistic infections, such as herpes simplex virus type 1 and/or type 2 (HSV-1/2). An estimated two-third of the 40 million individuals that have contracted HIV-1 worldwide are co-infected with HSV-1/2 viruses, the causative agents of ocular oro-facial and genital herpes. The highest prevalence of HIV and HSV-1/2 infections are confined to the same regions of Sub-Saharan Africa. HSV-1/2 infections affect HIV-1 immunity, and vice versa. While important research gains have been made in understanding herpes and HIV immunity, the cellular and molecular mechanisms underlying the crosstalk between HSV-1/2 and HIV co-infection remain to be fully elucidated. Understanding the mechanisms behind the apparent HSV/HIV negative immuno-synergy maybe the key to successful HSV and HIV vaccines; both are currently unavailable. An effective herpes immunotherapeutic vaccine would in turn - indirectly - contribute in reducing HIV epidemic. The purpose of this review is: (i) to summarize the current trends in understanding the negative immuno-crosstalk between HIV and HSV-1/2 infections; and (ii) to discuss the possibility of developing a novel mucosal herpes immunotherapeutic strategy or even a combined or chimeric immunotherapeutic vaccine that simultaneously targets HIV and HSV-1/2 infections. These new trends in immunology of HSV-1/2 and HIV co-infections should become part of current efforts in preventing sexually transmitted infections. The alternative is needed to balance the ethical and financial concerns associated with the rising number of unsuccessful mono-valent clinical vaccine trials.
Keywords: immuno-synergy, immuno-cross talk, T cells, dendritic cells, HIV-1, HSV-2, genital herpes, therapeutic vaccine
INTRODUCTION
In the current era of effective anti-retroviral therapy, immuno-compromised patients with HIV-1 infection do live long enough to suffer diseases caused by many other infections, such as herpes simplex virus type 1 and/or type 2 (HSV-1/2) [1]. An estimated two-third of individuals with HIV-1 are co-infected with HSV-1 and/or HSV-2, due to similar routes of infection [1–9]. More than two third of the world's population is infected with HSV-1/2, that cause mucocutaneous genital herpes disease in both immuno-competent and immuno-compromised individuals. Infection with HSV-1/2 has become an important consideration for the clinical management of AIDS, where 50–90% of HIV-1-infected individuals are seropositive for HSV-2 [5–9]. Both HSV-1/2 can cause genital herpes, leading to painful ulcerative genital lesions [10, 11]. The morbidity and socioeconomic burden associated with genital herpes, along with alarming negative immuno-crosstalk between genital herpes and HIV highlight the need for the development of an effective therapy or immunotherapeutic herpes vaccine. Despite significant advances made in antiviral therapy and education for safe sexual practices, genital herpes remains a significant public health problem as the prevalence of HSV infection has increased by an estimated 30%, since the late 1980's, when HIV infections emerged for the first time [7, 12, 13]. After a primary acute infection, HSV-1/2 remain latent in the local sensory ganglia and can spontaneously reactivate, leading to recurrent ocular, oro-facial (cold sores) and genital herpes with manifestations ranging from asymptomatic viral shedding to painful symptomatic blisters [14–19]. As illustrated in Figures 1, 2 and 3, in the absence of optimal mucosal T cell immunity, recurrent and frequent herpes virus reactivations from the sensory ganglia predisposes to increased risks of HIV-1 infection [20–23]. An immunotherapeutic vaccine strategy aiming at reducing the overall burden of herpes infections would therefore be highly efficacious in addressing the epidemic caused by HIV and HSV-1/2 infections [13, 24].
Figure 1. The natural routes of HSV-1/2 and HIV-1 transmission.
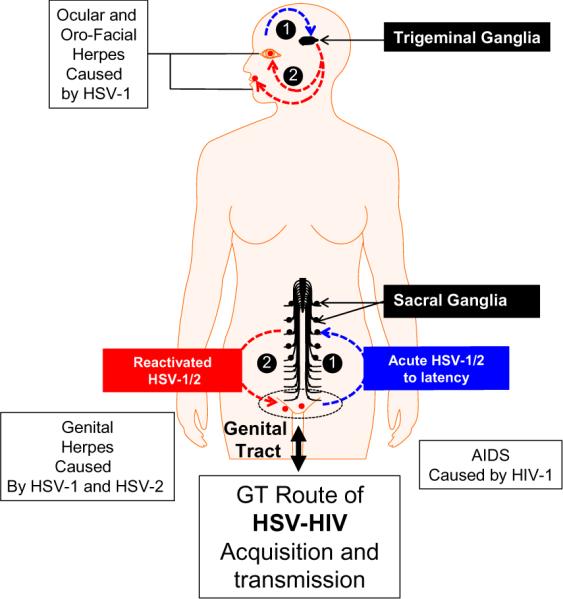
HSV-1/2 transmitted through the genital tract (GT) replicates locally in this primary mucocutaneous genital site of infection, enters and travels along the sensory nerves to the lumbosacral dorsal root gangli a (sacral ganglia or SG) where it establishes latency. Sporadic spontaneous reactivation of HSV-1/2 from the sensory neurons of SG leads to viral shedding in the genital tract, which can cause recurrent ulcerative genital herpes (blisters). While most cases of genital herpes are caused by HSV-2, reports of HSV-1 genital infection are on the rise. HSV-1 infects the cornea or the mucocutaneous oro-facial sites and then enters and travels along the sensory nerves to the trigeminal ganglia (TG) where it establishes latency. Sporadic spontaneous reactivation of HSV-1 from the sensory neurons of TG leads to viral shedding in the tears and saliva, which can then infect the oro-facial surfaces and cause recurrent ocular (e.g. blinding herpes stromal keratitis or HSK) or oro-facial herpes (e.g. cold sores). HIV-1 is mainly acquired and transmitted through the GT and replicate locally in this primary muco-cutaneous genital site of infection.
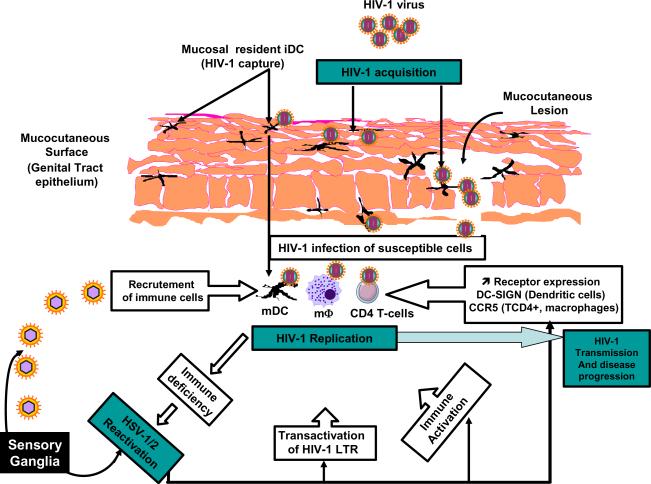
Figure 2. Schematic model of HSV-1/2 and HIV-1 cross talk, the negative HIV/HSV immuno-synergy and its impact on transmission and dissemination.
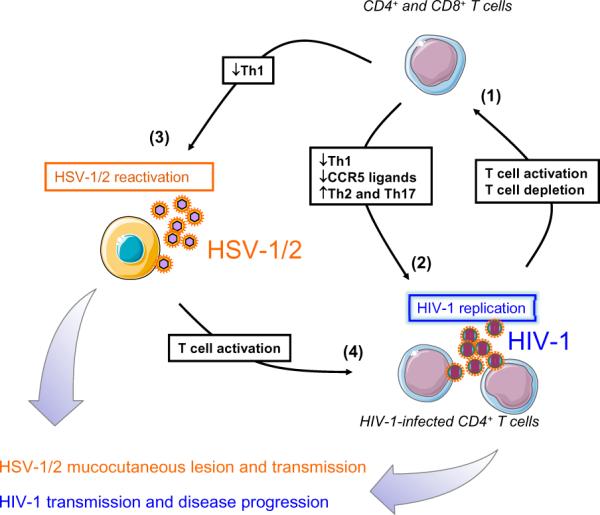
(1) In HIV-infected individuals, viral replication leads to T cell activation and impairment of mainly CD4+ T cell immunity. (2) Consequently, HIV-1-infected individuals have a reduction of Th1 cytokine and CCR5 ligand secretions associated to an increase of the Th2 and Th17 response contributing to ongoing HIV-1 replication of viral reactivation from latently infected cells. (3) The negative effect of HIV-infection on the HSV-specific CD4+ and CD8+ T cell immunity may facilitate HSV-1/2 reactivations. (4) More reactivated HSV-1/2 may lead to immune evasion (e.g. interference with the antigen presenting machinery) which, in turn, enhances ongoing HIV dissemination and induces viral replication from latently infected CD4+ T cells. Thus, HSV and HIV cross talk and the apparent negative immuno-synergy leads to dramatic transmission and dissemination of both viruses.
Figure 3.
Illustration of the Impact of HSV infection/reactivation on HIV-1 infection, replication, transmission, and disease progression (see details in [7]).
