Abstract
Internal ribosome entry sites/segments (IRESs) were first discovered over 20 years ago in picornaviruses, followed by the discovery of two other types of IRES in hepatitis C virus (HCV), and the dicistroviruses, which infect invertebrates. In the meantime, reports of IRESs in eukaryotic cellular mRNAs started to appear, and the list of such putative IRESs continues to grow to the point in which it now stands at ∼100, 80% of them in vertebrate mRNAs. Despite initial skepticism from some quarters, there now seems universal agreement that there is genuine internal ribosome entry on the viral IRESs. However, the same cannot be said for cellular mRNA IRESs, which continue to be shrouded in controversy. The aim of this article is to explain why vertebrate mRNA IRESs remain controversial, and to discuss ways in which these controversies might be resolved.
Internal ribosome entry sites/segments (IRESs) permit translation initiation in the middle of an mRNA sequence. While viral IRESs appear genuine, eukaryotic IRESs remain controversial.
The first part of this article reviews the current understanding of viral IRESs, mainly picornavirus IRESs, because they have strongly influenced thinking on putative cellular IRESs, and because picornavirus IRESs, especially the encephalomyocarditis virus (EMCV) IRES, and to a lesser extent the human rhinovirus (HRV) and poliovirus (PV) IRESs, are frequently used as positive controls in tests for putative cellular mRNA IRESs. All viral IRESs are readily classifiable into distinct families on the basis of sequence and secondary structure: (1) the intergenic IRES of invertebrate dicistroviruses, (2) the hepatitis C virus (HCV) and related animal virus IRESs, and (3) the picornavirus IRESs that can be further classified into several distinct subgroups, including one class (exemplified by porcine teschovirus 1 and simian virus 9) that is remarkably similar to the HCV-like IRESs in structure and initiation factor requirements (Hellen and de Breyne 2007; de Breyne et al. 2008). The predicted secondary structures of these RNA virus 5′-UTRs are particularly robust, because they are founded not only on direct structure probing but also on extensive phylogenetic comparisons. The very high error frequency of RNA replication results in enormous genetic drift, both within species and between species, and so there are numerous covariances validating the proposed base-pairing.
In contrast, the putative IRESs identified in cellular mRNAs defy classification because they are all different from one another in sequence and predicted secondary structures (Baird et al. 2006), which have necessarily been elucidated entirely from structure probing, as there is insufficient genetic drift, even between different animal species, to provide useful phylogenetic data. It may well be that the usual mammalian species for such comparisons (mainly primates, rodents, and ruminants) are too close in evolution, and it might be more informative to widen the comparison to include more distant vertebrates, such as birds, frogs, and marsupials, which has proved helpful in the discovery of other regulatory elements in mRNAs (Koeller et al. 1989; Sherrill and Lloyd 2008).
Putative cellular IRESs have been generally considered closest to the true picornavirus IRESs (mainly on the grounds of similar initiation factor requirements), although it will become apparent that any similarity is very remote. The dicistrovirus intergenic IRESs and the HCV-like IRESs differ from picornavirus IRESs in that they bind 40S ribosomal subunits directly in the absence of any canonical translation initiation factors. Initiation on the dicistrovirus intergenic IRESs does not even require Met-tRNAi and does not occur at an AUG codon (Sasaki and Nakashima 2000; Wilson et al. 2000). Although initiation on the HCV-like IRESs does require Met-tRNAi (usually as a ternary complex with eIF2 and GTP), the additional canonical initiation factor requirements are limited to eIF3, eIF5, and eIF5B, with no requirement for eIF4A, 4B, 4E, or 4G (Pestova et al.1998, 2008).
STRUCTURE AND FUNCTION OF PICORNAVIRUS IRESs (AND SOME COMPARISONS WITH PUTATIVE CELLULAR IRESs)
Classification of Picornavirus IRESs on the Basis of Sequence and Structure
Apart from one outlier, hepatitis A virus (HAV), every picornavirus IRES can be placed unambiguously into one of four distinct groups: the long-standing Type I IRESs, which include HRV, PV, and other enteroviruses, and Type II IRESs, which include foot-and-mouth disease virus (FMDV) and EMCV (Alexander et al. 1994; Jackson and Kaminski 1995); the more recently discovered Aichivirus (AV) group (Yu et al. 2011); and the HCV-like group already mentioned. Within each of the two major picornavirus IRES classes (Types I and II), there is quite strong conservation of primary sequence, particularly in unpaired loops or bulges, and even stronger conservation of predicted secondary structure (Jackson and Kaminski 1995). However, there is very little similarity between the different classes (including HAV and AV) apart from a ∼25 nt tract at the 3′-end (as defined by deletion analysis), which is G-poor throughout, pyrimidine-rich at its 5′-end, followed by a middle section that is hypervariable even between different strains and isolates of the same virus species (Pöyry et al. 1992), and ends in an AUG triplet (Fig. 1). The deletion mapping shows that the 5′-boundary of these IRESs is slightly “fuzzy” in that progressive deletion of the first ∼100 nt of the PV-2 5′-UTR, or the first ∼120 nt downstream from the polyC tract of EMCV, reduces internal initiation by up to ∼30% (Jang and Wimmer 1990; Nicholson et al. 1991). However, further deletion from the 5′-end results in a precipitous fall in activity, and the position in which this dramatic decrease occurs is usually taken as the 5′-boundary of the core IRES, which is typically ∼450 nt in length. The 3′-boundary is very sharp and coincides with the AUG immediately downstream from the oligopyrimidine tract. Any attempt to shorten the pyrimidine-rich tract and thereby move the AUG further upstream by more than a very few residues abrogates activity (Iizuka et al. 1989; Kaminski et al. 1994). Subdomains of the core IRES show no IRES activity. In contrast, for many putative cellular mRNA IRESs, several quite short segments have been reported to promote fairly efficient internal initiation, leading to the suggestion that such IRESs are composed of multiple short modules, which promote internal initiation by acting in combination (Stoneley and Willis 2004).
