Abstract
Cell polarity is fundamental for the architecture and function of epithelial tissues. Epithelial polarization requires the intervention of several fundamental cell processes, whose integration in space and time is only starting to be elucidated. To understand what governs the building of epithelial tissues during development, it is essential to consider the polarization process in the context of the whole tissue. To this end, the development of three-dimensional organotypic cell culture models has brought new insights into the mechanisms underlying the establishment and maintenance of higher-order epithelial tissue architecture, and in the dynamic remodeling of cell polarity that often occurs during development of epithelial organs. Here we discuss some important aspects of mammalian epithelial morphogenesis, from the establishment of cell polarity to epithelial tissue generation.
Epithelial cell membranes have different functional domains. Three main groups of proteins establish and maintain this polarity: the Crumbs (CRB)/PALS1/PATJ complex, the PAR system, and the Scribble (Scrib) module.
1. INTRODUCTION
Epithelia are cohesive sheets of cells lining exterior and interior surfaces of our bodies, constituting a selective barrier between the body and its environment. Some of our major organs, such as kidneys, lung, mammary gland, and liver, also contain hollow spaces—or lumens—lined by simple or stratified epithelial layers that selectively permit the exchange of nutrients, hormones, gases, and cells between different parts of the body. Those glandular organs are made of two kinds of building units: spherical cysts (also named acini in the mammary gland, alveoli in the lung, or follicles in the thyroid) and elongated tubules (or ducts) that assemble into complex branched tubular structures (O’Brien et al. 2002).
To achieve their specific functions, epithelial cells divide their plasma membrane into structurally and functionally different domains. Apical membranes line the lumen and constitute an exchange interface with other parts of the body. They contain most of the proteins necessary for the specific functions of organs, such as secretion. The lateral and basal surfaces interact with surrounding extracellular milieu and communicate with contacting epithelial cells and stromal cells. The unique functions of apical and basolateral membrane domains depend on oriented vesicle trafficking pathways that specifically segregate proteins and lipids into the domain in which they are required. Establishment of epithelial polarity is closely linked to the establishment of the apical junctional complex (AJC), which includes the tight junctions (also named zonula occludens) and adherens junctions (or zonula adherens). Maintenance of each domain identity is ensured by tight junctions, which are composed of three families of transmembrane proteins: occludin, claudins, and junctional adhesion molecules (JAM). These proteins are organized into a tight seal that prevents the diffusion of proteins and outer leaflet lipids between apical and lateral surfaces, and constitute an important selective barrier regulating the diffusion of molecules through the paracellular space. Basal to the tight junctions, adherens junctions form an adhesive belt that encircles each epithelial cell just underneath the apical surface. Adherens junction transmembrane components include cadherins, nectins, and nectin-like molecules, which provide cohesion between cells of the epithelial sheet (Shin et al. 2006; Wang and Margolis 2007; Martin-Belmonte and Perez-Moreno 2012).
Three evolutionary conserved groups of proteins play a major role in the establishment and maintenance of polarity in epithelial cells: the Crumbs (CRB)/PALS1/PATJ complex, the PAR system, and the Scribble (Scrib) module. Cross-regulation between members of the three groups leads to the segregation of each member to its appropriate apical or basal territory, a prerequisite for cell polarization (Fig. 1) (Nelson 2003; Goldstein and Macara 2007; St Johnston and Ahringer 2010). Although these proteins have long been known to participate in the establishment of apical–basal asymmetry, their role in the complex processes of epithelial morphogenesis, such as polarization of the cytoskeleton and membrane organelles, apical and basolateral membrane generation, and lumen formation, is just emerging.
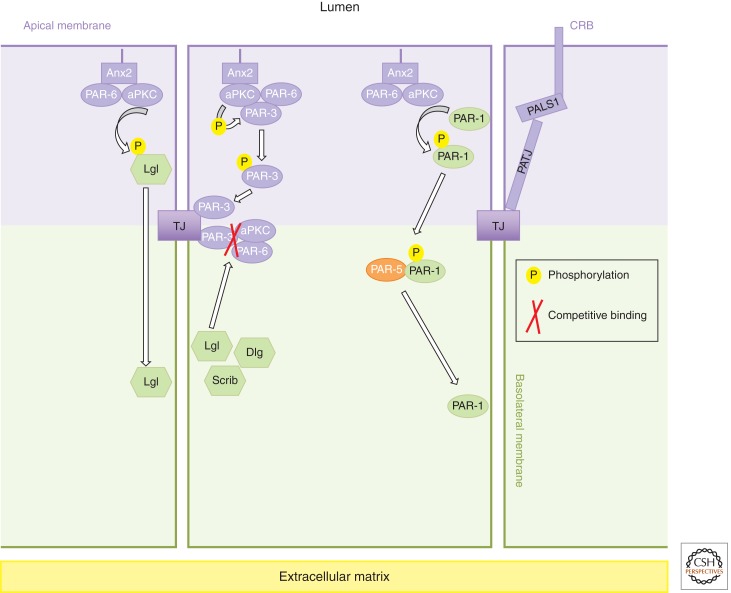
Figure 1.
Polarity complexes. The apical domain (purple) is specified by the Crumbs (CRB)/PALS1/PATJ complex. The mammalian CRB is an integral membrane protein whose intracellular domain contains conserved PDZ-binding and FERM-binding motifs. CRB3 localizes to the apical membrane of epithelial cells and is concentrated at tight junctions where it interacts with the PDZ domain of the cytoplasmic adaptor PALS1. One of the two L27 domains of PALS1 mediates the binding to PATJ, a multiple PDZ-domain-containing protein. PDZ domains of PATJ interact with tight junction (TJ) proteins such as claudins and zonula occludens-3 (ZO-3). The PAR (partitioning-defective) system in mammals contains three serine/threonine kinases (aPKC, PAR-1, and PAR-4), two PDZ-domain-containing scaffold proteins (PAR-3 and PAR-6), and a 14-3-3 protein (PAR-5). Cdc42, PAR6, aPKC (atypical protein kinase C) interact with each other and form a functional unit that localizes apically, whereas PAR-3 defines the apical–basal border. PAR-1 localizes to and defines basolateral membranes. The Scribble (Scrib) module, consisting of Scrib, Dlg, and Lgl proteins, acts as a determinant of the lateral membrane domain. Although Scrib and Lgl2 have been shown to physically interact in one model of polarized mammalian epithelial cells and in Drosophila epithelial cells, it is not clear if the three members of this complex might interact and act as a functional unit in other cell types. However, they depend on each other for correct subcellular localization. Studies in model organisms and mammalian cells revealed that mutually antagonistic interactions and phosphorylations between members of polarity complexes are fundamental for the formation of nonoverlapping apical and basolateral domains. In mammalian cells, aPKC phosphorylates and excludes Lgl and PAR-1 that diffused into the apical domain. Phosphorylation of PAR-1 induces its interaction with PAR-5 and its release into the cytoplasm. Inversely, restriction of PAR-6/aPKC complex at the apical membrane may involve a competition between PAR-3 and Lgl to bind the PAR-6/aPKC complex.
In vitro studies of epithelial monolayers generated important conceptual information about cell processes and molecular pathways required for cell polarization. However, the morphology of epithelial cells grown on plastic dishes differs considerably from the highly polarized morphology of epithelial cells in vivo. Also, cell–cell and cell–matrix adhesions, gene expression, and orchestration of signaling pathways are dramatically affected in the absence of a three-dimensional (3D) microenvironment. For example, mammary epithelial cells that are cultured on two-dimensional (2D) plastic fail to form acinar-like structures and lose tissue-specific milk protein expression. On the contrary, culturing those cells in 3D laminin-rich extracellular matrix (ECM) gels results in a morphology similar to in vivo acini, and restores several mammary-specific functions (Xu et al. 2009). Thus, efforts have been made during the last two decades to produce cell models more representative of physiological 3D cellular environments. 3D organotypic cell culture models of epithelial cells have been shown to recapitulate the key features of in vivo glandular epithelial morphogenesis. Indeed, when grown in appropriate 3D extracellular matrices, such as matrigel or collagen, epithelial cells are able to interpret signals originating from the matrix and neighboring cells to establish an axis of polarization and generate lumen-containing spherical structures resembling the in vivo architecture of tissues (Griffith and Swartz 2006; Yamada and Cukierman 2007). In addition, those structures are able to extend tubules, in a process mimicking in vivo tubulogenesis, in response to specific factors, such as hepatocyte growth factor to Madin-Darby canine kidney (MDCK) cysts (O’Brien et al. 2002). These observations together with the fact that cellular and molecular biology tools (i.e., antibody inhibition, cDNA overexpression, RNA interference, and high-resolution imaging) can be applied to those models makes 3D cell cultures powerful systems to decipher the molecular and cellular aspects of epithelial morphogenesis in a biologically relevant context.
One fundamental aspect of epithelial morphogenesis is how the polarity of each individual cell in a tissue is coordinated to generate specific tissue geometry and function. Some answers were obtained by studying how nonpolarized cells are able to coordinately orientate their axis of symmetry when completely surrounded by an isotropic ECM to form a lumen. Another important problem in epithelial morphogenesis, which can be appreciated with 3D culture models, is the molecular pathways regulating lumen formation and maintenance. During development, lumens can arise from an already polarized epithelium—or primordium—by wrapping or budding (reviewed in Lubarsky and Krasnow 2003; Andrew and Ewald 2010), or from nonpolarized precursors, sometimes after several cycles of polarization, depolarization, and repolarization (Lubarsky and Krasnow 2003; Bryant and Mostov 2008; Andrew and Ewald 2010). Study of de novo lumen formation using the 3D MDCK cell culture model notably revealed that cyst can switch between different mechanisms of lumen formation (hollowing and cavitation), depending on the extracellular context (Martin-Belmonte et al. 2008; Datta et al. 2011).
