Abstract
Neural crest cells (NCCs) comprise a multipotent, migratory cell population that generates a diverse array of cell and tissue types during vertebrate development. These include cartilage and bone, tendons, and connective tissue, as well as neurons, glia, melanocytes, and endocrine and adipose cells; this remarkable lineage potential persists into adult life. Taken together with a limited capacity for self-renewal, neural crest cells bear the hallmarks of stem and progenitor cells and are considered to be synonymous with vertebrate evolution. The neural crest has provided a system for exploring the mechanisms that govern developmental processes such as morphogenetic induction, cell migration, and fate determination. Today, much of the focus on neural crest cells revolves around their stem cell-like characteristics and potential for use in regenerative medicine. A thorough understanding of the signals and switches that govern mammalian neural crest patterning is central to potential therapeutic application of these cells and better appreciation of the role that neural crest cells play in vertebrate evolution, development, and disease.
Neural crest cells (NCCs) generate many cell types during vertebrate development (e.g., bone and neurons). NCC formation may involve Sox transcription factors; NCC differentiation involves Wnt, BMP, and FGF signaling.
1. INTRODUCTION
At the end of gastrulation, after generation of the three primary germ layers is complete, the ectoderm is subdivided into two distinct domains: the non-neural or surface ectoderm and the neural ectoderm. The surface ectoderm will eventually form placodes, skin, and dermis, whereas the neural ectoderm will ultimately give rise to the central nervous system. The neural ectoderm (also known as the neuroepithelium or neural plate) extends almost the entire length of the vertebrate axis, and during neurulation, the left and right halves elevate and fuse to form a neural tube. It is during this neurulation process that neural crest cells (NCCs) are formed within the dorsal-most part of the neuroepithelium at the junction with the surface ectoderm, a region termed the “neural plate border.” Explants of neural plate cultured in vitro do not endogenously generate neural crest cells. Thus, neural crest cell induction has been viewed as a multistep process, requiring an inducer (i.e., the ectoderm or paraxial mesoderm) and a competent receiving tissue (i.e., the neural plate). Furthermore, these interactions between non-neural and neural tissues are contact-mediated, suggesting that inductive signals pass to the neuroepithelium to induce neural crest cell formation (Selleck and Bronner-Fraser 1995).
Initially, neural crest cells are integrated within the neuroepithelium and are morphologically indistinguishable from the other neuroepithelial cells. However, in response to contact-mediated inductive signals from the surface ectoderm and underlying mesoderm, neural crest cells are born and undergo an epithelial-to-mesenchymal transition, after which they delaminate from the neuroepithelium. Some neural crest cells may also be derived from the surface ectoderm. Neural crest cells then migrate extensively to several different locations in the embryo (Fig. 1). Although the bone morphogenetic protein (BMP), fibroblast growth factor (FGF), and Wnt signaling families have each been identified as key signaling regulators of neural crest cell formation in diverse species such as avians, fish, and amphibians, there is no conclusive evidence that supports an absolute role for these same factors in mammalian neural crest cell induction (Crane and Trainor 2006). These signaling pathways appear to be more important for specifying cell-type differentiation within the mammalian neural crest cell lineage. Therefore, the signals and switches governing mammalian neural crest cell formation remain to be identified.
Figure 1.
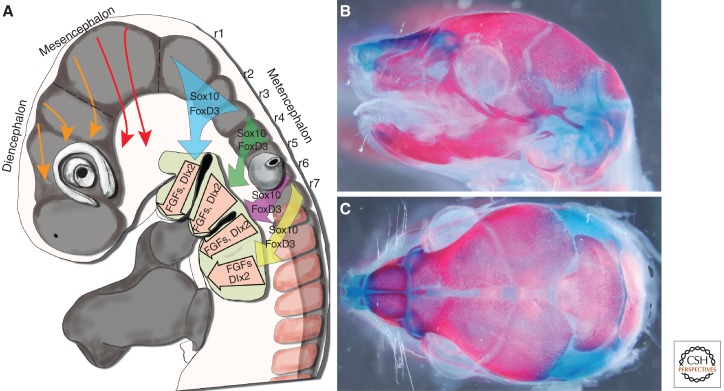
Cranial neural crest cell migration and differentiation. (A) Schematic representation of the pathways of mammalian cranial neural crest cell migration and the respective expression and interaction of Sox10, FoxD3, FGF, and Dlx signals and switches that govern neural crest cell differentiation. (B) Lateral, and (C) dorsal views of Alizarin Red- and Alcian Blue-stained bone and cartilage, respectively, in an embryonic day 18.5 (E18.5) mouse embryo.
The delamination of neural crest cells from the neural tube requires significant cytoarchitectural and cell-adhesive changes and is typically recognized by the activity of members of the Snail transcription factor gene family. Snail1, for example, demarcates neural crest cells in mouse embryos (Sefton et al. 1998). Snail1 and Snail2 can directly repress the cell adhesion molecule E-cadherin by binding to its promoter, which is thought to facilitate cell migration (Cano et al. 2000; Bolos et al. 2003). However, in contrast to avians, fish, and amphibians, a requirement for the Snail genes in mammalian neural crest cell induction is conspicuously absent. Conditional loss-of-function analyses of Snail1 and Snail2 either individually or in combination do not inhibit neural crest cell induction and delamination in mice (Jiang et al. 1998; Murray and Gridley 2006). To date, only mutations in Zfhx1b, which is also known as Smad-interacting protein 1 (SIP1), have been shown to affect neural crest cell formation and delamination in mammalian embryos (Van de Putte et al. 2003). Zfhx1b knockout mice do not develop post-otic vagal neural crest cells, and the delamination of cranial neural crest cells is perturbed. This is due to the persistent expression of E-cadherin throughout the epidermis and neural tube. Hence, appropriate regulation of cell adhesion is critical for formation, epithelial-to-mesenchymal transition (EMT), and subsequent delamination and migration of mammalian neural crest cells.
During normal mammalian embryogenesis, neural crest cell induction and delamination begin at the level of the midbrain and continue as a wave that extends progressively caudal toward the tail. Thus, neural crest cells are born along nearly the entire length of the neuraxis and, based on their axial level of origin, can be classified into distinct axial groups: cranial, cardiac, vagal, trunk, and sacral, each of which shows specific migration pathways and differentiation capacities. The cranial neural crest gives rise to the majority of the bone and cartilage of the head and face, as well as to nerve ganglia, smooth muscle, connective tissue, and pigment cells (Fig. 1A). The cardiac neural crest contributes to heart development by forming the aorticopulmonary septum and conotruncal cushions, whereas the vagal and sacral neural crest gives rise to enteric ganglia of the gut. Finally, the trunk neural crest give rise to neurons and glia that contribute to the peripheral nervous system, to secretory cells of the endocrine system, and to pigment cells of the skin. The remarkable capacity of neuroectoderm-derived neural crest cells to differentiate into both neuronal and mesenchymal derivatives has led to the neural crest being described as the fourth germ layer (Hall 1999). There are a couple of possible mechanisms that can account for the ability of neural crest cells to differentiate into such diverse cell types and tissues. Neural crest cells could comprise a heterogeneous mixture of progenitor cells, with each progenitor giving rise to a distinct cell type within the body. This would require some degree of neural crest cell specification before their emigration from neural tube and be largely dependent on intrinsic signals regulating their development. Alternatively, neural crest cells could be multipotent, with their differentiation into multiple distinct cell types being dependent on extrinsic signals emanating from the tissues with which they contact during their migration. The question of extrinsic versus intrinsic specification of neural crest cells and the appropriateness of their classification as true stem cells or progenitor cells has been addressed extensively elsewhere (Trainor and Krumlauf 2001; Trainor 2003; Trainor et al. 2003; Crane and Trainor 2006). Suffice it to say that differing opinions are attributable to semantic arguments and the context-dependent nature of specific experiments. Neural crest cells show some of the key hallmarks of stem and progenitor cells, and their development is governed by a balance between intrinsic and extrinsic signals; however, neural crest cells are only generated transiently during embryogenesis. Neural crest cell differentiation has thus proven to be a significant model for understanding cell signaling and remains relevant because of the importance of neural crest cells in vertebrate development, evolution, and disease. Therefore, in this article, we discuss the signals and switches that regulate mammalian neural crest cell differentiation with a particular emphasis on skeletogenic and neuronal specification, the primary derivatives of the head and trunk, respectively.
2. CRANIAL NEURAL CREST CELLS
The craniofacial complex is anatomically the most sophisticated structure of the vertebrate body. Composed of a neurocranium (brain case) and viscerocranium (components derived from the pharyngeal arches), the cranioskeletal complex houses and protects the brain as well as the majority of the sense organs (Fig. 1B,C). It is important to note that the craniofacial skeleton across all craniates is of dual origin, being derived from both neural crest cells and mesodermal cells; however, the majority of the bone, cartilage, and connective tissue is derived from the neural crest (Jiang et al. 2002; Yoshida et al. 2008). In the mammalian neurocranium, for example, the meninges and frontal bones are derived from neural crest cells as is the suture mesenchyme, whereas the parietal bone is mesoderm-derived (Jiang et al. 2002). Two types of bone formation occur in the head. Intramembranous bone formation occurs by the direct differentiation of mesenchymal condensations into osteoblasts that lay down a mineralized matrix. In contrast, in endochondral bone formation, chondrocytes derived from mesenchymal condensations produce a cartilaginous framework that subsequently becomes hypertrophic and is replaced by osteoblasts and bone matrix.
2.1. Hox-Positive versus Non-Hox-Negative Activity in Cranial Neural Crest Cells
Cranial neural crest cells in mouse embryos migrate in stereotypical patterns that are highly conserved across vertebrates (Kulesa et al. 2004) and use a complex array of intrinsic and extrinsic signaling cues (Trainor 2005). For craniofacial skeletogenesis, following colonization of the facial prominences and branchial arches, neural crest cells aggregate, condense, and differentiate from a common osteochondral progenitor toward more specific chondrogenic or osteogenic cell fates in response to signals from the surrounding epithelia, which include the neuroepithelium, endoderm, ectoderm, and mesoderm (Hall 1999; Trainor and Krumlauf 2001).
Neural crest cells have unique transcriptional identities correlating with their anterior–posterior axial origin within the neural plate. Most importantly, the cranial neural crest cells are subdivided into Hox-gene-negative- versus Hox-gene-positive-expressing cells. The first pharyngeal arch and more rostral populations of neural crest cells do not express Hox genes. Hox gene expression is associated with second and more caudal pharyngeal arch populations of neural crest cells. In mice, neural crest cells that colonize the first arch form skeletal tissue such as Meckel’s cartilage, the maxillae, and the dentary bones, whereas neural crest cells of the second arch form Reichert’s cartilage. The proximal region of Meckel’s cartilage develops into two of the middle ear bones, the malleus, and the incus, whereas Reichert’s cartilage forms the stapes (third bone of the middle ear), the styloid process of the temporal bone, the lesser horn, and part of the hyoid bone. Both endochondral and intramembranous ossification occurs during first pharyngeal arch differentiation, in contrast to primarily endochondral ossification in the second pharyngeal arch.
In mice, targeted inactivation of Hoxa2 results in lethality at birth and homeotic transformations of second arch neural crest-derived elements into proximal first arch derivatives, including a partial duplication of Meckel’s cartilage and ossification centers of the middle ear bones (Rijli et al. 1993). In these mutants, ectopic intramembranous ossification takes place in the second arch, resulting in duplicated jaw structures. Therefore, Hoxa2 is essential for proper patterning of neural crest cell differentiation and, in fact, functions as an inhibitor of intramembranous and endochondral ossification (Rijli et al. 1993; Kanzler et al. 1998). Consistent with this, overexpression of Hoxa2 in the first branchial arch of avian (Grammatopoulos et al. 2000) and frog (Pasqualetti et al. 2000) embryos suppresses jaw formation. Inroads have been made into the mechanisms by which Hoxa2 specifically influences cranial neural crest cell differentiation (Kanzler et al. 1998). During normal development, Hoxa2 is widely expressed in the second arch mesenchyme but is excluded from the chondrogenic condensations in the core of the arches. In the absence of Hoxa2, ectopic chondrogenesis coincides with an expansion of Sox9 expression into the normal Hoxa2 expression domain, where it is not normally expressed. Sox9 is a direct regulator of the cartilage-specific gene Col2a1 (Bell et al. 1997; Ng et al. 1997), and, using a mouse transgenic approach, it has been shown that changes in Sox9 expression are indeed responsible for the ectopic elements found in the second arch of Hoxa2 mutants. This is also supported by misexpression of Sox9 in the second arch, which produces a phenotype resembling that of the Hoxa2 mutants. Therefore, Hoxa2 acts very early in the chondrogenic pathway upstream of Sox9 during neural crest cell differentiation. In addition, Runx2 is up-regulated in the second branchial arch of Hoxa2 mutant embryos. Runx2 is required for bone formation, suggesting that the inhibition of Runx2 activity might mediate Hoxa2 suppression of intramembranous and endochondral bone formation.
Hox gene expression in cranial neural crest cells is considered to be inhibitory to skeletogenic differentiation and, in particular, incompatible with jaw formation (Trainor and Krumlauf 2001). Thus, the lack of expression of Hox genes in ectomesenchymal cells is imperative for proper patterning and skeletal development of the vertebrate face (Couly et al. 2002). Furthermore, the simultaneous inactivation of all Hoxa cluster genes leads to multiple jaw and first arch structures, partially replacing second, third, and fourth arch derivatives (Minoux et al. 2009). This pattern of Hox and non-Hox gene expression in cranial neural crest cells is conserved across vertebrate gnathostomes, and, interestingly, the vertebrate agnathan Lampetra fluviatilis shows expression of Hox6 in the first branchial arch (Cohn 2002), which may help to explain the absence of jaw formation in lampreys despite the presence of neural crest cells. Thus, jaw evolution may have coincided with suppression of Hox gene expression in the first branchial arch (Trainor 2003; Trainor et al. 2003). However, the presence or absence of Hox gene expression in the first branchial arch is likely too simplistic a model for jaw skeletal potency because another species of lamprey, Lethenteron japonicum, appears to maintain a Hox-expression-free mandible (Takio et al. 2004).