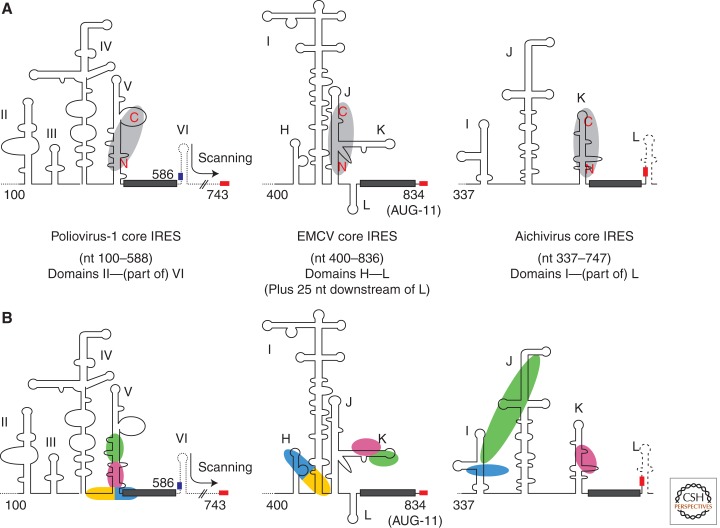
Figure 1.
Secondary structure models of the three main classes of picornavirus IRESs, with the eIF4G and PTB (polypyrimidine tract binding protein) binding sites also shown. (A) Secondary structures of the designated core IRESs, with individual structural domains labeled according to standard nomenclature. The dotted lines represent 5′-UTR sequences outside the core IRES boundaries, and the dashed line at 3′-end of the Aichivirus structure shows 5′-proximal viral coding sequences. The dark gray rectangle represents the ∼25 nt pyrimidine-rich tract at the 3′-end of the IRESs, the small red rectangle shows the authentic initiation site AUG, and the blue rectangle represents the putative ribosome recruitment AUG of Type I IRESs (at nt 586 in poliovirus type 1). The binding site of the central domain of eIF4G (p50 fragment—see Fig. 2A) on each IRES, as determined by footprinting and tethered hydroxyl radical probing, is shown in light gray, with the amino and carboxyl termini indicated (in red) to show the orientation of binding (Kolupaeva et al. 2003; de Breyne et al. 2009; Yu et al. 2011). (B) Sites and orientation of PTB binding, as determined by tethered hydroxyl radical probing. The interaction sites of each RBD (RNA-binding domain) of PTB-1 are shown on the three IRES secondary structure maps, using the same color coding as in Figure 2B, namely: RBD-1 in green, RBD-2 in pink, RBD-3 in blue and RBD-4 in yellow (Kafasla et al. 2009, 2010). The Aichivirus results showed that RBD-1 interacts strongly with the apical regions of both domains I and J, suggesting that these regions are closer to each other than can be shown on a two-dimensional diagram (Yu et al. 2011), which explains the elongated depiction of RBD-1. No contacts between RBD-4 and the Aichivirus IRES were detected.
Picornavirus 5′-UTRs have numerous AUG triplets at about the frequency expected for random occurrence (one per 64 nt), whereas a survey of the mammalian mRNA 5′-UTR database showed that AUG triplets occurred only slightly more frequently in the 66 mRNAs with putative IRESs (median frequency of one per 300 nt) than in the mRNA population at large (Baird et al. 2006). The AUG triplets in picornavirus 5′-UTRs are also subject to genetic drift, as shown by the fact that in 33 clinical isolates of poliovirus type 3 the number ranged from 5 to 15 (Pöyry et al. 1992); only three of them were absolutely conserved and the downstream short ORFs were not conserved in length. The only conserved AUG important for IRES activity is the one located at the 3′-end of the IRES. In the case of the EMCV IRES this AUG (AUG-11) is the authentic initiation codon, and there is very good evidence that 43S preinitiation complexes bind initially at, or extremely near, this AUG. Almost all translation initiation is at this AUG, with a little at AUG-12, situated four codons further downstream, but there is hardly any initiation at a nonconserved AUG present in some EMCV strains 8 nt upstream of AUG-11 (Kaminski et al. 1990, 1994).
In contrast, very little, if any, initiation occurs at the equivalent AUG located just downstream from the oligopyrimidine tract in Type I IRESs (Pestova et al. 1994; Kaminski et al. 2010), and the authentic initiation codon is the next AUG further downstream, at a distance of ∼35 nt in rhinoviruses and ∼160 nt in polioviruses and other enteroviruses. Despite its negligible activity as an initiation site, this AUG at the end of the pyrimidine-rich tract is nevertheless very important for efficient initiation at the correct downstream start site. Mutation of the near-silent AUG in HRV-2 and PV-2 reduced initiation at the authentic start site by ∼70% (Meerovitch et al. 1991; Kaminski et al. 2010), and conferred a small plaque phenotype in the PV-2 background (Pelletier et al. 1988). This has led to the suggestion that 43S preinitiation complexes are first recruited at this AUG, but instead of initiating there, they are transferred to the next AUG further downstream, most probably by a linear scanning process, or by a minor variant of linear scanning in which a few residues are by-passed (Kaminski et al. 2010).