2. INTIMATE LINK BETWEEN POLARITY COMPLEXES AND ADHESION COMPLEXES ESTABLISHES EPITHELIAL POLARITY
When searching for the molecular mechanisms leading to cell polarization, a central problem is the nature of the initiating polarity cue. A current, but still imprecise, model is that initiation of cell–cell contacts triggers the recruitment of polarity proteins. Then, complex interplay between polarity proteins generates molecular asymmetry along the apical–basal axis and regulates the maturation and maintenance of the AJC to reinforce cell polarization. The importance of polarity proteins for AJC formation is emphasized by the observation that disruption of any member of the PAR, CRB, or Scrib complexes leads to defects in tight junction formation (Bilder and Perrimon 2000; Joberty et al. 2000; Suzuki et al. 2001; Yamanaka et al. 2001; Hirose et al. 2002; Yamanaka et al. 2003; Lemmers et al. 2004; Michel et al. 2005; Qin et al. 2005; Shin et al. 2005; Ivanov et al. 2010; Van Campenhout et al. 2011). However, the precise hierarchy of recruitment and interplay between polarity proteins and AJC components during epithelial polarization remains poorly understood. Polarity proteins contain several protein–protein interaction domains; thus, these proteins likely act by recruiting multiprotein signaling complexes necessary for maturation of cell–cell adhesions.
Primordial cell–cell adhesions—resembling spotlike adherens junctions—are initiated by the contact of membrane protrusions extended from neighbor cells. The contact surface is then expanded through Rac-dependent actin polymerization and myosin II-driven contraction of actin bundles along the peripheral cortex (Vasioukhin et al. 2000; Vaezi et al. 2002; Yamada and Nelson 2007; Baum and Georgiou 2011). Generation of these adhesions involves the sequential recruitment of adherent junctions and tight junction components. Cell–cell contacts are engaged primarily by the nectin family of adhesion receptors, which then recruit E-cadherin and JAM-A to form adherens junctions, and next recruit claudins apically to adherens junction sites to form tight junctions (Ooshio et al. 2007; Sakisaka et al. 2007).
PAR-3 is recruited early to nectin adhesion complexes where it recruits afadin and is required for adherens junctions and tight junction formation (Ooshio et al. 2007). Members of the Scrib complex, Scrib and Dlg, are recruited to the basolateral membrane by E-cadherin and participate in E-cadherin-mediated adhesion (Laprise et al. 2004; Navarro et al. 2005). E-cadherin-mediated adhesion may also be promoted by PALS1, which enhances targeted delivery of E-cadherin at cell–cell contacts (Wang et al. 2007), and by aPKC, which is involved in maintenance of E-cadherin at the cell surface (Sato et al. 2011). This observation may suggest that early cell–cell contacts induce recruitment and activation of polarity proteins, which in turn promotes membrane delivery of adherent junction components to reinforce adhesion, which promotes further polarization.
Local activation and inactivation of Rho GTPases control polarity in various cellular models. In epithelial cells, evidence suggests that a complex interplay between polarity proteins and RhoGTPases regulates AJC formation. For instance, Cdc42 and Rac1 are locally activated at initial cell–cell contacts (Yap and Kovacs 2003) and activate the PAR-6/aPKC complex through binding of active Cdc42 and Rac1 to PAR-6 (Lin et al. 2000). aPKC activation is required for the maturation of tight junctions (Suzuki et al. 2002). Another binding partner of PAR-6 that might also be involved in junction maturation is the GTPase exchange factor ECT2 (epithelial cell transforming sequence 2). Coexpression of PAR6 and ECT2 activates Cdc42 in vivo and ECT2 increases the kinase activity of aPKC (Liu et al. 2004). PAR-3 binds the Rac GTPase exchange factor TIAM1 (T lymphoma invasion and metastasis-inducing protein 1) to regulate tight junction formation. Although there are conflicting results concerning the mechanism involved, this suggests a new role of the PAR complex in actin polymerization (Chen and Macara 2005; Mertens et al. 2005; Nishimura et al. 2005).
3. GENERATION OF APICAL AND BASOLATERAL MEMBRANES
Once cortical asymmetry is initiated by cell–cell contacts and recruitment of polarity proteins, which mechanisms lead to the establishment of mature apical and basolateral membrane domains? Although the answer remains elusive, the establishment of nonoverlapping apical and basolateral domains appears to depend on reciprocal exclusion mechanisms between polarity complexes (Fig. 1) (Goldstein and Macara 2007; St Johnston and Ahringer 2010). This is illustrated by the fact that loss of CRB3 or aPKC, as well as overexpression of Lgl, leads to expansion of basolateral markers to the apical domain (Chalmers et al. 2005). Conversely, overexpression of Crb3 leads to apical domain expansion (Roh et al. 2003). Exclusion of PAR-3 from the apical PAR-6/aPKC complex and its restriction to tight junctions at later stages of polarization is believed to limit the expansion of the basolateral domain (Martin-Belmonte et al. 2007; Morais-de-Sá et al. 2010; Walther and Pichaud 2010). This restriction involves on the one hand a competition between Lgl and PAR-3 to bind the PAR-6/aPKC complex (Yamanaka et al. 2003, 2006), and on the other hand, the phosphorylation of PAR-3 by aPKC, which inhibits its interaction with aPKC (Nagai-Tamai et al. 2002). Phosphorylation of Lgl and PAR-1 by aPKc is required to restrict their activity to the basolateral membrane (Yamanaka et al. 2003; Hurov et al. 2004; Suzuki et al. 2004). Further studies are required to reveal in more detail how polarity proteins cooperate or antagonize each other to establish membrane asymmetry.
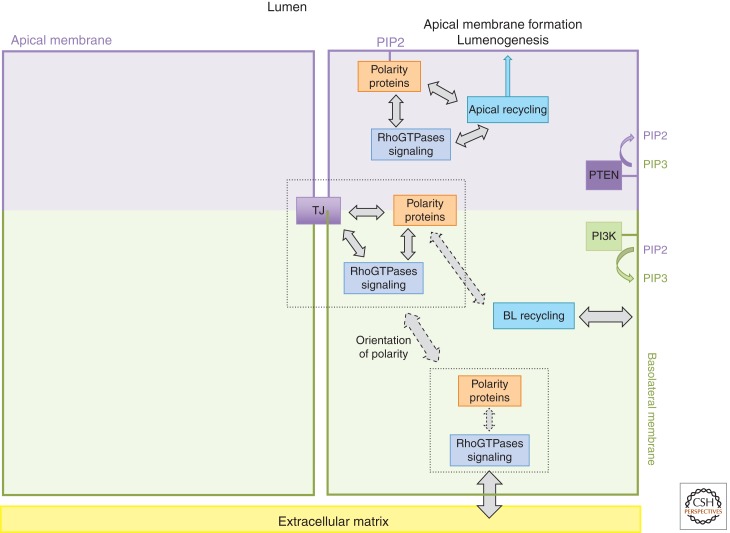
The unique functions of the apical and basolateral membrane domains rely on distinct protein and lipid compositions. Vesicle trafficking machineries play a fundamental role in the establishment of each membrane, by transporting lipids and proteins between different subcellular compartments and the cell surface. Most epithelial cells use biosynthetic sorting from the trans-Golgi network (TGN) as well as selective recycling/transcytosis to transport proteins to the correct surface. Different sorting motifs and cellular machineries are involved to sort proteins either to the apical or to the basolateral membranes (Mostov et al. 2003; Mellman and Nelson 2008). Little is known about how asymmetric partitioning of polarity determinants may control oriented vesicular trafficking pathways in epithelial cells. It is likely that polarity complexes interact in a direct and/or indirect manner with specific components of the trafficking machinery (Fig. 2). Thus, enrichment of polarity proteins into particular epithelial cell domains may orient the delivery of apical and basolateral proteins to their appropriate cortical domain.
Figure 2.
Major players and their interactions during establishment of epithelial polarity. This schematic highlights existing and hypothetical (dashed arrows) connections between cell–cell and cell–matrix interactions, polarity complexes, and oriented vesicular trafficking during establishment of polarity. Initiation of spatial asymmetry and orientation of the apical–basal axis involve, on one hand, a complex interplay between tight junctions (TJs), polarity proteins, and RhoGTPase signaling, and on the other hand, an interplay between extracellular matrix (ECM) signaling, ECM receptors, and RhoGTPase signaling. It is likely that the establishment of an apical–basal axis depends on a cross talk between cell–cell and cell–ECM junctions, possibly partly mediated by RhoGTPase signaling. Later, oriented vesicular trafficking generates apical and basolateral membranes and the lumen. This involves cooperation between trafficking machineries, polarity proteins, RhoGTPases, and membrane lipid composition.
Recent studies have provided some insights into how polarity pathways and vesicular trafficking pathways may be integrated to give rise to the fully polarized epithelial phenotype. Phospholipids regulate both endocytic and exocytic processes (Balla et al. 2009). For instance, phosphatidylinositol-(4,5)-bisphosphate (PIP2) controls targeting of the exocyst to the plasma membrane (He et al. 2007; Liu et al. 2007a), and SNARE-dependent vesicle fusion (Aoyagi et al. 2005; James et al. 2008). A possible link between cortical polarity and oriented trafficking may be the generation of an asymmetric apical–basolateral repartition of phospholipids on the cytosolic side of the plasma membrane (St Johnston and Ahringer 2010).