Studies have shown that most HSV-1/2-infected individuals, regardless of HIV-1 sero-status, shed HSV-1/2 in tears, oral or genital secretions, and, fortunately, most shedding is asymptomatic [1–9]. Interestingly, shedding of HSV occurs more frequently and with higher quantity in HIV-1 infected patients compared to HSV-infected/HIV-1-uninfected individuals [5–9]. Being seropositive for HSV-2 may increase the risk of HIV-1 acquisition among high-risk populations exposed to HIV-1 [25–27]. Likewise, the infectiousness of individuals positive for HIV-1 may increase during periods of HSV-1/2 reactivation. In fact, populations in Sub-Saharan regions with increased prevalence of HSV-2 infections have a threefold increased risk of HIV-1 infection [26, 28, 29]. Immuno-epidemiological studies have chronicled the “negative immuno-synergy” between HIV and HSV-1/2 infections [30, 31]. HIV adversely affects the natural history of HSV-1/2 and results in more frequent and severe herpes reactivation. Inversely, HSV-1/2 infections increase the risk of HIV-1 acquisition by 2 to 3 fold [32]. HSV-2 genital reactivation, including asymptomatic shedding, also increases the concentration of HIV-1 in plasma and genital secretions [33–35]. Our recent results point that during HIV-1 and HSV-1/2 co-infections, the subversion of the HSV-specific immune response, including Th1 cytokines and CCR5 ligand, leads to the facilitation of both HIV and HSV replication and transmission, demonstrating the immuno-synergistic crosstalk between HIV and HSV [12]. Thus, it may not be a coincidence that these two sexually transmitted infections (STIs) co-exist anatomically and geographically, with the highest prevalence confined to the same regions of Sub-Saharan Africa.
Concerted efforts have led to a dozen immunotherapeutic vaccine trials in the past two decades and yet there is no FDA-approved vaccine for either HSV or HIV at present. Although the standard antiviral drug regimens (e.g. acyclovir) reduce recurrent symptomatic disease to about 45%, asymptomatic shedding at mucosal sites continues to occur and contributes to herpes diseases and HIV-1 transmission [13, 24]. Rather than directly targeting HIV, as it has been done in the past, we propose to indirectly target HIV by first decreasing HSV-1/2 infection and reactivation, that leads to recurrent ocular, oro-facial and genital herpes disease.
The present report summarizes some of the current trends in understanding the negative immuno-crosstalk between HIV and HSV-1/2 infections; and discuss the possibility of developing a novel mucosal combined or chimeric immunotherapeutic vaccine (Figure 4), that would simultaneously targets HIV and HSV-1/2 infections. These new trends in immunology of HSV-1/2 and HIV co-infections should become part of current efforts in preventing sexually transmitted infections. The alternative is needed to balance the ethical and financial concerns associated with the rising number of unsuccessful mono-valent clinical vaccine trials.
Figure 4. Schematic representation of a prototype multi-epitope chimeric (bi-valent) HIV-HSV lipopeptide vaccine.
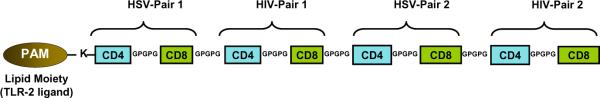
Several pairs of CD4–CD8 epitopes from both HSV-1 and HIV-1 proteins are synthesized in tandem with GPGPG sequences (spacers) and covalently linked at the N-terminal with a lysine (K) that is pre-coupled with a palmitic acid moiety (PAM).
A tale of war between HSV-2 and HIV-1 sexually transmitted viruses: An evolving negative immuno-synergy in transmission
As of the end of 2011, more than 40 million people worldwide have contracted human immunodeficiency virus (HIV), the causative agent of AIDS, and an estimated additional 14,000 people become infected every day [36–38]. Heterosexual transmission of HIV-1 through genital mucosal tissue is the primary route of worldwide HIV acquisition and dissemination [36–38]. Despite tremendous advances in our understanding of HIV-1 infection and immunity since the first cases were reported more than 25 years ago, an effective vaccine is yet to be developed.
HSV-2 is primarily responsible for genital herpes, one of the most common sexually transmitted disease worldwide [1, 10, 39–42] and a major co-factor in HIV-1 transmission [43]. HSV-2 enhances the susceptibility of HIV-1 infection [43–45] by (i) causing ulcerative lesions on mucosal tissues and (ii) inducing inflammatory responses via recruiting HIV-1 targeted hosting cells (macrophages and CD4+ T cells) [9, 46, 47]. HSV-2-infected tissues have increased chemokine receptor 5 (one of the main entry receptor for HIV-1) on CD4+ T cell surfaces. Several models have further emphasized the molecular mechanism underlying this negative synergistic relationship between HSV-2 and HIV-1 genital infections [48–53]. HIV-1 and HSV-2 co-infect and simultaneously replicate in the same human CD4+ [54–56] and dendritic cells [46, 51]. Recent study shows that infection of dendritic cells by HSV-2 amplifies a highly susceptible HIV-1 cell target by significantly increasing its α4β7 expression [43, 57–60]. Other studies demonstrate that viral HSV-2 co-infections disrupt the protective function of Langerhans cells (LCs) [43] HSV-2 enhanced HIV-1 infection of LCs then subsequently leads to HIV-1 transmission of T cells [43].
We recently found that HIV-1 infection impairs HSV-specific CD4+ and CD8+ T cell response by reducing Th1 cytokines and CCR5 ligand secretion [30, 61]. We used a method combining IFN-γ ELISpot and multiplex microbeads assay to investigate the impact of HIV infection on HSV-specific T-cell immunity. We analyzed the profile and magnitude of HSV-specific CD4+ and CD8+ T cell response after re-stimulation with HSV glycoprotein D peptide epitopes highly conserved between HSV-1 and HSV-2. The profiles were compared between individuals infected by both HIV and HSV and individuals infected by HSV only. We found that the T cell response is significantly reduced in co-infected individuals in comparison to HIV-uninfected/HSV-infected subjects. After stimulation with two of the immunodominant CD4+ and CD8+ epitopes, we also observed a reduction in the Th1 response and secretion of CCR5 ligands associated to an increase of Th17 cytokines in the HIV-infected group. The dysfunction in the HSV-specific Th1 response due to HIV-1 infection facilitates HSV reactivations that may induce CD4+ T-cell activation, which is required for the initiation of HIV-1 replication. Therefore the facilitation of HSV reactivation may increase, in turn, HIV-1 replication. In addition, the reduced secretion of CCR5 ligands and its imbalance with CCR5 receptors may increase the availability of CCR5 receptors at the cell surface, promoting also HIV-1 replication and accelerating HIV-1 disease progression [62, 63]. As part of a mutual cooperation between HIV-1 and HSV, the subversion of the cellular response by HIV-1 seems advantageous for the replication of both viruses (Figure 2). We thus speculate that successful HSV therapeutic strategies such as therapeutic vaccines could impact the vicious circle established by these virus in co-infected subjects, and therefore impact on HIV-1 transmission and disease progression.
Does herpes infection in female genital tract affect mucosal HIV-1 acquisition/transmission?
The Impact of HSV-1 and HSV-2 infection of the genital tract (GT) on HIV-1 acquisition/replication/transmission and disease progression is illustrated in Figure 3, and is described in detail in [7]. Logically, herpetic genital lesions causing breach of the mucosal surface integrity would be expected to “mechanically” lead to enhancing transmission of HIV-1. In addition, HSV contains several immunosuppressive genes, such as ICP47. If these herpes genes also interfere with the presentation of non-herpes antigens, the interference with HIV immunity would also be expected. Therefore, investigating the possibility that HSV-2 infection can decrease the immune response to HIV-1 by interfering with the presentation of HIV antigens would open doors for new therapeutic strategies to manage HIV infection. These studies would reveal novel immune mechanisms by which HSV-2 infection in the GT affect HIV-1 mucosal immunity and hence HIV-1 transmission and acquisition. This would implicate that HIV-1 infection and AIDS could be indirectly reduced by immunotherapeutic strategies that decrease genital herpes. In clinical trials, HSV suppressive drug therapy has decreased HIV RNA levels [8, 64–66] and reduced the progression of HIV [66] that might affect viral transmission. Nevertheless, a recent study of 3408 couples in Africa failed to demonstrate prevention of HIV acquisition by administrating acyclovir at 400 mg twice daily [64, 67]. Others showed ineffectiveness of anti-herpetic therapies in preventing genital HSV-2 reactivation, and therefore transmission [64, 67], which could be attributed to a persistent and constant low-level HSV replication as described in a recent infection model [13, 24]. Thus, efforts should focused on better understanding the natural mucosal immunity against such sexually transmitted virus in order to develop alternative immunotherapeutic strategies that could totally control HSV dynamic and reduce HIV-1 viral transmission.
Successful immunotherapies must induce local mucosal immunity to protect the eye, the oro-facial surface and the genital tract
Understanding the immunology of genital tract (GT) mucosal immune system will help to rationalize the design of an efficient HIV/HSV immunotherapeutic strategy that can induce a protective immunity against both HIV-1 and HSV infections. Adaptive mucosal immune responses are initiated and guided by the Langerhans cells (LCs), the dendritic cells (DCs) of the skin and mucosa. The lamina propria and epithelium of the GT contain DCs that serve as the interface between host and pathogens by extending dendrites into the surface epithelium to sample and respond to pathogenic stimuli. DC function is largely inferred from blood- and bone marrow-derived populations. The physical location of DCs gives them tremendous potential influence over the genital mucosal immune responses: (1) GT mucosal DCs are divided into two different subsets arising from the lamina propria, one of which samples the HIV and HSV antigens in the epithelium, and the other migrates through the local inguinal lymph nodes (ILN) to elicit an adaptive immune response that would protect the surface of GT from HIV and HSV infection; (2) intravaginal immunization with a chimeric HTL-CTL peptides bearing immunodominant epitopes from HIV and HSV associated to mucosal adjuvants (Figure 4), would induce T cell responses against both HIV and HSV antigens.