Canonical Initiation Factor Requirements for Picornavirus IRESs
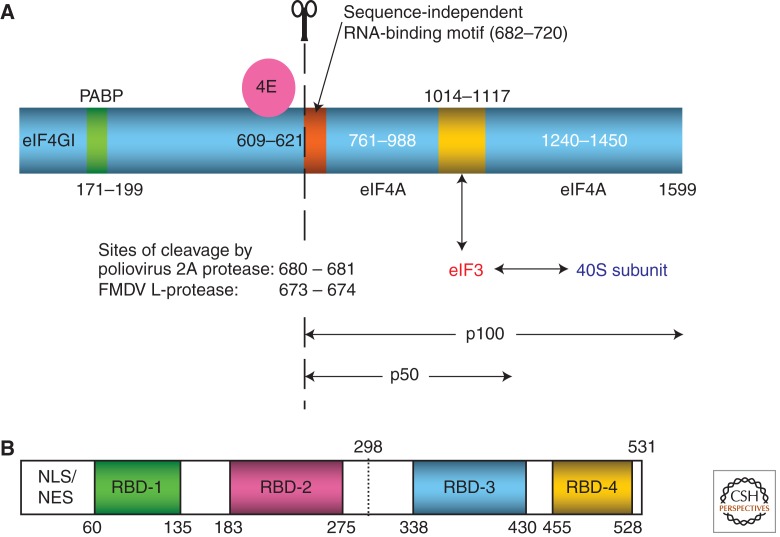
Initiation on Type I, Type II, and AV IRESs requires all the canonical initiation factors except that (1) eIF4E is completely redundant, and (2) the carboxy-terminal two-thirds fragment (p100) of eIF4G, or the central one-third fragment (p50), can substitute for full-length eIF4G and is usually somewhat better than the full-length protein. Both p50 and p100 retain the eIF3 interaction site and at least one of the two eIF4A interaction sites present in intact eIF4G (Fig. 2A). These eIF4G derivatives bind directly to the basal half of Domain V of Type 1 IRESs (de Breyne et al. 2009), the three-way junction between Domains J and K of the Type II IRESs (Kolupaeva et al. 1998; Lomakin et al. 2000), and Domain K of the AV IRESs (Yu et al. 2011), and in all cases binding is strongly enhanced by inclusion of eIF4A (Fig. 1). Although the amino terminus of p100 (or p50) has general RNA binding activity with little nucleotide sequence specificity (Prévôt et al. 2003), the high affinity binding to the viral IRESs appears to involve a more carboxy-terminal domain of eIF4G p100, because deletion of the amino terminus of p100 (or p50) has little effect on its activity in driving initiation on the EMCV IRES, but abrogates its ability to support scanning-dependent initiation (Ali and Jackson 2001; Prévôt et al. 2003).
Figure 2.
Domain structures of eIF4GI and polypyrimidine tract binding protein (PTB). (A) Domain structure of the longest isoform (1599 amino acids) of eIF4GI (blue) with associated eIF4E (magenta), showing the interaction sites of poly(A) binding protein (PABP) in green, and eIF3 (gold). The two sites of potential interaction with eIF4A are shown, although there is usually only a single bound eIF4A. The sites at which eIF4GI is cleaved by poliovirus 2A protease and FMDV L-protease are shown; the 2A cleavage site defines the amino termini of the carboxy-terminal two-thirds fragment (p100) of eIF4GI, and the central one-third domain (p50). The sequence-independent RNA-binding motif at the amino terminus of p100 (and p50) is highlighted in orange; this motif is necessary for scanning, but is not required for internal initiation on the EMCV IRES (Ali and Jackson 2001; Prévôt et al. 2003). (B) Domain structure of PTB-1. The amino-terminal ∼55 amino acid residues have nuclear import and export signals, but play no part in RNA-binding. The positions of the 4 RBDs (RNA-binding domains) are shown. RBDs-2 and -3 are longer than the other two RBDs because their RNA-binding surface has an additional β-strand. The linkers between the RBDs are flexible except for that between RBDs-3 and -4, which interact with each other in a back-to-back configuration, and act as a coordinated pair (Oberstrass et al. 2005). PTB-2 and PTB-4 differ from the canonical PTB-1 in having inserts (arising from alternative splicing) of 19 or 26 amino acids, respectively, at residue 298 of PTB-1.
Curiously, although there is some similarity between the p100 binding sites on Type II and AV IRESs, the binding site on Type I IRESs is completely different in sequence and secondary structure. Nevertheless, the positions of these binding sites suggest a common mechanism in which a 43S preinitiation complex (a 40S ribosomal subunit with associated eIF2/GTP/Met-tRNAi ternary complex, and other initiation factors including eIF3) could be initially recruited via the interaction of the eIF3 with the IRES-associated p100 (or p50), and then delivered to the AUG located immediately downstream from the oligopyrimidine tract (Fig. 1). Although the binding of eIF4G (or p100) to this internal site is a prerequisite for picornavirus IRES activity, it is clearly not sufficient, because the EMCV JK domain on its own (without the upstream H and I domains, and the downstream sequences extending to AUG-11) shows no significant activity in the standard bicistronic mRNA test for IRESs (described below).