2.2. Signals and Switches in Ectomesenchymal Fate
Ectomesenchymal differentiation of neural crest cells has been shown to vary temporally and spatially. Lineage tracing of neural crest cells in the zebrafish has revealed that there is a spatial correspondence between the time of emigration and ultimate fate of individual cells (Schilling and Kimmel 1994). Early-migrating neural crest cells typically become the skeletal and connective tissue of the face and pharyngeal region, whereas later-migrating neural crest cells take on primarily a neural fate. In mice and avian species, clonal neural crest cell differentiation assays and lineage tracing studies have shown the existence of neural crest cell progenitor cells with both ectomesenchymal and neural potential from the same cranial region, strongly supporting the multipotentiality of cranial neural crest cells that become fate-restricted as a product of the environmental signals encountered through migration into their target sites (Calloni et al. 2009). Consistent with this, upon delamination and migration, neural crest cells express glial determination factors such as Sox10 (also a migrating neural crest cell marker) (Britsch et al. 2001) and FoxD3 (Dottori et al. 2001) in accordance with their general glial potential (Fig. 2). However, after reaching the branchial arches, FoxD3 and Sox10 expression diminishes, and, instead, neural crest cells express ectomesenchymal markers such as Dlx2 and Dlx5 (Depew et al. 1999, 2005; Blentic et al. 2008), which are conserved in both mouse and chick. Recent work in avians has also shown that the expression of Dlx2 and Dlx5 may be responsible for initiating the formation or condensation of mesenchyme into cartilage and bone, a process common to all vertebrates (Gordon et al. 2010). Moreover, Dlx5 acts as a mediator downstream from Bmp2 signaling in the promotion of Runx2 expression (Lee et al. 2003, 2005). Furthermore, single and compound Dlx mouse mutants have revealed roles for this gene family in collectively regulating regional identity along the proximodistal axis of the pharyngeal arches and specifying their specific cranioskeletal derivatives (Robledo et al. 2002; Depew et al. 2005). Yet even at this stage, ectodermal versus neuroglial fate has not been irrevocably determined. Neural crest cells isolated from the branchial arches of avian embryos and transplanted back into the neural tube of younger embryos are still capable of colonizing the cranial ganglia and contributing to neuroglial derivatives. However, this plasticity in neural crest cell fate persists for only ∼72 h postcranial NCC migration; after this time point, pharyngeal arch NCCs transplanted back into younger embryos avoid the cranial ganglia territories (McKeown et al. 2003).
Figure 2.
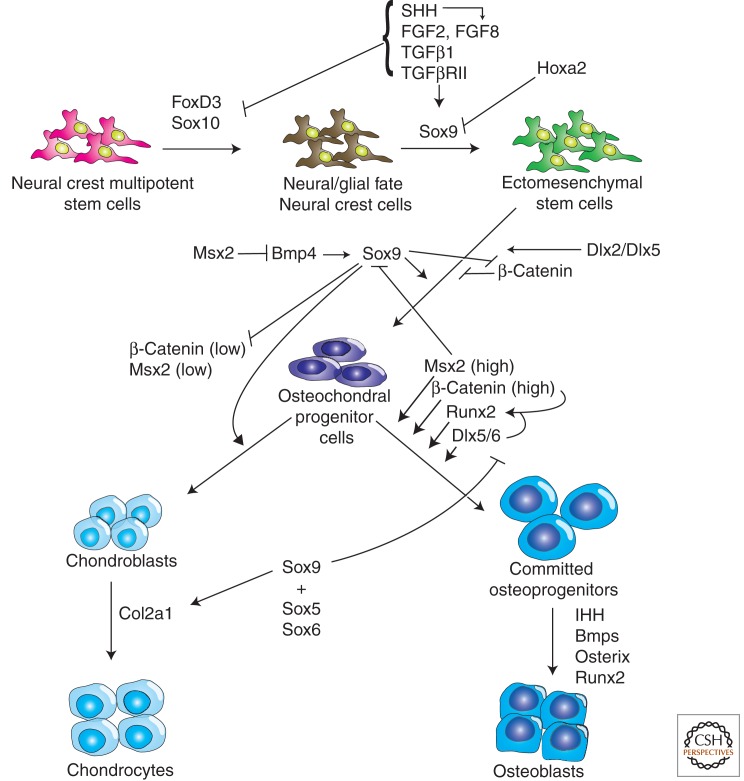
Signals and switches regulating cranial neural crest cell differentiation. Schematic representation of the signals and switches that govern neural crest cell segregation from a stem or progenitor cell into neuroglial or ectomesenchymal cells. This is then followed by differentiation of ectomesenchymal cells into an osteochondral progenitor cell and then bifurcation of potential into chondroblasts/chondrocytes or osteoprogenitors/osteoblasts.
2.2.1. Fibroblast Growth Factors
FGF signaling is known to play a role in promoting the fate of neural crest cells toward a skeletogenic type. In fact, FGF2 increases proliferation and skeletal fate (Li et al. 2010) of cranial neural crest cells in vitro (Sarkar et al. 2001; Abzhanov et al. 2003; Ido and Ito 2006) as well as in vivo (Abzhanov and Tabin 2004; Vitelli et al. 2006) in mouse and avian models. Interestingly, Fgf8 is expressed in the pharyngeal ectoderm, and its downstream signaling is mediated through the Fgfr1 receptor, which is expressed in neural crest cells entering the pharyngeal arches (Wada et al. 1997; Wilke et al. 1997; Walshe and Mason 2000; Blentic et al. 2008). Fgf8 can induce Sox9 expression and cartilage differentiation in vitro (John et al. 2011), and consistent with this, loss of FGF signaling leads to a failure of pharyngeal arch cartilage development (Sarkar et al. 2001; David et al. 2002; Walshe and Mason 2003; Ido and Ito 2006). Furthermore, Fgfr1 hypomorphic mice display craniofacial skeletal and other body defects (Partanen et al. 1998), whereas Fgfr1-nulls are embryonic-lethal (Deng et al. 1994). Conditional knockouts of Fgfr1 in NCCs display skeletal defects such as cleft palate and smaller skeletal elements (Trokovic et al. 2003), which may be a result of lower NCC proliferation or delay in differentiation. Electroporation of a dominant-negative Fgfr1 into chick embryonic neural crest cells results in neural crest cells that are entering the branchial arches failing to down-regulate Sox10. Thus, the failure to down-regulate glial markers such as Sox10 owing to the inability to respond to local FGF signaling cues supports a role for FGF signaling in the pharyngeal arch in promoting ectomesenchymal neural crest cell fate switching (Figs. 1A and 2). Consistent with this, FGFR2 is a well-known regulator of bone formation during embryonic development (Veistinen et al. 2009). Both gain- and loss-of-function studies in mice have shown that FGFR2 maintains a critical balance between the proliferation and differentiation of osteoprogenitor cells. Recently, de novo FGFR2 mutations were identified in a sporadically occurring peri-natal lethal skeletal disorder known as Bent Bone dysplasia, which is characterized by poor mineralization of the calvarium, craniosynostosis, dysmorphic facial features, osteopenia, and bent long bones (Merrill et al. 2012).
2.2.2. Transforming Growth Factors
Transforming growth factors (TGFs) have recently been shown to function as important switches mediating ectomesenchymal versus neural fate, both in vitro and in vivo (Fig. 2). Cranial and trunk neural crest cells exposed to TGFβ1 down-regulate Sox10 expression and differentiation into ectomesenchymal fates in mouse stem cell lines (John et al. 2011). Similar to FGF signaling, Tgfβ1 concomitantly induces Sox9 expression along with the formation of osteoblasts and chondrocytes as well as smooth muscle cells (McGonnell and Graham 2002). Consistent with Tgfβ1 signaling acting as a crucial switch in inducing ectomesenchymal fates, murine conditional inactivation of TgfβrII in neural crest cells in vivo led to a reduction of Sox9 expression in the pharyngeal arches and delayed ectomesenchymal fate acquisition (Mori-Akiyama et al. 2003). Furthermore, the skeletal elements that did develop were grossly malformed. In addition, the neural crest cells also failed to contribute to smooth muscle (Shah et al. 1996; Wurdak et al. 2005). Concomitant with the loss of Sox9, Sox10 expression was maintained in TgfβrII-null neural crest cells. Interestingly, overexpression of Sox10 in mouse neural crest stem cells (NCSCs) serves to maintain neurogenic potential at the expense of ectomesenchymal differentiation. Thus, Sox10 can counter the effects of Tgfβ1 (Kim et al. 2003). Sox10, however, is also important for smooth muscle differentiation, because inhibition of Sox10 leads to a failure of neural crest cell differentiation into smooth muscle in vivo and in vitro (Britsch et al. 2001; Dutton et al. 2001; Paratore et al. 2001, 2002). Collectively, this reveals TGFβ signaling as a fate switch selector for SoxE family members that govern divergent cranial neural crest cell differentiation fates (Fig. 2).
2.2.3. Hedgehog Signaling
Sonic Hedgehog (Shh), a morphogen with broad roles in organogenesis, has also been shown to play an important role in neural crest cell fate specification (Fig. 2). In vitro clonal culture of avian cranial neural crest cells in the presence of recombinant Shh not only induced ectomesenchymal fates but also was able to produce nodules of chondrogenic differentiation in cultures of both cranial and trunk neural crest cells (Dupin et al. 2010). The most common cell type obtained in this study (at 18.5%) was a multipotent cell with ectomesenchymal and neural fate, known as the GNMFC (glial, neuronal, melanoblastic, myofibroblastic, chondrocytic). Because of its wide differentiation capacity, the GNFMC progenitor is thought to comprise the apex of a hierarchy of neural crest cell progenitors that differentiate progressively. Sustained Shh treatment of neural crest progenitors in culture biases cell fates toward skeletal differentiation via the formation of chondrocytes and osteoblasts (Dupin et al. 2010). Consistent with this, Gli3 mutant mice show ectopic ossification in the interfrontal suture together with craniosysnostsis, which phenocopies the severe craniofacial malformations characteristic of Grieg cephalopolysyndactyly syndrome (Veistinen et al. 2012). Furthermore, the conditional disruption of HH signaling by removing the Smoothened receptor in neural crest cells results in decreased proliferation and increased apoptosis, revealing the importance of Hedgehog signaling in mouse cranial NCC survival (Jeong et al. 2004).
The foregut endoderm, facial ectoderm, and neuroepithelium are all sources of Shh signaling. Endodermal Shh signaling has been shown to influence the differentiation of ectomesenchyme toward a chondrogenic fate in the pharyngeal skeleton through both mouse and avian extirpation experiments (Epperlein 1974; Hall 1980; Couly et al. 2002; Aoto et al. 2009) as well as in mouse, chick, and zebrafish genetic studies (Piotrowski and Nusslein-Volhard 2000; Brito et al. 2008). Furthermore, extirpation of specific axial domains of endoderm during the neurula stage of avian embryogenesis disrupts specific cranial structures (Couly et al. 2002). Rotations and heterotopic transplantations of axial domains of endoderm affect the orientation of skeletogenic elements and induce ectopic skeletogenic elements, respectively. Shh-null mutant mice have shown the importance of this signal from the endoderm through its role in actively maintaining the expression of key differentiation genes such as Fgf8 and Sox9 in the first pharyngeal arch (Yamagishi et al. 2006).
The facial ectoderm also expresses Shh, particularly in a region termed the frontonasal ectodermal zone (FEZ) (Hu et al. 2003), a site where Shh expression abuts an Fgf8 domain and induces proliferation and outgrowth of the underlying NCC-derived mesenchyme as well as providing dorsoventral polarity. Ectopic grafting of the FEZ leads to formation of an ectopic upper beak (Hu and Marcucio 2009) in the upper jaw and ectopic lower jaws when transplanted to the developing mandible (Hu et al. 2003). These same patterning signals and structures are present in mice and humans, whose subtle morphological differences underlie changes in interactions between other tissues, such as the neural epithelium, neural crest mesenchyme, ectoderm, and endoderm, forming complex species-specific feedback signaling and phenotypes (Schneider and Helms 2003; Marcucio et al. 2005, 2011; Hu and Marcucio 2009, 2012). Miscommunication between these midline signaling centers leads to midline facial defects such as holoprosencephaly and hypertelorism as seen in humans and many other vertebrate species. In the frontonasal region, the anterior neural fold ectoderm has also been found to signal to the underlying ectomesenchyme to pattern the ectethmoid (dorsal) portion of the nasal capsule (Gitton et al. 2011) in avians. In contrast, the underlying mesethmoid cartilage of the avian nasal capsule is induced by endodermal Hedgehog signaling from the foregut (Benouaiche et al. 2008).
Indian hedgehog (Ihh) activity had previously been considered inhibitory to osteogenesis (Abzhanov et al. 2007); however, recent analyses show a strong pro-osteogenic role for IHH in calvarial development, because its loss leads to a reduction of osteogenic markers such as Bmp2/4, as well as a reduction in bone size and delay in matrix mineralization work (Lenton et al. 2011). Thus, similar to FGF and TGFβ, HH signaling also plays an important role in determining neural crest cell fate, particularly with respect to ectomesenchymal derivatives (Fig. 2).