Phosphatidylinositol phosphates have recently emerged as crucial determinants of apical and basolateral membrane identities and regulators of polarization in epithelial cells (Shewan et al. 2011). Studies of MDCK cysts revealed that PIP2 becomes enriched at the apical membrane domain delimiting the lumen during cyst formation. The importance of PIP2 in generating the apical surface is emphasized by the finding that exogenous insertion of PIP2 in the basolateral membrane of mature MDCK cysts induces relocalization of apical and tight junction components into the basolateral plasma membrane (Martin-Belmonte et al. 2007). PIP2 is a central determinant of apical identity by recruiting annexin 2 to the apical domain, which subsequently recruits Cdc42 to the apical plasma membrane. Apical Cdc42 binds and activates the PAR-6/aPKC complex, thereby promoting polarization (Martin-Belmonte et al. 2007). The lipid phosphatase PTEN (phosphatase and tensin homolog on chromosome 10) generates PIP2 by removing the phosphate at the third position on phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) (Maehama and Dixon 1998). PTEN thus functionally antagonizes phosphatidylinositol-3 kinase (PI3K), which increases the level of PIP3 by converting PIP2 to PIP3. PTEN strongly localizes to the apical membrane domain during cell polarization and lumen formation of MDCK cysts. Inhibition of its activity impairs PIP2 and PIP3 segregation and disrupts lumen formation and cyst architecture (Martin-Belmonte et al. 2007). In contrast, PIP3 is restricted to and specifies the basolateral surfaces of epithelial cells. This is supported by the abnormally short lateral surfaces observed when MDCK cells or intestinal epithelial cells (Caco-2/15) are grown in the presence of a PI3K inhibitor (Laprise et al. 2002; Gassama-Diagne et al. 2006). Furthermore, exogenous insertion of PIP3 into the apical plasma membrane rapidly transforms the apical surface into basolateral surface (Gassama-Diagne et al. 2006). It is important to note, however, that the partition of PIP2 and PIP3 in MDCK cells may not be true for all epithelial cells (Pinal et al. 2006), and also that other phosphoinositides and lipid phosphatases and kinases may also play a role in epithelial polarity (Datta et al. 2011; Shewan et al. 2011).
It is not clear how phosphoinositide asymmetry arises. Initial segregation between PIP2 and PIP3 is probably dependent on the recruitment and activation of PTEN and PI3K at the apical and basolateral membranes, respectively. How and when PTEN and PI3K become enriched to their specific cortical location during cyst morphogenesis is not clear. PTEN may be recruited to cell–cell junctions during their establishment by E-cadherin, and PAR-3-dependent recruitment of PTEN to cell–cell junctions is important for polarization of MDCK cells (Wu et al. 2007; Feng et al. 2008; Fournier et al. 2009). PI3K may be recruited to the basal surface following laminin signaling at the basal membrane, as PI3K is recruited and activated at the basal membrane of mammary cells when embedded in laminin-rich ECM (Xu et al. 2010). In addition, PI3K may be recruited and activated by Dlg at lateral membranes during assembly of E-cadherin-dependent cell–cell adhesions (Laprise et al. 2004).
Besides phosphoinositides, another type of lipid, glycosphingolipid, may control apical membrane generation. Glycosphingolipid has long been proposed to control apical protein sorting by forming lipid rafts with cholesterol (Simons and Ikonen 1997). However, little genetic evidence has been available until a recent report. In an unbiased genetic screen, glycosphingolipids and their biosynthetic pathway were found to be fundamental for the maintenance of apical polarity in the developing Caenorhabditis elegans intestine (Zhang et al. 2011). Depletion of enzymes involved in glycosphingolipid synthesis led to ectopic formation of apical surfaces in the lateral domain and gave rise to multiple lumens (Zhang et al. 2011). Whether and how glycosphingolipid helps to generate apical membranes of mammalian epithelial cells still awaits investigation (Hyenne and Labouesse 2011; Zhang et al. 2011).
The first indication that polarity proteins regulate vesicular trafficking came from a genome-wide RNA-mediated interference screen for genes regulating membrane traffic, in which PAR-3, PAR-6, PKC-3, and Cdc42 were identified as candidates (Balklava et al. 2007). Further investigations revealed that these factors were required for correct endocytic traffic in Caenorhabditis elegans coelomocytes and human HeLa cells and their mutation caused both reduced uptake of clathrin-dependent cargo and reduced recycling of clathrin-independent cargo (Balklava et al. 2007). These results strongly suggested a direct function of polarity proteins in the regulation of vesicular transport. This idea was further supported by a study of neuronal cells showing that interaction of PAR-3 and aPKC with the exocyst complex, a vesicle tethering complex comprising eight subunits (termed Sec6/8 in mammalian studies) (Whyte and Munro 2002), is required for neuronal polarization (Lalli 2009). Recent work revealed a strong collaboration between the polarity and trafficking machineries during early lumenogenesis (Martin-Belmonte et al. 2007; Bryant et al. 2010). De novo lumen formation in mammalian epithelial cells starts with the delivery of apical protein-containing vesicles to a small common landmark shared by contacting cells. This landmark is referred to as the apical membrane initiation site (Bryant et al. 2010). Studies of MDCK cyst formation suggested that vesicular traffic events are required for cortical localization of PAR-3 and Cdc42 at the apical membrane initiation site. Then, the PAR-3/aPKC complex cooperates with the exocyst complex to promote the delivery of Rab11a/Rab8a-positive vesicles that transport apical proteins (Bryant et al. 2010). Furthermore, the annexin 2/Cdc42 module, which interacts with PIP2 at the apical membrane (Martin-Belmonte et al. 2007), is also required for Rab11a-Rab8a-dependent transport of apical proteins and for apical delivery of aPKC/PAR-6 complex to the apical surface (Martin-Belmonte et al. 2007; Bryant and Mostov 2008). Cdc42 is elsewhere known to control vesicle dynamics at the cell cortex and at the Golgi in mammalian cells (for review, see Harris and Tepass 2010). Deregulation of Cdc42 activity in MDCK monolayers results in mistargeting of basolateral membrane proteins to the apical membrane. This effect may result from both defects in TGN and recycling pathways (Kroschewski et al. 1999; Cohen et al. 2001; Musch et al. 2001). In 3D MDCK cell cultures, knockdown of Cdc42 results in defects in apical membrane polarity and lumen formation, likely owing to a defect in apical trafficking (Martin-Belmonte et al. 2007). These studies highlight that Cdc42 participates to polarize trafficking in epithelial cells and suggest that Cdc42 is an interesting candidate to integrate polarity protein functions and vesicular trafficking machineries.
4. FROM INDIVIDUAL CELL POLARITY TO GENERATION OF EPITHELIAL TISSUE ARCHITECTURE
Establishment of polarity in individual cells is not sufficient by itself to build the tubular organization of glandular organs. For instance, impaired interaction of epithelial cells with their basement membrane (O’Brien et al. 2001; Myllymaki et al. 2011; Daley et al. 2012), or mutations in cell–cell adhesion receptors (Stephenson et al. 2010; Jia et al. 2011) can give rise to epithelial structures in which the cells are aberrantly polarized (some of them contain an apical surface) and do not give rise to a central lumen. Thus, the polarity of each cell must properly orientate to align with the higher-order tissue architecture to generate the specific geometry needed for tissue function. It is widely believed that cells determine their directionality of polarization by sensing the extracellular matrix and neighboring cells, through cell–matrix and cell–cell adhesion receptors, respectively. Signals from the ECM provide one axis from which to determine the orientation of apical–basal polarity, and cell–cell adhesions provide a second axis. Whereas the role of cell–cell adhesions in the orientation of polarity remains elusive, maybe owing to the redundancy of cell–cell adhesion receptors, recent investigations revealed the importance of cell–matrix interactions for establishment of tissue architecture.
Signaling from the ECM is a prerequisite for epithelial polarization in many developmental and 3D cell-based model systems (O’Brien et al. 2001; Li et al. 2003; Miner and Yurchenco 2004; Weir et al. 2006; Plachot et al. 2009; Rooney and Streuli 2011). The ECM is a complex, tissue-specific network made of collagens, proteoglycans, and glycoproteins such as fibronectins and laminins. In addition, a large number of ECM-modifying enzymes, ECM-binding growth factors, and other ECM-associated proteins interact and cooperate with ECM proteins to assemble and remodel ECM matrices (Hynes and Naba 2012). These matrices are actively remodeled by cells during development, normal tissue homeostasis, and in several disease processes such as cancer-associated desmoplasia or inflammation. Specialized cell surface-associated ECMs, named basement membranes (BMs), underline epithelial cells at their basal surfaces. BMs are composed of collagen IV, several types of laminins, nidogen, and proteoglycans. Laminin constitutes the first cell-anchored polymer required for BM assembly (Yurchenco 2011) and has long been implicated in epithelial polarity and morphogenesis (Li et al. 2003; Miner and Yurchenco 2004). Cells sense their surrounding ECM and BM through a variety of transmembrane receptors. The major receptors belong to the integrin family, which bind to collagen, laminin, and fibronectin. Different integrin isoforms ensure that epithelial cells adapt to various environmental conditions to generate appropriate apical–basal orientation (Myllymaki et al. 2011). Another well-characterized receptor is the heterodimeric glycoprotein dystroglycan, which binds several ECM proteins such as laminins, agrin, and perlecan (Michele and Campbell 2003). Dystroglycan plays a major role in the assembly and maintenance of laminin BMs (Barresi and Campbell 2006; Leonoudakis et al. 2010). The cytoplasmic domain of integrin and dystroglycan receptors assembles large and dynamic multiprotein complexes that relay signals from to the ECM to regulate cytoskeletal assembly and intracellular signaling pathways (Yurchenco 2011).
