Abstract
The rough endoplasmic reticulum is a major site of protein biosynthesis in all eukaryotic cells, serving as the entry point for the secretory pathway and as the initial integration site for the majority of cellular integral membrane proteins. The core components of the protein translocation machinery have been identified, and high-resolution structures of the targeting components and the transport channel have been obtained. Research in this area is now focused on obtaining a better understanding of the molecular mechanism of protein translocation and membrane protein integration.
The core components of the protein translocation machinery are known. However, specific molecular mechanisms of the translocation process (e.g., the integration of multispanning membrane proteins) are less understood.
Protein translocation across the rough endoplasmic reticulum (RER) is an ancient and evolutionarily conserved process that is analogous to protein export across the cytoplasmic membranes of eubacterial and archaebacterial cells both with respect to the mechanism and core components. The RER membrane of eukaryotic cells is contiguous with the nuclear envelope and is morphologically composed of interconnected cisternae and tubules. Electron microscope images of mammalian cells and tissues revealed that the cisternal regions of the cytoplasmic surface of the endoplasmic reticulum are densely studded by membrane-bound ribosomes (Palade 1955a,b), giving rise to the term “rough ER.” The RER-bound ribosomes in en face images are often arranged in spirals or hairpins (Palade 1955a; Christensen and Bourne 1999), indicative of polyribosomes that are actively engaged in protein translation.
Consistent with this high density of membrane-bound ribosomes, the RER is a major site of protein biosynthesis in eukaryotic cells. The nuclear envelope, the Golgi, lysosome, peroxisome, plasma membrane, and endosomes are biosynthetically derived from the rough ER. The three major groups of proteins that are synthesized by RER-bound ribosomes include secretory proteins, integral membrane proteins destined for ER-derived membranes, and the lumenal-resident proteins of the ER, Golgi, nuclear envelope, and lysosome. For those membranes that are not physically linked to the ER (e.g., the lysosome), integral membrane and lumenal proteins are delivered to their destination by vesicular transport pathways. Bioinformatics analysis of fully sequenced eukaryotic genomes indicates that roughly 30% of open reading frames encode integral membrane proteins (Wallin and von Heijne 1998); hence, a major role of the RER is the biosynthesis of membrane proteins. An important class of membrane proteins that are integrated into the RER has single carboxy-terminal TM spans and are known as tail-anchored (TA) membrane proteins. The posttranslational integration pathway for TA proteins has been a subject of several recent reviews (Borgese and Fasana 2011; Shao and Hegde 2011), thus we will not address the TA pathway in this article.
THE SIGNAL HYPOTHESIS
Biochemical experiments to address the role of membrane-bound ribosomes in secretory protein biosynthesis began in earnest in the 1960s (Redman and Sabatini 1966; Redman et al. 1966). In 1971, Gunter Blobel proposed that secretory protein mRNAs encode a signal that promotes targeting of ribosomes to the RER. The following year, Cesar Milstein and colleagues discovered that a secretory protein (IgG light chain) is synthesized as a higher-molecular-weight precursor (Milstein et al. 1972). In a landmark paper formally presenting the signal hypothesis, Blobel and Dobberstein showed that the IgG light chain could be synthesized in vitro, cotranslationally translocated across canine pancreas microsomal membranes, and proteolytically processed into the mature polypeptide (Blobel and Dobberstein 1975). During the next several years, protein sequence analysis showed that secretory proteins are synthesized as precursors that have an amino-terminal hydrophobic signal sequence and a processing site for an RER-localized signal peptidase (von Heijne 1983). The TM spans of integral membrane proteins, which are also hydrophobic, function as signal sequences when located near the amino terminus of membrane proteins (Friedlander and Blobel 1985) or when inserted into a signal sequence-deficient reporter protein (Mize et al. 1986). Thus, ribosomes synthesizing secretory or membrane proteins are targeted to the RER by nonidentical segments of hydrophobic amino acids.
TARGETING OF mRNAS AND TRANSLATING RIBOSOMES TO THE ROUGH ENDOPLASMIC RETICULUM
Although signal sequences provide a protein-based address code for the ER, what are the cellular proteins or lipids that decode a sequence composed of a string of hydrophobic amino acids? The identification of the signal recognition particle (SRP) by Walter and Blobel resolved this question (Walter and Blobel 1980). The SRP selectively binds to ribosomes translating mRNAs encoding presecretory proteins (Walter et al. 1981), reduces the protein synthesis elongation rate (Walter and Blobel 1981b), and mediates selective targeting of the ribosome–nascent chain complex (RNC) to the RER (Fig. 1) (Walter and Blobel 1981a).
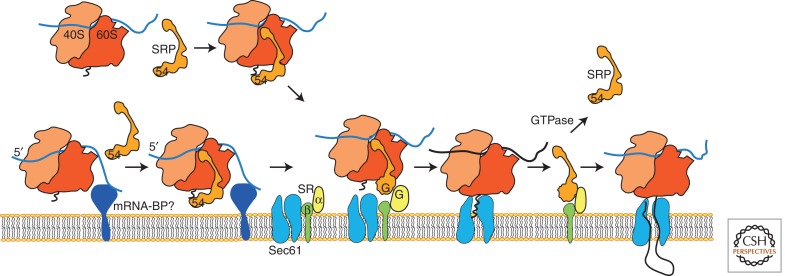
Figure 1.
Targeting of RNCs to the Sec61 complex. The mRNAs encoding proteins with ER signal sequences may be targeted to the vicinity of the RER by a translation-independent mechanism and bind to a currently unidentified mRNA-binding protein (mRNA-BP). The SRP particle binds to the 80S ribosome and mediates targeting to the ER via interaction with SRα. Cooperative GTP binding to SRP54 and SRα leads to dissociation of SRP from the RNC and attachment of the RNC to the Sec61 complex. Signal sequence insertion into the SSB site gates the translocation channel.
In mammalian cells, the SRP is composed of six protein subunits and the 7S RNA (Walter and Blobel 1982). An elongated architecture of the SRP was determined by mapping protein-binding sites onto the folded structure of the 7S RNA (Siegel and Walter 1988; Strub and Walter 1990), and by electron microscopy (Andrews et al. 1987). Of the protein subunits, SRP54 has received the most attention because it contains a methionine-rich domain that binds directly to the signal sequence and to the 7S RNA (Zopf et al. 1990). Cryoelectron microscopy of SRP–ribosome–nascent chain complexes (SRP–RNCs) revealed that SRP54 is positioned near the polypeptide exit site on the large ribosomal subunit, whereas the Alu subdomain of the SRP particle is positioned near the elongation factor-binding site (Halic et al. 2004). Recently, several groups have reported that SRP or the eubacterial homolog Ffh (see below) is recruited to the ribosome before the amino terminus of the protein emerges from the polypeptide exit tunnel (Bornemann et al. 2008; Berndt et al. 2009), thereby increasing the probability that SRP will discriminate between an authentic signal sequence and a nonsignal sequence shortly after the amino terminus of the protein emerges from the large ribosomal subunit. Recognition of the signal sequence by the SRP allows cotranslational delivery of RNCs to the RER. Cotranslational integration may be particularly important for the biosynthesis of multispanning membrane proteins because TM spans are prone to aggregation in aqueous environments.
Are certain classes of mRNAs targeted to the RER by translation-independent pathways? It has long been recognized that membrane-bound and free polysome fractions isolated from tissues synthesize different classes of proteins (Ramsey and Steele 1976). The use of high-throughput methods to analyze the partitioning of mRNAs between membrane-bound and free polysome fractions showed that nucleocytoplasmic proteins are primarily synthesized by free polysomes and that membrane-bound polysomes are enriched in mRNAs encoding endomembrane resident proteins (Diehn et al. 2000). Unexpectedly, mRNAs encoding certain nucleocytoplasmic proteins (e.g., Hsp90 and calcinuerin) are strongly enriched in the membrane-bound polysome fraction (Diehn et al. 2000; Lerner et al. 2003). Secondly, mRNAs encoding secretory proteins were not as enriched in the membrane-bound polysome fraction as mRNAs encoding endomembrane resident proteins (Chen et al. 2011). Evidence for translation-independent binding of mRNAs to the RER has also been obtained, suggesting that mRNA targeting to the vicinity of the RER may precede SRP-dependent targeting of RNCs to the protein translocation channel (Fig. 1) (Pyhtila et al. 2008).