Increasing neutralizing antibody titers in herpes clinical vaccine trials did not reduce viral reactivation; shedding and recurrent disease suggesting the necessity of elicitation of a strong cellular immune response [68–70]. (1) In mice, CD8+ T cells accumulate in the sensory ganglia from 7 to 10 days following herpes infection and become the predominant T-cell type during latency [71]. HSV-specific CD8+ T-cells secreting IFN-γ and GrB appear to suppress (or abort) induced viral reactivation in explanted mouse sensory ganglia [71, 72]. It has been proposed that CD8+ T-cells may similarly reduce detectable HSV reactivation in vivo [73–76]. Based on this model, a human therapeutic vaccine that increases HSV-specific IFN-γ+ and GrB+ CD8+ T cells in latently infected sensory ganglia should significantly reduce HSV spontaneous reactivation rate. (2) A significant number of activated CD8+ T cells producing IFN-γ and TNF-α were found in latently infected TG of human cadavers, likely indicating the existence of an antigen-driven immune response [77–79]. While HSV-specific CD8+ T-cells appear to be the key factor to prevent from spontaneous reactivation and recurrence, the challenge for herpes immunologists and vaccinologists remains “to identify a reliable and appropriate preclinical animal model to study the efficacy of human HLA-restricted T cell epitopes-based vaccine” [24, 80]. An ideal animal model should produce an immune response specific to human HSV epitopes (such as HLA-A*0201-restricted epitopes) while mimicking most if not all the aspects of viral pathogenesis, such as the establishment of latency that occurs in humans.
Shedding of reactivated HSV is estimated to occur at rates of 3 to 28% in seropositive adults who harbor latent HSV-2 in their sensory ganglia [39, 81–83]. However, the vast majority of immunocompetent individuals do not experience recurrent herpetic disease. The latest are described as “asymptomatic patients” [82, 84–86]. In contrast, in some immunocompetent individuals, reactivation of latent virus leads to recurrent symptomatic herpetic disease [82, 85, 86]. Recurrent disease ranges from rare episodes occurring once every 5–10 years to outbreaks occurring monthly or more frequently among a small proportion of subjects [86–88]. Individuals with a well-documented clinical history of at least 5 recurrent genital disease episodes per year are “symptomatic patients”. It is not known why genital HSV-2 infection is asymptomatic in some individuals and symptomatic in others, or why some individuals are symptomatic while others are not, or why the frequency and severity of recurrences vary among symptomatic individuals. Interestingly, both symptomatic and asymptomatic patients have similar virus-shedding rates (3.0% vs. 2.7%) [82, 84–86]. The immune mechanisms by which asymptomatic patients control the disease remain to be fully elucidated. Identifying these mechanisms, or at least the viral epitopes involved, is critical to understanding how to protect individuals against recurrent genital disease.
Several vaccine strategies have been tested in the last two decades [89]. A clinical vaccine trial using protein-in-adjuvant HSV-2 vaccine (glycoprotein D, gD) delivered parenterally in women from so-called discordant couples (for which only one of the two partners is infected) have reported in 2002 to be of a limited success [80, 90, 91]. When delivered intra-muscularly, the protein-in-adjuvant vaccine induced a transient immunity against HSV-2 disease in women who were seronegative for both HSV-1 & HSV-2 [90–92]. However, the vaccine failed to protect HSV-2 seropositive individuals despite a good neutralizing antibody response [90, 91]. A more recent clinical vaccine trial reported in 2012, based on the same glycoprotein gD vaccine, showed efficacy in preventing HSV-1, but not in preventing HSV-2 genital herpes [91]. The reasons for this discrepancy remain to be determined. A common conclusion could perhaps be drawn from these clinical trials [90, 91], together with our recently reported pre-clinical studies using the established murine model of intravaginal immunization [10, 11], that a T-cell based mucosal response, rather than the humoral response alone, may be necessary to protect against genital herpes. However, despite intensive research efforts, the progress toward a T-cell based vaccine continues to face major hurdles including the lack of an efficient Ag delivery system that would safely induce potent and sustained mucosal T cell immunity in and around the GT mucosa.
As a model antigen, we used a prototype CD4+ T helper-/CD8+ T cytotoxic-chimeric epitope lipopeptide consisting of several pairs of HSV glycoprotein B and D (gB & gD)-based CD8+ and CD4+ T cell “asymptomatic” immunodominant epitopes as illustrated in Figure 4. This prototype of lipopeptide was, in turn, linked to a palmitic acid moiety and designated Th-CTL lipopeptide [93]. IVAG delivery of Th-CTL lipopeptide in HLA-A2 transgenic mice elicited both local and systemic HSV-specific CD8+ T cell responses, leading to reduced virus replication in the GT with corresponding attenuated genital disease. Induction of IFN-γ-producing CD8+ T-cells induced by Th-CTL lipopeptide was partially dependent on TLR2. Our results highlight the potential of self-adjuvanting lipopeptides as a novel, noninvasive IVAG vaccine approach to prevent the transmission and/or limit the severity of sexually transmitted diseases. Figure 5 illustrates potential operating mechanisms for mucosal (e.g. intravaginal) immunization with herpes lipopeptides.
Figure 5. Illustration of mucosal (e.g. intravaginal) immunization with a mono-valent herpes lipopeptide.
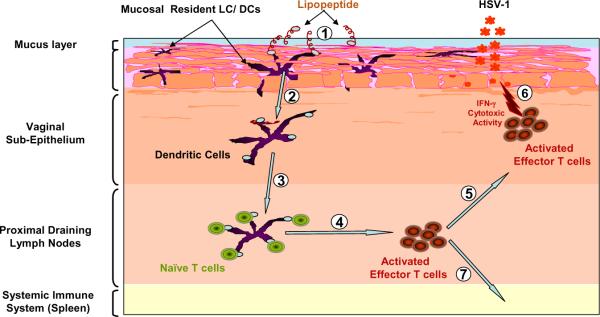
Lipopeptide antigens applied to intravaginal mucosal epithelium are taken up by mucosal resident APC (e.g. DC/LC), which are activated and migrate into the mucosal effector sites of the draining lymph nodes where antigen presentation to T cells occurs, resulting in subsequent local mucosal immune response.
A genital tract lipopeptide boost vaccine targeting toll-like receptor 2 efficiently protects against HSV challenge
Over the last five decades, numerous conventional candidates such as live attenuated and killed herpes vaccines that were efficacious in animal models failed to demonstrate adequate response in clinical trials (reviewed in [94, 95]). The majority of these vaccines is administered parenterally and can induce strong systemic immune responses. However, they do not generate significant immunity at the mucosal site of infection nor in the local draining lymph nodes that many experts see as necessary to prevent transmission or limit the severity of sexually transmitted diseases [96–103]. We hypothesize that an efficient sub-unit IVAG vaccine would induce a local immunity at -or at least close to - the site of genital infection, thus maximizing its ability to protect the GT from subsequent HSV-2 challenge. However, the progress towards an IVAG vaccine still faces significant challenges including: (i) the overall low immunogenicity of sub-unit formulations delivered into the GT compared to other mucosal routes (e.g. intranasal route) [96, 104–106]; (ii) the imperative requirement for a safe and effective mucosal adjuvant [94, 107, 108]; and (iii) a better understanding of key effector immune molecules of the vaginal mucosal immune system [109–111].
The initial host response to vaccination occurs after Toll-like receptors (TLRs) on dendritic cells (DC) are stimulated through specific TLR agonists. In the last decade, there has been an interest in targeting TLR in the GT to induce protective immunity against sexually transmitted diseases, including HSV-2 (reviewed in [96, 111]). Thus, recent studies have investigated the TLR expression patterns in the GT and reported that both DC and epithelial cells of the vaginal and cervical mucosa abundantly express bioactive TLR-2 [111–116]. In the meantime, we and others have established that parenteral delivery of self-adjuvanting peptides extended by a TLR-2 agonist (palmitic acid moiety), can induce significant protective immunity (reviewed in [94]). Moreover, intranasal administration of palmitoyl-tailed peptide epitopes induced strong local and systemic T cell responses [94, 117–120]. We have also found that, in vitro, antibody blocking of TLR-2, but not TLR-4, abrogates DC presentation of lipopeptide epitopes to T cells [121]. We therefore hypothesized that an IVAG lipopeptide vaccine targeting TLR-2 would induce local and systemic T cell immunity and protect the female GT against recurrent genital herpes.
Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes
The concept of using recombinant viral-vector vaccines to deliver an unrelated viral antigen was developed more than 25 years ago [122]. Recombinant adenoviruses (rAd) are both promising and safe due to their capacity to elicit potent CD8+ T cell responses to unrelated T cell epitopes (reviewed in [123] and [124, 125]). rAd5 vector-based vaccines have been recently tested successfully in animal models and several clinical trials are currently ongoing in the US (reviewed in [123] and [124] and http://clinicaltrials.gov/). We recently found that a Lipo/rAdv5 prime/boost vaccine delivered IVAG, induced a robust HSV-specific CD8+ T cell-dependent protective immunity against genital herpes in mice [1]. Viral replication in the GT was significantly lower in the Lipo/rAd5 vaccine group compared to a homologous Lipo/Lipo vaccine group. Moreover, mice immunized IVAG with the Lipo/rAdv5 prime/boost mucosal vaccine have decreased overt signs of genital herpes disease, and did not succumb to lethal infection following genital HSV-2 challenge when compared to mice immunized with the homologous Lipo/Lipo vaccine. The induced long-lasting memory CD8+ T cell responses persisted in the GT-DLN for up to 8 months post immunization. These long-lasting CD8+ memory T cells may play a critical role in immune surveillance and could rapidly respond and expand after sensing the virus to prevent genital herpes. The findings underscore the potential of Lipo/rAd5 prime/boost mucosal immunization as an efficient vaccination strategy for inducing genital CD8+ T cell immunity and its potential impact on the development of vaccines against STDs. However, one should keep in mind the high frequency of human anti-Ad5 neutralizing antibodies in the developing world, which will likely limit the immunogenicity and clinical utility of Ad5-vector based vaccines [126–131]. Thus, the ability of rAd5 to boost potent HSV-specific CD8+ T cell responses induced in a mouse model by a lipopeptide demonstrated in this study may not extrapolate well to human subjects. Nevertheless, this study constitutes a first proof-of-principle, showing a strong immunogenicity of a prime/boost mucosal vaccine strategy using a rAd vector. A future candidate Lipo/rAd5 prime/boost clinical vaccine will probably have to be constructed using a rAd vector derived from an adenovirus serotype that is rare in human populations and distinct from the Ad5 serotype, such as rAd26 or rAd35, which do not infect the same type of cells as Ad5 [80]. Neither Ad35 nor Ad26 are affected by anti-Ad5 immunity and rAd35 is not affected by anti-Ad26 immunity [132, 133].
The Lipo/rAdv5 prime/boost mucosal vaccine delivered IVAG stimulated potent and sustained HSV-specific CD8+ T cell responses, detected not only in the GT-DLN but also in the vaginal mucosal (VM) tissue. Clear differences were apparent in the kinetics with which CD8+ T cells are mobilized following Lipo/Lipo vs. Lipo/rAdv5 prime/boost mucosal vaccination. The success of Lipo/rAdv5 prime/boost mucosal vaccine is also highlighted by its ability to induce and facilitate the mobilization and establishment of local effector memory CD8+ T cells in the VM tissue, by promoting their migration. We found a cluster of IFN-γ-producing CD8+ T cells in the VM of Lipo/rAdv5 vaccinated mice, suggesting an establishment of memory cell foci in the genital mucosa (not shown). These clusters of CD8+ T cells appear to migrate from the GT-DLN to vaginal mucosa, since the vaginal sub-mucosa does not contain MALT in the steady state [134]. Several explanations are possible for the difference in mobilization and kinetics of CD8+ T cells by Lipo/Lipo vs. Lipo/rAdv5 prime/boost mucosal vaccines. Generally, effector memory T cells circulate throughout the peripheral tissues, such as the VM, whereas central memory T cells reside in the secondary lymphoid tissues, such as GT-DLN. Thus, regardless of the site of antigen encounter, HSV-specific memory CD8+ T cells must be found in various tissues, including the VM and GT-DLN. However, peripheral tissue distribution of memory CD8+ T cells occurs mainly after immunization with live replicating vectors, such as rAdv5. The mucosa of vaginal canal is drained by several lymph nodes, including the common iliac, interiliac, external iliac and inguinal femoral lymph nodes (in descending order, designated in this report as GT-DLN) (reviewed in [135]). It is likely that the IVAG immunogenicity of Lipo/rAdv5 occurs through vaginal sub-mucosal dendritic cells (DC), where they efficiently take up the rAdv5 vaccine and migrate to the GT-DLN, and present the gB498–505 peptide to cognate CD8+ T cells. The proportion of CD8+ T cells after lipopeptide priming is greater and these expand more prominently following heterologous rAdv5 boost. Since all the mechanisms above are not mutually exclusive, it is possible that they all play a role in our immunization scheme, but additional experiments will be necessary to assess the relative proportion of each mechanism in the observed immunogenicity.
HSV-2 infects the GT and then establishes latency in sensory neurons of the dorsal root ganglia (DRG). While sub-optimal “inherent” CD8+ T cells are detected in sensory ganglia and appear to provide an immune surveillance of the infected neurons, these cells cannot clear latent virus. It is generally assumed that prevention of HSV-2 reactivation from sensory ganglia requires a therapeutic vaccine that will boost a more vigorous and/or a different virus-specific CD8+ T cell response. This is because the quality and/or the magnitude of “inherent” CD8+ T cell responses from natural infection are not sufficient to reduce virus reactivation and recurrent genital herpes in symptomatic individuals [2, 19, 89, 136–138]. Following HSV-2 genital challenge, our Lipo/rAdv5 prime/boost mucosal vaccination appeared to significantly reduce the amount of virus that reactivated ex vivo from DRG. CD8+ T cells, as demonstrated by CD8-depletion studies, mediated protection and greater protection were apparent with cytotoxic CD8+ IFN-γ-producing cells present in both the GT-DLN and VM. As expected, mice immunized with thymidine kinase mutant HSV-2 TK(−), which is incapable of reactivation in the sacral DRG, also showed little reactivation from the DRG (positive control). The cluster of IFN-γ-producing CD8+ T cells found in the VM of Lipo/rAdv5 vaccinated B6 mice is likely important in providing an immediate response following reactivation of HSV-2 from DRG. In humans, memory CD8+ T cells are rapidly recruited following reactivation and persist near peripheral nerve endings at the dermal-epidermal junction for more than 60 days after reactivation and healing [139]. Importantly, subsequent virus reactivation at the site where CD8+ T cells are present did not result in lesion formation, indicating that HSV-2-specific CD8+ T cells at the site of genital herpes virus lesions contain local viral replication. Therefore, localized mucosal memory T cell populations seem to provide superior control of viral infection compared with circulating memory T cells. Our hope is that a therapeutic genital herpes prime-boost Lipo/rAdv5 vaccine would increase the number and function of HSV-specific CD8+ T cells in sensory ganglia of latently infected hosts, which should consequently decrease HSV-2 spontaneous reactivation (as measured by shedding in GT) and recurrent genital herpetic disease significantly. This hypothesis will be the subject of future investigation in a HSV-2 latently infected guinea pigs model, where the guinea pigs develop spontaneous reactivation that leads to recurrent genital herpetic disease similar to that in symptomatic human subjects (reviewed in [80]).
Potential of T-cell epitopes-based vaccines in decreasing HSV reactivation
The envelope glycoproteins gB and gD have been studied extensively and we and others have recently identified many T cell epitopes [140–147]. HSV-2 tegument proteins such as those encoded by UL41, UL46 (VP11/12), UL47 (VP13/14), UL48 (VP16) and UL49; and immediate early (IE) proteins such as ICP0 and ICP4 have also been identified as major targets for effector T cells [148–151]. However, until our recent studies with gB and gD [141], no HSV “symptomatic” or “asymptomatic” epitopes have been identified. Using the same approaches that we used to identify symptomatic and asymptomatic human epitopes from gB and gD, we also expect to find asymptomatic and symptomatic epitopes from HSV-2 tegument proteins. Using the same computer analyses previously used for gB and gD, we have already identified 10 potential human CD4+ and 10 potential human CD8+ T cell epitopes from the two major tegument proteins, VP11/12 and VP13/14 (data not shown).
HSV-specific CD4+ Th1 cells may be important in controlling the severity of HSV infection [143, 147, 152]. Individuals with severe immunodeficiency, usually with impaired CD4+ T cell functions, can have severe recurrent herpes with longer duration lesions [153]. Even though clearance of HSV-2 from recurrent genital lesions correlates with the infiltration of both HSV-2-specific CD4+ and CD8+ cytotoxic T cells [154, 155], the CD4+ T cells infiltrate first and are associated, time-wise, with a drop in infectious virus titer within the lesion [156, 157]. CD4+ T cells are thought to be one of the main mediators of protective immunity during recurrent episodes [143, 158–160]. CD4+ T-cell responses to HSV-2 appear to be directed against envelope glycoproteins, capsid proteins and regulatory elements within the tegument [149, 150, 155, 161]. Early in the development of recurrent lesions, HSV-2-specific IFN-γ-producing CD4+ T cells that display a cytotoxic activity predominate in the mononuclear infiltrate surrounding HSV-2 infected epidermal cells [143]. The role of effector CD4+ T cells in protecting murine female genital tract and in clearance of infectious HSV from sensory ganglia and spinal cords has been demonstrated by Milligan et al., [162–165].