When MDCK cells are grown in collagen I gels, activation of β1-integrins by collagen I induces Rac1 activity (outside-in signaling) (Yu et al. 2005). Then, activated Rac1 promotes the assembling of laminin basement membrane (inside-out signaling) (O’Brien et al. 2001; Yu et al. 2005), probably through regulation of dystroglycan (Barresi and Campbell 2006; Leonoudakis et al. 2010) or β1-integrins (Yu et al. 2005). Failure of laminin BM assembly by Rac1 inactivation or β1-integrin function-blocking antibody results in inversion of apical–basal polarity (the apical pole of these cysts localizes at the cyst periphery and the basolateral pole faces the center of the cyst) (O’Brien et al. 2001; Yu et al. 2005). These studies suggested that integrin-dependent interaction of cells with their ECM and subsequent generation of a basal membrane are required to orient the apical/luminal surface and generate the correct tissue architecture. Similarly, integrin-mediated signaling has recently been shown to be important for BM remodeling by mouse salivary gland epithelial cells grown in matrigel. This remodeling was shown to be a prerequisite for appropriate apical domain orientation (Daley et al. 2012). These findings suggested that ECM-dependent integrin activation and remodeling of BM may be a general mechanism for the coordinated orientation of epithelial cell polarization.
BM remodeling is controlled by the tight regulation of Rho-associated coiled-coil containing kinase (ROCK I), although different ROCK I-dependent mechanisms have been proposed. In MDCK cells, laminin remodeling and correct orientation of polarity require the inhibition of the RhoA-ROCK I-myosin II pathway by activated Rac1, suggesting that tension of the actin cytoskeleton may signal to matrix receptors to induce laminin remodeling (Yu et al. 2008). This is consistent with the observation that preferential laminin polymerization at cell surfaces depends on the actin cytoskeleton (Colognato et al. 1999). In mouse salivary glands, ROCK I ensures coordinated alignment of epithelial cells by restricting basement membrane positioning to the basal periphery of the developing salivary gland epithelium. In this model, ROCK I acts independently of myosin II by controlling PAR-1b localization to the basolateral surface of ECM-contacting cells (Daley et al. 2012). This is consistent with previous reports that PAR-1 is required for assembly of BM laminin at the basal surface of epithelial cells (Masuda-Hirata et al. 2009). It is yet unclear how PAR-1b regulates laminin organization, although it could involve the regulation of the microtubule cytoskeleton (Doerflinger et al. 2003; Cohen et al. 2004), or dystroglycan activity (Masuda-Hirata et al. 2009; Yamashita et al. 2010). Thus, these findings suggest that the correct tissue geometry required for tissue function is at least partly ensured by coordinating epithelial cells polarization with BM formation.
An intriguing question is how signaling from the BM orients epithelial cells with the apical surface opposite to the basal surface. Because of the major role of polarity complexes in cell polarization, it is tempting to speculate that assembled BM might impact the location and/or activity of those complexes. Interestingly, inhibition of β1-integrins in 3D cultures of MDCKII cells by function-blocking antibodies inhibits interaction of PAR-3 with the PAR-6/aPKC complex and leads to its mislocalization in the cytoplasm (Li and Pendergast 2011). Furthermore, Dlg, which is normally found at the basolateral membrane, is relocalized at the inverted apical membrane (Li and Pendergast 2011). Finally, PAR-3 expression and localization are regulated downstream from β1-integrins to establish endothelial cell polarity and arteriolar lumen formation (Zovein et al. 2010).
Another possible mechanism is that BM signaling may act upstream of cell–cell junction formation to regulate the segregation of the apical and basolateral plasma membrane. Indeed, BM-mediated outside-in signals are involved in the maturation of cell–cell contacts (Benton and St Johnston 2003; Li et al. 2003; Miner and Yurchenco 2004). In a 3D model of mouse salivary cells, this outside-in signaling is mediated by β1-integrins (Daley et al. 2012). A possible signaling intermediary between integrins and cadherins may be the Ras family GTPase Rap1, which transmits signals between cell–cell and cell–matrix adhesions (Retta et al. 2006). Supporting this idea, a dominant active Rap1 is able to revert the polarity inversion of MDCKII cells caused by dominant-negative Rac1, but not the defects in laminin assembly (Li and Pendergast 2011).
Besides the ECM itself, interaction of epithelial cells with their surrounding cells is also believed to participate in polarization. This idea was, for instance, exemplified by coculture experiments of luminal breast epithelial cells with mammary gland myoepithelial cells. When luminal cells were cultivated in collagen I gels, they formed structures with reverted polarity and devoid of lumens. But when those cells were cocultured with myoepithelial cells, normal polarized luminal structures were observed. The investigators further showed that this effect was related to the ability of myoepithelial cells to provide luminal cells with laminin I (Gudjonsson et al. 2002). Another example of the importance of surrounding cells for epithelial morphogenesis is given by studies of collective cell migration during mammary morphogenesis, in which myoepithelial cells control the elongation of ducts (Ewald et al. 2008). Thus, these studies underline to need to develop new 3D cell culture models of epithelial morphogenesis that recreate as much as possible the real organ features.
5. MAINTENANCE OF 3D ARCHITECTURE DURING CELL DIVISION
Symmetric cell division is required for expansion of luminal compartments and their maintenance during tissue turnover, but also for tissue elongation and shaping (Baena-Lopez et al. 2005; Segalen and Bellaiche 2009). The orientation of cell division is controlled by the position of mitotic spindle, which determines the cleavage plane of the mother cell. Epithelial cells usually place the mitotic spindle perpendicular to the apical–basal axis and divide symmetrically in the plane of the monolayer (Gillies and Cabernard 2011). Another type of cell division, asymmetrical cell division, has been studied extensively in model organisms. Asymmetrical cell division is essential to generate different cell fate and is commonly seen in epithelial progenitor or stem cells, e.g., in the skin, gut, mammary glands, lung, and heart (Neumuller and Knoblich 2009).
Studies of cell division in 3D structures showed that misoriented symmetric cell division causes multiple lumen formation, which not only supports the importance of oriented cell division but also makes 3D culture an ideal system to study its regulation (Jaffe et al. 2008; Zheng et al. 2010). In 3D cysts, mitosis occurs in the plane of the cyst surface, where the mitotic spindle is anchored to the lateral cell cortex and aligned perpendicular to the apical–basal axis (Yu et al. 2003; Zheng et al. 2010). At the interphase, the centrosome is localized apically in ciliated epithelial cells at the base of cilium (Reinsch and Karsenti 1994). To be oriented perpendicular to the apical–basal axis, the assembled mitotic spindle has to first undergo a planar rotation during metaphase (Reinsch and Karsenti 1994). The planar rotation is regulated by leucine-glycine-asparagine repeat protein (LGN), which orients the force exerted on the spindle poles (Peyre et al. 2011). LGN links the mitotic spindle to the cell cortex by binding to nuclear and mitotic apparatus protein (NuMA) and Gαi (inhibitory α subunits of heterotrimeric G proteins) (Zheng et al. 2010; Peyre et al. 2011). NuMA binds to microtubules and the dynein–dynactin motor complex, whereas Gαi is anchored at the cell membrane through myristoylation (Merdes et al. 1996; Siderovski et al. 1999; Merdes et al. 2000). In addition to their role in establishment of polarity, recent investigations revealed that polarity proteins control the formation and maintenance of epithelial tissue architecture by ensuring the proper orientation of mitotic spindles during symmetric cell division. Indeed, LGN binding to Gαi is restricted to the cell cortex and extruded from the apical surface by aPKC-mediated phosphorylation (Hao et al. 2010; Zheng et al. 2010). LGN is phosphorylated by aPKC at residue Ser401, which recruits 14-3-3 protein and inhibits LGN interaction with Gαi at the apical membrane (Hao et al. 2010). When either LGN expression or aPKC function is inhibited, mitotic spindles are inappropriately oriented and cause multiple lumen formation (Hao et al. 2010; Zheng et al. 2010). Maintenance of apical localization of aPKC through PAR-3, Cdc42, and PAR-6 signaling is important for correct orientation of cell division and single lumen formation (Jaffe et al. 2008; Hao et al. 2010; Durgan et al. 2011). Two Cdc42-specific guanine nucleotide exchange factors (GEFs), Tuba and Intersectin2, control localized Cdc42 activation (Qin et al. 2010; Rodriguez-Fraticelli et al. 2010). Tuba localizes to the apical membrane and may function to activate the PAR-6/PAR-3/aPKC pathway (Qin et al. 2010). Intersectin2 localizes to the centrosome, and likely activates Cdc42 in a pericentrosomal compartment, although it remains unclear what are the downstream effectors of Cdc42 at this site (Rodriguez-Fraticelli et al. 2010). Taken together, the concerted effort of polarity protein complexes and LGN regulate mitotic spindle orientation in symmetrical cell division.
6. DYNAMIC REARRANGEMENTS OF POLARITY DRIVE EPITHELIAL MORPHOGENESIS
Proper apical–basolateral polarity is not only important for the function and maintenance of epithelial tissues, but is required for epithelial morphogenesis during embryogenesis and tissue regeneration. In many cases during embryogenesis, cell fates are specified at locations distant to where they will ultimately reside, requiring cells to migrate either individually or collectively to their destination (Friedl and Gilmour 2009; Aman and Piotrowski 2010). To migrate collectively, some cells undergo an epithelial–mesenchymal transition (EMT) and only loosely interact and communicate through actin protrusions, such as the neural crest cells during emigration (Teddy and Kulesa 2004; Aman and Piotrowski 2010). In other cases, cells dynamically reorganize but maintain, at least some, cell–cell adhesions and apical–basolateral polarity, such as during the migration of the Drosophila border cell cluster (Pinheiro and Montell 2004; Friedl and Gilmour 2009). Cell–cell adhesions are important for cells to communicate cellular signals and mechanical forces during migration (Ilina and Friedl 2009; Rorth 2009), but what is the role of apical–basolateral polarity during this process? We are just starting to get some clues from model organisms, especially by observing the migration of Drosophila border cell clusters.