2.3. Skeletal Connective Tissue Fate Determination
2.3.1. Wnt, Sox9, and Runx2 Signaling: Regulators of Skeletogenic Fate
As described above, Sox9 is a transcription factor that directly targets the activity of collagen type II (Col2a1) in chondrocytes (Bell et al. 1997) and plays a critical role in skeletogenesis, particularly endochondral bone formation (Mori-Akiyama et al. 2003). Misexpression of Sox9 in mouse pharyngeal arches results in ectopic cartilage formation (Kanzler et al. 1998; Healy et al. 1999), such as in the case of Hoxa2−/− mice. Sox9 activity is thus sufficient for and indicative of cell fate commitment toward the chondrogenic pathway. Consistent with this, inhibiting Sox9 expression in neural crest cells before mesenchymal condensation alters Col2a1, Runx2, and Osterix activity, which prevents cartilage differentiation and thus endochondral ossification (Akiyama et al. 2002; Mori-Akiyama et al. 2003). This is consistent with Sox9 mutations in humans, which cause campomelic dysplasia, a skeletal syndrome characterized by skeletal malformation of the endochondral bones (Wagner et al. 1994). Thus, in mammalian embryos, Sox9 plays an important role in cranial neural crest cell fate determination. However, Sox9 alone is insufficient to induce ectomesenchymal fates. Sox9 is coexpressed with Runx2 in osteochondral progenitor cells (Akiyama et al. 2005), and Runx2 is considered a master regulator of the intramembranous mode of ossification (de Crombrugghe et al. 2001; Karsenty 2008). However, complete commitment toward an osteoblastic fate requires Osterix expression downstream from Runx2 (Nakashima et al. 2002).
Wnt signaling has emerged as a key regulatory pathway crucial to the fate choice between a chondrocytic or osteoblastic fate in mammalian studies (Hartmann 2006). The expression of Wnt signaling in the perichondria and calvaria where osteoblastogenesis is activated highlights a conserved role for this pathway in both endochondral and intramembranous ossification. Canonical Wnt signaling encompasses ligand binding to the receptor Frizzled and coreceptors Lrp5 and Lrp6, which inhibits Gsk-3β phosphorylation of β-catenin and leads to its stabilization. Stabilized β-catenin translocates to the nucleus, where it binds to the LEF/TCF transcription factors and leads to targeted gene expression (for review, see MacDonald et al. 2009). Conditional deletion of β-catenin in neural crest cells via Wnt1-Cre recombinase in mice leads to a loss of cranial bones (Brault et al. 2001). Conversely, enhanced activity of Lrp5 results in an increase in bone mass (Boyden et al. 2002), along with dental malformations and colon cancer (Logan and Nusse 2004). Although canonical Wnt signaling has been shown to promote bone formation, it simultaneously inhibits chondrogenesis (Boyden et al. 2002; Guo et al. 2004). Conditional inactivation of β-catenin in both dermis and chondrocytes (using Dermo1-Cre and Col2a1-Cre, respectively) suppresses osteoblast differentiation but concomitantly increases the generation of chondrocytes. In these conditional loss-of-β-catenin experiments, early osteoblastic markers such as Runx2, Collagen1a1, and alkaline phosphatase continued to be expressed in perichondria and periostia (Day et al. 2005; Glass et al. 2005; Hill et al. 2005); however, Osterix, which is involved in the definitive commitment to an osteoblastic lineage, failed to be expressed. Regardless of neural crest or mesodermal origin, mesenchyme with a conditional deletion of β-catenin generated cartilage at the expense of osteoblasts. Thus, canonical Wnt signaling is a key promoter of osteogenesis (Fig. 2) but, at the same time, is an important antagonist of the chondrogenic pathway (Kolpakova and Olsen 2005; Hartmann 2006). Interestingly, the conditional deletion of Wnt/β-catenin in the dermis gives rise to skeletogenic differentiation in mice (Day et al. 2005; Hill et al. 2005; Tran et al. 2010) and may thus provide some insight into the wide array of dermal armor and skeletal elements found across various extant and fossil vertebrates (Vickaryous and Sire 2009; Fraser et al. 2010), which may potentially be of neural crest origin.
How canonical Wnt signaling regulates both endochondral and intramembranous ossification has begun to be deciphered and appears to depend on a balance between Sox9 and Runx2. As mesenchyme begins to condense, Sox9 and Runx2 are coexpressed in a common osteochondral progenitor cell (Day et al. 2005). To proceed along the endochondral path, the ratio of Sox9 must initially be higher relative to Runx2. However, subsequently during hypertrophy, Wnt signaling and Runx2 activity become elevated at the expense of Sox9, which collectively promote osteoblastogenesis. Interestingly, in vitro evidence suggests that Sox9 binds Runx2 directly, suppressing its activity (Zhou et al. 2006). During intramembranous ossification, osteochondral mesenchymal condensation begins with high levels of Wnt activity, which promotes elevated Runx2 to give rise to the intramembranous skeleton of the skull. Interestingly, SOX9 can bind to the TCF/LEF-binding region of β-catenin and influence its degradation, which blocks downstream canonical signaling (Akiyama et al. 2002). This shows the reciprocal antagonism that exists between the chondrogenic and osteogenic pathways and the importance of the precise regulation of Wnt/β-catenin levels that is required in order to control cell fates. Canonical Wnt signaling, like Runx2 and Osterix, is therefore a key regulator of osteogenesis, while also serving as an inhibitor of chondrogenesis.
Chondrocyte differentiation through Sox9 is therefore achieved by the inhibition of osteoblast-promoting genes such as β-catenin (Day et al. 2005). Indeed, expression of a stable form of β-catenin in mice inhibits chondrogenesis, mimicking the loss of Sox9. Consistent with this, the conditional deletion of β-catenin in chondrocytes mimics overexpression of Sox9 (Akiyama et al. 2004). Thus, an antagonistic relationship exists between Sox9 and β-catenin in the regulation of cartilage and bone development (Mori-Akiyama et al. 2003). Similar to Sox9, β-catenin signaling plays multiple roles during NCC differentiation by influencing chondrogenesis as well as sensory neurogenesis. Collectively, this illustrates the reiteration of the same signaling pathways during multiple stages of NCC development, and this is a common theme during embryogenesis.
2.3.2. Muscle Segment Homeobox Genes
Muscle segment homeobox transcription factors Msx1 and Msx2 also play a role in fate determination for controlling cranial neural crest cells, specifically during craniofacial development (Satokata and Maas 1994; Bei and Maas 1998; Satokata et al. 2000; Ishii et al. 2005). Msx1 and Msx2 are strongly expressed in migrating neural crest cells, whose expression continues during their colonization of the facial prominences and branchial arches (Hill et al. 1989; MacKenzie et al. 1991; Catron et al. 1996). Mouse Msx1 loss of function results in multiple craniofacial abnormalities, including frontal bone development defects (Satokata and Maas 1994). Similarly, Msx2-null mutant mice show defective skull ossification and persistent calvarial foramen, suggesting that Msx2 plays a critical role in regulating calvarial morphogenesis (Ishii et al. 2003). Msx2 loss of function therefore causes pleiotropic defects in bone growth (Satokata et al. 2000) that result from osteoprogenitor anomalies.
Interestingly, Msx2 and Sox9 are coexpressed in a subpopulation of cranial neural crest cells within the branchial arches (Semba et al. 2000). Because Sox9 is a transactivator of chondrogenesis and Msx genes can act as transcriptional repressors, it was hypothesized that Sox9 expression was indicative of cranial neural crest-derived chondrogenic cell lineages and that Msx2 represses chondrogenic differentiation until cranial neural crest cell migration was completed. In mice, Msx2 diminishes after the completion of migration, at which point Sox9-mediated chondrogenic differentiation proceeds. Consistent with this, inhibition of Msx2 in migrating neural crest cells accelerates the rate and extent of chondrogenesis, as indicated by increased expression of Col2a1, aggrecan, and Alcian Blue staining. This suggests that Msx2 is a repressor of chondrogenic differentiation and that an important early event in craniofacial morphogenesis is transient coexpression of Sox9 and Msx2 during cranial neural crest cell migration, which is followed by restricted expression of Sox9 within cranial neural crest cell-derived chondroprogenitor cells (Fig. 2).
Msx genes are critical for osteogenic lineage differentiation, and they control osteogenesis by regulating Runx2 (Satokata et al. 2000). Recent work has shown a synergistic interaction between Msx1 and Dlx5 in the osteogenesis of the mouse frontal bone (Chung et al. 2010). In agreement with this, mutations in MSX1 in humans have been associated with tooth agenesis and orofacial clefting conditions on their own or as part of syndromes such as Witkop syndrome (Jumlongras et al. 2001). MSX2 mutations underlie craniosynostosis type 2 (Jabs et al. 1993), parietal foramina (Wilkie et al. 2000), and cleidocranial dysplasia (Garcia-Minaur et al. 2003) syndromes. Significantly, the entire ossified calvaria is missing in Msx1−/−;Msx2−/− double mutants, indicating that Msx1 and Msx2 function together to regulate osteogenesis during calvarial development (Ishii et al. 2005; Han et al. 2007), where neural crest cell migration was found to be normal with delayed migration; mispatterning along the neural tube and mixing of crest cells between streams were also noted. Thus, Msx genes control multiple steps in neural crest cell patterning and differentiation into skeletogenic tissues.
2.4. Ectomesenchymal Fate—Differences between Cranial and Trunk Neural Crest
An important feature that distinguishes cranial neural crest from trunk neural crest is their ability to differentiate into mesenchymal tissues. Mammalian cranial NCCs contribute to cartilage and bone (Fig. 1B,C), the articulating disk of the temporomandibular joint, and odontoblasts and mesenchyme that support tooth development (Chai et al. 2000). In contrast, mammalian trunk NCCs do not appear to generate bone and cartilage endogenously. However, this property may have been lost during evolution, because avian trunk neural crest progenitors cultured in media that promotes bone differentiation will, in fact, form both bone and cartilage cells, albeit at low frequency (McGonnell and Graham 2002). Furthermore, if these same trunk NCCs are transplanted into the developing avian head, experiments not yet amenable within mammalian embryos, they will also contribute appropriately to cranioskeletal components such as the scleral cartilages of the orbit and Meckel’s cartilage of the lower jaw.
This implies that NCCs from all axial levels can perhaps generate the full repertoire of neural crest derivatives under appropriate conditions. Consistent with this, quail cranial neural plate transplanted in place of a chick trunk neural plate generates NCCs that contribute to all typical trunk neural crest cell derivatives, and in addition produce ectopic cartilage (Le Douarin and Teillet 1974; Le Lievre and Le Douarin 1975). Similarly, mouse neural crest cells transplanted in ovo into avian hosts maintain their species-specific molecular identity and character (Trainor and Krumlauf 2000; Fontaine-Perus and Cheraud 2005; Tokita and Schneider 2009). Donor murine NCCs migrate normally within their avian hosts; however, their long-term differentiation has not yet been explored with respect to bone and cartilage. Nonetheless, it is known that mouse NCC transplants can support tooth development in avian hosts (Mitsiadis et al. 2003). Interestingly, the turtle plastron, whose nine bones have been homologized to the exoskeletal components of the clavicles, interclavicular bone, and gastralia, has recently been shown to be derived from late-migrating trunk NCCs (Cebra-Thomas et al. 2007; Gilbert et al. 2007). The skeletogenic potential of trunk neural crest cells is of considerable evolutionary significance, because early vertebrates, many of which have been identified in the Burgess Shale in Canada, together with fossilized fish had extensive postcranial exoskeletal coverings, and this external armor is likely to have been trunk neural crest–derived.
3. TRUNK NEURAL CREST CELLS
Cranial NCCs differentiate primarily into bone, cartilage, and connective tissues, but they also generate neurons and glia. In contrast, trunk NCCs give rise primarily to sensory neurons and glia that are central to formation of the peripheral nervous system (Fig. 3A). The primary function of the peripheral nervous system (PNS) is to sense and react to stimuli from the viscera, smooth muscles, skin, and the exocrine glands of the body. Accordingly, the PNS is loosely categorized into two anatomical and functional classifications, the sensory and autonomic nervous systems.
Figure 3.