THE SRP54 AND SRα GTPase REGULATE THE DELIVERY OF RNCs TO THE TRANSLOCATION CHANNEL
The discovery that SRP delivers RNCs to the RER provided the foundation for identifying an RER-localized SRP receptor (SR) for the SRP–RNC complex (Gilmore et al. 1982a,b). The heterodimeric SR (SRα + SRβ) is localized to the ER by integration of the β-subunit (Lauffer et al. 1985). Dissociation of the SRP–SR complex from the signal sequence precedes RNC binding to the protein translocation channel (Gilmore and Blobel 1983).
SRα, SRP54, and the 7S RNA are evolutionarily conserved; FtsY and the Ffh–4.5S RNA complex are the eubacterial equivalents of the SR and SRP (Poritz et al. 1988; Poritz et al. 1990; Miller et al. 1994). In the eubacterial organism Escherichia coli, the SRP–SR targeting pathway is primarily involved in the biosynthesis of inner membrane proteins (Ulbrandt et al. 1997; Koch et al. 1999). Most periplasmic proteins and β-barrel outer membrane proteins are translocated by a posttranslational SecA–SecYEG-dependent pathway (for a recent review, see Park and Rapoport 2012). Disruption of the budding yeast (Saccharomyces cerevisiae) genes encoding SR or SRP subunits or the SRP RNA yielded slow-growing strains that have severe, yet transient, defects in protein translocation of a subset of proteins with RER signal sequences (Hann and Walter 1991; Ogg et al. 1992). In contrast to budding yeast, the SRP54 and SRP RNA genes are essential in Schizosaccharomyces pombe (Brennwald et al. 1988; Althoff et al. 1994).
The interaction between the SRP receptor and the SRP–RNC complex is regulated by a GTPase cycle (Connolly and Gilmore 1986, 1989) that results in dissociation of SRP54 from the signal sequence (Connolly et al. 1991) and attachment of the RNC to the protein translocation channel (Fig. 1). Protein sequence analysis and GTPase assays using purified SRP and SR led to the conclusion that SRα, SRβ, and SRP54 are members of the GTPase superfamily (Connolly and Gilmore 1993; Miller et al. 1993). The minimal components for the SR–SRP GTPase cycle are SRα, SRP54, and the 7S RNA (Miller et al. 1993). The most thorough kinetic analysis of the SRP–SR GTPase cycle has been achieved using bacterially expressed derivatives of Ffh, FtsY, and 4.5S RNA (Peluso et al. 2000; Shan and Walter 2003).
Unlike many GTPases, the hydrolysis cycle of the SRP–SR complex is not regulated by conventional guanine-nucleotide exchange factors (GEFs) or GTPase-activating proteins (GAPs). The requirement for the SRP RNA in the GTPase cycle is explained by the finding that the 4.5S RNA increases the rate of Ffh–FtsY complex formation and disassembly following hydrolysis (Peluso et al. 2000). The SRP and SR GTPases have a low affinity for guanine ribonucleotides compared with many other GTPases, and a lower affinity for GTP than for GDP (Connolly and Gilmore 1993; Rapiejko and Gilmore 1997). Indeed, before formation of the SRP–SR complex, GTP binding to Ffh and FtsY is reversible, and the binding specificity (GTP vs. ATP) is surprisingly weak for FtsY (Shan and Walter 2003). GTP hydrolysis by SRP and SR are catalytically linked and dependent on nucleotide occupancy of both sites (Powers and Walter 1995; Rapiejko and Gilmore 1997).
Several roles have been proposed for the SRP–SR GTPase cycle. One role is to control the assembly and disassembly of the SRP–SR complex; nonhydrolyzable GTP analogs stabilize the SRP–SR complex, whereas GTP hydrolysis promotes complex dissociation (Fig. 1) (Connolly et al. 1991). Recent evidence indicates that additional proofreading steps occur after signal sequence recognition by SRP to increase the fidelity of the protein translocation reaction (Zhang et al. 2010). When reconstituted into proteoliposomes, signal sequence dissociation from SRP54 and GTP hydrolysis by the SRP–SR complex is blocked unless an active Sec61 complex is present to serve as a receptor for the RNC complex (Song et al. 2000). Thus, the GTPase cycle regulates multiple steps in the delivery of an RNC to the protein translocation channel.
STRUCTURAL BIOLOGY OF THE SRP–SR TARGETING PATHWAY
SRP54 and Ffh are composed of an amino-terminal domain (N-domain), the central GTPase (G-domain), and the carboxy-terminal methionine-rich M-domain (Bernstein et al. 1989). Homologous N- and G-domains are also present in SRα and FtsY. The simpler composition of the eubacterial SR and SRP facilitated structural analysis of the SRP family of GTPases. High-resolution structures of the nucleotide-free forms of FfhNG and FtsYNG (Freymann et al. 1997; Montoya et al. 1997) and the GDP-Mg2+-bound form of FfhNG (Freymann et al. 1999) highlighted the homologous architecture of these GTPases and helped explain their low affinity for ribonucleotides. The structure of the FfhNG–FtsYNG complex obtained in the presence of a nonhydrolyzable GTP analog revealed a composite active site formed upon heterodimerization (Egea et al. 2004; Focia et al. 2004) that helped explain why Ffh and FtsY act as reciprocal GAPS (Powers and Walter 1995). A cocrystal structure of FfhNGM bound to domain IV of the 4.5S RNA revealed that the hydrophobic signal sequence-binding groove in the M-domain of Ffh terminates at the RNA-binding interface (Batey et al. 2000) and suggests that signal sequence binding to the M-domain may be communicated directly to the G-domain via the 4.5S RNA (Rosendal et al. 2003).
TRANSLOCATION CHANNELS AND RIBOSOME-BINDING SITES
After the discovery of SRP and the SR, several laboratories focused their efforts on the identification of the protein translocation channel. A yeast screen for gene products that were required for translocation of a secretory protein led to the identification of the essential SEC61, SEC62, and SEC63 genes (Deshaies and Schekman 1987; Rothblatt et al. 1989). Subsequent analysis showed that all three genes encode ER-localized integral membrane proteins that assemble into the SEC complex (Deshaies and Schekman 1989; Deshaies et al. 1991; Feldheim et al. 1992). Mutations in Sec61p inhibited translocation of secreted proteins and integration of membrane proteins, thereby providing the first evidence that Sec61 was the core subunit of the protein translocation channel (Stirling et al. 1992).
The mammalian translocation channel was initially detected by cross-linking an in vitro-assembled translocation intermediate to several different ER membrane proteins in the 30- to 40-kDa range (Wiedmann et al. 1989; Kellaris et al. 1991). Purification of the cross-linking targets resulted in identification of the TRAM protein (Görlich et al. 1992a) and the Sec61α protein (Görlich et al. 1992b). Mammalian Sec61α is homologous to yeast Sec61 and to the E. coli SecY protein (Görlich et al. 1992b), showing that protein translocation channels are conserved between eukaryotic and eubacterial organisms. Fungal genomes (e.g., S. cerevisiae) do not encode an obvious TRAM homolog.
Proteoliposomes containing the mammalian Sec61 complex (Sec61α, Sec61β, and Sec61γ) and the SR are active in translocation of a subset of secretory proteins (Görlich and Rapoport 1993). Incorporation of TRAM into Sec61–SR proteoliposomes stimulates translocation of all substrates tested, consistent with an accessory role for TRAM at an early stage in protein translocation (Voigt et al. 1996). Unlike TRAM, which contacts only the amino-terminal regions of nascent secretory proteins, Sec61 can be cross-linked to a photoreactive amino acid analog incorporated at any site in the nascent polypeptide (Mothes et al. 1994). Thus, Sec61 forms the aqueous transport pore that had been detected by a variety of biophysical and biochemical approaches (Gilmore and Blobel 1985; Simon and Blobel 1991; Crowley et al. 1993,1994).
Long before the identification of Sec61, multiple proteins had been proposed to be ER-localized ribosome receptors. The Sec61 complex binds nontranslating ribosomes with an affinity comparable to ribosome-stripped ER membranes (Kalies et al. 1994). Proteolytic digestion of canine Sec61 in intact membranes inhibits ribosome-binding activity by severing the carboxyl terminus and two cytosolically exposed loops (L6 and L8) (Fig. 2B) (Raden et al. 2000). Charge reversal substitutions at conserved basic residues in L6 (e.g., R275E) and L8 (R406E) of S. cerevisiae Sec61p cause a cotranslational translocation defect by interfering with RNC attachment to Sec61 (Cheng et al. 2005).