The Drosophila ovary consists of strings of egg chambers. Each egg chamber contains one oocyte and 15 nurse cells surrounded by a monolayer of follicle epithelial cells. The border cell cluster is specified from polarized follicle epithelial cells and consists of two cell types, the border and polar cells (Montell et al. 1992). Once the cluster is formed, it delaminates from the follicular epithelium and begins to move between the nurse cells toward the posterior pole of the egg chamber until reaching the oocyte (Geisbrecht and Montell 2002). The detachment of the border cell cluster from the follicular epithelium requires proper apical–basolateral polarity of the border cells. Border cell detachment is directly regulated by the polarity protein PAR-1 (McDonald et al. 2008). Loss of PAR-1 disrupts the cell polarity of border cells and adhesions between border cells and follicle cells, resulting in the failure of border cell cluster to detach (McDonald et al. 2008). After detachment, border cells start to move toward the oocyte, while retaining their polarity as evident by the asymmetrical localization of the PAR complex (PAR-3/PAR-6/aPKC) and Crumbs (Pinheiro and Montell 2004). Aberrant PAR-3 or PAR-6 expression disrupts the apical–basolateral polarity as well as E-cadherin localization, resulting in dissociation of the border cell cluster (Pinheiro and Montell 2004). These studies show that apical–basolateral polarity is retained and required for collective border cell migration. However, it seems contradictory that polarity is required for both the dissociation (during detachment) and maintenance (during migration) of cell–cell adhesions. It is possible that polarity is simply needed for the reorganization of cell–cell adhesions, and that other signals control whether the adhesion is maintained or disrupted. Alternatively, different combinations of polarity proteins are retained under different contexts, which could dictate different dynamics of cell–cell adhesions.
Besides the type of migration exemplified by border cells (migrate as a free group), several other types of collective cell migration have been described, including sheet, streams, sprouting, and branching (Rorth 2009). In many of these cases, apical–basolateral polarity is also dynamically regulated during cell movement, although its role is less clear (Revenu and Gilmour 2009). For example, during the development of the zebrafish posterior lateral line primordium, the apical membrane constriction (enriched in ZO-1, aPKC, and actin) in epithelial cells is regulated by the polarity protein Lgl and is required for the deposition of proneuromast rosettes (Hava et al. 2009).
Recently, two mammalian in vitro 3D models have been developed that were proved useful to study branching morphogenesis. During puberty, the mammary gland extends tubular network into the surrounding stroma by the branching morphogenesis of terminal end buds (TEBs) (Hinck and Silberstein 2005). This branching morphogenesis process can be visualized and analyzed in vitro by culturing organoids isolated from mice mammary gland in Matrigel, and in the presence of fibroblast growth factor 2 (Fata et al. 2007; Ewald et al. 2008). New ducts extend from the organoid through the collective migration of luminal epithelial cells and of myoepithelial cells, and it requires cell proliferation, Rac, and myosin light-chain kinase (MLCK) (Fata et al. 2007; Ewald et al. 2008). During this process, luminal epithelial cells reorganize into a multilayered epithelium, which closely mimics TEB structure in vivo (Ewald et al. 2008; Gray et al. 2010). Within the multilayered epithelium, luminal epithelial cells partially lose apical polarity, as evident by the lateral localization of aPKC and Scrib and cytoplasmic localization of PAR-3 (Ewald et al. 2008, 2012). Adherens junctions are also largely absent, although E-cadherin and β-catenin remain at cell surface (Ewald et al. 2008, 2012). Distinct from other branching morphogenesis events, mammary ducts elongate without extending actin-dependent membrane protrusions at the leading edge and the cells continuously exchange their positions during migration (Ewald et al. 2008, 2012). Eventually, the multilayered epithelium converts to a bilayered epithelium with single-layered myoepithelial cells surrounding one layer of luminal epithelial cells (Ewald et al. 2008). The luminal epithelial cells reestablish polarity at this stage, which requires the Rho kinase (ROCK) (Ewald et al. 2008). Future studies may focus on defining the molecular mechanisms that enable epithelial cells to reversibly reduce and reestablish polarity and how it is regulated in space and time.
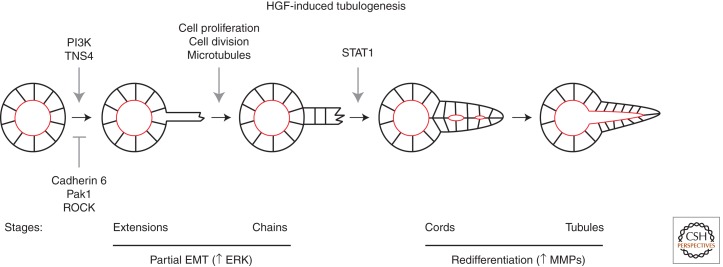
Hepatocyte growth factor (HGF)-induced tubulogenesis in 3D MDCK cells provides another system to study remodeling of epithelial polarity during epithelial morphogenesis (Fig. 3). Treatment of MDCK cysts with HGF causes cells to undergo four morphologically distinct steps to produce tubules, termed as extensions, chains, cords, and tubules (Pollack et al. 1998; Zegers et al. 2003). First, cells send out large extensions from the basolateral surface, while retaining the apical domain (Pollack et al. 1998). Extension formation requires the down-regulation of cadherin-6 and Pak1 (p21-activated kinase 1), activation of PI3K, and up-regulation of TNS4 (tensin 4), whereas the small GTPase Rho and its effector ROCK control the length and number of extensions (Yu et al. 2003; Kong et al. 2009; Hunter and Zegers 2010; Jia et al. 2011; Kwon et al. 2011). Next, chains of 1–3 cells protrude and migrate out of the cyst wall (Pollack et al. 1998). This process requires cell proliferation and changes in the plane of cell division (Yu et al. 2003; Wang et al. 2005). Cells in chains lose the apical domain, but maintain E-cadherin at the cell surface (Pollack et al. 1998). Next, chains transform into cords that are 2–3 cells thick, and this transition is regulated by STAT1 (signal transducer and activator of transcription signaling) (Pollack et al. 1998; Kim et al. 2010). At this step, cells regain epithelial polarity and form small lumens lined by a newly established apical surface (Pollack et al. 1998). Finally, lumens expand to become contiguous with the central lumen of the cyst, marking the completion of mature tubules (Pollack et al. 1998). Because MDCK cells transiently lose polarity in chains (step two) and then repolarize and differentiate in subsequent steps, the tubulogenesis process can also be described by two phases: a partial EMT and the redifferentiation phase. The first phase is regulated by the activation of extracellular-regulated kinase (ERK) and the down-regulation of myosin activity, whereas matrix metalloproteases (MMPs) are necessary for the second phase (O’Brien et al. 2004; Hellman et al. 2005, 2008; Liu et al. 2007b; Raghavan et al. 2010).
Figure 3.
Tubulogenesis process in MDCK cells. After MDCK cyst is treated with hepatocyte growth factor (HGF), new tubules initiate through four morphologically distinct steps, termed as extensions, chains, cords, and tubules. The signaling pathways identified to regulate each step are labeled. The HGF-induced tubulogenesis process can also be described by two phases: partial EMT and redifferentiation phase. The first phase requires activation of ERK (extracellular-regulated kinase), whereas the second phase requires MMP activity. (Adapted from O’Brien 2002.)
7. CONCLUDING REMARKS
In recent decades, important progress has been made in identifying and characterizing how polarity complexes regulate epithelial polarization in model organisms. However, how those polarity complexes physically and/or functionally interact in mammalian epithelial cells is not completely clear. Studies have been confounded by the existence of several isoforms of polarity proteins in mammalian cells compared to model organisms. A particularly challenging question is how cortical asymmetry of polarity complexes is transduced to give rise to the fully polarized phenotype of epithelial cells. Elucidating the interplay between polarity complexes, the cytoskeleton and vesicular trafficking machineries may provide some answers to this question.
Another aspect of epithelial morphogenesis that has been barely studied until recently is the effect of the 3D microenvironment on epithelial morphogenesis. Hopefully, the investigation of 3D cell culture models of increasing complexity will be useful. We highlighted here how ECM membrane receptors are able to interact and remodel the surrounding matrix to generate tissue architecture. Nevertheless, there is more to be learned about the nature and function of membrane receptors acting as bidirectional transmitters of signaling between ECM and cells. It is worth noting that not only the nature of ECM components can affect the response of membrane receptors, but also forces applied on these receptors may regulate their activity and the final architecture of epithelia. Indeed, in multicellular tissues, cells are subjected to a myriad of forces, including compressive, tensile, fluid shear stress, and hydrostatic pressure. Understanding how mechanical signals are sensed and transduced by polarizing cells, and how these signals might talk to polarity machineries, may be of great interest.
Cancers of epithelial origin (carcinomas) account for 80% of all cancers. Most primary human carcinomas retain epithelial characteristics such as intercellular adhesions and tight junctions, whereas high-grade epithelial tumors usually display loss of apical–basal polarity and architectural disorganization. Loss of polarity has first been viewed as a side effect of abnormal proliferation of tumor cells, but it is now becoming clear that not only polarity pathways often play an active role in promoting tumor development but also that epithelial cell polarity acts as a major gatekeeper against cancer initiation and metastasis. Expression, activity, and subcellular localization of core-polarity proteins are generally deregulated in carcinoma, and polarity pathways are often direct targets of oncogenes, proto-oncogenes, and tumor suppressors. Dysregulation of polarity pathways affect several cancer-relevant biological processes such as proliferation, apoptosis, polarity, and epithelial–mesenchymal transition (for recent reviews, see Aranda et al. 2008; Huang and Muthuswamy 2010; McCaffrey and Macara 2011; Royer and Lu 2011). A concept is emerging that 3D tissue architecture itself plays an important tumor-suppressive role. This hypothesis arises from observations that it is more difficult to induce transformation in a tissue than in single cells grown in culture dishes. For instance, expression of Ras oncogene is sufficient to cause transformed growth of established cell lines in culture, but activation of Ras in vivo in normal tissue is not sufficient to induce the clonal development of cells without additional protumoral modifications (Frame and Balmain 2000). Also, activation of c-Myc in quiescent but structurally unorganized 3D mammary acinar structures provokes abnormal cell proliferation, although activation of the same oncogene in mature quiescent acini with established architecture has no effect (Partanen et al. 2007). It has been proposed that the internal cell-polarity mechanisms of the normal cells function as a noncell autonomous tumor suppressor by using cell–cell junctions to “force” the mutant cell to maintain a polarized structure, thus attenuating its malignant phenotype (Lee and Vasioukhin 2008). Therefore, an understanding of the interaction of isolated transformed cells with neighboring normal cells would be crucial in dissecting the role of cell interaction on the acquisition and maintenance of cell polarity (Hogan et al. 2009; Kajita et al. 2010).