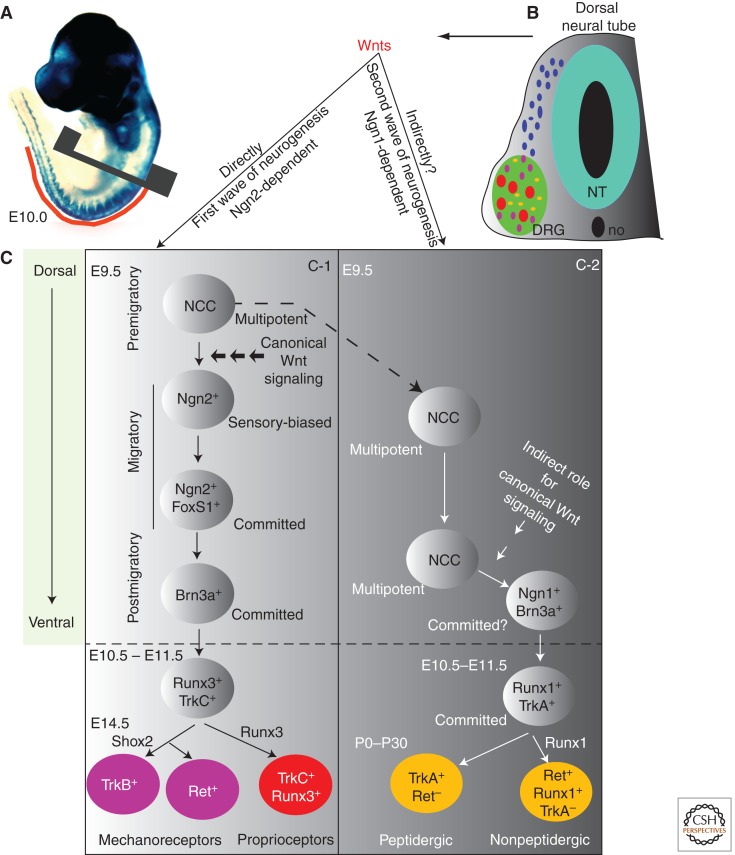
Neural crest differentiation into trunk sensory neurons. (A) Wnt1Cre;R26RlacZ labels all of the migrating neural crest cells (NCCs) at E10.0. (B) A cross section through the trunk reveals the ventromedial path of NCC migration that populates the dorsal root ganglia (DRG). Differentiation of NCC during and postmigration leads to formation of three morphologically distinct types of sensory neurons: small-diameter nocipeptive (peptidergic and nonpeptidergic) (shown in yellow) and large-diameter mechanoreceptors (red) and proprioceptors shown in red and (pink), respectively. (C) Trunk sensory neurogenesis occurs in two waves, both of which are directly or indirectly dependent on canonical Wnt signals emanating from the dorsal neural tube. (C-1) During the first wave of sensory neurogenesis, Wnt signals act on migratory NC cells to induce the expression of Neurogenin2 (Ngn2). Ngn2 expression biases the neural crest fate toward sensory neurogenesis. Postmigratory cells of the first wave express Brn3a and differentiate into large-diameter proprioceptor (TrkC+, shown in red) and mechanoreceptor (TrkB+ or Ret+, shown in purple) neurons. Runx3 plays an important role in formation of TrkC+ proprioceptors, whereas Shox2 has an important role in regulating expression of TrkB in mechanoreceptors. (C-2) The second wave of neurogenesis occurs subsequently in embryogenesis and gives rise to nociceptor neurons. Neurogenin1 and Brn3a expression in the postmigratory second-wave cells marks them for sensory differentiation. Brn3a directly activates TrkA in these cells, which then, based on the differential expression of Runx1, differentiate into nonpeptidergic (Runx1+;Ret+) and peptidergic (TrkA+) nociceptor neurons (peptidergic cells are shown in yellow). E, Embryonic day; P, postnatal day; NT, neural tube; no, notochord; DRG, dorsal root ganglion; NCC, neural crest cell. (Figure adapted and modified based on Marmigere and Ernfors 2007.)
The sensory nervous system (SNS) comprises afferent sensory neurons that sense stimuli and convey information to the central nervous system (CNS; brain and spinal cord) and in the trunk consists of a metameric series of ganglia called dorsal root ganglia (DRG). The DRGs are composed of a variety of sensory neuron cell bodies whose axons enable the sensation of touch (low-threshold mechanoreceptor neurons), temperature (thermal sensory neurons), pain (nociceptive neurons), movement, and spatial position (proprioceptive neurons), all of which are derived from trunk NCCs. Each functional type of sensory neuron is characterized by its own unique set of receptors and ion channels, and their differentiation depends on unique sets of transcription factors (Table 1).
Table 1.
DRG neurons are identified with the expression of specific neurotrophic receptors
| Sense | Sensory neuronal subtype | Diameter | Receptor(s) expressed | Neurotrophic requirement | References |
|---|---|---|---|---|---|
| Temperature | Thermal | Small | TrkA Ret | NGF BDNF | Snider and Wright 1996; Chen et al. 2006; Luo et al. 2007 |
| Pain | Nociceptive | Small | TrkA Ret | NGF BDNF | |
| Touch | Low-threshold mechanoreceptor | Large | TrkB | BDNF | Gonzalez-Martinez et al. 2004; Shimizu et al. 2007; Perez-Pinera et al. 2008 |
| Movement and spatial position | Proprioceptive | Large | TrkC | NT-3 | Snider and Wright 1996; Hasegawa and Wang 2008 |
The autonomic nervous system (ANS) largely comprises efferent motor neurons that carry information from the CNS to the various organs of the body and is loosely defined as the motor component of PNS. It provides a way for the CNS to send commands to the rest of the body and provides involuntary control of the visceral organs. The ANS consists of three major components: the sympathetic (SyNS) and parasympathetic (PSNS) nervous systems, which function together to maintain body homeostasis, and the enteric nervous system (ENS), which controls gut motility. Axons from CNS neurons (brainstem and spinal cord) project and synapse with the ganglionic cell bodies located paravertebrally (for SyNS) or inside the target organs (for PSNS). In the gut, the noradrenergic and cholinergic ganglia form the enteric nervous system (ENS), which exclusively innervates the gastrointestinal (GI) tract. Even though usually grouped as a subdivision of the ANS, the ENS comprises local neural circuits consisting of sensory, inter-, and motor neurons, capable of autonomous control without CNS input. Consistent with this, it is also called the second brain of the body (Gershon 1998). Neural crest cells contribute to all of the neurons and glia of the PNS, except for some cranial sensory neurons. The signals that regulate the individual contributions of NCCs to each of the different components and sublineages of the PNS are described below.
3.1. Sensory Neuron Differentiation
3.1.1. Neurogenins Regulate Trunk Sensory Neuron Differentiation
Trunk sensory neurogenesis occurs in waves (Fig. 3). The first wave is characterized by migrating NCCs forming large-diameter touch and movement neurons, whereas the second wave occurs after the DRGs coalesce, producing small pain as well as large touch and movement neurons. A third wave of sensory neurogenesis has also been described that generates primarily nociceptive (TrkA+) neurons from neural crest-derived boundary cap cells (Maro et al. 2004). The first two waves of NCC differentiation are regulated by Neurogenin1 (Ngn1) (Ma et al. 1996) and Neurogenin2 (Ngn2) (Fode et al. 1998), which are vertebrate homologs of the Drosophila bHLH (helix–loop–helix) proneural gene atonal. Analyses of individual Ngn1 and Ngn2 mouse mutant embryos suggest that Ngn2, which is expressed in early-migratory neural crest, regulates the first wave of sensory neurogenesis, whereas Ngn1, which is expressed in the coalesced DRG, regulates the second wave (Fig. 3B) (Ma et al. 1999; Marmigere and Ernfors 2007). Ngn1 appears to play a predominant role in formation of small-diameter nociceptive (TrkA+) neurons with a minor requirement in the formation of large-diameter mechanoreceptor (TrkB+) and proprioceptive (TrkC+) neurons (Fig. 3B,C). In contrast, Ngn2 plays a transient role in formation of large-diameter mechanoreceptor (TrkB+) and proprioceptive (TrkC+) neurons; Ngn2+ cells also contribute to a small but significant fraction of nociceptive (TrkA+) neurons (Zirlinger et al. 2002).
Ngn1 and Ngn2 control expression of other bHLH transcription factors such as NeuroD that function as neural differentiation factors. Ngn1;Ngn2 double-mutant embryos show a complete lack of NeuroD expression and a concomitant absence of DRGs. Because both Ngn1 and Ngn2 play a role in the formation of TrkA+, TrkB+, and TrkC+ neurons, there is no doubt that neurogenins promote sensory lineage determination of NCCs (Fig. 3C). However, it is not yet clear whether individual neurogenins specify the subtypes of sensory neurons. The neurogenins expressed in sensory progenitors turn on expression of Delta1 cell-autonomously. Consequently, the Ngn+;Delta1+ cell acquires a neuronal fate, and through lateral inhibition, Delta–Notch signaling prohibits neurogenesis in the surrounding cells. These surrounding cells subsequently form the glia of the sensory ganglion (Wakamatsu et al. 2000). Consistent with this, mutations in the Notch signaling antagonist Numb cause a defect in trunk sensory neuron differentiation despite normal migration of neural cells into the DRG anlage (Zilian et al. 2001).
3.1.2. Wnt Signaling Regulates Neurogenin Expression and Activation in Sensory Neuron Progenitors
Avian in vitro studies have revealed that although neural tube cultures consisting of premigratory NCCs can generate sensory neurons, cultures consisting exclusively of migratory neural crest cells lose this potential (Le Douarin and Kalcheim 1999; Bronner-Fraser 2004). Consistent with this, two reports have defined a necessary and sufficient role for dorsal neural tube-derived canonical Wnt signaling in differentiation of trunk sensory neurons (Fig. 3B) (Hari et al. 2002; Lee et al. 2004). Genetic ablation of β-catenin specifically in premigratory neural crest cells results in the complete absence of DRGs and melanocytes, and inhibition of β-catenin function leads to loss of both the early and late waves of sensory neurogenesis, observed by the absence of Ngn2 and Ngn1 expression (Hari et al. 2002). However, the lack of active Wnt signaling in postmigratory NCCs in DRG and sympathetic ganglia (SG) at E12.5 and subsequently, suggests that Wnt signaling is not directly involved in the induction of the second wave of DRG neurogenesis (Kleber et al. 2005). Whether Wnt signaling has an indirect role in regulation of late sensory neurogenesis in the DRG, via an intermediary factor, remains to be determined.
Interestingly, constitutive activation of canonical Wnt signaling in neural crest progenitor cells in the dorsal neural tube in vivo promotes sensory neuron differentiation at the expense of other NCC progeny. Sustained β-catenin activation leads to ectopic sensory lineage differentiation, marked by presence of Ngn2+ cells, even at sites of sympathetic neurons and glia formation, as well as the anterior region of the embryo, which is usually devoid of NCC-derived sensory neurons. In vitro clonal analysis proved that this effect of canonical Wnt signaling in sensory cell differentiation is not caused by Wnt-mediated “sensory progenitor” cell proliferation or expansion but, rather, represents true determination of cell fate specification (Lee et al. 2004). Therefore, the differentiation or lack of sensory neurons following constitutive activation or inhibition of canonical Wnt signaling, respectively, is mediated via Neurogenins. Wnt signaling also has a well-documented role in melanocyte differentiation; however, whether a neural crest cell differentiates into sensory neuron or melanocyte is dependent on the stage of NCC development at which it is exposed to active Wnt signaling.
3.1.3. Transcription Factors Determine the Sensory Neuronal Subtype
The expression of various receptors and ion channels specific to each subtype of sensory neuron requires a sequential expression of cohort of transcription factors. The POU-domain transcription factor Brn3a (aka Pou4f1) is expressed in terminally differentiating sensory neurons of DRGs and is important for the correct development and/or survival of a subpopulation of proprioceptive, nociceptive, and mechanoceptive sensory neurons (McEvilly et al. 1996). Brn3a binds directly upstream of NGF receptor TrkA (Ma et al. 2003), which is expressed in all nociceptors during embryonic development (Fig. 3C). The expression of TrkA in the nociceptive sensory neurons of the DRG also requires the transcription factor Klf7 (Lei et al. 2005). Postnatally, however, some nociceptors down-regulate TrkA expression and concomitantly activate the GDNF receptor Ret, thus segregating the nociceptors into inflammation-sensing TrkA+ (aka peptidergic neurons) and neuropathic pain-sensing Ret+ neurons (nonpeptidergic neurons) (Molliver and Snider 1997; Snider and McMahon 1998; Huang and Reichardt 2001). These two neuron types express a distinct set of ion channels and receptors (Bradbury et al. 1998; Dong et al. 2001; Potrebic et al. 2003; Zylka et al. 2003), and the Runt-related transcription factor Runx1 is important for this segregation (Chen et al. 2006; Kramer et al. 2006; Marmigere and Ernfors 2007; Inoue et al. 2008; Abdel Samad et al. 2010). The specification of subsets of TrkB-expressing mechanoreceptor neurons requires Shox2 (Short statured homeobox 2) (Scott et al. 2011), whereas the maintenance of TrkC+ proprioceptive neurons, which are required for movement, depends on Runx3 (Levanon et al. 2002). Runx3 loss of function leads to a lack of motor coordination (Inoue et al. 2002; Levanon et al. 2002).
3.2. Autonomic Neuron Differentiation
3.2.1. Sympathetic Neuron Differentiation
The sympathetic nervous system (SyNS) is made up of a ventrally located metameric series of ganglia aligned parallel to the vertebrae on both sides of the embryo. Although the signals that regulate NCC differentiation into sympathetic neurons are site-specific, the trunk neural tube and the path of trunk neural crest migration confer competency to the trunk NCCs for sympathetic differentiation. The long-standing perception of sympathetic differentiation suggests the presence of a common sympatho-adrenal (SA) progenitor cell within the trunk neural crest that, based on the local environmental cues, can differentiate into three related cell types: sympathetic neurons, neuro-endocrine chromaffin cells, and small intensely fluorescent (SIF) cells, all of which show catecholaminergic (ability to produce adrenaline and noradrenaline) traits (Unsicker 1973; Unsicker et al. 1978a; Vogel and Weston 1990). Sympathetic progenitor NCCs migrate along the ventro-medial path and reach the dorsal aorta, initially forming a continuous sympathetic chain that subsequently segregates into discrete ganglia. The cells then undergo a second migration to para-aortic sites, where secondary sympathetic ganglia are formed. Some of these cells from the primary sympathetic ganglia migrate deeper into the embryo toward the kidney, where they differentiate into predominantly neuro-endocrine cells (chromaffin cells) of the adrenal gland (Fig. 4A,B) (for review, see Le Douarin and Kalcheim 1999). The ventro-medial path taken by sympathetic precursor neural crest cells is shared with the trunk DRG precursor crest, and the sympathetic precursors pass through the DRG to reach the sites of primary sympathetic ganglia. Mouse knockout studies have shown that the growth factor Neuregulin-1 and its receptor tyrosine kinase heterodimer ErbB2/ErbB3 are important positive regulators of neural crest cell migration beyond the developing DRG (Britsch et al. 1998).
Figure 4.
