Abstract
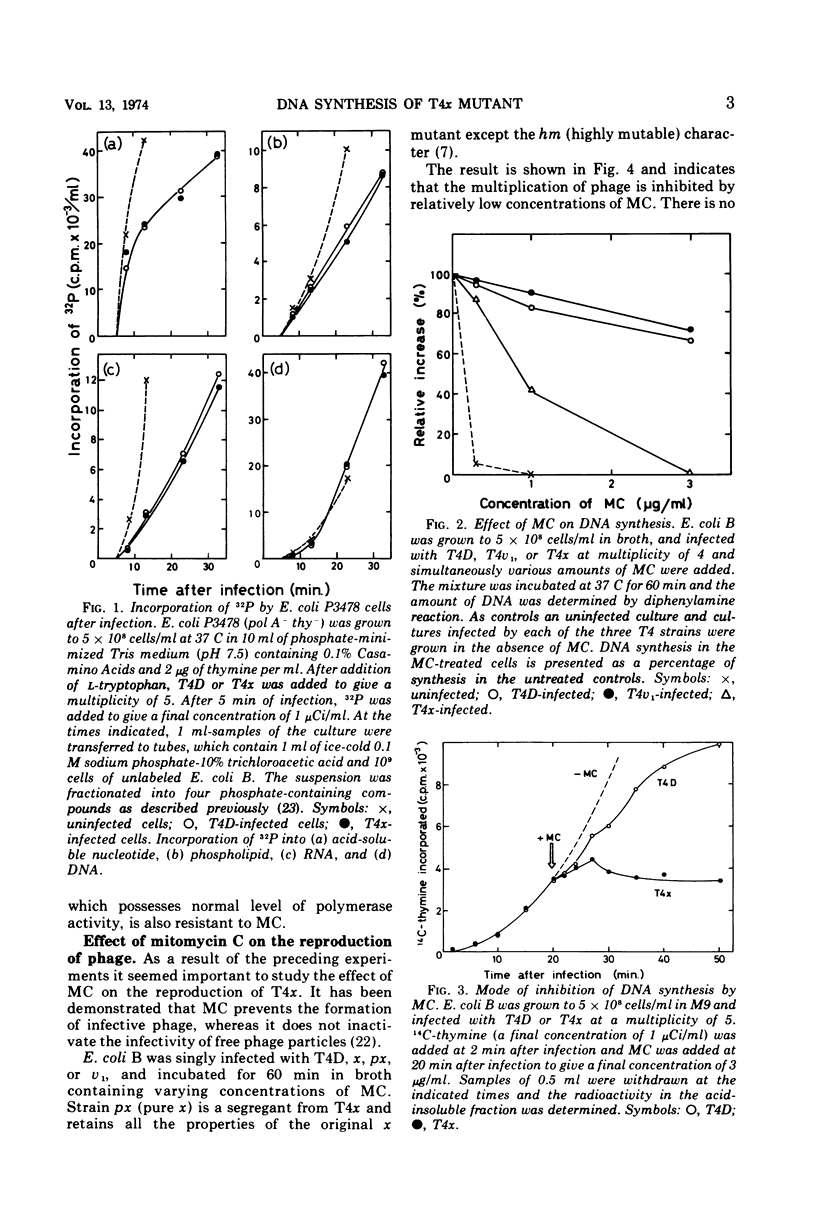
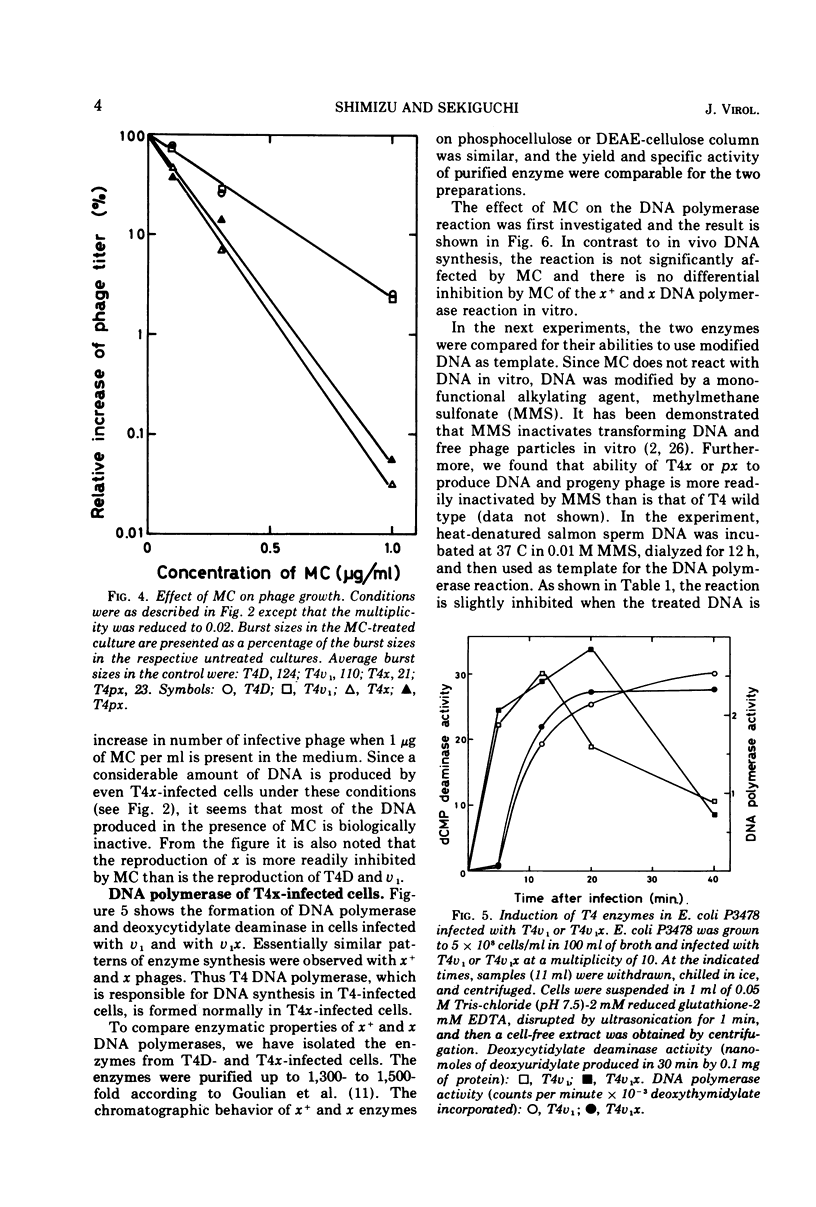
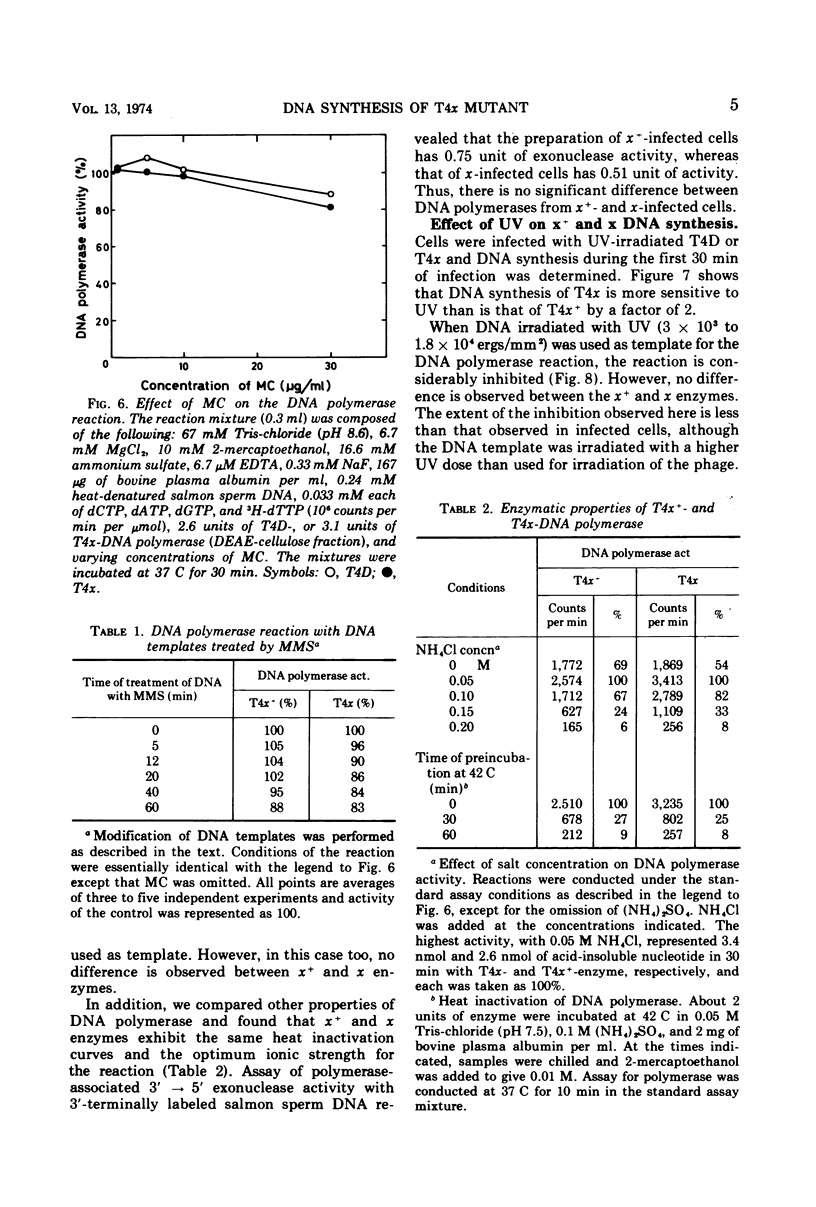
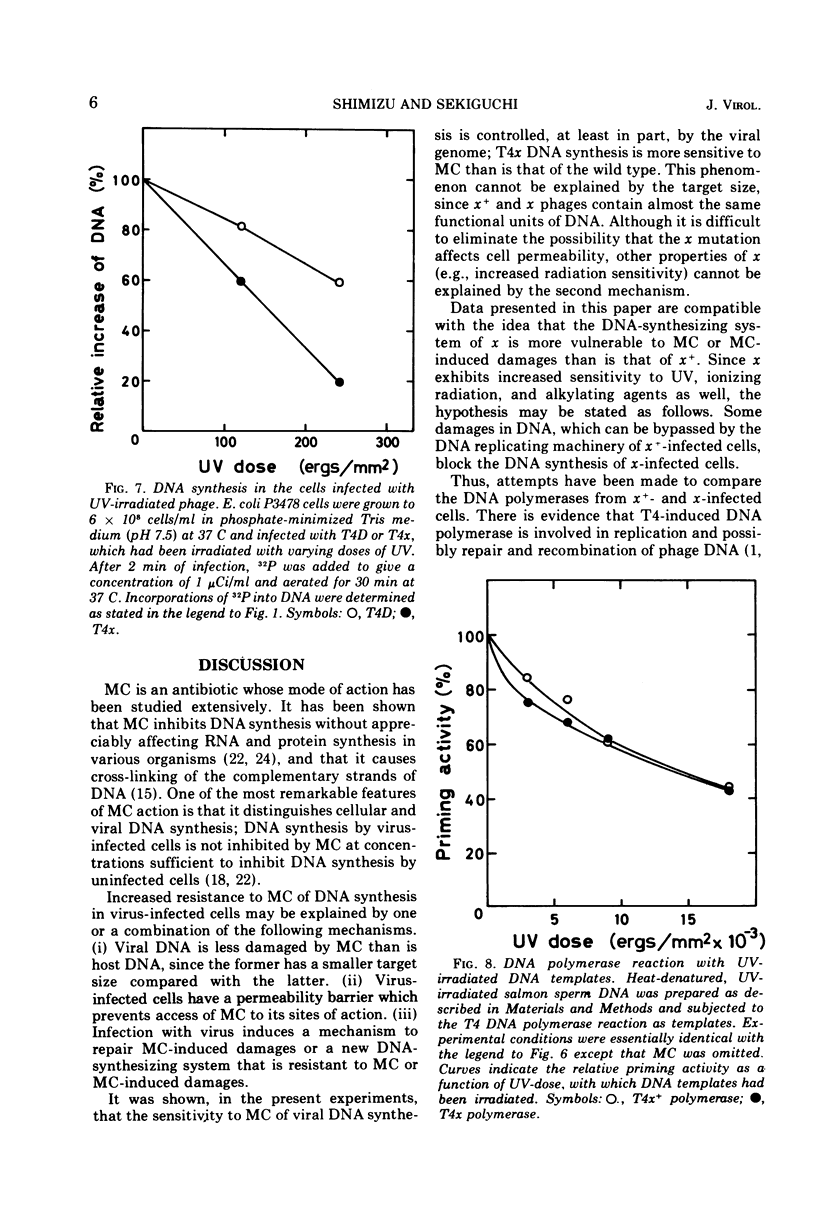
Biochemical studies were carried out to determine the effect of χ mutation on T4 DNA synthesis. The rate and final extent of DNA synthesis are almost the same with T4D- and T4χ-infected cells, although the burst size of T4χ is about one-sixth that of the wild type. The DNA synthesis of T4χ-infected cells is more readily inhibited by mitomycin C than is that of T4 wild type. When mitomycin C was added during active phage growth, DNA synthesis of T4χ halted almost immediately. T4 DNA polymerases isolated from χ+- and χ-infected cells, however, exhibit no difference with regard to their sensitivities to mitomycin C, priming activities with alkylated or ultraviolet light-irradiated templates and other enzymatic properties.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOLLUM F. J., SETLOW R. B. Ultraviolet inactivation of DNA primer activity. I. Effects of different wavelengths and doses. Biochim Biophys Acta. 1963 Apr 30;68:599–607. doi: 10.1016/0006-3002(63)90189-6. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldy M. W., Strom B., Bernstein H. Repair of alkylated bacteriophage T4 deoxyribonucleic acid by a mechanism involving polynucleotide ligase. J Virol. 