Based on the above observations, we believe that the clinical spectrum of herpes, ranging from asymptomatic to frequently distressing outbreaks, may be attributed to CD4+ and CD8+ T cell recognition of different sets of epitopes from one or several HSV protein antigens (Ags). Thus, recognition by CD4+ and/or CD8+ T-cells of a set of viral epitopes designated as “symptomatic” might be associated with severe and frequent immuno-pathologic diseases while recognition of “asymptomatic” epitopes might, in turn, lead to immuno-protection. Thus, the identification of HSV-specific T cell epitopes recognized by “asymptomatic” vs. “symptomatic” CD4+ and CD8+ T cells is likely to be important for the rational design of an efficient HSV-2 vaccine.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
There is increasing evidence that HSV-1/2 infection contributed to the global HIV-1 epidemic. (1) In recent years, it has become increasingly clear that HSV-1/2 infections are associated with HIV-1 acquisition. (2) Co-infection with HSV-1/2 may contribute to faster HIV-1 disease progression and heightened risk of HIV-1 transmission. (3) Inversely, an enhancement in the genital HSV-2 shedding, among HIV-1-coinfected individuals, may in turn likely increase the risk of HSV-2 transmission. In light of the current trends in an evolving negative immuno-synergy between HSV-1/2 and HIV-1 and the availability of effective antiviral therapy, proper diagnosis and management of HSV becomes increasingly important. (3) The cellular and molecular mechanisms and the exact role of genital ulcers or asymptomatic reactivations from HSV-1/2 in HIV-1 acquisition remain to be fully elucidated. Current ongoing research in our laboratory and others are promising in providing more critical information for the immune evasion of HSV and hence the control of HIV infection. (4) Recent studies have shown that HSV-2 shedding and asymptomatic reactivations still occur in drug treated patients, confirming the ineffectiveness of current HSV suppressive therapies and the need to develop new immunotherapeutic vaccine strategies boosting HSV-specific mucosal T cell response. (5) During HIV-1 and HSV-1/2 co-infections, the subversion of the HSV-specific immune response, including Th1 cytokines and CCR5 ligand, leads to the facilitation of both HIV and HSV replication and transmission, demonstrating the immuno-synergistic crosstalk between HIV and HSV. (6) Because persistent effort in developing herpes therapeutic vaccine using mouse as a preclinical model have only shown increase in failure rate, it is worth considering the possibility that immune response elicited in a mouse may not correspond well to that in humans. (7) The new model in question is between HLA transgenic rabbit and HLA transgenic guinea pig, both of which are more difficult to generate and study compared to the readily available mouse immunology. (8) We hope that future advances over the next several years of research on HIV-1 and HSV-1/2 infection and immunity will provide us with important insights into several aspects of these infections, including: (i) how these sexually transmitted viruses invade the mucocutaneous tissues; which host immune factors can limit virus replication; and (iii) what are the target cells that the viruses use for initial infection and subsequent replication in the mucocutaneous mucosa of the eye, the mouth and the genital tract.
ACKNOWLEDGEMENTS
This work was supported by Public Health Service NIH grants EY14017, EY14900 and EY019896 to LBM, The Discovery Eye Foundation, The Henry L. Guenther Foundation and an unrestricted Research to Prevent Blindness Challenge grant.
REFERENCES
- 1.Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, BenMohamed L. The Journal of Immunololgy. 2012;189:4049. doi: 10.4049/jimmunol.1201121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dervillez X, Wechsler S, Nesburn AB, BenMohamed L. Future Virology. 2012;4:371. doi: 10.2217/fvl.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dasgupta G, Chentoufi AA, Kalantari M, Falatoonzadeh P, Chun S, Lim CH, Felgner PL, Davies DH, BenMohamed L. J. Virol. 2012;86:4358. doi: 10.1128/JVI.07107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chentoufi AA, Kritzer E, Yu DM, Nesburn AB, BenMohamed L. Clin. Dev. Immunol. 2012;2012:187585. doi: 10.1155/2012/187585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strick LB, Wald A, Celum C. Clin. Infect. Dis. 2006;43:347. doi: 10.1086/505496. [DOI] [PubMed] [Google Scholar]
- 6.Baeten JM, Strick LB, Lucchetti A, Whittington WL, Sanchez J, Coombs RW, Magaret A, Wald A, Corey L, Celum C. J. Infect. Dis. 2008;198:1804. doi: 10.1086/593214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van de Perre P, Segondy M, Foulongne V, Ouedraogo A, Konate I, Huraux JM, Mayaud P, Nagot N. Lancet. Infect. Dis. 2008;8:490. doi: 10.1016/S1473-3099(08)70181-6. [DOI] [PubMed] [Google Scholar]
- 8.Nagot N, Ouedraogo A, Foulongne V, Konate I, Weiss HA, Vergne L, Defer MC, Djagbare D, Sanon A, Andonaba JB, Becquart P, Segondy M, Vallo R, Sawadogo A, Van de Perre P, Mayaud P. N. Engl. J. Med. 2007;356:790. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 9.Mayaud P, Nagot N, Konate I, Ouedraogo A, Weiss HA, Foulongne V, Defer MC, Sawadogo A, Segondy M, Van de Perre P. Sex Transm. Infect. 2008;84:332. doi: 10.1136/sti.2007.027987. [DOI] [PubMed] [Google Scholar]
- 10.Bettahi I, Zhang X, Afifi RE, BenMohamed L. Viral Immunol. 2006;19:220. doi: 10.1089/vim.2006.19.220. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. Mucosal Immunol. 2009;2:129. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubbo PA, Tuaillon E, Nagot N, Chentoufi AA, Bollore K, Reynes J, Vendrell JP, BenMohamed L, Van De Perre P. Journal of acquired immune deficiency syndromes. 2011;58:9. doi: 10.1097/QAI.0b013e318224d0ad. [DOI] [PubMed] [Google Scholar]
- 13.Rubbo PA, Tuaillon E, Bollore K, Foulongne V, Bourdin A, Nagot N, Van de Perre P, Desgranges C, Israel-Biet D, Vendrell JP. Clin. Immunol. 2011;139:142. doi: 10.1016/j.clim.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Vagvala SP, Thebeau LG, Wilson SR, Morrison LA. J. Virol. 2009;83:953. doi: 10.1128/JVI.02022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison LA. Virology. 2008;376:205. doi: 10.1016/j.virol.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chentoufi AA, Kritzer E, Tran M, Dasgupta G, EA R, Xianzhi J, Carpenter D, Osorio O, Nesburn AB, Wechsler SL, BenMohamed L. J. Virology. 2011;85:9127. doi: 10.1128/JVI.00587-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen SR, Hamrah P, Gate DM, Mott KR, Mantopoulos D, Zheng L, Town T, Jones C, von Andrian UH, Freeman GJ, Sharpe AH, BenMohamed L, Ahmed R, Wechsler SL, Ghiasi H. J. Virology. 2011;85:4184. doi: 10.1128/JVI.02290-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang X, Chentoufi AA, Hsiang C, Carpenter D, Osorio N, BenMohamed L, Fraser NW, Jones C, Wechsler SL. Journal of Virology. 2010;85:2325. doi: 10.1128/JVI.01791-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasgupta G, Nesburn AB, Wechsler SL, BenMohamed L. Future Microbiol. 2010;5:1–4. doi: 10.2217/fmb.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Launay O, Durier C, Desaint C, Silbermann B, Jackson A, Pialoux G, Bonnet B, Poizot-Martin I, Gonzalez-Canali G, Cuzin L, Figuereido S, Surenaud M, Ben Hamouda N, Gahery H, Choppin J, Salmon D, Guerin C, Bourgault Villada I, Guillet JG. PLoS ONE. 2007;2:13. doi: 10.1371/journal.pone.0000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pialoux G, Hocini H, Perusat S, Silberman B, Salmon-Ceron D, Slama L, Journot V, Mathieu E, Gaillard C, Petitprez K, Launay O, Chene G. Vaccine. 2007;12:43. doi: 10.1016/j.vaccine.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 22.Danve-Szatanek C, Aymard M, Thouvenot D, Morfin F, Agius G, Bertin I, Billaudel S, Chanzy B, Coste-Burel M, Finkielsztejn L, Fleury H, Hadou T, Henquell C, Lafeuille H, Lafon ME, Le Faou A, Legrand MC, Maille L, Mengelle C, Morand P, Morinet F, Nicand E, Omar S, Picard B, Pozzetto B, Puel J, Raoult D, Scieux C, Segondy M, Seigneurin JM, Teyssou R, Zandotti C. J. Clin. Microbiol. 2004;42:242. doi: 10.1128/JCM.42.1.242-249.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wald A, Link K. J. Infect. Dis. 2002;185:45. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 24.Rubbo PA, Tuaillon E, Nagot N, Chentoufi AA, Bollore K, Reynes J, Vendrell JP, BenMohamed L, Van De Perre P. J. Acquir. Immune Defic. Syndr. 2011;58:9. doi: 10.1097/QAI.0b013e318224d0ad. [DOI] [PubMed] [Google Scholar]