Aknowledgments
Supported by National Institutes of Health grants 5R01 DK074398, 5R37 AI25144, and 5R01 DK091530 to K.M.
Footnotes
Editors: Patrick P.L. Tam, W. James Nelson, and Janet Rossant
Additional Perspectives on Mammalian Development available at www.cshperspectives.org
REFERENCES
- Aman A, Piotrowski T 2010. Cell migration during morphogenesis. Dev Biol 341: 20–33 [DOI] [PubMed] [Google Scholar]
- Andrew DJ, Ewald AJ 2010. Morphogenesis of epithelial tubes: Insights into tube formation, elongation, and elaboration. Dev Biol 341: 34–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi K, Sugaya T, Umeda M, Yamamoto S, Terakawa S, Takahashi M 2005. The activation of exocytotic sites by the formation of phosphatidylinositol 4,5-bisphosphate microdomains at syntaxin clusters. J Biol Chem 280: 17346–17352 [DOI] [PubMed] [Google Scholar]
- Aranda V, Nolan ME, Muthuswamy SK 2008. Par complex in cancer: A regulator of normal cell polarity joins the dark side. Oncogene 27: 6878–6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-Lopez LA, Baonza A, Garcia-Bellido A 2005. The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol 15: 1640–1644 [DOI] [PubMed] [Google Scholar]
- Balklava Z, Pant S, Fares H, Grant BD 2007. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol 9: 1066–1073 [DOI] [PubMed] [Google Scholar]
- Balla T, Szentpetery Z, Kim YJ 2009. Phosphoinositide signaling: New tools and insights. Physiology (Bethesda) 24: 231–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi R, Campbell KP 2006. Dystroglycan: From biosynthesis to pathogenesis of human disease. J Cell Sci 119: 199–207 [DOI] [PubMed] [Google Scholar]
- Baum B, Georgiou M 2011. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol 192: 907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, St Johnston D 2003. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115: 691–704 [DOI] [PubMed] [Google Scholar]
- Bilder D, Perrimon N 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403: 676–680 [DOI] [PubMed] [Google Scholar]
- Bryant DM, Mostov KE 2008. From cells to organs: Building polarized tissue. Nat Rev Mol Cell Biol 9: 887–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE 2010. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol 12: 1035–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers AD, Pambos M, Mason J, Lang S, Wylie C, Papalopulu N 2005. aPKC, Crumbs3 and Lgl2 control apicobasal polarity in early vertebrate development. Development 132: 977–986 [DOI] [PubMed] [Google Scholar]
- Chen X, Macara IG 2005. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol 7: 262–269 [DOI] [PubMed] [Google Scholar]
- Cohen D, Musch A, Rodriguez-Boulan E 2001. Selective control of basolateral membrane protein polarity by cdc42. Traffic 2: 556–564 [DOI] [PubMed] [Google Scholar]
- Cohen D, Brennwald PJ, Rodriguez-Boulan E, Musch A 2004. Mammalian PAR-1 determines epithelial lumen polarity by organizing the microtubule cytoskeleton. J Cell Biol 164: 717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Winkelmann DA, Yurchenco PD 1999. Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol 145: 619–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Gervais EM, Centanni SW, Gulfo KM, Nelson DA, Larsen M 2012. ROCK1-directed basement membrane positioning coordinates epithelial tissue polarity. Development 139: 411–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Bryant DM, Mostov KE 2011. Molecular regulation of lumen morphogenesis. Curr Biol 21: R126–R136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger H, Benton R, Shulman JM, St Johnston D 2003. The role of PAR-1 in regulating the polarised microtubule cytoskeleton in the Drosophila follicular epithelium. Development 130: 3965–3975 [DOI] [PubMed] [Google Scholar]
- Durgan J, Kaji N, Jin D, Hall A 2011. Par6B and atypical PKC regulate mitotic spindle orientation during epithelial morphogenesis. J Biol Chem 286: 12461–12474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z 2008. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell 14: 570–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald AJ, Huebner RJ, Palsdottir H, Lee JK, Perez MJ, Jorgens DM, Tauscher AN, Cheung KJ, Werb Z, Auer M 2012. Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium. J Cell Sci 125 (Pt 11): 2638–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata JE, Mori H, Ewald AJ, Zhang H, Yao E, Werb Z, Bissell MJ 2007. The MAPK(ERK-1,2) pathway integrates distinct and antagonistic signals from TGFα and FGF7 in morphogenesis of mouse mammary epithelium. Dev Biol 306: 193–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Wu H, Chan LN, Zhang M 2008. Par-3-mediated junctional localization of the lipid phosphatase PTEN is required for cell polarity establishment. J Biol Chem 283: 23440–23449 [DOI] [PubMed] [Google Scholar]
- Fournier MV, Fata JE, Martin KJ, Yaswen P, Bissell MJ 2009. Interaction of E-cadherin and PTEN regulates morphogenesis and growth arrest in human mammary epithelial cells. Cancer Res 69: 4545–4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame S, Balmain A 2000. Integration of positive and negative growth signals during ras pathway activation in vivo. Curr Opin Genet Dev 10: 106–113 [DOI] [PubMed] [Google Scholar]
- Friedl P, Gilmour D 2009. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10: 445–457 [DOI] [PubMed] [Google Scholar]
- Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, Mostov K 2006. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol 8: 963–970 [DOI] [PubMed] [Google Scholar]
- Geisbrecht ER, Montell DJ 2002. Myosin VI is required for E-cadherin-mediated border cell migration. Nat Cell Biol 4: 616–620 [DOI] [PubMed] [Google Scholar]
- Gillies TE, Cabernard C 2011. Cell division orientation in animals. Curr Biol 21: R599–R609 [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG 2007. The PAR proteins: Fundamental players in animal cell polarization. Dev Cell 13: 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RS, Cheung KJ, Ewald AJ 2010. Cellular mechanisms regulating epithelial morphogenesis and cancer invasion. Curr Opin Cell Biol 22: 640–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith LG, Swartz MA 2006. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol 7: 211–224 [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW 2002. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci 115: 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Du Q, Chen X, Zheng Z, Balsbaugh JL, Maitra S, Shabanowitz J, Hunt DF, Macara IG 2010. Par3 controls epithelial spindle orientation by aPKC-mediated phosphorylation of apical Pins. Curr Biol 20: 1809–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KP, Tepass U 2010. Cdc42 and vesicle trafficking in polarized cells. Traffic 11: 1272–1279 [DOI] [PubMed] [Google Scholar]
- Hava D, Forster U, Matsuda M, Cui S, Link BA, Eichhorst J, Wiesner B, Chitnis A, Abdelilah-Seyfried S 2009. Apical membrane maturation and cellular rosette formation during morphogenesis of the zebrafish lateral line. J Cell Sci 122: 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Xi F, Zhang X, Zhang J, Guo W 2007. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J 26: 4053–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman NE, Greco AJ, Rogers KK, Kanchagar C, Balkovetz DF, Lipschutz JH 2005. Activated extracellular signal-regulated kinases are necessary and sufficient to initiate tubulogenesis in renal tubular MDCK strain I cell cysts. Am J Physiol Renal Physiol 289: F777–F785 [DOI] [PubMed] [Google Scholar]
- Hellman NE, Spector J, Robinson J, Zuo X, Saunier S, Antignac C, Tobias JW, Lipschutz JH 2008. Matrix metalloproteinase 13 (MMP13) and tissue inhibitor of matrix metalloproteinase 1 (TIMP1), regulated by the MAPK pathway, are both necessary for Madin-Darby canine kidney tubulogenesis. J Biol Chem 283: 4272–4282 [DOI] [PubMed] [Google Scholar]
- Hinck L, Silberstein GB 2005. Key stages in mammary gland development: The mammary end bud as a motile organ. Breast Cancer Res 7: 245–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose T, Izumi Y, Nagashima Y, Tamai-Nagai Y, Kurihara H, Sakai T, Suzuki Y, Yamanaka T, Suzuki A, Mizuno K, et al. 2002. Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J Cell Sci 115: 2485–2495 [DOI] [PubMed] [Google Scholar]
- Hogan C, Dupre-Crochet S, Norman M, Kajita M, Zimmermann C, Pelling AE, Piddini E, Baena-Lopez LA, Vincent JP, Itoh Y, et al. 2009. Characterization of the interface between normal and transformed epithelial cells. Nat Cell Biol 11: 460–467 [DOI] [PubMed] [Google Scholar]
- Huang L, Muthuswamy SK 2010. Polarity protein alterations in carcinoma: A focus on emerging roles for polarity regulators. Curr Opin Genet Dev 20: 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MP, Zegers MM 2010. Pak1 regulates branching morphogenesis in 3D MDCK cell culture by a PIX and β1-integrin-dependent mechanism. Am J Physiol Cell Physiol 299: C21–C32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurov JB, Watkins JL, Piwnica-Worms H 2004. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol 14: 736–741 [DOI] [PubMed] [Google Scholar]
- Hyenne V, Labouesse M 2011. Making sense of glycosphingolipids in epithelial polarity. Nat Cell Biol 13: 1185–1187 [DOI] [PubMed] [Google Scholar]
- Hynes RO, Naba A 2012. Overview of the matrisome—An inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol 4: a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilina O, Friedl P 2009. Mechanisms of collective cell migration at a glance. J Cell Sci 122: 3203–3208 [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Young C, Den Beste K, Capaldo CT, Humbert PO, Brennwald P, Parkos CA, Nusrat A 2010. Tumor suppressor scribble regulates assembly of tight junctions in the intestinal epithelium. Am J Pathol 176: 134–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Kaji N, Durgan J, Hall A 2008. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol 183: 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James DJ, Khodthong C, Kowalchyk JA, Martin TF 2008. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J Cell Biol 182: 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Liu F, Hansen SH, Ter Beest MB, Zegers MM 2011. Distinct roles of cadherin-6 and E-cadherin in tubulogenesis and lumen formation. Mol Biol Cell 22: 2031–2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty G, Petersen C, Gao L, Macara IG 2000. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol 2: 531–539 [DOI] [PubMed] [Google Scholar]