The HAV IRES is exceptional in that it appears to require all the canonical initiation factors including the complete eIF4F complex (consisting of eIF4G, the RNA helicase eIF4A, and the cap-binding factor eIF4E), because it is inhibited by cap analogs, or 4E-BP, or cleavage of eIF4G by the poliovirus 2A protease or the FMDV L-protease (Ali et al. 2001; Borman et al. 2001). Although these publications proposed two alternative reasons for this unusual requirement of the HAV IRES, the true explanation remains unknown.
IRES Trans-Acting Factor (ITAF) Requirements
In addition to the canonical initiation factors, the activity of picornaviral IRESs is also dependent on other RNA-binding proteins, or ITAFs, but the requirements differ quite markedly for different IRESs, even between closely related IRESs. For example, although the HRV and FMDV IRESs have a very strong requirement for polypyrimidine tract binding protein (PTB) (Hunt and Jackson 1999; Pilipenko et al. 2000), the EMCV IRES generally shows high activity in the absence of any ITAFs and is only slightly stimulated by PTB (Pestova et al. 1996; Kaminski and Jackson 1998), whereas the PV and AV IRESs occupy an intermediate position between the two extremes of FMDV and EMCV (Hunt and Jackson 1999; Yu et al. 2011). The FMDV IRES also requires another RNA-binding protein of 45 kDa known as ITAF 45 (Pilipenko et al. 2000). All Type I IRESs tested so far require polyC binding protein-2 (Walter et al. 1999), which seems to act in conjunction with SRp20 (Bedard et al. 2007). In addition, the type I HRV IRES has a very strong requirement for Unr (upstream of N-ras), whereas this ITAF has little effect on the PV IRES in vitro (Hunt et al. 1999), although a strong dependency was seen in transfection assays (Boussadia et al. 2003). The PV IRES is also reported to be strongly stimulated by the autoantigen La (Svitkin et al. 1994; Costa-Mattioli et al. 2004).
The list of ITAFs reported to affect the activity of various putative cellular mRNA IRESs is even longer (reviewed in King et al. 2010); in addition to PTB (also known as hnRNP I), PCBP-2, Unr and La, it includes several other hnRNP proteins and other RNA-binding proteins that are predominantly located in the nucleus. As with the viral IRESs, PTB is the most “promiscuous” in the sense that it stimulates more putative cellular IRESs than any other ITAF (King et al. 2010).
As for the underlying mechanism, there is no evidence that any of the ITAFs play a direct role in recruiting 43S preinitiation complexes to the IRES. The idea of a more indirect effect is also consistent with the rather puzzling finding that closely related viral IRESs (e.g., EMCV and FMDV) differ quite markedly in their dependency on ITAFs. A frequent suggestion is that ITAF binding might remodel the secondary and tertiary structure of the IRES into a form that is more optimal for internal initiation, and this receives some support from the observation that ITAF binding not only protects some IRES residues against enzymatic and chemical attack (as would be expected), but also makes some residues more susceptible to these reagents (Pilipenko et al. 2000). Further clues have emerged from recent studies using tethered hydroxyl radical probing to determine which of the four RNA-binding domains (RBDs) of PTB (Fig. 2B) binds to which site on the IRES. This has shown that the core EMCV IRES binds a single PTB at widely dispersed sites (Fig. 1), such that PTB binding could constrain the three-dimensional flexibility of the IRES (Kafasla et al. 2009). In contrast, however, PTB binding to the PV-1 IRES was highly localized to the basal half of Domain V and the single-stranded flanking linkers (Fig. 1). The PTB binding site overlapped the eIF4G-binding site on the PV-1 IRES, and it was shown that PTB binding subtly repositions eIF4G (Kafasla et al. 2010), which might provide an explanation for how PTB activates Type I IRESs.
ALTERNATIVES TO THE CAP-DEPENDENT SCANNING MECHANISM OF INITIATION OF CELLULAR mRNA TRANSLATION
Because cellular mRNAs are the products of transcription by RNA Pol II, they all have m7G-caps and so they are all potentially translatable by the cap-dependent scanning mechanism; and although AUG triplets in 5′-UTRs can attenuate scanning or even act as barriers (depending on their position, context and the length of the following ORF), such AUGs occur only slightly more frequently in 5′-UTRs with putative IRESs than in the mRNA population at large (Baird et al. 2006). The question, therefore, is whether some cellular mRNAs can be translated by alternative mechanisms, which might operate in parallel with the scanning mechanism under normal conditions, but could predominate under conditions when cap-dependent (scanning) initiation is compromised.
Although the focus of this review is IRES-dependent initiation, it is worth briefly noting that there are two other “nonstandard” initiation mechanisms (excluding special mechanisms for leaderless mRNAs or mRNAs with extremely short 5′-UTRs, which are irrelevant to this article). One is ribosome shunting, which is considered a form of discontinuous scanning that has been seen with the adenovirus tripartite leader 5′-UTR and the Hsp 70 5′-UTR, and appears to be favored over scanning when eIF4F availability or activity is reduced (Yueh and Schneider 1996, 2000).