1971 Mar;7(3):407–408. doi: 10.1128/jvi.7.3.407-408.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldy M. W. The UV sensitivity of some early-function temperature-sensitive mutants of phage T4. Virology. 1970 Feb;40(2):272–287. doi: 10.1016/0042-6822(70)90403-4. [DOI] [PubMed] [Google Scholar]
- Boyle J. M., Symonds N. Radiation-sensitive mutants of T4D. I. T4y: a new radiation-sensitive mutant; effect of the mutation on radiation survival, growth and recombination. Mutat Res. 1969 Nov-Dec;8(3):431–439. doi: 10.1016/0027-5107(69)90060-8. [DOI] [PubMed] [Google Scholar]
- De Waard A., Paul A. V., Lehman I. R. The structural gene for deoxyribonucleic acid polymerase in bacteriophages T4 and T5. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1241–1248. doi: 10.1073/pnas.54.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J. W. The genetic control of spontaneous and induced mutation rates in bacteriophage T4. Genetics. 1973 Apr;73(Suppl):45–64. [PubMed] [Google Scholar]
- Friedberg E. C., King J. J. Dark repair of ultraviolet-irradiated deoxyribonucleic acid by bacteriophage T4: purification and characterization of a dimer-specific phage-induced endonuclease. J Bacteriol. 