- Kajita M, Hogan C, Harris AR, Dupre-Crochet S, Itasaki N, Kawakami K, Charras G, Tada M, Fujita Y 2010. Interaction with surrounding normal epithelial cells influences signalling pathways and behaviour of Src-transformed cells. J Cell Sci 123: 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, O’Brien LE, Kwon SH, Mostov KE 2010. STAT1 is required for redifferentiation during Madin-Darby canine kidney tubulogenesis. Mol Biol Cell 21: 3926–3933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong T, Xu D, Yu W, Takakura A, Boucher I, Tran M, Kreidberg JA, Shah J, Zhou J, Denker BM 2009. Gα12 inhibits α2β1 integrin-mediated Madin-Darby canine kidney cell attachment and migration on collagen-I and blocks tubulogenesis. Mol Biol Cell 20: 4596–4610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroschewski R, Hall A, Mellman I 1999. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol 1: 8–13 [DOI] [PubMed] [Google Scholar]
- Kwon SH, Nedvetsky PI, Mostov KE 2011. Transcriptional profiling identifies TNS4 function in epithelial tubulogenesis. Curr Biol 21: 161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli G 2009. RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC. J Cell Sci 122: 1499–1506 [DOI] [PubMed] [Google Scholar]
- Laprise P, Chailler P, Houde M, Beaulieu JF, Boucher MJ, Rivard N 2002. Phosphatidylinositol 3-kinase controls human intestinal epithelial cell differentiation by promoting adherens junction assembly and p38 MAPK activation. J Biol Chem 277: 8226–8234 [DOI] [PubMed] [Google Scholar]
- Laprise P, Viel A, Rivard N 2004. Human homolog of disc-large is required for adherens junction assembly and differentiation of human intestinal epithelial cells. J Biol Chem 279: 10157–10166 [DOI] [PubMed] [Google Scholar]
- Lee M, Vasioukhin V 2008. Cell polarity and cancer—Cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci 121: 1141–1150 [DOI] [PubMed] [Google Scholar]
- Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi MH, Medina E, Arsanto JP, Le Bivic A 2004. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell 15: 1324–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonoudakis D, Singh M, Mohajer R, Mohajer P, Fata JE, Campbell KP, Muschler JL 2010. Dystroglycan controls signaling of multiple hormones through modulation of STAT5 activity. J Cell Sci 123: 3683–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Pendergast AM 2011. Arg kinase regulates epithelial cell polarity by targeting β1-integrin and small GTPase pathways. Curr Biol 21: 1534–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Edgar D, Fassler R, Wadsworth W, Yurchenco PD 2003. The role of laminin in embryonic cell polarization and tissue organization. Dev Cell 4: 613–624 [DOI] [PubMed] [Google Scholar]
- Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T 2000. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol 2: 540–547 [DOI] [PubMed] [Google Scholar]
- Liu XF, Ishida H, Raziuddin R, Miki T 2004. Nucleotide exchange factor ECT2 interacts with the polarity protein complex Par6/Par3/protein kinase Cζ (PKCζ) and regulates PKCζ activity. Mol Cell Biol 24: 6665–6675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zuo X, Yue P, Guo W 2007a. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol Biol Cell 18: 4483–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Greco AJ, Hellman NE, Spector J, Robinson J, Tang OT, Lipschutz JH 2007b. Intracellular signaling via ERK/MAPK completes the pathway for tubulogenic fibronectin in MDCK cells. Biochem Biophys Res Commun 353: 793–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubarsky B, Krasnow MA 2003. Tube morphogenesis: Making and shaping biological tubes. Cell 112: 19–28 [DOI] [PubMed] [Google Scholar]
- Maehama T, Dixon JE 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Perez-Moreno M 2012. Epithelial cell polarity, stem cells and cancer. Nat Rev Cancer 12: 23–38 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K 2007. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128: 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, Yu W, Rodriguez-Fraticelli AE, Ewald AJ, Werb Z, Alonso MA, Mostov K 2008. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr Biol 18: 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda-Hirata M, Suzuki A, Amano Y, Yamashita K, Ide M, Yamanaka T, Sakai M, Imamura M, Ohno S 2009. Intracellular polarity protein PAR-1 regulates extracellular laminin assembly by regulating the dystroglycan complex. Genes Cells 14: 835–850 [DOI] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG 2011. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol 21: 727–735 [DOI] [PubMed] [Google Scholar]
- McDonald JA, Khodyakova A, Aranjuez G, Dudley C, Montell DJ 2008. PAR-1 kinase regulates epithelial detachment and directional protrusion of migrating border cells. Curr Biol 18: 1659–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ 2008. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol 9: 833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A, Ramyar K, Vechio JD, Cleveland DW 1996. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 87: 447–458 [DOI] [PubMed] [Google Scholar]
- Merdes A, Heald R, Samejima K, Earnshaw WC, Cleveland DW 2000. Formation of spindle poles by dynein/dynactin-dependent transport of NuMA. J Cell Biol 149: 851–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG 2005. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol 170: 1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel D, Arsanto JP, Massey-Harroche D, Beclin C, Wijnholds J, Le Bivic A 2005. PATJ connects and stabilizes apical and lateral components of tight junctions in human intestinal cells. J Cell Sci 118: 4049–4057 [DOI] [PubMed] [Google Scholar]
- Michele DE, Campbell KP 2003. Dystrophin-glycoprotein complex: Post-translational processing and dystroglycan function. J Biol Chem 278: 15457–15460 [DOI] [PubMed] [Google Scholar]
- Miner JH, Yurchenco PD 2004. Laminin functions in tissue morphogenesis. Ann Rev Cell Dev Biol 20: 255–284 [DOI] [PubMed] [Google Scholar]
- Montell DJ, Rorth P, Spradling AC 1992. Slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP. Cell 71: 51–62 [DOI] [PubMed] [Google Scholar]
- Morais-de-Sá E, Mirouse V, St Johnston D 2010. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell 141: 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostov K, Su T, ter Beest M 2003. Polarized epithelial membrane traffic: Conservation and plasticity. Nat Cell Biol 5: 287–293 [DOI] [PubMed] [Google Scholar]
- Musch A, Cohen D, Kreitzer G, Rodriguez-Boulan E 2001. cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. EMBO J 20: 2171–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllymaki SM, Teravainen TP, Manninen A 2011. Two distinct integrin-mediated mechanisms contribute to apical lumen formation in epithelial cells. PloS ONE 6: e19453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai-Tamai Y, Mizuno K, Hirose T, Suzuki A, Ohno S 2002. Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells 7: 1161–1171 [DOI] [PubMed] [Google Scholar]
- Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, Ginestier C, Marchetto S, Jacquemier J, Isnardon D, Le Bivic A, et al. 2005. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene 24: 4330–4339 [DOI] [PubMed] [Google Scholar]
- Nelson WJ 2003. Adaptation of core mechanisms to generate cell polarity. Nature 422: 766–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumuller RA, Knoblich JA 2009. Dividing cellular asymmetry: Asymmetric cell division and its implications for stem cells and cancer. Genes Dev 23: 2675–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K 2005. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol 7: 270–277 [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Jou TS, Pollack AL, Zhang Q, Hansen SH, Yurchenco P, Mostov KE 2001. Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat Cell Biol 3: 831–838 [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Zegers MM, Mostov KE 2002. Opinion: Building epithelial architecture: Insights from three-dimensional culture models. Nat Rev Mol Cell Biol 3: 531–537 [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Tang K, Kats ES, Schutz-Geschwender A, Lipschutz JH, Mostov KE 2004. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Dev Cell 7: 21–32 [DOI] [PubMed] [Google Scholar]
- Ooshio T, Fujita N, Yamada A, Sato T, Kitagawa Y, Okamoto R, Nakata S, Miki A, Irie K, Takai Y 2007. Cooperative roles of Par-3 and afadin in the formation of adherens and tight junctions. J Cell Sci 120: 2352–2365 [DOI] [PubMed] [Google Scholar]
- Partanen JI, Nieminen AI, Makela TP, Klefstrom J 2007. Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proc Natl Acad Sci 104: 14694–14699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyre E, Jaouen F, Saadaoui M, Haren L, Merdes A, Durbec P, Morin X 2011. A lateral belt of cortical LGN and NuMA guides mitotic spindle movements and planar division in neuroepithelial cells. J Cell Biol 193: 141–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinal N, Goberdhan DC, Collinson L, Fujita Y, Cox IM, Wilson C, Pichaud F 2006. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr Biol 16: 140–149 [DOI] [PubMed] [Google Scholar]
- Pinheiro EM, Montell DJ 2004. Requirement for Par-6 and Bazooka in Drosophila border cell migration. Development 131: 5243–5251 [DOI] [PubMed] [Google Scholar]