The other is the mechanism of “cap-independent” initiation of translation of several different types of plant viral RNAs (listed in Miller et al. 2007; Mokrejs et al. 2010), which all have uncapped positive strand RNA genomes. Initiation is strictly dependent on a 3′-UTR motif known as a cap-independent translation enhancer (CITE, or simply TE), which binds eIF4F through its eIF4G and/or eIF4E subunits, and promotes initiation by a mechanism of scanning from the uncapped 5′-end. This mechanism shows that a 5′-cap (and therefore, by implication, also eIF4E) is not necessary for efficient initiation by scanning from the 5′-end, provided there is an effective means of eIF4G recruitment to compensate for absence of the eIF4E-cap interaction. Like the EMCV JK domain, these TEs do not show significant IRES activity in the standard bicistronic mRNA test (described below). Although the natural position is always in the 3′-UTR, many such TEs function equally well when repositioned to replace the endogenous 5′-UTR. In view of the similarity between plant and vertebrate translation mechanisms, it seems quite possible that this initiation mechanism could also operate in vertebrates (reviewed in Shatsky et al. 2010).
CELLULAR mRNA IRESs
Indicators of a Possible IRES in a Cellular mRNA
Because there is presently no high throughput screening method for revealing cellular mRNA IRESs, they have to be identified by testing each candidate on a case-by-case basis. Two features are often taken as preliminary indicators of candidates that would be worth testing: (1) an unusually long and GC-rich 5′-UTR that is predicted to be highly structured; and (2) persistence of translation in stress conditions, or in mitosis (Qin and Sarnow 2004), when eIF4F activity or availability is compromised. However, there are caveats associated with both these indicators. All too often, a high negative ΔG value for the predicted 5′-UTR secondary structure is taken as strong evidence that initiation via cap-dependent scanning will be inevitably inefficient. However, this overlooks the fact that the ΔG value is related to the equilibrium constant for the transition from the folded to the completely unfolded forms, and is inversely related to the probability of spontaneous and unassisted complete unwinding of the whole 5′-UTR, yet scanning requires only localized step-wise unwinding of the 5′-UTR rather than a complete unwinding of the whole sequence. Thus, it is the stability of local elements of secondary structure that determines whether they are significant barriers to scanning (Özeş et al. 2011), rather than the ΔG value of the complete 5′-UTR. Indeed it has been shown that in a Krebs-2 in vitro system, or in an RNA transfection assay, an mRNA with the ∼900 nt (60% GC) LINE-1 5′-UTR, is translated by the cap-dependent scanning mechanism at 50% relative efficiency compared to the β-globin 5′-UTR (Dmitriev et al. 2007). It should also be noted that some long 5′-UTRs with apparent IRES activity have subsequently been shown to be incompletely spliced variants with retained introns (Baranick et al. 2008), for example the original eIF4GI 5′-UTR studied by Gan and Rhoads (1996), in contrast to the eIF4GI 5′-UTRs described by Johannes and Sarnow (1998) and Byrd et al. (2005).
As for the second indicator, the “persistence” of translation of some mRNAs under conditions of reduced eIF4F activity is often relative rather than absolute. For example, nucleophosmin mRNA translation does decrease in mitosis, but by only half as much as global mRNA translation is reduced (Qin and Sarnow 2004). Similarly, there is actually a decrease in c-myc synthesis during apoptosis, but the decrease is delayed by ∼2 h with respect to global protein synthesis (Bushell et al. 2006). Admittedly there are some intriguing cases of real increases in translation efficiency, for example vimentin mRNA translation during mitosis (Qin and Sarnow 2004). On the other hand, the increased VEGF expression in hypoxia (0.1% O2 for 6 h) was found to be largely ascribable to increased mRNA abundance (Young et al. 2008), although different hypoxia regimes in other cell types have been reported to activate IRES-dependent translation of VEGF mRNA (Braunstein et al. 2007).
It would be naïve to assume that the affinity of eIF4F for the 5′-end of all mRNAs is the same. It is much more likely that there is differential affinity for different mRNAs, in which case those mRNAs with high affinity will continue to be translated moderately efficiently via the scanning mechanism even when eIF4F activity has been significantly reduced. A likely reflection of this can be found in the observation that addition of cap analog (m7GTP) or especially 4E-BP1 to a Krebs-2 in vitro system resulted in less inhibition of a reporter with the Apaf-1 (especially), c-myc or Hsp70 5′-UTRs than with the actin 5′-UTR (Andreev et al. 2009). In addition, it should be appreciated that the relief of competition consequent on inhibition of bulk mRNA translation following partial eIF4F inactivation will increase the availability of other initiation factors and ribosomal subunits for those mRNAs with high functional affinity for the residual eIF4F.
It is also worth noting some other observations that are somewhat at variance (but maybe not directly contradictory) with the idea that low eIF4F activity might favor IRES-dependent translation. First, it has been found that a 90% knock-down of eIF4GI, which reduced global translation by only ∼20%, preferentially decreased translation of low abundance mRNAs that have short upstream ORFs (Ramírez-Valle et al. 2008). In contrast, the translation of several mRNAs with putative IRESs, including p120 catenin, seemed to be promoted by the overexpression of eIF4GI that commonly occurs in inflammatory breast cancer (Silvera et al. 2009). Finally, the example of the picornaviral HAV IRES shows that some bona fide IRESs may actually require an intact eIF4F complex; and the existence of CITE elements shows that “cap-independent initiation” does not necessarily equate with IRES-dependent initiation.