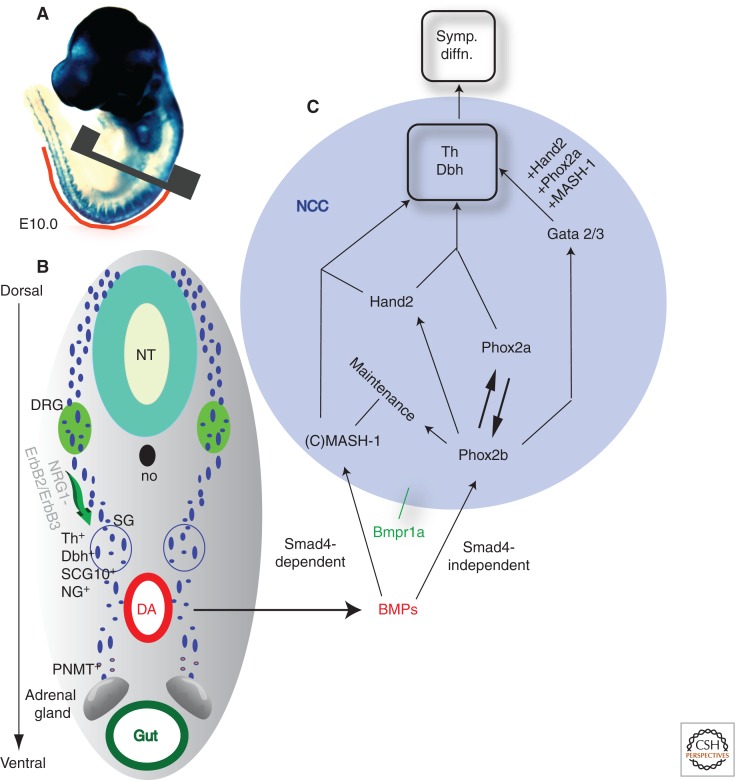
Neural crest differentiation into sympathetic neurons. (A) Wnt1Cre;R26RlacZ labels all the migrating neural crest cells (NCCs) at E10.0. (B) A cross section through the trunk reveals the ventro-medial path of NCC migration. NCCs migrate beyond the dorsal root ganglia (DRG) to coalesce at the dorso-lateral side of the dorsal aorta (DA) to form sympathetic ganglia (SG). Neuregulin1–ErbB2/ErbB3 signaling has a role in regulating the ventral migration of NCCs beyond DRG. A subpopulation of cells from the SG migrates further ventrally to the adrenal gland to differentiate into the neuro-endocrine chromaffin cells expressing PNMT (phenylethanolamine N-methyltransferase). (C) BMP signaling from the dorsal aorta plays a very important role in differentiation of the crest cells coalescing at the sites of SG formation. BMP ligands through Smad4-dependent and -independent mechanisms activate expression of Mash1 and Phox2b, respectively. Phox2b regulates the expression of Hand2, Phox2a, and Gata factors and maintains Mash1 expression within the differentiating crest cells. The combinatorial activity of all of these factors leads to activation of two enzymes—Th (tyrosine hydroxylase) and Dbh (dopamine β-hydroxylase)—crucial for noradrenaline synthesis. Symp. diffn., sympathetic differentiation; NT, neural tube; no, notochord.
3.2.2. BMPs Regulate Induction of Sympathetic Neuron Differentiation
Avian studies defined the requirement for embryonic tissues like the notochord, neural tube, and somitic mesoderm for priming the competency of migrating NCCs to differentiate into sympathetic neurons (Cohen 1972; Norr 1973; Teillet and Le Douarin 1983; Howard and Bronner-Fraser 1986). Furthermore, in vitro and in vivo work in avian models revealed BMPs to be sufficient to induce adrenergic differentiation (Varley et al. 1995; Reissmann et al. 1996; Shah et al. 1996; Varley and Maxwell 1996). Overexpression of Bmp4 and Bmp7 in migrating avian neural crest increases the size of sympathetic ganglia and forms ectopic ganglia, whereas blocking BMP signaling prevents sympathetic neuron generation. Consistent with this, NCC-specific inactivation of the type I BMP receptor (Bmpr1a) Alk3 in mice does not affect neural crest cell migration or recognition of the SyNS site, but leads to failure of NCCs to aggregate and express sympathetic neuron markers. The influence of BMP signaling is on differentiation (but not migration) and the initial coalescence of the sympathetic progenitor crest (Schneider et al. 1999). The expression of Bmp2 and 4 in mouse dorsal aorta, the site of primary (SG) chain formation, supports an endogenous role for BMP signaling in sympathetic neuron differentiation (Fig. 4B) (Shah et al. 1996). BMPs belong to the transforming growth factor β (TGFβ) family of secreted ligands and signal via activation of cytoplasmic coreceptor proteins SMADs (R-SMADs, SMAD 1,5,8 for BMPs), which, along with co-Smad and SMAD4, regulate gene expression. Although Smad4 is not involved in colonization or survival of sympathetic ganglia (Buchmann-Moller et al. 2009; Morikawa et al. 2009), NCC-specific inactivation of Smad4 leads to defects in murine SyNS development (Nie et al. 2008). Treatment of these mutant embryos with noradrenaline extends the life span of the mutants, showing a requirement for Smad4 in sustaining the proliferation of NCC-derived sympathetic neurons (Morikawa et al. 2009).
3.2.3. Sympathetic Neuron Differentiation Involves a Linear Hierarchal Genetic Cascade
Sympathetic differentiation is unique such that there is no single master regulator of sympathetic fate. Rather, multiple players such as Mash1, Phox2a/2b, Hand2, and Gata3, which are all capable of cross-regulating each other, comprise a network that collectively governs sympathetic differentiation (Fig. 4C). Combinatorial expression of each of these factors defines the different sympathetic neuronal subtypes. Most of these factors depend on BMP signaling for their activity (Shah et al. 1996; Lo et al. 1997, 1998; Schneider et al. 1999; Howard et al. 2000; Tsarovina et al. 2004). Phox2 (Phox2a, Phox2b) genes are expressed in sympathetic, parasympathetic, and enteric ganglia of the developing PNS and are necessary and sufficient for specification of sympathetic neurons from trunk neural crest cells (Pattyn et al. 1999; Stanke et al. 1999). Mice lacking Phox2b die before birth because of the failure of autonomic ganglia formation leading to lack of noradrenaline in the body. The initial expressions of Phox2b and Mash1 are independent of each other (Schneider et al. 1999); however, Phox2b is required for the maintenance of Mash1 expression, because Mash1 expression is lost in the ganglia of Phox2b mutant mice (Pattyn et al. 1999, 2000; Brunet and Pattyn 2002). Phox2a and Phox2b can induce transcription of each other and independently induce transcription of the noradrenaline synthesis enzymes Th (tyrosine hydroxylase) and Dbh (dopamine β-hydroxylase) (Zellmer et al. 1995; Kim et al. 1998; Yang et al. 1998; Lo et al. 1999). Adding to this complexity, MASH-1 has been shown to regulate the expression of Phox2a (Hirsch et al. 1998; Lo et al. 1998) and Hand1 (Ma et al. 1997), and interactions between Hand2 and Phox2a are capable of inducing Dbh transcription (Fig. 4C) (Rychlik et al. 2003; Xu et al. 2003). During the normal course of sympathetic ganglia development, Hand2 expression follows that of Phox2b and Mash1. Hand2 expression has not been analyzed in Mash1 mutants; hence, it remains unclear if Mash1 and/or Phox2b have any role in regulating Hand2. Phox2b, however, regulates expression of Gata3 (Tsarovina et al. 2004). Gata3-null mouse embryos display drastic reductions in Th and Dbh expression in the developing sympathetic ganglia (Lim et al. 2000) and die at mid-gestation (Pandolfi et al. 1995) of drastic noradrenergic differentiation defects.
3.2.4. Sympatho-Adrenal Progenitor Differentiation into Distinct Cell Types Is Based on Environmental Cues
Trunk neural crest cells generate both noradrenergic and cholinergic neurons (fewer in number compared with the noradrenergic). Neurotropins NGF and NT3 play a general role in survival and differentiation of sympathetic neuroblasts, and both are capable of inducing Th and Dbh expression in vivo. However, analyses of superior cervical ganglion (SCG) neurons of the SyNS in the NGF and NT3 loss-of-function mouse embryos indicate a differential requirement for NGF and NT3 in cholinergic and adrenergic differentiation. NGF seems to have a specific role in expression of acetylcholine receptor, whereas NT3 is important for the expression of Th and Dbh enzymes (Andres et al. 2008). This differential in vivo requirement for NGF and NT3 neurotrophins might be due to differences in spatial availability of the factors to the progenitor neural crest and be an added way to regulate cholinergic versus adrenergic differentiation.
Chromaffin cells are neuro-endocrine cells present in the medullary region of the adrenal gland. Derived from the NCCs, these cells are postsynaptic sympathetic neurons modified such that upon electrical sympathetic input they secrete catecholamine neurotransmitters (predominantly adrenaline and in limited amounts noradrenaline) into the blood circulatory system. For differentiation into chromaffin cells, sympathetic precursors turn off Th expression while concomitantly activating the enzyme phenylethanolamine N-methyltransferase (PNMT) (Fig. 4B). PNMT catalyzes the conversion of noradrenaline to adrenaline. Sympathetic progenitors can be differentiated in vitro into sympathetic neurons or chromaffin cells depending on whether they are exposed to NGF or glucocorticoids, respectively (Anderson 1993; Francis and Landis 1999), and treatment of postnatal chromaffin cells with NGF causes them to trans-differentiate into neurons (Unsicker et al. 1978b; Doupe et al. 1985). Glucocorticoid receptor (GR) mutant mice (GRα−/−) lack the adrenaline-synthesizing enzyme PNMT, however this fails to affect development of adrenal chromaffin cells, and no transformation into a neuronal phenotype is observed (Finotto et al. 1999). This suggests that sympatho-adrenal progenitors can differentiate into distinct cell types in response to environmental molecular cues.
4. CONCLUSIONS
Neural crest cells show a remarkable capacity for differentiation, and in this article, we highlighted the complexity of signals and switches, which govern cranial neural crest cell differentiation into bone and cartilage and the differentiation of trunk NCCs into primarily neuronal derivatives. NCCs continue to fascinate scientists, and, although clearly important to embryonic development and disease pathogenesis, much of the current focus resides in their evolution and their stem cell-like characteristics and application to regenerative medicine.
Neural crest cells are synonymous with vertebrate evolution, and there is tremendous interest in identifying their specific origin and the acquisition of their incredible properties. There is no doubt that the properties of NCCs were acquired gradually over time. Understanding the signals and switches that govern NCC differentiation may eventually reveal how and when the NCCs and their distinctive properties came to be.
The discovery that NCCs show multipotency and self-renewal capacity was pioneering for the field of stem cell biology. The stem and progenitor cell properties of NCCs, combined with their remarkable diversity of cell type and tissue differentiation, have made NCCs an attractive proposition in regenerative medicine. Even though NCC progenitor cells are generated transiently in the embryo, many NCCs appear to retain their capacity throughout embryogenesis and even into adulthood. Consequently, there have been concerted efforts to isolate embryonic and adult NCCs as well as derive NCCs from human and mouse embryonic stem cell (ESCs) and induced pluripotent stem cells (iPSCs). More research, however, is needed to further our knowledge regarding the capacity of NCC progenitors to differentiate into cells of several different lineages. More importantly, this needs to be performed in vivo, and a better appreciation of the precise signals and switches that dictate the survival, proliferation, and differentiation of neural crest cells into their distinct derivatives will facilitate their application in therapeutic and regenerative medicine.
Currently, however, very little is known regarding the key factors or signaling cascades and switches that are essential for mammalian NCC induction. The Wnt, BMP, and FGF signaling pathways that show well-established and characterized roles in NCC formation in avians, fish, and amphibians appear to be required primarily for lineage specification of NCCs in mammalian embryos. It is important to note that as neural stem cells differentiate into NCCs, there is a clear switch in Sox expression state with Sox2 being inactivated in concert with the activation of Sox9 and then Sox10 in progenitors and migrating NCCs, respectively (Remboutsika et al. 2011). These parameters may present avenues for ultimately identifying the key signals required for mammalian NCC formation, which will provide insights into the evolution of neural crest cells and their properties as well as facilitate ways to generate neural crest cells from hESCs and iPS cells, both of which will be enormously beneficial in the application of NCCs to tissue engineering and regenerative medicine.