1971 May;106(2):500–507. doi: 10.1128/jb.106.2.500-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg E. C. Studies on the substrate specificity of the T 4 excision repair endonuclease. Mutat Res. 1972 Jun;15(2):113–123. doi: 10.1016/0027-5107(72)90024-3. [DOI] [PubMed] [Google Scholar]
- Goulian M., Lucas Z. J., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXV. Purification and properties of deoxyribonucleic acid polymerase induced by infection with phage T4. J Biol Chem. 1968 Feb 10;243(3):627–638. [PubMed] [Google Scholar]
- HARM W. Mutants of phage T4 with increased sensitivity to ultraviolet. Virology. 1963 Jan;19:66–71. doi: 10.1016/0042-6822(63)90025-4. [DOI] [PubMed] [Google Scholar]
- Harm W. Recovery of UV-inactivated E. coli cells by the v-gene action of phage T4. Mutat Res. 1968 Jul-Aug;6(1):175–179. doi: 10.1016/0027-5107(68)90115-2. [DOI] [PubMed] [Google Scholar]
- IYER V. N., SZYBALSKI W. A MOLECULAR MECHANISM OF MITOMYCIN ACTION: LINKING OF COMPLEMENTARY DNA STRANDS. Proc Natl Acad Sci U S A. 1963 Aug;50:355–362. doi: 10.1073/pnas.50.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KECK K., MAHLER H. R., FRASER D. Synthesis of deoxycytidine-5'-phosphate deaminase in Escherichia coli infected by T2 bacteriophage. Arch Biochem Biophys. 1960 Jan;86:85–88. doi: 10.1016/0003-9861(60)90373-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGEE W. E., MILLER O. V. Dissociation of the synthesis of host and viral deoxyribonucleic acid. Biochim Biophys Acta. 1962 Jun 11;55:818–826. doi: 10.1016/0006-3002(62)90894-6. [DOI] [PubMed] [Google Scholar]