The Standard Bicistronic Plasmid Test for Cellular mRNA IRESs
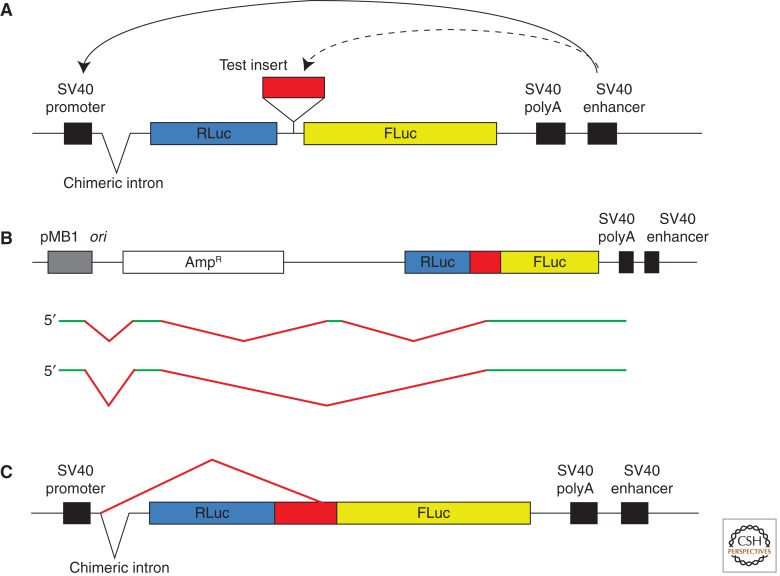
Because all cellular mRNAs have at least the potential to be translated by a cap-dependent scanning mechanism, this potential needs to be suppressed to test for possible IRES activity. The usual test follows the same approach as originally used to show the poliovirus IRES (Pelletier and Sonenberg 1988), namely to insert the candidate 5′-UTR as the intercistronic spacer of a bicistronic DNA plasmid construct, although monocistronic constructs with a very stable 5′-proximal hairpin provide a valid alternative. Although many different bicistronic expression constructs have been used (most of them listed in Kozak 2003, 2005), the majority of tests have employed a dual luciferase construct, with Renilla luciferase (RLuc) as the upstream cistron, and firefly (Photinus) luciferase (FLuc) in the downstream position (Fig. 3A). IRES activity is indicated by a significant increase in the FLuc/RLuc expression ratio as compared with a control bicistronic construct lacking the putative IRES, though the raw data for RLuc and FLuc expression should also be presented so that we can see whether a decrease in RLuc expression, which is by no means unknown (van Eden et al. 2004), has contributed significantly to the IRES-dependent increase in FLuc/RLuc expression ratio. (If insertion of the test 5′-UTR does significantly decrease RLuc expression, this could be a strong warning that splicing may have removed part or all of the RLuc cistron from some transcripts.) The intercistronic spacer in the control construct is usually a short multiple cloning site, and it could be argued that a better control would have an intercistronic spacer more similar in length to the putative IRES, such as the reverse complement of the test 5′-UTR sequence, as has been used in some publications.
Figure 3.
Mechanisms by which monocistronic mRNAs can arise from the dual luciferase plasmid construct commonly used to test for IRESs. In all three subpanels the Renilla luciferase (RLuc) ORF is shown in blue, the Photinus luciferase (FLuc) ORF in yellow, and the cellular mRNA 5′-UTR under test for IRES activity is in red. Panel (B) is on a reduced scale. (A) Configuration of the dual luciferase construct and its essential elements (promoter, chimeric intron, polyA addition signal, and enhancer). The downstream SV40 enhancer strongly activates transcription from the SV40 promoter, but can also activate transcription from any cryptic promoter element present in the putative IRES (as shown by the dashed line), thereby generating a monocistronic FLuc mRNA. (B) Transcription from a promoter in the vector backbone, near the pMB1 origin, also gives rise to monocistronic FLuc mRNA if the putative IRES has 3′-splice sites. The diagram shows two of the most abundant spliced RNA products found (Lemp et al. 2012), with the retained (exon) sequences shown in green, and the spliced out introns in red. About half of such monocistronic FLuc mRNAs are devoid of upstream AUG triplets, and so could give rise to high FLuc expression via cap-dependent scanning. These unanticipated mRNAs are equally abundant irrespective of whether the SV40 promoter is absent (as depicted) or present (Lemp et al. 2012). (C) Generation of a monocistronic FLuc mRNA by splicing from the 5′-splice donor site of the chimeric intron to 3′-splice site(s) fortuitously present in the putative IRES.
Many 5′-UTR sequences tested in this way promote a huge increase in the FLuc/RLuc ratio, often a few hundred-fold and significantly greater than the typical ∼20-fold increase observed with the bona fide EMCV IRES. The current record appears to be the triose phosphate isomerase (TPI) 5′-UTR, which elicits almost a 1000-fold increase in the FLuc/RLuc ratio (Young et al. 2008). Impressive though these stimulations may appear at first sight, it should be remembered that FLuc expression from the control (no IRES) construct should, in principle, be zero, and so a 100-fold increase in what is probably close to zero could still be quite small in absolute terms. This highlights a major weakness in this method of determining IRES activity, because it does not give any indication of the efficiency of the apparent IRES-dependent translation in comparison with the efficiency of translation of (1) a monocistronic FLuc mRNA with the same 5′-UTR, and (2) the upstream RLuc cistron (i.e., the molar ratio of FLuc/RLuc synthesis rates). The first issue could be addressed by simply deleting the RLuc 5′-UTR and ORF sequences from the bicistronic construct depicted in Figure 3A, yet this control seems to be never included in DNA transfection assays, although it is common practice in RNA transfections (discussed below). For the second issue, there seems to be no instance where the actual molar ratio of FLuc/Ruc expression has been calculated from the luciferase activity data, yet the luciferase activities per mole of each protein under the particular assay conditions that were used must surely be known, or could be quite easily determined experimentally.