- 25.Lingappa JR, Celum C. Drugs. 2007;67:155. doi: 10.2165/00003495-200767020-00001. [DOI] [PubMed] [Google Scholar]
- 26.Corey L, Wald A, Celum CL, Quinn TC. J. Acquir. Immune Defic. Syndr. 2004;35:435. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 27.Celum C, Levine R, Weaver M, Wald A. Bull World Health Organ. 2004;82:447. [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, Corey L. Nat. Med. 2009;15:886. doi: 10.1038/nm.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keet IP, Lee FK, van Griensven GJ, Lange JM, Nahmias A, Coutinho RA. Genitourin Med. 1990;66:330. doi: 10.1136/sti.66.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubbo PA, Tuaillon E, Nagot N, Chentoufi AA, Bollore K, Reynes J, Vendrell JP, BenMohamed L, PV DEP. J. Acquir. Immune Defic. Syndr. 2011;58:9. doi: 10.1097/QAI.0b013e318224d0ad. [DOI] [PubMed] [Google Scholar]
- 31.Hoots BE, Hudgens MG, Cole SR, King CC, Klein RS, Mayer KH, Rompalo AM, Sobel JD, Jamieson DJ, Smith JS. Am. J. Epidemiol. 2011;173:837. doi: 10.1093/aje/kwq432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. AIDS. 2006;20:73. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 33.Schacker T, Zeh J, Hu H, Shaughnessy M, Corey L. J. Infect. Dis. 2002;186:1718. doi: 10.1086/345771. [DOI] [PubMed] [Google Scholar]
- 34.Schacker T, Ryncarz AJ, Goddard J, Diem K, Shaughnessy M, Corey L. Jama. 1998;280:61. doi: 10.1001/jama.280.1.61. [DOI] [PubMed] [Google Scholar]
- 35.Schacker T, Hu HL, Koelle DM, Zeh J, Saltzman R, Boon R, Shaughnessy M, Barnum G, Corey L. Ann. Intern. Med. 1998;128:21. doi: 10.7326/0003-4819-128-1-199801010-00004. [DOI] [PubMed] [Google Scholar]
- 36.Aral SO. Curr. Infect. Dis. Rep. 2010;12:134. doi: 10.1007/s11908-010-0087-2. [DOI] [PubMed] [Google Scholar]
- 37.Block SL, Yogev R, Waldmeier F, Hamed K. Pediatr. Infect. Dis. 2010;4:24. doi: 10.1097/INF.0b013e3182067cee. [DOI] [PubMed] [Google Scholar]
- 38.Andersson KM, Paltiel AD, Owens DK. Vaccine. 2011;56:34. doi: 10.1016/j.vaccine.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wald A, Corey L, Cone R, Hobson A, Davis G, Zeh J. J. Clin. Invest. 1997;99:1092. doi: 10.1172/JCI119237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta R, Warren T, Wald A. Lancet. 2007;370:2127. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 41.Corey L. J. Infect. Dis. 2002;186:29. doi: 10.1086/342971. [DOI] [PubMed] [Google Scholar]
- 42.Chan T, Barra NG, Lee AJ, Ashkar AA. J. Reprod. Immunol. 2011;88:210. doi: 10.1016/j.jri.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 43.de Jong MA, de Witte L, Taylor ME, Geijtenbeek TB. J. Immunol. 2010;185:1633. doi: 10.4049/jimmunol.0904137. [DOI] [PubMed] [Google Scholar]
- 44.Rebbapragada A, Wachihi C, Pettengell C, Sunderji S, Huibner S, Jaoko W, Ball B, Fowke K, Mazzulli T, Plummer FA, Kaul R. Aids. 2007;21:589. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 45.Posavad CM, Koelle DM, Shaughnessy MF, Corey L. Proc. Natl. Acad. Sci. USA. 1997;94:10289. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnson KE, Redd AD, Quinn TC, Collinson-Streng AN, Cornish T, Kong X, Sharma R, Tobian AA, Tsai B, Sherman ME, Kigozi G, Serwadda D, Wawer MJ, Gray RH. J. Infect. Dis. 2011;203:602. doi: 10.1093/infdis/jiq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minami R, Yamamoto M, Takahama S, Ando H, Miyamura T, Suematsu E. AIDS Res. Hum. Retroviruses. 2009;25:1. doi: 10.1089/aid.2007.0260. [DOI] [PubMed] [Google Scholar]
- 48.Yudin MH, Kaul R. Infect. Dis. Obstet. Gynecol. 2008;592:12. doi: 10.1155/2008/592532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheth PM, Sunderji S, Shin LY, Rebbapragada A, Huibner S, Kimani J, Macdonald KS, Ngugi E, Bwayo JJ, Moses S, Kovacs C, Loutfy M, Kaul R. J. Infect. Dis. 2008;197:1394. doi: 10.1086/587697. [DOI] [PubMed] [Google Scholar]
- 50.Pagani JM, Duan JQ. Optometry. 2011;82:77. doi: 10.1016/j.optm.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 51.Gorantla S, Santos K, Meyer V, Dewhurst S, Bowers WJ, Federoff HJ, Gendelman HE, Poluektova L. J. Virol. 2005;79:2124. doi: 10.1128/JVI.79.4.2124-2132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Couppie P, Sarazin F, Clyti E, El Guedj M, Vaz T, Sainte-Marie D, Marty C, Nacher M. AIDS Patient Care STDS. 2006;20:143. doi: 10.1089/apc.2006.20.143. [DOI] [PubMed] [Google Scholar]
- 53.Baltzer H, Chege D, Rebbapragada A, Wachihi C, Shin LY, Kimani J, Ball TB, Jaoko W, Plummer FA, Kaul R. Curr. HIV Res. 2009;7:504. doi: 10.2174/157016209789346336. [DOI] [PubMed] [Google Scholar]
- 54.Wright PW, Hoesley CJ, Squires KE, Croom-Rivers A, Weiss HL, Gnann JW., Jr. Clin. Infect. Dis. 2003;36:207. doi: 10.1086/345440. [DOI] [PubMed] [Google Scholar]
- 55.Kucera LS, Leake E, Iyer N, Raben D, Myrvik QN. AIDS Res. Hum. Retroviruses. 1990;6:641. doi: 10.1089/aid.1990.6.641. [DOI] [PubMed] [Google Scholar]
- 56.Bagdades EK, Pillay D, Squire SB, O'Neil C, Johnson MA, Griffiths PD. AIDS. 1992;6:1317. doi: 10.1097/00002030-199211000-00012. [DOI] [PubMed] [Google Scholar]
- 57.Smit C, Pfrommer C, Mindel A, Taylor J, Spaargaren J, Berkhout B, Coutinho R, Dukers NH. Eur. J. Epidemiol. 2007;22:937. doi: 10.1007/s10654-007-9178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Farrell N. Sex Transm. Infect. 1999;75:377. doi: 10.1136/sti.75.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagot N, Ouedraogo A, Konate I, Weiss HA, Foulongne V, Defer MC, Sanon A, Becquart P, Segondy M, Sawadogo A, Van de Perre P, Mayaud P. J. Infect. Dis. 2008;198:241. doi: 10.1086/589621. [DOI] [PubMed] [Google Scholar]
- 60.Jin F, Prestage GP, Mao L, Kippax SC, Pell CM, Donovan B, Templeton DJ, Taylor J, Mindel A, Kaldor JM, Grulich AE. J. Infect. Dis. 2006;194:561. doi: 10.1086/506455. [DOI] [PubMed] [Google Scholar]
- 61.Rubbo PA, Tuaillon E, Nagot N, Chentoufi AA, Bollore K, REYNES J, Vendrell JP, BenMohamed L, Van De Perre P. AIDS. 2011;12:45. doi: 10.1097/QAI.0b013e318224d0ad. [DOI] [PubMed] [Google Scholar]
- 62.Kaul R, Pettengell C, Sheth PM, Sunderji S, Biringer A, MacDonald K, Walmsley S, Rebbapragada A. J. Reprod. Immunol. 2008;77:32. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Rebbapragada A, Wachihi C, Pettengell C, Sunderji S, Huibner S, Jaoko W, Ball B, Fowke K, Mazzulli T, Plummer FA, Kaul R. AIDS. 2007;21:589. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 64.Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, Mujugira A, Baeten JM, Mullins JI, Hughes JP, Bukusi EA, Cohen CR, Katabira E, Ronald A, Kiarie J, Farquhar C, Stewart GJ, Makhema J, Essex M, Were E, Fife KH, de Bruyn G, Gray GE, McIntyre JA, Manongi R, Kapiga S, Coetzee D, Allen S, Inambao M, Kayitenkore K, Karita E, Kanweka W, Delany S, Rees H, Vwalika B, Stevens W, Campbell MS, Thomas KK, Coombs RW, Morrow R, Whittington WL, McElrath MJ, Barnes L, Ridzon R, Corey L. N. Engl. J. Med. 2010;362:427. [Google Scholar]