- Otsuji N., Murayama I. Deoxyribonucleic acid damage by monofunctional mitomycins and its repair in Escherichia coli. J Bacteriol. 1972 Feb;109(2):475–483. doi: 10.1128/jb.109.2.475-483.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poddar R. K., Sinsheimer R. L. Nature of the complementary strands synthesized in vitro upon the single-stranded circular DNA of bacteriophage phiX174 after ultraviolet irradiation. Biophys J. 1971 Apr;11(4):355–369. doi: 10.1016/s0006-3495(71)86220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray U., Bartenstein L., Drake J. W. Inactivation of bacteriophage T4 by ethyl methanesulfonate: influence of host and viral genotypes. J Virol. 1972 Mar;9(3):440–447. doi: 10.1128/jvi.9.3.440-447.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEKIGUCHI M., TAKAGI Y. Effect of mitomycin C on the synthesis of bacterial and viral deoxyribonucleic acid. Biochim Biophys Acta. 1960 Jul 15;41:434–443. doi: 10.1016/0006-3002(60)90040-8. [DOI] [PubMed] [Google Scholar]
- SHIBA S., TERAWAKI A., TAGUCHI T., KAWAMATA J. Selective inhibition of formation of deoxyribonucleic acid in Escherichia coli by mitomycin C. Nature. 1959 Apr 11;183(4667):1056–1057. doi: 10.1038/1831056a0. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M. Studies on the physiological defect in rII mutants of bacteriophage T4. J Mol Biol. 1966 Apr;16(2):503–522. doi: 10.1016/s0022-2836(66)80188-2. [DOI] [PubMed] [Google Scholar]
- Smith S. M., Symonds N. The unexpected location of a gene conferring abnormal radiation sensitivity on phage T4. Nature. 1973 Feb 9;241(5389):395–396. doi: 10.1038/241395a0. [DOI] [PubMed] [Google Scholar]
- Taketo A., Yasuda S., Sekiguchi M. Initial step of excision repair in Escherichia coli: replacement of defective function of uvr mutants by T4 endonuclease V. J Mol Biol. 1972 Sep 14;70(1):1–14. doi: 10.1016/0022-2836(72)90160-x. [DOI] [PubMed] [Google Scholar]
- Wood W. B., Edgar R. S. Building a bacterial virus. Sci Am. 1967 Jul;217(1):61–passim. [PubMed] [Google Scholar]
- Yasuda S., Sekiguchi M. T4 endonuclease involved in repair of DNA. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1839–1845. doi: 10.1073/pnas.67.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ende P., Symonds N. The isolation and characterization of a T4 mutant partially defective in recombination. Mol Gen Genet. 1972;116(3):239–247. doi: 10.1007/BF00269768. [DOI] [PubMed] [Google Scholar]