Some IRESs Are Only Active if Expressed via Transcription by RNA Polymerase II
One reason for the widespread use of DNA transfection assays is that many putative cellular mRNA IRESs do not function efficiently, if at all, in cell-free translation systems (not even in extracts from HeLa or other tissue culture cells), nor in direct RNA transfections, nor in DNA transfections in which mRNA synthesis occurs in the cytoplasm because it is driven by T7 RNA polymerase generated via infection of the cells with a recombinant vaccinia virus that expresses T7 polymerase (Stoneley et al. 2000; Shiroki et al. 2002; Holcik et al. 2003). Thus, in these cases IRES activity is manifest only if the mRNA is generated in the nucleus via Pol II. This has led to the suggestion that a “nuclear experience” is required for these IRESs to be active.
One obvious and plausible explanation for this requirement is that the apparent IRES activity might actually be attributable to the production of a monocistronic FLuc mRNA, either from a cryptic Pol II promoter sequence or through a minor splicing event. When the “nuclear experience” requirement was first observed, it was claimed that such artefacts had been ruled out, and the proposed explanation was that IRES activity is absolutely dependent on certain ITAFs being deposited on the nascent bicistronic mRNA in the nucleus, and coexported to the cytoplasm. The presumption was that such ITAFs are either (normally) restricted to the nuclear compartment, or that they can only be deposited appropriately during transcription or subsequent pre-mRNA processing. However, no direct evidence supporting this hypothesis has been forthcoming in the many years since it was first proposed. Meanwhile, in the past ∼8 years there have been so many reports of monocistronic mRNA artefacts arising from cryptic promoters or from splicing that this must now surely be taken as the default explanation for the “nuclear experience,” unless or until convincing evidence to the contrary is produced.
Essential Controls to Screen for Monocistronic mRNAs Arising from Cryptic Promoters and/or Splicing
It has long been common practice to screen for possible monocistronic FLuc mRNA production by using Northern blots, or, more recently, qPCR measurement of the relative quantity of RLuc and FLuc ORF sequences. However, it is very doubtful whether these methods would detect monocistronic FLuc mRNA present at a level of less than 2%, which is the likely required threshold suggested by the relative FLuc expression from monocistronic and bicistronic mRNAs with the same test 5′-UTR in RNA transfection assays (discussed in a subsequent section). In any case, this approach is rather unsatisfactory because it is indirect and involves two controversial issues: (1) what is the required sensitivity, and (2) whether the assay achieves this level of stringency (Kozak 2003, 2005). As discussed below, better methods are now available for revealing more directly whether monocistronic mRNAs are giving rise to significant FLuc expression. Many investigators mistakenly consider the test of inserting a very stable hairpin upstream of the RLuc cistron to be a decisive control. As expected, this drastically reduces RLuc synthesis, usually with little or no effect on FLuc expression, but this outcome does not constitute proof of an IRES, because precisely the same result could be obtained if virtually all FLuc expression was from an unanticipated monocistronic mRNA.
Although some investigators screen for cryptic promoters by testing for FLuc expression when the bicistronic construct with the putative IRES sequence is transferred into a completely different plasmid background lacking known promoters (and in some such tests also lacking an enhancer), a more rigorous approach is to delete the SV40 promoter but retain the SV40 enhancer of the expression plasmid construct (Fig. 3A) actually used to assay IRES activity. This invariably results in an 80%–90% reduction in RLuc expression, and a similar percentage reduction in FLuc yield is seen in the case of the EMCV IRES and the no IRES control. However, for a great many 5′-UTRs with putative IRES activity (HIF-1α, HIF-2α, XIAP, c-myc, VEGF, VEGF receptor1, EGR-1, Glut-1, PIM-1, TPI, p27Kip1, and p57Kip2) there was, at best, only a slight reduction in downstream cistron translation, and in many cases a significant increase in FLuc expression was seen (Liu et al. 2005; Wang et al. 2005; Bert et al. 2006; Young et al. 2008). These results provide strong evidence that a cryptic promoter is producing FLuc mRNAs that certainly lack a complete RLuc ORF and may well be monocistronic. Although there is a report (Vopalensky et al. 2008) of weak cryptic promoter activity in the 3′-proximal part of the FLuc ORF (that would be troublesome only when FLuc is used as the upstream cistron), no such activity has been found in the RLuc ORF, and so the usual presumption is that the cryptic promoter lies in the putative IRES sequence itself. However, it has recently been shown that there is a cryptic promoter within the backbone (near the origin of replication) of virtually all expression vectors in current use (Lemp et al. 2012), and although the primary transcripts from this promoter are polycistronic, they can give rise to monocistronic FLuc mRNAs lacking upstream AUG triplets (and therefore translatable by cap-dependent scanning) if the tested 5′-UTR has potential 3′-splice sites (Fig. 3B). A detailed RNA analysis (e.g., 5′- RACE) is needed to determine whether the ultimate origin of the monocistronic FLuc mRNA is this vector backbone promoter or a cryptic promoter in the putative IRES (Lemp et al. 2012).