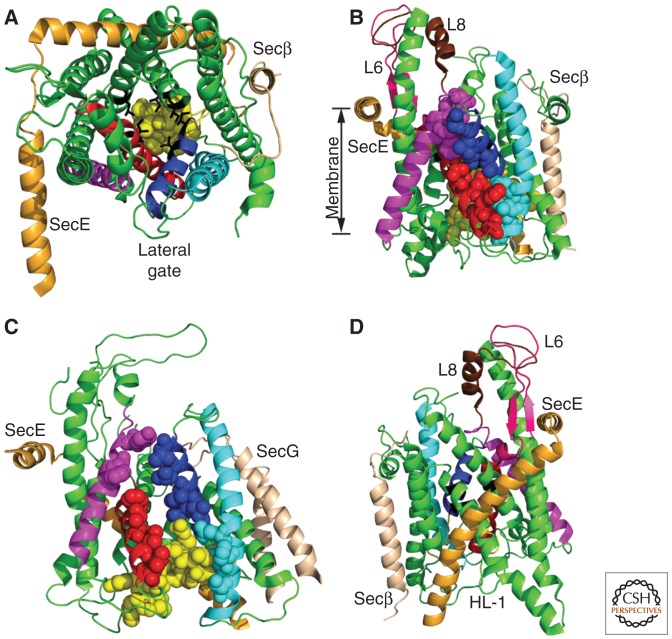
Figure 2.
SecYEβ and SecYEG translocation channels. TM spans of SecY are color coded as follows: TMs 1, 4–6, 9–10 (green), TM2 (blue), TM3 (cyan), TM7 (red), and TM8 (magenta). (Yellow spheres) The plug domain. Cytosolic loops 6 and 8 are pink and chocolate, respectively, in panels B and D. (A) The cytosolic face of the Methanocaldococcus jannaschii SecYEβ complex in the closed conformation. (Black sticks) Pore ring residues. (B) Lateral gate of M. jannaschii SecYEβ viewed from the plane of the membrane. (Spheres) Lateral gate contact residues (LGCRs). (C) The partially open conformation of the Thermotoga maritima SecYEG complex. (D) The hinge domain of M. jannaschii SecYEβ. The HL-1 hinge loop is labeled. All structure views were generated using PyMOL and PDB files 1RHZ and 3DIN.
TRANSFER OF RNCS FROM THE SRP–SR COMPLEX TO THE SEC61 COMPLEX
The ability of SRP to reduce the protein synthesis elongation rate is necessary in vivo both in budding yeast and in mammalian cells (Mason et al. 2000; Lakkaraju et al. 2008) to allow sufficient time for targeting of SRP–RNC to the SR. In addition to binding SRP54, SRα has a high affinity for the large ribosomal subunit, thereby facilitating formation of the SR–SRP–RNC complex (Mandon et al. 2003). Cryo-EM structures of the SRP–RNC complex and the Sec61–RNC complex have shown that the SRP and Sec61 have overlapping binding sites near the polypeptide exit tunnel (Halic et al. 2004). Upon formation of the SR–SRP–RNC complex, movement of the M-domain of SRP54 exposes the Sec61-binding site (Halic et al. 2004) for subsequent attachment of the RNC to the Sec61 complex.
The GTP-bound conformation of SRβ forms the membrane-binding site for SRα (Ogg et al. 1998; Legate et al. 2000; Schwartz and Blobel 2003). Interestingly, deletion of the SRβ-TM span has a less severe impact on SR function than inactivation of the SRβ GTP-binding site (Ogg et al. 1998). Yeast genetic experiments using SRβ-ΔTM cells provided evidence that the SR interacts with translocation channels via Sec61β subunits (Jiang et al. 2008), thereby providing a mechanism to position the SRP–RNC adjacent to a vacant protein translocation channel (Fig. 1).
STRUCTURES OF PROTEIN TRANSLOCATION CHANNELS
The first cryo-EM structures of the yeast Sec61–RNC complex (Beckmann et al. 1997, 2001) and the mammalian Sec61–80S complex (Hanein et al. 1996; Menetret et al. 2000; Morgan et al. 2002) were thought to contain three to four Sec61 heterotrimers. An oligomeric interface was proposed to form a large-diameter (∼25 Å) transport pore (Hanein et al. 1996; Beckmann et al. 1997; Morgan et al. 2002). Biophysical studies supported the concept of a large pore (∼40 Å) (Hamman et al. 1997) that was sealed by the ribosome on the cytoplasmic face of the ER and by BiP in the ER lumen (Hamman et al. 1998).
The X-ray crystal structure of Methanocaldococcus jannaschii SecYEβ (Van den Berg et al. 2004) was obtained in the absence of a ribosome or a nascent polypeptide, hence the structure is in a closed conformation. The membrane-exposed surface of SecYEβ complex lacks polar residues, arguing strongly against a transport pore formed by oligomer formation. SecE and Secβ, like their eukaryotic homologs Sec61β and Sec61γ, are C-tail-anchored membrane proteins with a single TM span (Fig. 2A). The 10 TM spans of SecY are arranged in two five-helix bundles (TM1-5 and TM6-10) to form an hourglass-shaped channel with a polar interior (Fig. 2A). A central constriction or pore ring is formed by side chains of hydrophobic residues projecting from TM spans closest to the channel center (Fig. 2A, side chains shown as black sticks). The exoplasmic face of the channel is blocked by a reentrant loop referred to as the plug domain (Fig. 2A, yellow spheres). Most point mutations in E. coli SecYEG that cause the prl phenotype, which corresponds to enhanced translocation of precursors with signal sequence mutations, map to the plug domain or the pore ring (Smith et al. 2005). Disulfides formed between a secretory protein precursor and a cysteine residue located near the pore ring provide evidence that secretory proteins are transported through the central pore (Cannon et al. 2005).
Budding yeast express an auxiliary protein translocation channel known as the Ssh1 complex (Finke et al. 1996) that is exclusively involved in the cotranslational translocation pathway (Wittke et al. 2002). Higher-resolution cryo-EM structures showed that single copies of mammalian Sec61, yeast Ssh1, or E. coli SecYEG form protein-conducting channels when bound to an RNC (Becker et al. 2009) or a 70S ribosome (Menetret et al. 2007). The two primary contact sites on the cytoplasmic surface of Sec61 or Ssh1 for the RNC are loops 6 and 8 (Becker et al. 2009), consistent with previous mutagenesis experiments (Cheng and Gilmore 2006). Cytosolic loop 8 undergoes a conformational change upon RNC binding to project into the polypeptide exit tunnel of the large ribosomal subunit.
TM2 and TM7 (blue and red α-helices in Fig. 2) of yeast Sec61 can be photocross-linked to the signal sequence of a nascent polypeptide (Plath et al. 1998), so this region has been termed the signal sequence-binding (SSB) site. Integration of a membrane protein necessitates lateral passage of the TM span from the central pore of SecY or Sec61 into the lipid bilayer. The lateral gate (TM2, TM3, TM7, and TM8) (Fig. 2A,B) is the only site where a TM span could exit the channel interior without crossing a cytosolic or lumenal loop joining two SecY TM spans (Fig. 2A,B).
The Thermatoga maritima SecYEG–SecA complex (Zimmer et al. 2008), the Thermus thermophilus SecYE–Fab complex (Tsukazaki et al. 2008), and the Pyrococcus furiosus SecYEβ complex (Egea and Stroud 2010) provided high-resolution structures of partially open protein conducting channels. SecA-dependent opening of SecYEG occurs by rigid-body movement of TMs 6–10 relative to TMs 1–5 (Zimmer et al. 2008) and is accompanied by movement of the plug domain away from the pore ring (Fig. 2C). In the fully open conformation, a translocation channel could accommodate a signal sequence in the SSB site and a nascent polypeptide in the central pore. The segment labeled HL-1 in loop 5 (Fig. 2D) is thought to be a hinge that allows the channel to open (Gumbart and Schulten 2007). Interestingly, the yeast sec61-2 point mutations map to a conserved glycine in HL-1 (Nishikawa et al. 2001), highlighting the importance of the hinge in Sec61 function. Several yeast sec61 alleles, including sec61-3, that cause a general defect in protein translocation can be suppressed by prl alleles, indicating that the transition between the closed and open conformations of the channel controls translocation efficiency and fidelity (Trueman et al. 2011).