Footnotes
Editors: Patrick P.L. Tam, W. James Nelson, and Janet Rossant
Additional Perspectives on Mammalian Development available at www.cshperspectives.org
REFERENCES
- Abdel Samad O, Liu Y, Yang FC, Kramer I, Arber S, Ma Q 2010. Characterization of two Runx1-dependent nociceptor differentiation programs necessary for inflammatory versus neuropathic pain. Mol Pain 6: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abzhanov A, Tabin CJ 2004. Shh and Fgf8 act synergistically to drive cartilage outgrowth during cranial development. Dev Biol 273: 134–148 [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Tzahor E, Lassar AB, Tabin CJ 2003. Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development 130: 4567–4579 [DOI] [PubMed] [Google Scholar]
- Abzhanov A, Rodda SJ, McMahon AP, Tabin CJ 2007. Regulation of skeletogenic differentiation in cranial dermal bone. Development 134: 3133–3134 [DOI] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B 2002. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 16: 2813–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Behringer RR, Rowitch DH, Schedl A, Epstein JA, de Crombrugghe B 2004. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc Natl Acad Sci 101: 6502–6507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T, et al. 2005. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci 102: 14665–14670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DJ 1993. Molecular control of cell fate in the neural crest: The sympathoadrenal lineage. Annu Rev Neurosci 16: 129–158 [DOI] [PubMed] [Google Scholar]
- Andres R, Herraez-Baranda LA, Thompson J, Wyatt S, Davies AM 2008. Regulation of sympathetic neuron differentiation by endogenous nerve growth factor and neurotrophin-3. Neurosci Lett 431: 241–246 [DOI] [PubMed] [Google Scholar]
- Aoto K, Shikata Y, Imai H, Matsumaru D, Tokunaga T, Shioda S, Yamada G, Motoyama J 2009. Mouse Shh is required for prechordal plate maintenance during brain and craniofacial morphogenesis. Dev Biol 327: 106–120 [DOI] [PubMed] [Google Scholar]
- Bei M, Maas R 1998. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development 125: 4325–4333 [DOI] [PubMed] [Google Scholar]
- Bell DM, Leung KK, Wheatley SC, Ng LJ, Zhou S, Ling KW, Sham MH, Koopman P, Tam PP, Cheah KS 1997. SOX9 directly regulates the type-II collagen gene. Nat Genet 16: 174–178 [DOI] [PubMed] [Google Scholar]
- Benouaiche L, Gitton Y, Vincent C, Couly G, Levi G 2008. Sonic hedgehog signalling from foregut endoderm patterns the avian nasal capsule. Development 135: 2221–2225 [DOI] [PubMed] [Google Scholar]
- Blentic A, Tandon P, Payton S, Walshe J, Carney T, Kelsh RN, Mason I, Graham A 2008. The emergence of ectomesenchyme. Dev Dyn 237: 592–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A 2003. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: A comparison with Snail and E47 repressors. J Cell Sci 116: 499–511 [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP 2002. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med 346: 1513–1521 [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB 1998. The expression of P2X3 purinoreceptors in sensory neurons: Effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci 12: 256–268 [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R 2001. Inactivation of the β-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128: 1253–1264 [DOI] [PubMed] [Google Scholar]
- Brito JM, Teillet MA, Le Douarin NM 2008. Induction of mirror-image supernumerary jaws in chicken mandibular mesenchyme by Sonic Hedgehog-producing cells. Development 135: 2311–2319 [DOI] [PubMed] [Google Scholar]
- Britsch S, Li L, Kirchhoff S, Theuring F, Brinkmann V, Birchmeier C, Riethmacher D 1998. The ErbB2 and ErbB3 receptors and their ligand, neuregulin-1, are essential for development of the sympathetic nervous system. Genes Dev 12: 1825–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M 2001. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev 15: 66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner-Fraser M 2004. Development. Making sense of the sensory lineage. Science 303: 966–968 [DOI] [PubMed] [Google Scholar]
- Brunet JF, Pattyn A 2002. Phox2 genes—From patterning to connectivity. Curr Opin Genet Dev 12: 435–440 [DOI] [PubMed] [Google Scholar]
- Buchmann-Moller S, Miescher I, John N, Krishnan J, Deng CX, Sommer L 2009. Multiple lineage-specific roles of Smad4 during neural crest development. Dev Biol 330: 329–338 [DOI] [PubMed] [Google Scholar]
- Calloni GW, Le Douarin NM, Dupin E 2009. High frequency of cephalic neural crest cells shows coexistence of neurogenic, melanogenic, and osteogenic differentiation capacities. Proc Natl Acad Sci 106: 8947–8952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA 2000. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2: 76–83 [DOI] [PubMed] [Google Scholar]
- Catron KM, Wang H, Hu G, Shen MM, Abate-Shen C 1996. Comparison of MSX-1 and MSX-2 suggests a molecular basis for functional redundancy. Mech Dev 55: 185–199 [DOI] [PubMed] [Google Scholar]
- Cebra-Thomas JA, Betters E, Yin M, Plafkin C, McDow K, Gilbert SF 2007. Evidence that a late-emerging population of trunk neural crest cells forms the plastron bones in the turtle Trachemys scripta. Evol Dev 9: 267–277 [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM 2000. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development 127: 1671–1679 [DOI] [PubMed] [Google Scholar]
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q 2006. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron 49: 365–377 [DOI] [PubMed] [Google Scholar]
- Chung IH, Han J, Iwata J, Chai Y 2010. Msx1 and Dlx5 function synergistically to regulate frontal bone development. Genesis 48: 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AM 1972. Factors directing the expression of sympathetic nerve traits in cells of neural crest origin. J Exp Zool 179: 167–182 [DOI] [PubMed] [Google Scholar]
- Cohn MJ 2002. Evolutionary biology: Lamprey Hox genes and the origin of jaws. Nature 416: 386–387 [DOI] [PubMed] [Google Scholar]
- Couly G, Creuzet S, Bennaceur S, Vincent C, Le Douarin NM 2002. Interactions between Hox-negative cephalic neural crest cells and the foregut endoderm in patterning the facial skeleton in the vertebrate head. Development 129: 1061–1073 [DOI] [PubMed] [Google Scholar]
- Crane JF, Trainor PA 2006. Neural crest stem and progenitor cells. Annu Rev Cell Dev Biol 22: 267–286 [DOI] [PubMed] [Google Scholar]
- David NB, Saint-Etienne L, Tsang M, Schilling TF, Rosa FM 2002. Requirement for endoderm and FGF3 in ventral head skeleton formation. Development 129: 4457–4468 [DOI] [PubMed] [Google Scholar]
- Day TF, Guo X, Garrett-Beal L, Yang Y 2005. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 8: 739–750 [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B, Lefebvre V, Nakashima K 2001. Regulatory mechanisms in the pathways of cartilage and bone formation. Curr Opin Cell Biol 13: 721–727 [DOI] [PubMed] [Google Scholar]
- Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P 1994. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev 8: 3045–3057 [DOI] [PubMed] [Google Scholar]
- Depew MJ, Liu JK, Long JE, Presley R, Meneses JJ, Pedersen RA, Rubenstein JL 1999. Dlx5 regulates regional development of the branchial arches and sensory capsules. Development 126: 3831–3846 [DOI] [PubMed] [Google Scholar]
- Depew MJ, Simpson CA, Morasso M, Rubenstein JL 2005. Reassessing the Dlx code: The genetic regulation of branchial arch skeletal pattern and development. J Anat 207: 501–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ 2001. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell 106: 619–632 [DOI] [PubMed] [Google Scholar]
- Dottori M, Gross MK, Labosky P, Goulding M 2001. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development 128: 4127–4138 [DOI] [PubMed] [Google Scholar]
- Doupe AJ, Landis SC, Patterson PH 1985. Environmental influences in the development of neural crest derivatives: Glucocorticoids, growth factors, and chromaffin cell plasticity. J Neurosci 5: 2119–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupin E, Calloni GW, Le Douarin NM 2010. The cephalic neural crest of amniote vertebrates is composed of a large majority of precursors endowed with neural, melanocytic, chondrogenic and osteogenic potentialities. Cell Cycle 9: 238–249 [DOI] [PubMed] [Google Scholar]
- Dutton KA, Pauliny A, Lopes SS, Elworthy S, Carney TJ, Rauch J, Geisler R, Haffter P, Kelsh RN 2001. Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates. Development 128: 4113–4125 [DOI] [PubMed] [Google Scholar]
- Epperlein HH 1974. The ectomesenchymal–endodermal interaction-system (EEIS) of Triturus alpestris in tissue culture. I. Observations on attachment, migration and differentiation of neural crest cells. Differentiation 2: 151–168 [DOI] [PubMed] [Google Scholar]
- Finotto S, Krieglstein K, Schober A, Deimling F, Lindner K, Bruhl B, Beier K, Metz J, Garcia-Arraras JE, Roig-Lopez JL, et al. 1999. Analysis of mice carrying targeted mutations of the glucocorticoid receptor gene argues against an essential role of glucocorticoid signalling for generating adrenal chromaffin cells. Development 126: 2935–2944 [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F 1998. The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron 20: 483–494 [DOI] [PubMed] [Google Scholar]
- Fontaine-Perus J, Cheraud Y 2005. Mouse–chick neural chimeras. Int J Dev Biol 49: 349–353 [DOI] [PubMed] [Google Scholar]
- Francis NJ, Landis SC 1999. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci 22: 541–566 [DOI] [PubMed] [Google Scholar]
- Fraser GJ, Cerny R, Soukup V, Bronner-Fraser M, Streelman JT 2010. The odontode explosion: The origin of tooth-like structures in vertebrates. Bioessays 32: 808–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Minaur S, Mavrogiannis LA, Rannan-Eliya SV, Hendry MA, Liston WA, Porteous MEM, Wilkie AOM 2003. Parietal foramina with cleidocranial dysplasia is caused by mutation in MSX2. Eur J Hum Genet 11: 892–895 [DOI] [PubMed] [Google Scholar]
- Gershon MD 1998. V. Genes, lineages, and tissue interactions in the development of the enteric nervous system. Am J Physiol 275: G869–G873 [DOI] [PubMed] [Google Scholar]
- Gilbert SF, Bender G, Betters E, Yin M, Cebra-Thomas JA 2007. The contribution of neural crest cells to the nuchal bone and plastron of the turtle shell. Integr Comp Biol 47: 401–408 [DOI] [PubMed] [Google Scholar]
- Gitton Y, Benouaiche L, Vincent C, Heude E, Soulika M, Bouhali K, Couly G, Levi G 2011. Dlx5 and Dlx6 expression in the anterior neural fold is essential for patterning the dorsal nasal capsule. Development 138: 897–903 [DOI] [PubMed] [Google Scholar]
- Glass DA 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, et al. 2005. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8: 751–764 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Martinez T, Germana GP, Monjil DF, Silos-Santiago I, de Carlos F, Germana G, Cobo J, Vega JA 2004. Absence of Meissner corpuscles in the digital pads of mice lacking functional TrkB. Brain Res 1002: 120–128 [DOI] [PubMed] [Google Scholar]
- Gordon CT, Brinas IM, Rodda FA, Bendall AJ, Farlie PG 2010. Role of Dlxgenes in craniofacial morphogenesis: Dlx2 influences skeletal patterning by inducing ectomesenchymal aggregation in ovo. Evol Dev 12: 459–473 [DOI] [PubMed] [Google Scholar]
- Grammatopoulos GA, Bell E, Toole L, Lumsden A, Tucker AS 2000. Homeotic transformation of branchial arch identity after Hoxa2 overexpression. Development 127: 5355–5365 [DOI] [PubMed] [Google Scholar]
- Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y 2004. Wnt/β-catenin signaling is sufficient and necessary for synovial joint formation. Genes Dev 18: 2404–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall BK 1980. Tissue interactions and the initiation of osteogenesis and chondrogenesis in the neural crest-derived mandibular skeleton of the embryonic mouse as seen in isolated murine tissues and in recombinations of murine and avian tissues. J Embryol Exp Morphol 58: 251–264 [PubMed] [Google Scholar]
- Hall BK 1999. The neural crest in development and evolution. Springer, New York [Google Scholar]
- Han J, Ishii M, Bringas P Jr, Maas RL, Maxson RE Jr, Chai Y 2007. Concerted action of Msx1 and Msx2 in regulating cranial neural crest cell differentiation during frontal bone development. Mech Dev 124: 729–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari L, Brault V, Kleber M, Lee HY, Ille F, Leimeroth R, Paratore C, Suter U, Kemler R, Sommer L 2002. Lineage-specific requirements of β-catenin in neural crest development. J Cell Biol 159: 867–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann C 2006. A Wnt canon orchestrating osteoblastogenesis. Trends Cell Biol 16: 151–158 [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Wang F 2008. Visualizing mechanosensory endings of TrkC-expressing neurons in HS3ST-2–hPLAP mice. J Comp Neurol 511: 543–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy C, Uwanogho D, Sharpe PT 1999. Regulation and role of Sox9 in cartilage formation. Dev Dyn 215: 69–78 [DOI] [PubMed] [Google Scholar]
- Hill RE, Jones PF, Rees AR, Sime CM, Justice MJ, Copeland NG, Jenkins NA, Graham E, Davidson DR 1989. A new family of mouse homeo box-containing genes: Molecular structure, chromosomal location, and developmental expression of Hox-7.1. Genes Dev 3: 26–37 [DOI] [PubMed] [Google Scholar]
- Hill TP, Spater D, Taketo MM, Birchmeier W, Hartmann C 2005. Canonical Wnt/β-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell 8: 727–738 [DOI] [PubMed] [Google Scholar]
- Hirsch MR, Tiveron MC, Guillemot F, Brunet JF, Goridis C 1998. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development 125: 599–608 [DOI] [PubMed] [Google Scholar]