- 65.Delany S, Mlaba N, Clayton T, Akpomiemie G, Capovilla A, Legoff J, Belec L, Stevens W, Rees H, Mayaud P. AIDS. 2009;23:461. doi: 10.1097/QAD.0b013e32831db217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tanton C, Weiss HA, Rusizoka M, Legoff J, Changalucha J, Baisley K, Mugeye K, Everett D, Belec L, Clayton TC, Ross DA, Hayes RJ, Watson-Jones D. J. Infect. Dis. 2010;201:1285. doi: 10.1086/651696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Celum CL. Top HIV Med. 2010;18:138. [PubMed] [Google Scholar]
- 68.Corey L, Langenberg AG, Ashley R, Sekulovich RE, Izu AE, Douglas JM, Jr., Handsfield HH, Warren T, Marr L, Tyring S, DiCarlo R, Adimora AA, Leone P, Dekker CL, Burke RL, Leong WP, Straus SE. Jama. 1999;282:331. doi: 10.1001/jama.282.4.331. [DOI] [PubMed] [Google Scholar]
- 69.Hartley C. Jama. 2000;283:746. [PubMed] [Google Scholar]
- 70.Friedman HM. Jama. 2000;746:746. doi: 10.1001/jama.283.6.746. [DOI] [PubMed] [Google Scholar]
- 71.Sheridan BS, Cherpes TL, Urban J, Kalinski P, Hendricks RL. J. Virol. 2009;83:2237. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. J. Exp. Med. 2000;191:1459. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu T, Khanna KM, Carriere BN, Hendricks RL. J. Virol. 2001;75:11178. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Divito S, Cherpes TL, Hendricks RL. Immunol. Res. 2006;36:119. doi: 10.1385/IR:36:1:119. [DOI] [PubMed] [Google Scholar]
- 75.Khanna KM, Lepisto AJ, Hendricks RL. Trends Immunol. 2004;25:230. doi: 10.1016/j.it.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 76.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Science. 2008;322:268. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. Proc. Natl. Acad. Sci. USA. 2007;104:3496. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Theil D, Arbusow V, Derfuss T, Strupp M, Pfeiffer M, Mascolo A, Brandt T. Brain Pathol. 2001;11:408. doi: 10.1111/j.1750-3639.2001.tb00408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Derfuss T, Segerer S, Herberger S, Sinicina I, Hufner K, Ebelt K, Knaus HG, Steiner I, Meinl E, Dornmair K, Arbusow V, Strupp M, Brandt T, Theil D. Brain Pathol. 2007;17:389. doi: 10.1111/j.1750-3639.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dasgupta G, BenMohamed L. Vaccine. 2011;29:5824. doi: 10.1016/j.vaccine.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Knaup B, Schunemann S, Wolff MH. Oral Microbiol. Immunol. 2000;15:281. doi: 10.1034/j.1399-302x.2000.150502.x. [DOI] [PubMed] [Google Scholar]
- 82.Wald A, Zeh J, Selke S, Warren T, Ryncarz AJ, Ashley R, Krieger JN, Corey L. N. Engl. J. Med. 2000;342:844. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 83.Wald A, Zeh J, Selke S, Warren T, Ashley R, Corey L. J. Infect. Dis. 2002;186(Suppl. 1):S34. doi: 10.1086/342969. [DOI] [PubMed] [Google Scholar]
- 84.Leigh JF, Acharya N, Cevallos V, Margolis TP. Br. J. Ophthalmol. 2008;92:435. doi: 10.1136/bjo.2007.122150. [DOI] [PubMed] [Google Scholar]
- 85.Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. J. Immunol. 2007;179:322. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.HEDS N. Engl. J. Med. 1998;339:300. [Google Scholar]
- 87.Hobbs MR, Jones BB, Otterud BE, Leppert M, Kriesel JD. J. Infect. Dis. 2008;197:340. doi: 10.1086/525540. [DOI] [PubMed] [Google Scholar]
- 88.Stamm WE, Handsfield HH, Rompalo AM, Ashley RL, Roberts PL, Corey L. Jama. 1988;260:1429. [PubMed] [Google Scholar]
- 89.Chentoufi AA, Kritzer E, D. YM, Nesburn AB, BenMohamed L. Clinical and Developmental Immunology. 2012;5:456. doi: 10.1155/2012/187585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G. N. Engl. J. Med. 2002;347:1652. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 91.Belshe PB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RLA, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. N. Engl. J. Med. 2012;366:34. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cohen J. Science. 2010;330:304. doi: 10.1126/science.330.6002.304. [DOI] [PubMed] [Google Scholar]
- 93.Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L. Journal of Virology. 2005;79:15289. doi: 10.1128/JVI.79.24.15289-15301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.BenMohamed L, Wechsler SL, Nesburn AB. Lancet Infect. Dis. 2002;2:425. doi: 10.1016/s1473-3099(02)00318-3. [DOI] [PubMed] [Google Scholar]
- 95.Koelle DM. Curr. Opin. Investig. Drugs. 2006;7:136. [PubMed] [Google Scholar]
- 96.Russell MW. Am. J. Reprod. Immunol. 2002;47:265. doi: 10.1034/j.1600-0897.2002.01099.x. [DOI] [PubMed] [Google Scholar]
- 97.Kaul R, Pettengell C, Sheth PM, Sunderji S, Biringer A, Macdonald K, Walmsley S, Rebbapragada A. J. Reprod. Immunol. 2007;56:43. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 98.MasCasullo V, Fam E, Keller MJ, Herold BC. Viral Immunol. 2005;18:595. doi: 10.1089/vim.2005.18.595. [DOI] [PubMed] [Google Scholar]
- 99.Milligan GN, Dudley-McClain KL, Chu CF, Young CG. Virology. 2004;318:507–515. doi: 10.1016/j.virol.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 100.Kwant A, Rosenthal KL. Vaccine. 2004;22:3098. doi: 10.1016/j.vaccine.2004.01.059. [DOI] [PubMed] [Google Scholar]
- 101.Hamajima K, Hoshino Y, Xin KQ, Hayashi F, Tadokoro K, Okuda K. Clin. Immunol. 2002;102:12. doi: 10.1006/clim.2001.5141. [DOI] [PubMed] [Google Scholar]
- 102.Gallichan WS, Rosenthal KL. J. Infect. Dis. 1998;177:1155. doi: 10.1086/515286. [DOI] [PubMed] [Google Scholar]
- 103.Schleiss MR, Bourne N, Jensen NJ, Bravo F, Bernstein DI. Viral Immunol. 2000;13:155. doi: 10.1089/vim.2000.13.155. [DOI] [PubMed] [Google Scholar]
- 104.Gherardi MM, Perez-Jimenez E, Najera JL, Esteban M. J. Immunol. 2004;172:6209. doi: 10.4049/jimmunol.172.10.6209. [DOI] [PubMed] [Google Scholar]
- 105.Tengvall S, Lundqvist A, Eisenberg RJ, Cohen GH, Harandi AM. Journal of virology. 2006;80:5283. doi: 10.1128/JVI.02013-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rosenthal KL, Gallichan WS. Semin. Immunol. 1997;9:303. doi: 10.1006/smim.1997.0086. [DOI] [PubMed] [Google Scholar]
- 107.Toka FN, Pack CD, Rouse BT. Immunol. Rev. 2004;199:100. doi: 10.1111/j.0105-2896.2004.0147.x. [DOI] [PubMed] [Google Scholar]
- 108.Stanberry LR. Herpes. 2004;11(Suppl. 3):161. [PubMed] [Google Scholar]
- 109.Mestecky J, Moldoveanu Z, Russell MW. Am. J. Reprod. Immunol. 2005;53:208. doi: 10.1111/j.1600-0897.2005.00267.x. [DOI] [PubMed] [Google Scholar]
- 110.Moldoveanu Z, Huang WQ, Kulhavy R, Pate MS, Mestecky J. J. Immunol. 2005;175:4127. doi: 10.4049/jimmunol.175.6.4127. [DOI] [PubMed] [Google Scholar]
- 111.Gill N, Davies EJ, Ashkar AA. Am. J. Reprod. Immunol. 2008;59:35. doi: 10.1111/j.1600-0897.2007.00558.x. [DOI] [PubMed] [Google Scholar]
- 112.Herbst-Kralovetz MM, Quayle AJ, Ficarra M, Greene S, Rose WA, 2nd, Chesson R, Spagnuolo RA, Pyles RB. Am. J. Reprod. Immunol. 2008;59:212. doi: 10.1111/j.1600-0897.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 113.Soboll G, Schaefer TM, Wira CR. Am. J. Reprod. Immunol. 2006;55:434. doi: 10.1111/j.1600-0897.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 114.Zariffard MR, Novak RM, Lurain N, Sha BE, Graham P, Spear GT. J. Infect. Dis. 2005;191:1913. doi: 10.1086/429922. [DOI] [PubMed] [Google Scholar]
- 115.Lund JM, Linehan MM, Iijima N, Iwasaki A. J. Immunol. 2006;177:7510. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- 116.Fichorova RN, Cronin AO, Lien E, Anderson DJ, Ingalls RR. J. Immunol. 2002;168:2424. doi: 10.4049/jimmunol.168.5.2424. [DOI] [PubMed] [Google Scholar]
- 117.BenMohamed L, Krishnan R, Auge C, Primus JF, Diamond DJ. Immunology. 2002;106:113. doi: 10.1046/j.1365-2567.2002.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.BenMohamed L, Belkaid Y, Loing E, Brahimi K, Gras-Masse H, Druilhe P. Eur. J. Immunol. 2002;32:2274. doi: 10.1002/1521-4141(200208)32:8<2274::AID-IMMU2274>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 119.Borsutzky S, Ebensen T, Link C, Becker PD, Fiorelli V, Cafaro A, Ensoli B, Guzman CA. Vaccine. 2006;24:2049. doi: 10.1016/j.vaccine.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 120.Batzloff MR, Hartas J, Zeng W, Jackson DC, Good MF. J. Infect. Dis. 2006;194:325–330. doi: 10.1086/505146. [DOI] [PubMed] [Google Scholar]