How is the membrane permeability seal maintained when a translocation channel is in the open or closed conformation? Biophysical studies have suggested that BiP seals the lumenal face of the translocon during membrane protein integration (Hamman et al. 1998; Haigh and Johnson 2002). A second hypothesis was that the plug domain of SecY forms the membrane permeability seal (Van den Berg et al. 2004). Deletion of the plug domain in yeast Sec61p (Junne et al. 2006) or in E. coli SecY (Maillard et al. 2007) causes a minor growth defect and the prl phenotype (Junne et al. 2007). The membrane permeability barrier is reduced in E. coli cells expressing a SecY plug-deletion mutant (Saparov et al. 2007; Park and Rapoport 2011) when the channel is in the closed state (Park and Rapoport 2011). Replacement of three or more pore ring residues in E. coli SecYEG with alanine or glycine residues caused a disruption of the membrane permeability seal and a severe growth defect (Park and Rapoport 2011). In contrast, replacement of all six pore residues in yeast Sec61p with alanine or glycine yields viable strains that display the prl phenotype (Junne et al. 2010).
POSTTRANSLATIONAL TRANSLOCATION—ROLE OF THE SEC62/SEC63 COMPLEX
Translocation assays conducted using microsomes and translation extracts prepared from budding yeast provided overwhelming evidence for an ATP-dependent posttranslational translocation pathway (Hansen et al. 1986; Rothblatt and Meyer 1986; Waters and Blobel 1986). ATP dependence is explained by the involvement of cytoplasmic (Chirico et al. 1988) and lumenal (Vogel et al. 1990) Hsc70 proteins. The Ssa family of cytosolic Hsc70s help maintain precursor proteins in a translocation-competent conformation (Deshaies et al. 1988).
Partitioning of yeast translocation substrates between the cotranslational and posttranslational translocation pathways is dependent on the hydrophobicity of the signal sequence (Ng et al. 1996). Most integral membrane proteins that lack cleavable signal sequences use the SRP-dependent pathway. Proteins with less hydrophobic cleavable signal sequences are translocated by the SEC complex. The heptameric SEC complex is composed of a heterotrimeric Sec61 complex (Sec61p, Sbh1p, and Sss1p) combined with a Sec62/Sec63 complex (Sec62p, Sec63p, Sec66p, and Sec72p) (Esnault et al. 1993,1994; Feldheim et al. 1993; Fang and Green 1994; Feldheim and Schekman 1994; Panzner et al. 1995). Mutations in yeast BiP (Kar2p), Sec62p, and Sec63p inhibit posttranslational translocation in vivo (Rothblatt et al. 1989) and in vitro (Deshaies and Schekman 1989; Sanders et al. 1992; Brodsky et al. 1995).
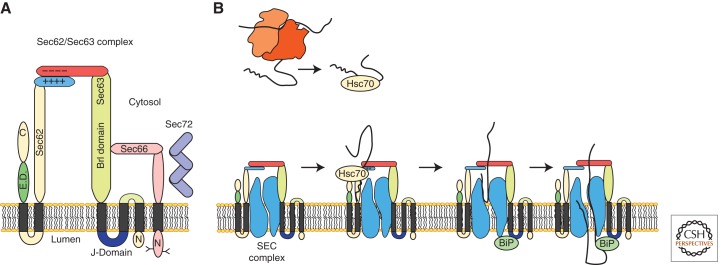
The Sec62/Sec63 complex has functionally important cytoplasmic and lumenal domains (Fig. 3A). A point mutation in the sec63-1 allele (Rothblatt et al. 1989) alters a critical residue in the lumenal J-domain of Sec63, thereby inhibiting the interaction between Sec63p and Kar2p (Lyman and Schekman 1995). Binding of Kar2 to the precursor provides a driving force for posttranslational translocation (Matlack et al. 1999). Truncation of a carboxy-terminal 27-residue acidic segment of Sec63p, which interacts with the basic amino terminus of Sec62 (Fig. 3A) (Wittke et al. 2000), promotes dissociation of Sec62p (Ng and Walter 1996) and causes a translocation defect (Ng et al. 1996). Carboxy-terminal deletions of Sec62 that remove a poorly understood effecter domain (Fig. 3B, segment labeled E.D.) are lethal (Wittke et al. 2000).
Figure 3.
Posttranslational translocation pathway in yeast. (A) A diagram of the yeast Sec62/Sec63 complex. (B) Posttranslational translocation through the SEC complex. Fungi-specific subunits (Sec66 and Sec71) are not shown for clarity. Substrate delivery to the Sec62/Sec63 complex by Hsc70 precedes signal sequence insertion into the SSB site. BiP is recruited to the SEC complex by the lumenal J-domain of Sec63. BiP binding to substrates promotes posttranslational translocation.
Photocross-linking experiments have shown that the signal sequence of a secretory protein can be inserted into the SSB site of Sec61 in the absence of ATP. The mature region of the precursor is then in contact with Sec62 (Müsch et al. 1992; Plath et al. 1998). Subsequent transport of the mature region of the protein through the Sec61 is ATP and Kar2p dependent. It is not known whether the Sec62/Sec63 complex simply serves as a targeting site for posttranslational substrates or, instead, promotes lateral gate separation of Sec61 to allow signal sequence insertion into the SSB site.
POTENTIAL ROLES FOR THE SEC62/SEC63 COMPLEX IN COTRANSLATIONAL TRANSLOCATION
Fully assembled SEC complexes as well as Sec61 heterotrimers are readily detected upon solubilization of yeast microsomes (Panzner et al. 1995). SEC complexes lack ribosome-binding activity (Prinz et al. 2000), suggesting that the cytosolic domains of the Sec62/Sec63 complex occlude the RNC-binding site on Sec61p (Harada et al. 2011). According to one viewpoint, Sec61 and Ssh1 heterotrimers mediate cotranslational translocation (Panzner et al. 1995; Cheng et al. 2005). An alternative hypothesis is that the Sec61 complex is assembled into either the heptameric SEC complex or a hexameric SEC′ complex (SEC complexes lacking Sec62) (Jermy et al. 2006). Formation of both the SEC and SEC′ complexes is proposed to depend on an interaction between the BRL domain of Sec63 (Jermy et al. 2006) and cytosolic loops of Sec61 (Fig. 3A) (Harada et al. 2011). The role of the Sec62/Sec63 complex in cotranslational integration of membrane proteins has not been resolved, despite an intriguing report that yeast Sec66 and Sec72 are involved (Green et al. 1992). Evidence has been presented that Sec63p (in cooperation with Kar2p) provides an essential driving force for all protein translocation reactions in yeast (Young et al. 2001; Willer et al. 2003; Jermy et al. 2006).
Homologs of Sec62 and Sec63 are encoded by the genomes of metazoan organisms, unlike Sec66 and Sec72, which are fungi-specific. Mammalian Sec62 and Sec63 are abundant ER proteins; biochemical experiments indicate that a portion of the ER pool of Sec62 and Sec63 will copurify with the Sec61 complex (Meyer et al. 2000; Tyedmers et al. 2000; Guth et al. 2004). The physiological role of mammalian Sec62 and Sec63 is unclear because protein translocation primarily occurs by a cotranslational pathway in mammalian cells. Evidence that the mammalian Sec62/Sec63 complex is dispensable for cotranslational translocation of several standard secretory proteins has been provided by in vitro translocation assays using SR–Sec61 proteoliposomes supplemented with the TRAM or TRAP complexes (Görlich and Rapoport 1993; Voigt et al. 1996; Hegde et al. 1998; Fons et al. 2003).
Small secretory proteins (less than 75 residues) are translocated by a posttranslational pathway in mammalian cells (Muller and Zimmermann 1987; Schlenstedt and Zimmermann 1987; Shao and Hegde 2012). Mammalian cells treated with siRNAs specific for Sec62 or Sec63 show reduced translocation or integration of several proteins including preprocecropin A, a small secretory protein (Lang et al. 2012). Thus, one documented role for mammalian Sec62 and Sec63 is posttranslational translocation of proteins that are too small to be targeted by the SRP pathway.