- Howard MJ, Bronner-Fraser M 1986. Neural tube-derived factors influence differentiation of neural crest cells in vitro: Effects on activity of neurotransmitter biosynthetic enzymes. Dev Biol 117: 45–54 [DOI] [PubMed] [Google Scholar]
- Howard MJ, Stanke M, Schneider C, Wu X, Rohrer H 2000. The transcription factor dHAND is a downstream effector of BMPs in sympathetic neuron specification. Development 127: 4073–4081 [DOI] [PubMed] [Google Scholar]
- Hu D, Marcucio RS 2009. A SHH-responsive signaling center in the forebrain regulates craniofacial morphogenesis via the facial ectoderm. Development 136: 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio RS 2012. Neural crest cells pattern the surface cephalic ectoderm during FEZ formation. Dev Dyn 241: 732–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Marcucio RS, Helms JA 2003. A zone of frontonasal ectoderm regulates patterning and growth in the face. Development 130: 1749–1758 [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF 2001. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci 24: 677–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ido A, Ito K 2006. Expression of chondrogenic potential of mouse trunk neural crest cells by FGF2 treatment. Dev Dyn 235: 361–367 [DOI] [PubMed] [Google Scholar]
- Inoue K, Ozaki S, Shiga T, Ito K, Masuda T, Okado N, Iseda T, Kawaguchi S, Ogawa M, Bae SC, et al. 2002. Runx3 controls the axonal projection of proprioceptive dorsal root ganglion neurons. Nat Neurosci 5: 946–954 [DOI] [PubMed] [Google Scholar]
- Inoue K, Shiga T, Ito Y 2008. Runx transcription factors in neuronal development. Neural Dev 3: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii M, Merrill AE, Chan Y-S, Gitelman I, Rice DPC, Sucov HM, Maxson RE 2003. Msx2 and Twist cooperatively control the development of the neural crest-derived skeletogenic mesenchyme of the murine skull vault. Development 130: 6131–6142 [DOI] [PubMed] [Google Scholar]
- Ishii M, Han J, Yen HY, Sucov HM, Chai Y, Maxson RE Jr 2005. Combined deficiencies of Msx1 and Msx2 cause impaired patterning and survival of the cranial neural crest. Development 132: 4937–4950 [DOI] [PubMed] [Google Scholar]
- Jabs EW, Muller U, Li X, Ma L, Luo W, Haworth IS, Klisak I, Sparkes R, Warman ML, Mulliken JB, et al. 1993. A mutation in the homeodomain of the human MSX2 gene in a family affected with autosomal dominant craniosynostosis. Cell 75: 443–450 [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP 2004. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev 18: 937–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Lan Y, Norton CR, Sundberg JP, Gridley T 1998. The Slug gene is not essential for mesoderm or neural crest development in mice. Dev Biol 198: 277–285 [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM 2002. Tissue origins and interactions in the mammalian skull vault. Dev Biol 241: 106–116 [DOI] [PubMed] [Google Scholar]
- John N, Cinelli P, Wegner M, Sommer L 2011. Transforming growth factor β-mediated Sox10 suppression controls mesenchymal progenitor generation in neural crest stem cells. Stem Cells 29: 689–699 [DOI] [PubMed] [Google Scholar]
- Jumlongras D, Bei M, Stimson JM, Wang W-F, DePalma SR, Seidman CE, Felbor U, Maas R, Seidman JG, Olsen BR 2001. A nonsense mutation in MSX1 causes Witkop syndrome. Am J Hum Genet 69: 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzler B, Kuschert SJ, Liu Y-H, Mallo M 1998. Hoxa2 restricts the chondrogenic domain and inhibits bone formation during development of the branchial area. Development 125: 2587–2597 [DOI] [PubMed] [Google Scholar]
- Karsenty G 2008. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet 9: 183–196 [DOI] [PubMed] [Google Scholar]
- Kim HS, Seo H, Yang C, Brunet JF, Kim KS 1998. Noradrenergic-specific transcription of the dopamine β-hydroxylase gene requires synergy of multiple cis-acting elements including at least two Phox2a-binding sites. J Neurosci 18: 8247–8260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lo L, Dormand E, Anderson DJ 2003. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron 38: 17–31 [DOI] [PubMed] [Google Scholar]
- Kleber M, Lee HY, Wurdak H, Buchstaller J, Riccomagno MM, Ittner LM, Suter U, Epstein DJ, Sommer L 2005. Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. J Cell Biol 169: 309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpakova E, Olsen BR 2005. Wnt/β-catenin—A canonical tale of cell-fate choice in the vertebrate skeleton. Dev Cell 8: 626–627 [DOI] [PubMed] [Google Scholar]
- Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, Arber S 2006. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron 49: 379–393 [DOI] [PubMed] [Google Scholar]
- Kulesa P, Ellies DL, Trainor PA 2004. Comparative analysis of neural crest cell death, migration, and function during vertebrate embryogenesis. Dev Dyn 229: 14–29 [DOI] [PubMed] [Google Scholar]
- Le Douarin N, Kalcheim C 1999. The neural crest. Cambridge University Press, Cambridge, UK [Google Scholar]
- Le Douarin N, Teillet M-A 1974. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev Biol 41: 162–184 [DOI] [PubMed] [Google Scholar]
- Lee MH, Kim YJ, Kim HJ, Park HD, Kang AR, Kyung HM, Sung JH, Wozney JM, Ryoo HM 2003. BMP-2-induced Runx2 expression is mediated by Dlx5, and TGF-β1 opposes the BMP-2-induced osteoblast differentiation by suppression of Dlx5 expression. J Biol Chem 278: 34387–34394 [DOI] [PubMed] [Google Scholar]
- Lee HY, Kleber M, Hari L, Brault V, Suter U, Taketo MM, Kemler R, Sommer L 2004. Instructive role of Wnt/β-catenin in sensory fate specification in neural crest stem cells. Science 303: 1020–1023 [DOI] [PubMed] [Google Scholar]
- Lee MH, Kim YJ, Yoon WJ, Kim JI, Kim BG, Hwang YS, Wozney JM, Chi XZ, Bae SC, Choi KY, et al. 2005. Dlx5 specifically regulates Runx2 type II expression by binding to homeodomain-response elements in the Runx2 distal promoter. J Biol Chem 280: 35579–35587 [DOI] [PubMed] [Google Scholar]
- Lei L, Laub F, Lush M, Romero M, Zhou J, Luikart B, Klesse L, Ramirez F, Parada LF 2005. The zinc finger transcription factor Klf7 is required for TrkA gene expression and development of nociceptive sensory neurons. Genes Dev 19: 1354–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Lievre CS, Le Douarin NM 1975. Mesenchymal derivatives of the neural crest: Analysis of chimaeric quail and chick embryos. J Embryol Exp Morphol 34: 125–154 [PubMed] [Google Scholar]
- Lenton K, James AW, Manu A, Brugmann SA, Birker D, Nelson ER, Leucht P, Helms JA, Longaker MT 2011. Indian hedgehog positively regulates calvarial ossification and modulates bone morphogenetic protein signaling. Genesis 49: 784–796 [DOI] [PubMed] [Google Scholar]
- Levanon D, Bettoun D, Harris-Cerruti C, Woolf E, Negreanu V, Eilam R, Bernstein Y, Goldenberg D, Xiao C, Fliegauf M, et al. 2002. The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J 21: 3454–3463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Quarto N, Longaker MT 2010. Activation of FGF signaling mediates proliferative and osteogenic differences between neural crest derived frontal and mesoderm parietal derived bone. PLoS ONE 5: e14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KC, Lakshmanan G, Crawford SE, Gu Y, Grosveld F, Engel JD 2000. Gata3 loss leads to embryonic lethality due to noradrenaline deficiency of the sympathetic nervous system. Nat Genet 25: 209–212 [DOI] [PubMed] [Google Scholar]
- Lo L, Sommer L, Anderson DJ 1997. MASH1 maintains competence for BMP2-induced neuronal differentiation in post-migratory neural crest cells. Curr Biol 7: 440–450 [DOI] [PubMed] [Google Scholar]
- Lo L, Tiveron MC, Anderson DJ 1998. MASH1 activates expression of the paired homeodomain transcription factor Phox2a, and couples pan-neuronal and subtype-specific components of autonomic neuronal identity. Development 125: 609–620 [DOI] [PubMed] [Google Scholar]
- Lo L, Morin X, Brunet JF, Anderson DJ 1999. Specification of neurotransmitter identity by Phox2 proteins in neural crest stem cells. Neuron 22: 693–705 [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R 2004. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Luo W, Wickramasinghe SR, Savitt JM, Griffin JW, Dawson TM, Ginty DD 2007. A hierarchical NGF signaling cascade controls Ret-dependent and Ret-independent events during development of nonpeptidergic DRG neurons. Neuron 54: 739–754 [DOI] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ 1996. Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87: 43–52 [DOI] [PubMed] [Google Scholar]
- Ma Q, Sommer L, Cserjesi P, Anderson DJ 1997. Mash1 and neurogenin1 expression patterns define complementary domains of neuroepithelium in the developing CNS and are correlated with regions expressing notch ligands. J Neurosci 17: 3644–3652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ 1999. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev 13: 1717–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Lei L, Eng SR, Turner E, Parada LF 2003. Brn3a regulation of TrkA/NGF receptor expression in developing sensory neurons. Development 130: 3525–3534 [DOI] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X 2009. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev Cell 17: 9–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie A, Ferguson M, Sharpe P 1991. Hox-7 expression during murine craniofacial development. Development 113: 601–612 [DOI] [PubMed] [Google Scholar]
- Marcucio RS, Cordero DR, Hu D, Helms JA 2005. Molecular interactions coordinating the development of the forebrain and face. Dev Biol 284: 48–61 [DOI] [PubMed] [Google Scholar]
- Marcucio RS, Young NM, Hu D, Hallgrimsson B 2011. Mechanisms that underlie co-variation of the brain and face. Genesis 49: 177–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmigere F, Ernfors P 2007. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat Rev Neurosci 8: 114–127 [DOI] [PubMed] [Google Scholar]
- Maro GS, Vermeren M, Voiculescu O, Melton L, Cohen J, Charnay P, Topilko P 2004. Neural crest boundary cap cells constitute a source of neuronal and glial cells of the PNS. Nat Neurosci 7: 930–938 [DOI] [PubMed] [Google Scholar]
- McEvilly RJ, Erkman L, Luo L, Sawchenko PE, Ryan AF, Rosenfeld MG 1996. Requirement for Brn-3.0 in differentiation and survival of sensory and motor neurons. Nature 384: 574–577 [DOI] [PubMed] [Google Scholar]
- McGonnell IM, Graham A 2002. Trunk neural crest has skeletogenic potential. Curr Biol 12: 767–771 [DOI] [PubMed] [Google Scholar]
- McKeown SJ, Newgreen DF, Farlie PG 2003. Temporal restriction of migratory and lineage potential in rhombomere 1 and 2 neural crest. Dev Biol 255: 62–76 [DOI] [PubMed] [Google Scholar]
- Merrill AE, Sarukhanov A, Krejci P, Idoni B, Camacho N, Estrada KD, Lyons KM, Deixler H, Robinson H, Chitayat D, et al. 2012. Bent bone dysplasia-FGFR2 type, a distinct skeletal disorder, has deficient canonical FGF signaling. Am J Hum Genet 90: 550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoux M, Antonarakis GS, Kmita M, Duboule D, Rijli FM 2009. Rostral and caudal pharyngeal arches share a common neural crest ground pattern. Development 136: 637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiadis TA, Cheraud Y, Sharpe P, Fontaine-Perus J 2003. Development of teeth in chick embryos after mouse neural crest transplantations. Proc Natl Acad Sci 100: 6541–6545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Snider WD 1997. Nerve growth factor receptor TrkA is down-regulated during postnatal development by a subset of dorsal root ganglion neurons. J Comp Neurol 381: 428–438 [DOI] [PubMed] [Google Scholar]
- Mori-Akiyama Y, Akiyama H, Rowitch DH, de Crombrugghe B 2003. Sox9 is required for determination of the chondrogenic cell lineage in the cranial neural crest. Proc Natl Acad Sci 100: 9360–9365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y, Zehir A, Maska E, Deng C, Schneider MD, Mishina Y, Cserjesi P 2009. BMP signaling regulates sympathetic nervous system development through Smad4-dependent and -independent pathways. Development 136: 3575–3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SA, Gridley T 2006. Snail family genes are required for left–right asymmetry determination, but not neural crest formation, in mice. Proc Natl Acad Sci 103: 10300–10304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B 2002. The novel zinc finger–containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108: 17–29 [DOI] [PubMed] [Google Scholar]
- Ng LJ, Wheatley S, Muscat GE, Conway-Campbell J, Bowles J, Wright E, Bell DM, Tam PP, Cheah KS, Koopman P 1997. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol 183: 108–121 [DOI] [PubMed] [Google Scholar]
- Nie X, Deng CX, Wang Q, Jiao K 2008. Disruption of Smad4 in neural crest cells leads to mid-gestation death with pharyngeal arch, craniofacial and cardiac defects. Dev Biol 316: 417–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norr SC 1973. In vitro analysis of sympathetic neuron differentiation from chick neural crest cells. Dev Biol 34: 16–38 [DOI] [PubMed] [Google Scholar]
- Pandolfi PP, Roth ME, Karis A, Leonard MW, Dzierzak E, Grosveld FG, Engel JD, Lindenbaum MH 1995. Targeted disruption of the GATA3 gene causes severe abnormalities in the nervous system and in fetal liver haematopoiesis. Nat Genet 11: 40–44 [DOI] [PubMed] [Google Scholar]
- Paratore C, Goerich DE, Suter U, Wegner M, Sommer L 2001. Survival and glial fate acquisition of neural crest cells are regulated by an interplay between the transcription factor Sox10 and extrinsic combinatorial signaling. Development 128: 3949–3961 [DOI] [PubMed] [Google Scholar]
- Paratore C, Hagedorn L, Floris J, Hari L, Kleber M, Suter U, Sommer L 2002. Cell-intrinsic and cell-extrinsic cues regulating lineage decisions in multipotent neural crest-derived progenitor cells. Int J Dev Biol 46: 193–200 [PubMed] [Google Scholar]