- Plachot C, Chaboub LS, Adissu HA, Wang L, Urazaev A, Sturgis J, Asem EK, Lelievre SA 2009. Factors necessary to produce basoapical polarity in human glandular epithelium formed in conventional and high-throughput three-dimensional culture: Example of the breast epithelium. BMC Biol 7: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack AL, Runyan RB, Mostov KE 1998. Morphogenetic mechanisms of epithelial tubulogenesis: MDCK cell polarity is transiently rearranged without loss of cell-cell contact during scatter factor/hepatocyte growth factor-induced tubulogenesis. Dev Biol 204: 64–79 [DOI] [PubMed] [Google Scholar]
- Qin Y, Capaldo C, Gumbiner BM, Macara IG 2005. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol 171: 1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Meisen WH, Hao Y, Macara IG 2010. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J Cell Biol 189: 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S, Shen CJ, Desai RA, Sniadecki NJ, Nelson CM, Chen CS 2010. Decoupling diffusional from dimensional control of signaling in 3D culture reveals a role for myosin in tubulogenesis. J Cell Sci 123: 2877–2883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsch S, Karsenti E 1994. Orientation of spindle axis and distribution of plasma membrane proteins during cell division in polarized MDCKII cells. J Cell Biol 126: 1509–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retta SF, Balzac F, Avolio M 2006. Rap1: A turnabout for the crosstalk between cadherins and integrins. Eur J Cell Biol 85: 283–293 [DOI] [PubMed] [Google Scholar]
- Revenu C, Gilmour D 2009. EMT 2.0: Shaping epithelia through collective migration. Curr Opin Genet Dev 19: 338–342 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli AE, Vergarajauregui S, Eastburn DJ, Datta A, Alonso MA, Mostov K, Martin-Belmonte F 2010. The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J Cell Biol 189: 725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh MH, Fan S, Liu CJ, Margolis B 2003. The Crumbs3-Pals1 complex participates in the establishment of polarity in mammalian epithelial cells. J Cell Sci 116: 2895–2906 [DOI] [PubMed] [Google Scholar]
- Rooney N, Streuli CH 2011. How integrins control mammary epithelial differentiation: A possible role for the ILK-PINCH-Parvin complex. FEBS Lett 585: 1663–1672 [DOI] [PubMed] [Google Scholar]
- Rorth P 2009. Collective cell migration. Annu Rev Cell Dev Biol 25: 407–429 [DOI] [PubMed] [Google Scholar]
- Royer C, Lu X 2011. Epithelial cell polarity: A major gatekeeper against cancer? Cell Death Differ 18: 1470–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T, Ikeda W, Ogita H, Fujita N, Takai Y 2007. The roles of nectins in cell adhesions: Cooperation with other cell adhesion molecules and growth factor receptors. Curr Opin Cell Biol 19: 593–602 [DOI] [PubMed] [Google Scholar]
- Sato K, Watanabe T, Wang S, Kakeno M, Matsuzawa K, Matsui T, Yokoi K, Murase K, Sugiyama I, Ozawa M, et al. 2011. Numb controls E-cadherin endocytosis through pp120 catenin with aPKC. Mol Biol Cell 22: 3103–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalen M, Bellaiche Y 2009. Cell division orientation and planar cell polarity pathways. Semin Cell Dev Biol 20: 972–977 [DOI] [PubMed] [Google Scholar]
- Shewan A, Eastburn DJ, Mostov K 2011. Phosphoinositides in cell architecture. Cold Spring Harb Perspect Biol 3: a004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Straight S, Margolis B 2005. PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J Cell Biol 168: 705–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B 2006. Tight junctions and cell polarity. Annu Rev Cell Dev Biol 22: 207–235 [DOI] [PubMed] [Google Scholar]
- Siderovski DP, Diverse-Pierluissi M, De Vries L 1999. The GoLoco motif: A Galphai/o binding motif and potential guanine-nucleotide exchange factor. Trends Biochem Sci 24: 340–341 [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E 1997. Functional rafts in cell membranes. Nature 387: 569–572 [DOI] [PubMed] [Google Scholar]
- Stephenson RO, Yamanaka Y, Rossant J 2010. Disorganized epithelial polarity and excess trophectoderm cell fate in preimplantation embryos lacking E-cadherin. Development 137: 3383–3391 [DOI] [PubMed] [Google Scholar]
- St Johnston D, Ahringer J 2010. Cell polarity in eggs and epithelia: Parallels and diversity. Cell 141: 757–774 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S 2001. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol 152: 1183–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Ishiyama C, Hashiba K, Shimizu M, Ebnet K, Ohno S 2002. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J Cell Sci 115: 3565–3573 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N, et al. 2004. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol 14: 1425–1435 [DOI] [PubMed] [Google Scholar]
- Teddy JM, Kulesa PM 2004. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development 131: 6141–6151 [DOI] [PubMed] [Google Scholar]
- Vaezi A, Bauer C, Vasioukhin V, Fuchs E 2002. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell 3: 367–381 [DOI] [PubMed] [Google Scholar]
- Van Campenhout CA, Eitelhuber A, Gloeckner CJ, Giallonardo P, Gegg M, Oller H, Grant SG, Krappmann D, Ueffing M, Lickert H 2011. Dlg3 trafficking and apical tight junction formation is regulated by nedd4 and nedd4–2 e3 ubiquitin ligases. Dev Cell 21: 479–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E 2000. Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell 100: 209–219 [DOI] [PubMed] [Google Scholar]
- Walther RF, Pichaud F 2010. Crumbs/DaPKC-dependent apical exclusion of Bazooka promotes photoreceptor polarity remodeling. Curr Biol 20: 1065–1074 [DOI] [PubMed] [Google Scholar]
- Wang Q, Margolis B 2007. Apical junctional complexes and cell polarity. Kidney Int 72: 1448–1458 [DOI] [PubMed] [Google Scholar]
- Wang CZ, Hsu YM, Tang MJ 2005. Function of discoidin domain receptor I in HGF-induced branching tubulogenesis of MDCK cells in collagen gel. J Cell Physiol 203: 295–304 [DOI] [PubMed] [Google Scholar]
- Wang Q, Chen XW, Margolis B 2007. PALS1 regulates E-cadherin trafficking in mammalian epithelial cells. Mol Biol Cell 18: 874–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir ML, Oppizzi ML, Henry MD, Onishi A, Campbell KP, Bissell MJ, Muschler JL 2006. Dystroglycan loss disrupts polarity and β-casein induction in mammary epithelial cells by perturbing laminin anchoring. J Cell Sci 119: 4047–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte JR, Munro S 2002. Vesicle tethering complexes in membrane traffic. J Cell Sci 115: 2627–2637 [DOI] [PubMed] [Google Scholar]
- Wu H, Feng W, Chen J, Chan LN, Huang S, Zhang M 2007. PDZ domains of Par-3 as potential phosphoinositide signaling integrators. Mol Cell 28: 886–898 [DOI] [PubMed] [Google Scholar]
- Xu R, Boudreau A, Bissell MJ 2009. Tissue architecture and function: Dynamic reciprocity via extra- and intra-cellular matrices. Cancer Metast Rev 28: 167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Spencer VA, Groesser DL, Bissell MJ 2010. Laminin regulates PI3K basal localization and activation to sustain STAT5 activation. Cell Cycle 9: 4315–4322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Cukierman E 2007. Modeling tissue morphogenesis and cancer in 3D. Cell 130: 601–610 [DOI] [PubMed] [Google Scholar]
- Yamada S, Nelson WJ 2007. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J Cell Biol 178: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, Nagai Y, Yamashita A, Hirose T, Ishikawa H, et al. 2001. PAR-6 regulates aPKC activity in a novel way and mediates cell–cell contact-induced formation of the epithelial junctional complex. Genes Cells 6: 721–731 [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T, Iwamatsu A, Shinohara A, Ohno S 2003. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol 13: 734–743 [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Izumi N, Suzuki A, Mizuno K, Ohno S 2006. Lgl mediates apical domain disassembly by suppressing the PAR-3-aPKC-PAR-6 complex to orient apical membrane polarity. J Cell Sci 119: 2107–2118 [DOI] [PubMed] [Google Scholar]
- Yamashita K, Suzuki A, Satoh Y, Ide M, Amano Y, Masuda-Hirata M, Hayashi YK, Hamada K, Ogata K, Ohno S 2010. The 8th and 9th tandem spectrin-like repeats of utrophin cooperatively form a functional unit to interact with polarity-regulating kinase PAR-1b. Biochem Biophys Res Commun 391: 812–817 [DOI] [PubMed] [Google Scholar]
- Yap AS, Kovacs EM 2003. Direct cadherin-activated cell signaling: A view from the plasma membrane. J Cell Biol 160: 11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, O’Brien LE, Wang F, Bourne H, Mostov KE, Zegers MM 2003. Hepatocyte growth factor switches orientation of polarity and mode of movement during morphogenesis of multicellular epithelial structures. Mol Biol Cell 14: 748–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Datta A, Leroy P, O’Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM 2005. β1-Integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell 16: 433–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Shewan AM, Brakeman P, Eastburn DJ, Datta A, Bryant DM, Fan QW, Weiss WA, Zegers MM, Mostov KE 2008. Involvement of RhoA, ROCK I and myosin II in inverted orientation of epithelial polarity. EMBO Rep 9: 923–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenco PD 2011. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol 3: a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers MM, O'Brien LE, Yu W, Datta A, Mostov KE 2003. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol 13: 169–176 [DOI] [PubMed] [Google Scholar]
- Zhang H, Abraham N, Khan LA, Hall DH, Fleming JT, Gobel V 2011. Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat Cell Biol 13: 1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Zhu H, Wan Q, Liu J, Xiao Z, Siderovski DP, Du Q 2010. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J Cell Biol 189: 275–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein AC, Luque A, Turlo KA, Hofmann JJ, Yee KM, Becker MS, Fassler R, Mellman I, Lane TF, Iruela-Arispe ML 2010. β1 Integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell 18: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]