CONCLUDING REMARKS
Mid- to high-resolution structures of most of the core components of the protein translocation machinery have now been obtained both in isolation and as part of larger complexes. A noteworthy exception is the lack of mid- to high-resolution structural information regarding the SEC complex. Although we have a reasonable understanding of secretory protein translocation, there remain important knowledge gaps in terms of molecular mechanism. The targeting mechanism for small secretory and membrane proteins is not well understood, nor is it known whether small integral membrane proteins are integrated by the Sec61 complex or by the SEC complex. The mechanism of integration of multispanning membrane proteins, because of its greater complexity, is an area of considerable interest. Interactions between the Sec61–RNC complex and adjacent membrane-embedded proteins like TRAM, TRAP, the signal peptidase, and the oligosaccharyltransferase have been detected and are likely to be important. These larger assemblies are often referred to as translocons to reflect the coordination and temporal links between protein translocation, nascent chain modification, and protein folding.
ACKNOWLEDGMENTS
Research in R.G.’s laboratory is supported by grants from the National Institutes of Health.
Footnotes
Editors: Susan Ferro-Novick, Tom A. Rapoport, and Randy Schekman
Additional Perspectives on The Endoplasmic Reticulum available at www.cshperspectives.org
REFERENCES
- Althoff SM, Stevens SW, Wise JA 1994. The SRP54 GTPase is essential for protein export in the fission yeast Schizosaccharomyces pombe. Mol Cell Biol 14: 7839–7854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews D, Walter P, Ottensmeyer FP 1987. Evidence for an extended 7SL RNA structure in the signal recognition particle. EMBO J 6: 3471–3477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batey RT, Rambo RP, Lucast L, Rha B, Doudna JA 2000. Crystal structure of the ribonucleoprotein core of the signal recognition particle. Science 287: 1232–1239 [DOI] [PubMed] [Google Scholar]
- Becker T, Bhushan S, Jarasch A, Armache JP, Funes S, Jossinet F, Gumbart J, Mielke T, Berninghausen O, Schulten K, et al. 2009. Structure of monomeric yeast and mammalian Sec61 complexes interacting with the translating ribosome. Science 326: 1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann R, Bubeck D, Grassucci R, Penczek P, Verschoor A, Blobel G, Frank J 1997. Alignment of conduits for the nascent polypeptide chain in the ribosome–Sec61 complex. Science 278: 2123–2126 [DOI] [PubMed] [Google Scholar]
- Beckmann R, Spahn CM, Eswar N, Helmers J, Penczek PA, Sali A, Frank J, Blobel G 2001. Architecture of the protein-conducting channel associated with the translating 80S ribosome. Cell 107: 361–372 [DOI] [PubMed] [Google Scholar]
- Berndt U, Oellerer S, Zhang Y, Johnson AE, Rospert S 2009. A signal-anchor sequence stimulates signal recognition particle binding to ribosomes from inside the exit tunnel. Proc Natl Acad Sci 106: 1398–1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein HD, Poritz MA, Strub K, Hoben PJ, Brenner S, Walter P 1989. Model for signal sequence recognition from amino-acid sequence of 54K subunit of signal recognition particle. Nature 340: 482–486 [DOI] [PubMed] [Google Scholar]
- Blobel G, Dobberstein B 1975. Transfer of proteins across membranes. I. Presence of proteolytic processed and unprocessed nascent immunoglobulin light chains on membrane bound ribosomes of murine myeloma. J Cell Biol 67: 835–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N, Fasana E 2011. Targeting pathways of C-tail-anchored proteins. Biochim Biophys Acta 1808: 937–946 [DOI] [PubMed] [Google Scholar]
- Bornemann T, Jockel J, Rodnina MV, Wintermeyer W 2008. Signal sequence-independent membrane targeting of ribosomes containing short nascent peptides within the exit tunnel. Nat Struct Mol Biol 15: 494–499 [DOI] [PubMed] [Google Scholar]
- Brennwald P, Liao X, Holm K, Porter G, Wise JA 1988. Identification of an essential Schizosaccharomyces pombe RNA homologous to the 7SL component of signal recognition particle. Mol Cell Biol 8: 1580–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL, Goeckeler J, Schekman R 1995. BiP and Sec63p are required for both co- and posttranslational protein translocation into the yeast endoplasmic reticulum. Proc Natl Acad Sci 92: 9643–9646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon KS, Or E, Clemons WM Jr, Shibata Y, Rapoport TA 2005. Disulfide bridge formation between SecY and a translocating polypeptide localizes the translocation pore to the center of SecY. J Cell Biol 169: 219–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Jagannathan S, Reid DW, Zheng T, Nicchitta CV 2011. Hierarchical regulation of mRNA partitioning between the cytoplasm and the endoplasmic reticulum of mammalian cells. Mol Biol Cell 22: 2646–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Gilmore R 2006. Slow translocon gating causes cytosolic exposure of transmembrane and lumenal domains during membrane protein integration. Nat Struct Mol Biol 13: 930–936 [DOI] [PubMed] [Google Scholar]
- Cheng Z, Jiang Y, Mandon EC, Gilmore R 2005. Identification of cytoplasmic residues of Sec61p involved in ribosome binding and cotranslational translocation. J Cell Biol 168: 67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirico WJ, Waters MG, Blobel G 1988. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature 322: 805–810 [DOI] [PubMed] [Google Scholar]
- Christensen AK, Bourne CM 1999. Shape of large bound polysomes in cultured fibroblasts and thyroid epithelial cells. Anat Rec 255: 116–129 [DOI] [PubMed] [Google Scholar]
- Connolly T, Gilmore R 1986. Formation of a functional ribosome–membrane junction during translocation requires the participation of a GTP-binding protein. J Cell Biol 103: 2253–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T, Gilmore R 1989. The signal recognition particle receptor mediates the GTP-dependent displacement of SRP from the signal sequence of the nascent polypeptide. Cell 57: 599–610 [DOI] [PubMed] [Google Scholar]
- Connolly T, Gilmore R 1993. GTP hydrolysis by complexes of the signal recognition particle and the signal recognition particle receptor. J Cell Biol 123: 799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly T, Rapiejko PJ, Gilmore R 1991. Requirement of GTP hydrolysis for dissociation of the signal recognition particle from its receptor. Science 252: 1171–1173 [DOI] [PubMed] [Google Scholar]
- Crowley KS, Reinhart GD, Johnson AE 1993. The signal sequence moves through a ribosomal tunnel into a noncytoplasmic aqueous environment at the ER membrane early in translocation. Cell 73: 1101–1115 [DOI] [PubMed] [Google Scholar]
- Crowley KS, Liao S, Worrell VE, Reinhart GD, Johnson AE 1994. Secretory proteins move through the endoplasmic reticulum via an aqueous, gated pore. Cell 78: 461–471 [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Schekman R 1987. A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J Cell Biol 105: 633–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies RJ, Schekman R 1989. Sec62 encodes a putative membrane protein required for protein translocation into the yeast endoplasmic reticulum. J Cell Biol 109: 2653–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies RJ, Koch BD, Werner-Washburne M, Craig EA, Schekman R 1988. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature 332: 800–805 [DOI] [PubMed] [Google Scholar]
- Deshaies RJ, Sanders SL, Feldheim DA, Schekman R 1991. Assembly of yeast Sec proteins involved in translocation into the endoplasmic reticulum into a membrane-bound multisubunit complex. Nature 349: 806–808 [DOI] [PubMed] [Google Scholar]
- Diehn M, Eisen MB, Botstein D, Brown PO 2000. Large-scale identification of secreted and membrane-associated gene products using DNA microarrays. Nat Genet 25: 58–62 [DOI] [PubMed] [Google Scholar]
- Egea PF, Stroud RM 2010. Lateral opening of a translocon upon entry of protein suggests the mechanism of insertion into membranes. Proc Natl Acad Sci 107: 17182–17187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egea PF, Shan SO, Napetschnig J, Savage DF, Walter P, Stroud RM 2004. Substrate twinning activates the signal recognition particle and its receptor. Nature 427: 215–221 [DOI] [PubMed] [Google Scholar]