- Partanen J, Schwartz L, Rossant J 1998. Opposite phenotypes of hypomorphic and Y766 phosphorylation site mutations reveal a function for Fgfr1 in anteroposterior patterning of mouse embryos. Genes Dev 12: 2332–2344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualetti M, Ori M, Nardi I, Rijli FM 2000. Ectopic Hoxa2 induction after neural crest migration results in homeosis of jaw elements in Xenopus. Development 127: 5367–5378 [DOI] [PubMed] [Google Scholar]
- Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF 1999. The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399: 366–370 [DOI] [PubMed] [Google Scholar]
- Pattyn A, Goridis C, Brunet JF 2000. Specification of the central noradrenergic phenotype by the homeobox gene Phox2b. Mol Cell Neurosci 15: 235–243 [DOI] [PubMed] [Google Scholar]
- Perez-Pinera P, Garcia-Suarez O, Germana A, Diaz-Esnal B, de Carlos F, Silos-Santiago I, del Valle ME, Cobo J, Vega JA 2008. Characterization of sensory deficits in TrkB knockout mice. Neurosci Lett 433: 43–47 [DOI] [PubMed] [Google Scholar]
- Piotrowski T, Nusslein-Volhard C 2000. The endoderm plays an important role in patterning the segmented pharyngeal region in zebrafish (Danio rerio). Dev Biol 225: 339–356 [DOI] [PubMed] [Google Scholar]
- Potrebic S, Ahn AH, Skinner K, Fields HL, Basbaum AI 2003. Peptidergic nociceptors of both trigeminal and dorsal root ganglia express serotonin 1D receptors: Implications for the selective antimigraine action of triptans. J Neurosci 23: 10988–10997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissmann E, Ernsberger U, Francis-West PH, Rueger D, Brickell PM, Rohrer H 1996. Involvement of bone morphogenetic protein-4 and bone morphogenetic protein-7 in the differentiation of the adrenergic phenotype in developing sympathetic neurons. Development 122: 2079–2088 [DOI] [PubMed] [Google Scholar]
- Remboutsika E, Elkouris M, Iulianella A, Andoniadou CL, Poulou M, Mitsiadis TA, Trainor PA, Lovell-Badge R 2011. Flexibility of neural stem cells. Front Physiol 2: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijli FM, Mark M, Lakkaraju S, Dierich A, Dolle P, Chambon P 1993. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell 75: 1333–1349 [DOI] [PubMed] [Google Scholar]
- Robledo RF, Rajan L, Li X, Lufkin T 2002. The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev 16: 1089–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychlik JL, Gerbasi V, Lewis EJ 2003. The interaction between dHAND and Arix at the dopamine β-hydroxylase promoter region is independent of direct dHAND binding to DNA. J Biol Chem 278: 49652–49660 [DOI] [PubMed] [Google Scholar]
- Sarkar S, Petiot A, Copp A, Ferretti P, Thorogood P 2001. FGF2 promotes skeletogenic differentiation of cranial neural crest cells. Development 128: 2143–2152 [DOI] [PubMed] [Google Scholar]
- Satokata I, Maas R 1994. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet 6: 348–356 [DOI] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, Maeda T, Takano Y, Uchiyama M, Heaney S, et al. 2000. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat Genet 24: 391–395 [DOI] [PubMed] [Google Scholar]
- Schilling TF, Kimmel CB 1994. Segment and cell type lineage restrictions during pharyngeal arch development in the zebrafish embryo. Development 120: 483–494 [DOI] [PubMed] [Google Scholar]
- Schneider RA, Helms JA 2003. The cellular and molecular origins of beak morphology. Science 299: 565–568 [DOI] [PubMed] [Google Scholar]
- Schneider C, Wicht H, Enderich J, Wegner M, Rohrer H 1999. Bone morphogenetic proteins are required in vivo for the generation of sympathetic neurons. Neuron 24: 861–870 [DOI] [PubMed] [Google Scholar]
- Scott A, Hasegawa H, Sakurai K, Yaron A, Cobb J, Wang F 2011. Transcription factor short stature homeobox 2 is required for proper development of tropomyosin-related kinase B-expressing mechanosensory neurons. J Neurosci 31: 6741–6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton M, Sanchez S, Nieto MA 1998. Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development 125: 3111–3121 [DOI] [PubMed] [Google Scholar]
- Selleck MA, Bronner-Fraser M 1995. Origins of the avian neural crest: The role of neural plate-epidermal interactions. Development 121: 525–538 [DOI] [PubMed] [Google Scholar]
- Semba I, Nonaka K, Takahashi I, Takahashi K, Dashner R, Shum L, Nuckolls GH, Slavkin HC 2000. Positionally-dependent chondrogenesis induced by BMP4 is co-regulated by Sox9 and Msx2. Dev Dyn 217: 401–414 [DOI] [PubMed] [Google Scholar]
- Shah NM, Groves AK, Anderson DJ 1996. Alternative neural crest cell fates are instructively promoted by TGFβ superfamily members. Cell 85: 331–343 [DOI] [PubMed] [Google Scholar]
- Shimizu S, Ichikawa H, Nakagawa H, Kiyomiya K, Matsuo S 2007. Effect of BDNF depletion on the formation of Ruffini endings in vibrissa follicles and the survival of their mechanoreceptive neurons in trigeminal ganglion. Brain Res 1154: 95–104 [DOI] [PubMed] [Google Scholar]
- Snider WD, McMahon SB 1998. Tackling pain at the source: New ideas about nociceptors. Neuron 20: 629–632 [DOI] [PubMed] [Google Scholar]
- Snider WD, Wright DE 1996. Neurotrophins cause a new sensation. Neuron 16: 229–232 [DOI] [PubMed] [Google Scholar]
- Stanke M, Junghans D, Geissen M, Goridis C, Ernsberger U, Rohrer H 1999. The Phox2 homeodomain proteins are sufficient to promote the development of sympathetic neurons. Development 126: 4087–4094 [DOI] [PubMed] [Google Scholar]
- Takio Y, Pasqualetti M, Kuraku S, Hirano S, Rijli FM, Kuratani S 2004. Evolutionary biology: Lamprey Hox genes and the evolution of jaws. Nature 429: 263. [DOI] [PubMed] [Google Scholar]
- Teillet MA, Le Douarin NM 1983. Consequences of neural tube and notochord excision on the development of the peripheral nervous system in the chick embryo. Dev Biol 98: 192–211 [DOI] [PubMed] [Google Scholar]
- Tokita M, Schneider RA 2009. Developmental origins of species-specific muscle pattern. Dev Biol 331: 311–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor PA 2003. Making headway: The roles of Hox genes and neural crest cells in craniofacial development. ScientificWorldJournal 3: 240–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor PA 2005. Specification of neural crest cell formation and migration in mouse embryos. Semin Cell Dev Biol 16: 683–693 [DOI] [PubMed] [Google Scholar]
- Trainor P, Krumlauf R 2000. Plasticity in mouse neural crest cells reveals a new patterning role for cranial mesoderm. Nat Cell Biol 2: 96–102 [DOI] [PubMed] [Google Scholar]
- Trainor PA, Krumlauf R 2001. Hox genes, neural crest cells and branchial arch patterning. Curr Opin Cell Biol 13: 698–705 [DOI] [PubMed] [Google Scholar]
- Trainor PA, Melton KR, Manzanares M 2003. Origins and plasticity of neural crest cells and their roles in jaw and craniofacial evolution. Int J Dev Biol 47: 541–553 [PubMed] [Google Scholar]
- Tran TH, Jarrell A, Zentner GE, Welsh A, Brownell I, Scacheri PC, Atit R 2010. Role of canonical Wnt signaling/ss-catenin via Dermo1 in cranial dermal cell development. Development 137: 3973–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trokovic R, Trokovic N, Hernesniemi S, Pirvola U, Vogt Weisenhorn DM, Rossant J, McMahon AP, Wurst W, Partanen J 2003. FGFR1 is independently required in both developing mid- and hindbrain for sustained response to isthmic signals. EMBO J 22: 1811–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarovina K, Pattyn A, Stubbusch J, Muller F, van der Wees J, Schneider C, Brunet JF, Rohrer H 2004. Essential role of Gata transcription factors in sympathetic neuron development. Development 131: 4775–4786 [DOI] [PubMed] [Google Scholar]
- Unsicker K 1973. Fine structure and innervation of the avian adrenal gland. I. Fine structure of adrenal chromaffin cells and ganglion cells. Z Zellforsch Mikrosk Anat 145: 389–416 [DOI] [PubMed] [Google Scholar]
- Unsicker K, Habura-Fluh O, Zwarg U 1978a. Different types of small granule-containing cells and neurons in the guinea-pig adrenal medulla. Cell Tissue Res 189: 109–130 [DOI] [PubMed] [Google Scholar]
- Unsicker K, Krisch B, Otten U, Thoenen H 1978b. Nerve growth factor-induced fiber outgrowth from isolated rat adrenal chromaffin cells: Impairment by glucocorticoids. Proc Natl Acad Sci 75: 3498–3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Putte T, Maruhashi M, Francis A, Nelles L, Kondoh H, Huylebroeck D, Higashi Y 2003. Mice lacking ZFHX1B, the gene that codes for Smad-interacting protein-1, reveal a role for multiple neural crest cell defects in the etiology of Hirschsprung disease-mental retardation syndrome. Am J Hum Genet 72: 465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varley JE, Maxwell GD 1996. BMP-2 and BMP-4, but not BMP-6, increase the number of adrenergic cells which develop in quail trunk neural crest cultures. Exp Neurol 140: 84–94 [DOI] [PubMed] [Google Scholar]
- Varley JE, Wehby RG, Rueger DC, Maxwell GD 1995. Number of adrenergic and islet-1 immunoreactive cells is increased in avian trunk neural crest cultures in the presence of human recombinant osteogenic protein-1. Dev Dyn 203: 434–447 [DOI] [PubMed] [Google Scholar]
- Veistinen L, Aberg T, Rice DP 2009. Convergent signalling through Fgfr2 regulates divergent craniofacial morphogenesis. J Exp Zool B Mol Dev Evol 312B: 351–360 [DOI] [PubMed] [Google Scholar]
- Veistinen L, Takatalo M, Tanimoto Y, Kesper DA, Vortkamp A, Rice DP 2012. Loss-of-function of Gli3 in mice causes abnormal frontal bone morphology and premature synostosis of the interfrontal suture. Front Physiol 3: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickaryous MK, Sire JY 2009. The integumentary skeleton of tetrapods: Origin, evolution, and development. J Anat 214: 441–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitelli F, Zhang Z, Huynh T, Sobotka A, Mupo A, Baldini A 2006. Fgf8 expression in the Tbx1 domain causes skeletal abnormalities and modifies the aortic arch but not the outflow tract phenotype of Tbx1 mutants. Dev Biol 295: 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KS, Weston JA 1990. The sympathoadrenal lineage in avian embryos. I. Adrenal chromaffin cells lose neuronal traits during embryogenesis. Dev Biol 139: 1–12 [DOI] [PubMed] [Google Scholar]
- Wada H, Holland PW, Sato S, Yamamoto H, Satoh N 1997. Neural tube is partially dorsalized by overexpression of HrPax-37: The ascidian homologue of Pax-3 and Pax-7. Dev Biol 187: 240–252 [DOI] [PubMed] [Google Scholar]
- Wagner T, Wirth J, Meyer J, Zabel B, Held M, Zimmer J, Pasantes J, Bricarelli FD, Keutel J, Hustert E, et al. 1994. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79: 1111–1120 [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Maynard TM, Weston JA 2000. Fate determination of neural crest cells by NOTCH-mediated lateral inhibition and asymmetrical cell division during gangliogenesis. Development 127: 2811–2821 [DOI] [PubMed] [Google Scholar]
- Walshe J, Mason I 2000. Expression of FGFR1, FGFR2 and FGFR3 during early neural development in the chick embryo. Mech Dev 90: 103–110 [DOI] [PubMed] [Google Scholar]
- Walshe J, Mason I 2003. Fgf signalling is required for formation of cartilage in the head. Dev Biol 264: 522–536 [DOI] [PubMed] [Google Scholar]
- Wilke TA, Gubbels S, Schwartz J, Richman JM 1997. Expression of fibroblast growth factor receptors (FGFR1, FGFR2, FGFR3) in the developing head and face. Dev Dyn 210: 41–52 [DOI] [PubMed] [Google Scholar]
- Wilkie AO, Tang Z, Elanko N, Walsh S, Twigg SR, Hurst JA, Wall SA, Chrzanowska KH, Maxson RE Jr 2000. Functional haploinsufficiency of the human homeobox gene MSX2 causes defects in skull ossification. Nat Genet 24: 387–390 [DOI] [PubMed] [Google Scholar]
- Wurdak H, Ittner LM, Lang KS, Leveen P, Suter U, Fischer JA, Karlsson S, Born W, Sommer L 2005. Inactivation of TGFβ signaling in neural crest stem cells leads to multiple defects reminiscent of DiGeorge syndrome. Genes Dev 19: 530–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Firulli AB, Zhang X, Howard MJ 2003. HAND2 synergistically enhances transcription of dopamine-β-hydroxylase in the presence of Phox2a. Dev Biol 262: 183–193 [DOI] [PubMed] [Google Scholar]
- Yamagishi C, Yamagishi H, Maeda J, Tsuchihashi T, Ivey K, Hu T, Srivastava D 2006. Sonic hedgehog is essential for first pharyngeal arch development. Pediatr Res 59: 349–354 [DOI] [PubMed] [Google Scholar]
- Yang C, Kim HS, Seo H, Kim CH, Brunet JF, Kim KS 1998. Paired-like homeodomain proteins, Phox2a and Phox2b, are responsible for noradrenergic cell-specific transcription of the dopamine β-hydroxylase gene. J Neurochem 71: 1813–1826 [DOI] [PubMed] [Google Scholar]
- Yoshida T, Vivatbutsiri P, Morriss-Kay G, Saga Y, Iseki S 2008. Cell lineage in mammalian craniofacial mesenchyme. Mech Dev 125: 797–808 [DOI] [PubMed] [Google Scholar]
- Zellmer E, Zhang Z, Greco D, Rhodes J, Cassel S, Lewis EJ 1995. A homeodomain protein selectively expressed in noradrenergic tissue regulates transcription of neurotransmitter biosynthetic genes. J Neurosci 15: 8109–8120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Zheng Q, Engin F, Munivez E, Chen Y, Sebald E, Krakow D, Lee B 2006. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proc Natl Acad Sci 103: 19004–19009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilian O, Saner C, Hagedorn L, Lee HY, Sauberli E, Suter U, Sommer L, Aguet M 2001. Multiple roles of mouse Numb in tuning developmental cell fates. Curr Biol 11: 494–501 [DOI] [PubMed] [Google Scholar]
- Zirlinger M, Lo L, McMahon J, McMahon AP, Anderson DJ 2002. Transient expression of the bHLH factor neurogenin-2 marks a subpopulation of neural crest cells biased for a sensory but not a neuronal fate. Proc Natl Acad Sci 99: 8084–8089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylka MJ, Dong X, Southwell AL, Anderson DJ 2003. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci 100: 10043–10048 [DOI] [PMC free article] [PubMed] [Google Scholar]