- 121.Zhu X, Ramos TV, Gras-Masse H, Kaplan BE, BenMohamed L. Eur. J. Immunol. 2004;34:1142. doi: 10.1002/eji.200425166. [DOI] [PubMed] [Google Scholar]
- 122.Chentoufi AA, BenMohamed L. Future Virology. 2010;5:525. doi: 10.2217/fvl.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lu S. Curr. Opin. Immunol. 2009;21:346. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barouch DH. Curr. Opin. HIV AIDS. 2010;5:386. doi: 10.1097/COH.0b013e32833cfe4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kaufman DR, Bivas-Benita M, Simmons NL, Miller D, Barouch DH. J. Virol. 2010;84:5986. doi: 10.1128/JVI.02563-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Qureshi H, Ma ZM, Huang Y, Hodge G, Thomas MA, DiPasquale J, DeSilva V, Fritts L, Bett AJ, Casimiro DR, Shiver JW, Robert-Guroff M, Robertson MN, McChesney MB, Gilbert PB, Miller CJ. J. Virol. 2012;86:2239. doi: 10.1128/JVI.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bolton DL, Song K, Wilson RL, Kozlowski PA, Tomaras GD, Keele BF, Lovingood RV, Rao S, Roederer M. Mucosal immunology. 2012;5:41. doi: 10.1038/mi.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cheng-Mayer C, Huang Y, Gettie A, Tsai L, Ren W, Shakirzyanova M, Sina ST, Trunova N, Blanchard J, Jenkins LM, Lo Y, Schito ML, Appella E. AIDS. 2011;25:1833. doi: 10.1097/QAD.0b013e32834a1d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ledgerwood JE, Costner P, Desai N, Holman L, Enama ME, Yamshchikov G, Mulangu S, Hu Z, Andrews CA, Sheets RA, Koup RA, Roederer M, Bailer R, Mascola JR, Pau MG, Sullivan NJ, Goudsmit J, Nabel GJ, Graham BS. Vaccine. 2010;29:304. doi: 10.1016/j.vaccine.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 130.Cheng C, Gall JG, Nason M, King CR, Koup RA, Roederer M, McElrath MJ, Morgan CA, Churchyard G, Baden LR, Duerr AC, Keefer MC, Graham BS, Nabel GJ. Journal of Virology. 2010;84:630. doi: 10.1128/JVI.00866-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wilks AB, Christian EC, Seaman MS, Sircar P, Carville A, Gomez CE, Esteban M, Pantaleo G, Barouch DH, Letvin NL, Permar SR. J. Immunol. 2010;185:7097. doi: 10.4049/jimmunol.1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, Clark S, Ng'ang'a D, Brandariz KL, Abbink P, Sinangil F, de Bruyn G, Gray GE, Roux S, Bekker LG, Dilraj A, Kibuuka H, Robb ML, Michael NL, Anzala O, Amornkul PN, Gilmour J, Hural J, Buchbinder SP, Seaman MS, Dolin R, Baden LR, Carville A, Mansfield KG, Pau MG, Goudsmit J. Vaccine. 2011;29:5203. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bradley RR, Maxfield LF, Lynch DM, Iampietro MJ, Borducchi EN, Barouch DH. J. Virol. 2012;86:1267. doi: 10.1128/JVI.06165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, Iwasaki A. J. Exp. Med. 2008;205:3041. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Iwasaki A. Nat. Rev. Immunol. 2010;10:699. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chentoufi AA, BenMohamed L. Future Virol. 2010;5:525. doi: 10.2217/fvl.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, Maillere B, BenMohamed L. J. Virol. 2008;82:11792. doi: 10.1128/JVI.00692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dasgupta G, Chentoufi AA, Kalantari-Dehaghi M, Falatoonzadeh P, Chun S, H. LC, Felgner PL, H. HD, BenMohamed L. J. Virology. 2012;54:98. doi: 10.1128/JVI.07107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. J. Exp. Med. 2007;44:204. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, Wechsler SL, Nesburn AB, BenMohamed L. J. Immunol. 2008;180:426. doi: 10.4049/jimmunol.180.1.426. [DOI] [PubMed] [Google Scholar]
- 141.Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, Maillère B, BenMohamed L. Journal Virology. 2008;453:23. [Google Scholar]
- 142.BenMohamed L, Bertrand G, McNamara CD, Gras-Masse H, Hammer J, Wechsler SL, Nesburn AB. J. Virol. 2003;77:9463. doi: 10.1128/JVI.77.17.9463-9473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mikloska Z, Cunningham AL. J. Gen. Virol. 1998;79(Pt. 2):353. doi: 10.1099/0022-1317-79-2-353. [DOI] [PubMed] [Google Scholar]
- 144.Koelle DM, Chen HB, Gavin MA, Wald A, Kwok WW, Corey L. J. Immunol. 2001;166:4049. doi: 10.4049/jimmunol.166.6.4049. [DOI] [PubMed] [Google Scholar]
- 145.Koelle DM, Liu Z, McClurkan CL, Cevallos RC, Vieira J, Hosken NA, Meseda CA, Snow DC, Wald A, Corey L. Proc. Natl. Acad. Sci. USA. 2003;100:12899. doi: 10.1073/pnas.2131705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Posavad CM, Wald A, Hosken N, Huang ML, Koelle DM, Ashley RL, Corey L. J. Immunol. 2003;170:4380. doi: 10.4049/jimmunol.170.8.4380. [DOI] [PubMed] [Google Scholar]
- 147.Mikloska Z, Kesson AM, Penfold ME, Cunningham AL. J. Infect. Dis. 1996;173:7–17. doi: 10.1093/infdis/173.1.7. [DOI] [PubMed] [Google Scholar]
- 148.Hosken N, McGowan P, Meier A, Koelle DM, Sleath P, Wagener F, Elliott M, Grabstein K, Posavad C, Corey L. J. Virol. 2006;80:5509. doi: 10.1128/JVI.02659-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Carmack MA, Yasukawa LL, Chang SY, Tran C, Saldana F, Arvin AM, Prober CG. J. Infect. Dis. 1996;174:899. doi: 10.1093/infdis/174.5.899. [DOI] [PubMed] [Google Scholar]
- 150.Koelle DM, Abbo H, Peck A, Ziegweid K, Corey L. J. Infect. Dis. 1994;169:956. doi: 10.1093/infdis/169.5.956. [DOI] [PubMed] [Google Scholar]
- 151.Koelle DM, Corey L, Burke RL, Eisenberg RJ, Cohen GH, Pichyangkura R, Triezenberg SJ. J. Virol. 1994;68:2803. doi: 10.1128/jvi.68.5.2803-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aurelian L. Clin. Diagn. Lab Immunol. 2004;11:437. doi: 10.1128/CDLI.11.3.437-445.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Siegal FP, Lopez C, Hammer GS, Brown AE, Kornfeld SJ, Gold J, Hassett J, Hirschman SZ, Cunningham-Rundles C, Adelsberg BR, Parham DM, Siegal M, Cunningham-Rundles S, Armstrong D. N. Engl. J. Med. 1981;305:1439. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- 154.Koelle DM, Frank JM, Johnson ML, Kwok WW. J. Virol. 1998;72:7476. doi: 10.1128/jvi.72.9.7476-7483.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Koelle DM, Posavad CM, Barnum GR, Johnson ML, Frank JM, Corey L. J. Clin. Invest. 1998;101:1500. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bassett I, Donovan B, Bodsworth NJ, Field PR, Ho DW, Jeansson S, Cunningham AL. Med. J. Aust. 1994;160:697. [PubMed] [Google Scholar]
- 157.Spruance SL, Overall JC, Jr., Kern ER, Krueger GG, Pliam V, Miller W. N. Engl. J. Med. 1977;297:69. doi: 10.1056/NEJM197707142970201. [DOI] [PubMed] [Google Scholar]
- 158.Koelle DM, Benedetti J, Langenberg A, Corey L. Ann. Intern. Med. 1992;116:433. doi: 10.7326/0003-4819-116-6-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zarling JM, Moran PA, Brewer L, Ashley R, Corey L. J. Virol. 1988;62:4481. doi: 10.1128/jvi.62.12.4481-4485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zarling JM, Moran PA, Burke RL, Pachl C, Berman PW, Lasky LA. J. Immunol. 1986;136:4669. [PubMed] [Google Scholar]
- 161.Koelle DM, Wald A. J. Antimicrob. Chemother. 2000;45(Suppl. T3):1–8. doi: 10.1093/jac/45.suppl_4.1. [DOI] [PubMed] [Google Scholar]
- 162.Johnson AJ, Chu CF, Milligan GN. J. Virol. 2008;56:343. doi: 10.1128/JVI.01159-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Milligan GN, Bernstein DI. Virology. 1995;212:481. doi: 10.1006/viro.1995.1506. [DOI] [PubMed] [Google Scholar]
- 164.Milligan GN, Bernstein DI. Virology. 1997;229:259. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 165.Milligan GN, Bernstein DI, Bourne N. J. Immunol. 1998;160:6093. [PubMed] [Google Scholar]