- Esnault Y, Blondel M-O, Deshaies R, Schekman R, Képes F 1993. The yeast SSS1 gene is essential for secretory protein translocation and encodes a conserved protein of the endoplasmic reticulum. EMBO J 12: 4083–4093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault Y, Feldheim D, Blondel M-O, Schekman R, Képes F 1994. SSS1 encodes a stabilizing component of the Sec61 subcomplex of the yeast protein translocation apparatus. J Biol Chem 269: 27478–27485 [PubMed] [Google Scholar]
- Fang H, Green N 1994. Nonlethal sec71-1 and sec72-1 mutations eliminate proteins associated with the Sec63p–BiP complex from S. cerevisiae. Mol Biol Cell 5: 933–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim D, Schekman R 1994. Sec72p contributes to the selective recognition of signal peptides by the secretory polypeptide translocation complex. J Cell Biol 126: 935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim D, Rothblatt J, Schekman R 1992. Topology and functional domains of Sec63p, an endoplasmic reticulum membrane protein required for secretory protein translocation. Mol Cell Biol 12: 3288–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldheim DA, Yoshimura K, Admon A, Schekman R 1993. Structural and functional characterization of Sec66p, a new subunit of the polypeptide translocation apparatus in yeast endoplasmic reticulum. Mol Biol Cell 4: 931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke K, Plath K, Panzer S, Prehn S, Rapoport TA, Hartmann E, Sommer T 1996. A second trimeric complex containing homologues of the Sec61p complex functions in protein transport across the ER membrane of S. cerevisiae. EMBO J 15: 1482–1494 [PMC free article] [PubMed] [Google Scholar]
- Focia PJ, Shepotinovskaya IV, Seidler JA, Freymann DM 2004. Heterodimeric GTPase core of the SRP targeting complex. Science 303: 373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fons RD, Bogert BA, Hegde RS 2003. Substrate-specific function of the translocon-associated protein complex during translocation across the ER membrane. J Cell Biol 160: 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freymann DM, Keenan RJ, Stroud RM, Walter P 1997. Structure of the conserved GTPase domain of the signal recognition particle. Nature 385: 361–364 [DOI] [PubMed] [Google Scholar]
- Freymann DM, Keenan RJ, Stroud RM, Walter P 1999. Functional changes in the structure of the SRP GTPase on binding GDP and Mg2+ GDP. Nat Struct Biol 6: 793–801 [DOI] [PubMed] [Google Scholar]
- Friedlander M, Blobel G 1985. Bovine opsin has more than one signal sequence. Nature 318: 338–343 [DOI] [PubMed] [Google Scholar]
- Gilmore R, Blobel G 1983. Transient involvement of signal recognition particle and its receptor in the microsomal membrane prior to protein translocation. Cell 35: 677–685 [DOI] [PubMed] [Google Scholar]
- Gilmore R, Blobel G 1985. Translocation of secretory proteins across the microsomal membrane occurs through an environment accessible to aqueous perturbants. Cell 42: 497–505 [DOI] [PubMed] [Google Scholar]
- Gilmore R, Blobel G, Walter P 1982a. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol 95: 463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R, Walter P, Blobel G 1982b. Protein translocation across the endoplasmic reticulum. II. Isolation and characterization of the signal recognition particle receptor. J Cell Biol 95: 470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlich D, Rapoport TA 1993. Protein translocation into proteoliposomes reconstituted from purified components of the ER membrane. Cell 75: 615–630 [DOI] [PubMed] [Google Scholar]
- Görlich D, Hartmann E, Prehn S, Rapoport T 1992a. A protein of the endoplasmic reticulum involved early in polypeptide translocation. Nature 357: 47–52 [DOI] [PubMed] [Google Scholar]
- Görlich D, Prehn S, Hartmann E, Kalies K-U, Rapoport TA 1992b. A mammalian homologue of Sec61p and SecYp is associated with ribosomes and nascent polypeptides during translocation. Cell 71: 489–503 [DOI] [PubMed] [Google Scholar]
- Green N, Fang H, Walter P 1992. Mutants in three novel complementation groups inhibit membrane protein insertion into and soluble protein translocation across the endoplasmic reticulum membrane of Saccharomyces cerevisiae. J Cell Biol 116: 597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbart J, Schulten K 2007. Structural determinants of lateral gate opening in the protein translocon. Biochemistry 46: 11147–11157 [DOI] [PubMed] [Google Scholar]
- Guth S, Volzing C, Muller A, Jung M, Zimmermann R 2004. Protein transport into canine pancreatic microsomes: A quantitative approach. Eur J Biochem 271: 3200–3207 [DOI] [PubMed] [Google Scholar]
- Haigh NG, Johnson AE 2002. A new role for BiP: Closing the aqueous translocon pore during protein integration into the ER membrane. J Cell Biol 156: 261–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halic M, Becker T, Pool MR, Spahn CM, Grassucci RA, Frank J, Beckmann R 2004. Structure of the signal recognition particle interacting with the elongation-arrested ribosome. Nature 427: 808–814 [DOI] [PubMed] [Google Scholar]
- Hamman BD, Chen J-C, Johnson EE, Johnson AE 1997. The aqueous pore through the translocon has a diameter of 40–60 Å during cotranslational protein translocation at the ER membrane. Cell 89: 535–544 [DOI] [PubMed] [Google Scholar]
- Hamman BD, Hendershot LM, Johnson AE 1998. BiP maintains the permeability barrier of the ER membrane by sealing the lumenal end of the translocation pore before and early in translocation. Cell 92: 747–758 [DOI] [PubMed] [Google Scholar]
- Hanein D, Matlack KES, Jungnickel B, Plath K, Kalies K-U, Miller KR, Rapoport TA, Akey CW 1996. Oligomeric rings of the Sec61p complex induced by ligands required for protein translocation. Cell 87: 721–732 [DOI] [PubMed] [Google Scholar]
- Hann BC, Walter P 1991. The signal recognition particle in S. cerevisiae. Cell 67: 131–144 [DOI] [PubMed] [Google Scholar]
- Hansen W, Garcia PD, Walter P 1986. In vitro protein translocation across the yeast endoplasmic reticulum. ATP-dependent posttranslational translocation of the prepro-α-factor. Cell 45: 397–406 [DOI] [PubMed] [Google Scholar]
- Harada Y, Li H, Wall JS, Lennarz WJ 2011. Structural studies and the assembly of the heptameric post-translational translocon complex. J Biol Chem 286: 2956–2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde RS, Voigt S, Rapoport TA, Lingappa VR 1998. TRAM regulates the exposure of nascent secretory proteins to the cytosol during translocation into the endoplasmic reticulum. Cell 92: 621–631 [DOI] [PubMed] [Google Scholar]
- Jermy AJ, Willer M, Davis E, Wilkinson BM, Stirling CJ 2006. The Brl domain in Sec63p is required for assembly of functional endoplasmic reticulum translocons. J Biol Chem 281: 7899–7906 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Cheng Z, Mandon EC, Gilmore R 2008. An interaction between the SRP receptor and the translocon is critical during cotranslational protein translocation. J Cell Biol 180: 1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junne T, Schwede T, Goder V, Spiess M 2006. The plug domain of yeast Sec61p is important for efficient protein translocation, but is not essential for cell viability. Mol Biol Cell 17: 4063–4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junne T, Schwede T, Goder V, Spiess M 2007. Mutations in the Sec61p channel affecting signal sequence recognition and membrane protein topology. J Biol Chem 282: 33201–33209 [DOI] [PubMed] [Google Scholar]
- Junne T, Kocik L, Spiess M 2010. The hydrophobic core of the Sec61 translocon defines the hydrophobicity threshold for membrane integration. Mol Biol Cell 21: 1662–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalies K-U, Görlich D, Rapoport TA 1994. Binding of ribosomes to the rough endoplasmic reticulum is mediated by the Sec61p-complex. J Cell Biol 126: 925–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellaris KV, Bowen S, Gilmore R 1991. ER translocation intermediates are adjacent to a nonglycosylated 34-kD integral membrane protein. J Cell Biol 114: 21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HG, Hengelage T, Neumann-Haefelin C, MacFarlane J, Hoffschulte HK, Schimz KL, Mechler B, Muller M 1999. In vitro studies with purified components reveal signal recognition particle (SRP) and SecA/SecB as constituents of two independent protein-targeting pathways of Escherichia coli. Mol Biol Cell 10: 2163–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkaraju AK, Mary C, Scherrer A, Johnson AE, Strub K 2008. SRP keeps polypeptides translocation-competent by slowing translation to match limiting ER-targeting sites. Cell 133: 440–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang S, Benedix J, Fedeles SV, Schorr S, Schirra C, Schauble N, Jalal C, Greiner M, Hassdenteufel S, Tatzelt J, et al. 2012. Different effects of Sec61α, Sec62 and Sec63 depletion on transport of polypeptides into the endoplasmic reticulum of mammalian cells. J Cell Sci 125: 1958–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffer L, Garcia PD, Harkins RN, Coussens L, Ullrich A, Walter P 1985. Topology of signal recognition particle receptor in endoplasmic reticulum membrane. Nature 318: 334–338 [DOI] [PubMed] [Google Scholar]
- Legate KR, Falcone D, Andrews DW 2000. Nucleotide-dependent binding of the GTPase domain of the signal recognition particle receptor β-subunit to the α-subunit. J Biol Chem 275: 27439–27446 [DOI] [PubMed] [Google Scholar]
- Lerner RS, Seiser RM, Zheng T, Lager PJ, Reedy MC, Keene JD, Nicchitta CV 2003. Partitioning and translation of mRNAs encoding soluble proteins on membrane-bound ribosomes. RNA 9: 1123–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman SK, Schekman R 1995. Interaction between BiP and Sec63 is required for the completion of protein translocation across the ER of Saccharomyces cerevisiae. J Cell Biol 131: 1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard AP, Lalani S, Silva F, Belin D, Duong F 2007. Deregulation of the SecYEG translocation channel upon removal of the plug domain. J Biol Chem 282: 1281–1287 [DOI] [PubMed] [Google Scholar]
- Mandon EC, Jiang Y, Gilmore R 2003. Dual recognition of the ribosome and the signal recognition particle by the SRP receptor during protein targeting to the endoplasmic reticulum. J Cell Biol 162: 575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason N, Ciufo LF, Brown JD 2000. Elongation arrest is a physiologically important function of signal recognition particle. EMBO J 19: 4164–4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlack KE, Misselwitz B, Plath K, Rapoport TA 1999. BiP acts as a molecular ratchet during posttranslational transport of prepro-α factor across the ER membrane. Cell 97: 553–564 [DOI] [PubMed] [Google Scholar]
- Menetret J, Neuhof A, Morgan DG, Plath K, Radermacher M, Rapoport TA, Akey CW 2000. The structure of ribosome–channel complexes engaged in protein translocation. Mol Cell 6: 1219–1232 [DOI] [PubMed] [Google Scholar]
- Menetret JF, Schaletzky J, Clemons WM Jr, Osborne AR, Skanland SS, Denison C, Gygi SP, Kirkpatrick DS, Park E, Ludtke SJ, et al. 2007. Ribosome binding of a single copy of the SecY complex: Implications for protein translocation. Mol Cell 28: 1083–1092 [DOI] [PubMed] [Google Scholar]
- Meyer HA, Grau H, Kraft R, Kostka S, Prehn S, Kalies KU, Hartmann E 2000. Mammalian Sec61 is associated with Sec62 and Sec63. J Biol Chem 275: 14550–14557 [DOI] [PubMed] [Google Scholar]
- Miller JD, Wilhelm H, Gierasch L, Gilmore R, Walter P 1993. GTP binding and hydrolysis by the signal recognition particle during initiation of protein translocation. Nature 366: 351–354 [DOI] [PubMed] [Google Scholar]
- Miller JD, Bernstein HD, Walter P 1994. Interaction of E. coli Ffh/4.5S ribonucleoprotein and FtsY mimics that of mammalian signal recognition particle and its receptor. Nature 367: 657–659 [DOI] [PubMed] [Google Scholar]
- Milstein C, Brownlee GG, Harrison TM, Mathews MB 1972. A possible precursor of immunoglobulin light chains. Nat New Biol 239: 117–120 [DOI] [PubMed] [Google Scholar]
- Mize NK, Andrews DW, Lingappa VR 1986. A stop transfer sequence recognizes receptors for nascent chain translocation across the endoplasmic reticulum. Cell 47: 711–719 [DOI] [PubMed] [Google Scholar]
- Montoya G, Svensson C, Luirink J, Sinning I 1997. Crystal structure of the NG domain from the signal recognition particle receptor FtsY. Nature 385: 365–368 [DOI] [PubMed] [Google Scholar]
- Morgan DG, Menetret JF, Neuhof A, Rapoport TA, Akey CW 2002. Structure of the mammalian ribosome–channel complex at 17 Å resolution. J Mol Biol 324: 871–886 [DOI] [PubMed] [Google Scholar]
- Mothes W, Prehn S, Rapoport TA 1994. Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J 13: 3973–3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller G, Zimmermann R 1987. Import of honeybee prepromelittin into the endoplasmic reticulum: Structural basis for independence of SRP and docking protein. EMBO J 6: 2099–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müsch A, Wiedmann M, Rapoport TA 1992. Yeast Sec proteins interact with polypeptides traversing the endoplasmic reticulum membrane. Cell 69: 343–352 [DOI] [PubMed] [Google Scholar]
- Ng DTW, Walter P 1996. ER membrane protein complex required for nuclear fusion. J Cell Biol 132: 499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DTW, Brown JD, Walter P 1996. Signal sequences specify the targeting route to the endoplasmic reticulum. J Cell Biol 134: 269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S-I, Fewell SW, Kato Y, Brodsky JL, Endo T 2001. Molecular chaperones in the yeast endoplasmic reticulum maintain the solubility of proteins for retrotranslocation and degradation. J Cell Biol 153: 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg SC, Poritz MA, Walter P 1992. Signal recognition particle receptor is important for cell growth and protein secretion in Saccharomyces cerevisiae. Mol Biol Cell 3: 895–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg SC, Barz WP, Walter P 1998. A functional GTPase domain, but not its transmembrane domain, is required for function of the SRP receptor β-subunit. J Cell Biol 142: 341–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade GE 1955a. A small particulate component of the cytoplasm. J Biophys Biochem Cytol 1: 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade GE 1955b. Studies on the endoplasmic reticulum. II. Simple dispositions in cells in situ. J Biophys Biochem Cytol 1: 567–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzner S, Dreier L, Hartmann E, Kostka S, Rapoport TA 1995. Posttranslational protein transport in yeast reconstituted with a purified complex of Sec proteins and Kar2p. Cell 81: 561–570 [DOI] [PubMed] [Google Scholar]
- Park E, Rapoport TA 2011. Preserving the membrane barrier for small molecules during bacterial protein translocation. Nature 473: 239–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E, Rapoport TA 2012. Mechanisms of Sec61/SecY-mediated protein translocation across membranes. Annu Rev Biophys 41: 21–40 [DOI] [PubMed] [Google Scholar]
- Peluso P, Herschlag D, Nock S, Freymann DM, Johnson AE, Walter P 2000. Role of 4.5S RNA in assembly of the bacterial signal recognition particle with its receptor. Science 288: 1640–1643 [DOI] [PubMed] [Google Scholar]
- Plath K, Mothes W, Wilkinson BM, Stirling CJ, Rapoport TA 1998. Signal sequence recognition in posttranslational protein transport across the yeast ER membrane. Cell 94: 795–807 [DOI] [PubMed] [Google Scholar]
- Poritz MA, Strub K, Walter P 1988. Human SRP RNA and E. coli 4.5S RNA contain a highly homologous structural domain. Cell 55: 4–6 [DOI] [PubMed] [Google Scholar]
- Poritz MA, Bernstein HD, Strub K, Zopf D, Wilhelm H, Walter P 1990. An E. coli ribonucleoprotein containing 4.5S RNA resembles mammalian signal recognition particle. Science 250: 1111–1117 [DOI] [PubMed] [Google Scholar]
- Powers T, Walter P 1995. Reciprocal stimulation of GTP hydrolysis by two directly interacting GTPases. Science 269: 1422–1424 [DOI] [PubMed] [Google Scholar]
- Prinz A, Hartmann E, Kalies KU 2000. Sec61p is the main ribosome receptor in the endoplasmic reticulum of Saccharomyces cerevisiae. Biol Chem 381: 1025–1029 [DOI] [PubMed] [Google Scholar]
- Pyhtila B, Zheng T, Lager PJ, Keene JD, Reedy MC, Nicchitta CV 2008. Signal sequence- and translation-independent mRNA localization to the endoplasmic reticulum. RNA 14: 445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raden D, Song W, Gilmore R 2000. Role of the cytoplasmic segments of Sec61α in the ribosome-binding and translocation-promoting activities of the Sec61 complex. J Cell Biol 150: 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JC, Steele WJ 1976. A procedure for the quantitative recovery of homogenous populations of undegraded free and bound polysomes from rat liver. Biochemistry 15: 1704–1712 [DOI] [PubMed] [Google Scholar]
- Rapiejko PJ, Gilmore R 1997. Empty site forms of the SRP54 and SR α GTPases mediate targeting of ribosome–nascent chain complexes to the endoplasmic reticulum. Cell 89: 703–713 [DOI] [PubMed] [Google Scholar]
- Redman C, Sabatini DD 1966. Vectorial discharge of peptides released by puromycin from attached ribosomes. Proc Natl Acad Sci 56: 608–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman C, Siekevitz P, Palade GE 1966. Synthesis and transfer of amylase in pigeon pancreatic microsomes. J Biol Chem 241: 1150–1158 [PubMed] [Google Scholar]
- Rosendal KR, Wild K, Montoya G, Sinning I 2003. Crystal structure of the complete core of archaeal signal recognition particle and implications for interdomain communication. Proc Natl Acad Sci 100: 14701–14706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblatt JA, Meyer DI 1986. Secretion in yeast: Translocation and glycosylation of prepro-α-factor in vitro can occur via an ATP-dependent post-translational mechanism. EMBO J 5: 1031–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblatt JA, Deshaies RJ, Sanders SL, Daum G, Schekman R 1989. Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J Cell Biol 109: 2641–2652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SL, Whitfield KM, Vogel JP, Rose MD, Schekman RW 1992. Sec61p and Bip directly facilitate polypeptide translocation into the ER. Cell 69: 353–365 [DOI] [PubMed] [Google Scholar]
- Saparov SM, Erlandson K, Cannon K, Schaletzky J, Schulman S, Rapoport TA, Pohl P 2007. Determining the conductance of the SecY protein translocation channel for small molecules. Mol Cell 26: 501–509 [DOI] [PubMed] [Google Scholar]
- Schlenstedt G, Zimmermann R 1987. Import of frog prepropeptide GLa into microsomes requires ATP but does not involve docking protein or ribosomes. EMBO J 6: 699–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz T, Blobel G 2003. Structural basis for the function of the β subunit of the eukaryotic signal recognition particle receptor. Cell 112: 793–803 [DOI] [PubMed] [Google Scholar]
- Shan SO, Walter P 2003. Induced nucleotide specificity in a GTPase. Proc Natl Acad Sci 100: 4480–4485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Hegde RS 2011. Membrane protein insertion at the endoplasmic reticulum. Annu Rev Cell Dev Biol 27: 25–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao S, Hegde RS 2012. A calmodulin-dependent translocation pathway for small secretory proteins. Cell 147: 1576–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel V, Walter P 1988. Binding sites of the 19-kDa and 68/72-kDa signal recognition particle (SRP) proteins on SRP RNA as determined by protein–RNA “footprinting.” Proc Natl Acad Sci 85: 1801–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SM, Blobel G 1991. A protein-conducting channel in the endoplasmic reticulum. Cell 65: 371–380 [DOI] [PubMed] [Google Scholar]
- Smith MA, Clemons WM Jr, DeMars CJ, Flower AM 2005. Modeling the effects of prl mutations on the Escherichia coli SecY complex. J Bacteriol 187: 6454–6465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Raden D, Mandon EC, Gilmore R 2000. Role of Sec61α in the regulated transfer of the ribosome–nascent chain complex from the signal recognition particle to the translocation channel. Cell 100: 333–343 [DOI] [PubMed] [Google Scholar]
- Stirling CJ, Rothblatt J, Hosobuchi M, Deshaies R, Schekman R 1992. Protein translocation mutants defective in the insertion of integral membrane proteins into the endoplasmic reticulum. Mol Biol Cell 3: 129–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strub K, Walter P 1990. Assembly of the Alu domain of the signal recognition particle (SRP): Dimerization of the two protein components is required for efficient binding to SRP RNA. Mol Cell Biol 10: 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueman SF, Mandon EC, Gilmore R 2011. Translocation channel gating kinetics balances protein translocation efficiency with signal sequence recognition fidelity. Mol Biol Cell 22: 2983–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukazaki T, Mori H, Fukai S, Ishitani R, Mori T, Dohmae N, Perederina A, Sugita Y, Vassylyev DG, Ito K, et al. 2008. Conformational transition of Sec machinery inferred from bacterial SecYE structures. Nature 455: 988–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers J, Lerner M, Bies C, Dudek J, Skowronek MH, Haas IG, Heim N, Nastainczyk W, Volkmer J, Zimmermann R 2000. Homologs of the yeast Sec complex subunits Sec62p and Sec63p are abundant proteins in dog pancreas microsomes. Proc Natl Acad Sci 97: 7214–7219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrandt ND, Newitt JA, Bernstein HD 1997. The E. coli signal recognition particle is required for the insertion of a subset of inner membrane proteins. Cell 88: 187–196 [DOI] [PubMed] [Google Scholar]
- Van den Berg B, Clemons WM Jr, Collinson I, Modis Y, Hartmann E, Harrison SC, Rapoport TA 2004. X-ray structure of a protein-conducting channel. Nature 427: 36–44 [DOI] [PubMed] [Google Scholar]
- Vogel JP, Misra LM, Rose MD 1990. Loss of BiP/GRP78 function blocks translocation of secretory proteins in yeast. J Cell Biol 110: 1885–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt S, Jungnickel B, Hartmann E, Rapoport TA 1996. Signal sequence-dependent function of the TRAM protein during early phases of protein transport across the endoplasmic reticulum. J Cell Biol 134: 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G 1983. Patterns of amino acids near signal sequence cleavage sites. Eur J Biochem 133: 17–21 [DOI] [PubMed] [Google Scholar]
- Wallin E, von Heijne G 1998. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci 7: 1029–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Blobel G 1980. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci 77: 7112–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Blobel G 1981a. Translocation of proteins across the endoplasmic reticulum. II. Signal recognition protein (SRP) mediates the selective binding to microsomal membranes of in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol 91: 551–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Blobel G 1981b. Translocation of proteins across the endoplasmic reticulum. III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol 91: 557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P, Blobel G 1982. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature 299: 691–698 [DOI] [PubMed] [Google Scholar]
- Walter P, Ibrahimi I, Blobel G 1981. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol 91: 545–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MG, Blobel G 1986. Secretory protein translocation in a yeast cell-free system can occur posttranslationally and requires ATP hydrolysis. J Cell Biol 102: 1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann M, Goerlich D, Hartmann E, Kurzchalia TV, Rapoport TA 1989. Photocrosslinking demonstrates proximity of a 34 kDa membrane protein to different portions of preprolactin during translocation through the endoplasmic reticulum. FEBS Lett 257: 263–268 [DOI] [PubMed] [Google Scholar]
- Willer M, Jermy AJ, Steel GJ, Garside HJ, Carter S, Stirling CJ 2003. An in vitro assay using overexpressed yeast SRP demonstrates that cotranslational translocation is dependent upon the J-domain of Sec63p. Biochemistry 42: 7171–7177 [DOI] [PubMed] [Google Scholar]
- Wittke S, Dunnwald M, Johnsson N 2000. Sec62p, A component of the endoplasmic reticulum protein translocation machinery, contains multiple binding sites for the sec-complex. Mol Biol Cell 11: 3859–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittke S, Dunnwald M, Albertsen M, Johnsson N 2002. Recognition of a subset of signal sequences by Ssh1p, a Sec61p-related protein in the membrane of endoplasmic reticulum of yeast Saccharomyces cerevisiae. Mol Biol Cell 13: 2223–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young BP, Craven RA, Reid PJ, Willer M, Stirling CJ 2001. Sec63p and Kar2p are required for the translocation of SRP-dependent precursors into the yeast endoplasmic reticulum in vivo. EMBO J 20: 262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Rashid R, Wang K, Shan SO 2010. Sequential checkpoints govern substrate selection during cotranslational protein targeting. Science 328: 757–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer J, Nam Y, Rapoport TA 2008. Structure of a complex of the ATPase SecA and the protein-translocation channel. Nature 455: 936–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zopf D, Bernstein HD, Johnson AE, Walter P 1990. The methionine-rich domain of the 54 kD protein subunit of the signal recognition particle contains an RNA binding site and can be crosslinked to a signal sequence. EMBO J 9: 4511–4517 [DOI] [PMC free article] [PubMed] [Google Scholar]