Even if (almost) all transcription were from the intended (SV40) promoter, splicing can still cause artefacts, as almost all expression vectors in current use have an intron in the 5′-UTR (Fig. 3), because introns have been shown to stimulate the synthesis, processing and nucleo-cytoplasmic transport of mRNA (Le Hir et al. 2003), but an intron in the 3′-UTR located more than ∼50 nt downstream from the stop codon will trigger nonsense mediated decay (Nagy and Maquat 1998). However, the propensity for alternative splicing in vertebrates is so extremely high that the system will likely attempt all possible permutations, not just the intended splicing pattern. So this ubiquitous presence of a 5′-proximal splice donor site poses a strong risk of mis-splicing to any cryptic 3′-splice site present in the putative IRES under test (Fig. 3C), as shown by the finding that when all potential 5′-splice sites in the 5′-UTR and the upstream cistron (in this case coding for Gaussia luciferase) were inactivated by mutations, the BiP, NRF, VEGF, and XIAP 5′-UTRs, as well as the eIF4GI 5′-UTR reported by Gan and Rhoads (1996), failed to show significant IRES activity above the “no IRES” control, or the β-globin 5′-UTR, although the EMCV IRES positive control yielded high activity (Baranick et al. 2008). In the same publication a sensitive assay involving incorporating the IRES and a GFP reporter into a murine leukaemia virus proviral DNA (Fig. 4) revealed that the same eIF4GI 5′-UTR, as well as the NRF and XIAP 5′-UTRs all had 3′-splice sites, which accounted for the apparent IRES activity, although here again the EMCV IRES scored as a bona fide IRES. Moreover, a database search revealed ESTs in which these 3′-splice sites in all three putative IRES sequences had been used in their endogenous cellular mRNA context. A survey of 81 cellular 5′-UTR sequences with claimed IRESs, revealed EST evidence for functional 3′-splice sites in a small minority, including BCL2, DAP5, HIAP2, MTG8a, ODC, UNR, and utrophin A (Baranick et al. 2008). Another salutary warning of how splicing-dependent artefacts can give highly misleading results is the fact that a 42 nt sequence corresponding to a 3′-splice site in the β-globin gene masquerades as an exceptionally potent IRES when tested in the dual luciferase plasmid system (Lemp et al. 2012). In view of these potential problems arising from splicing, perhaps it would be worth investing time in constructing an alternative expression vector in which the customary 5′-proximal intron (Fig. 3A) is replaced either by an intron in the FLuc ORF itself or just downstream from its stop codon, or by an intron that is spliced by the alternative U11/U12snRNA-dependent pathway.
Figure 4.
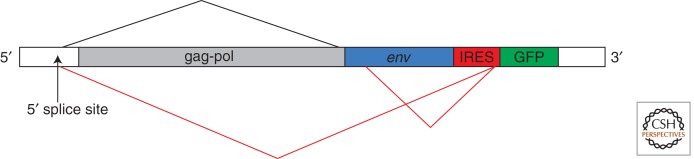
Highly sensitive murine leukaemia virus test for the presence of 3′-splice sites in putative IRESs (Baranick et al. 2008). The putative IRES and GFP reporter are inserted into the proviral DNA (driven by a CMV promoter) downstream from the env gene. The normal MLV splicing pattern (in the absence of the IRES-GFP insert) is shown in black, above the gene map. The presence of a 3′-splice site in the putative IRES promotes the alternative splicing pattern shown in red below the gene map. Consequently, there is very high GFP expression (i.e., apparent IRES activity) from the resulting capped monocistronic mRNA, but viral replication is severely impaired because of the decrease in full-length unspliced RNA for packaging, coupled with the reduction in gag, pol, and env protein synthesis. In contrast, with the EMCV IRES, GFP production is significantly lower (though nevertheless 10- to 20-fold greater than background), but there is no inhibitory effect on viral replication. The sensitivity of this assay is because of the double readout of (1) GFP expression (i.e., apparent IRES activity), and (2) viral replication.
siRNA Screen for FLuc Expression from Unanticipated mRNA Species
Although the retroviral test is undoubtedly very sensitive, a more generally accessible screen, developed by Rick Lloyd's group, has the advantage of detecting FLuc expression from unanticipated mRNA species irrespective of whether they arise from cryptic promoters or from splicing. It involves cotransfection or coexpression of an siRNA targeted against RLuc coding sequences (van Eden et al. 2004), which usually results in a 70%–80% decrease in RLuc expression, a level of reduction that is necessary if the results are to be readily interpretable. On the plausible assumption that the 3′-fragment of siRNA-mediated endonucleolytic cleavage is rapidly degraded to completion, the percentage reductions in FLuc and RLuc expression should be nearly equal if all the FLuc expression is from the anticipated bicistronic mRNA (i.e., dependent on an IRES), and this outcome is observed in tests of the EMCV and HRV picornavirus IRESs (Bert et al. 2006; Andreev et al. 2009). If there are stable degradation intermediates, the reduction in FLuc expression could be less than that of RLuc, but decay intermediates of sufficient stability to seriously prejudice the interpretation should be detectable by Northern blotting (even though 5′-RACE would be needed to map the 5′-ends). In the one case where this Northern blotting was performed, no decay intermediates were detected (Saffran and Smiley 2009).
If the reduction in FLuc expression is significantly less than that of RLuc, and yet no stable decay intermediates can be detected, the inescapable conclusion is that some FLuc must be expressed from unanticipated mRNAs, which lack the siRNA target site. The numerous possible configurations for such aberrant mRNAs can be broadly categorized according to whether the RLuc ORF is completely missing or present only in a truncated form, and whether the whole or only part of the test 5′-UTR is retained. If the whole RLuc ORF is missing, FLuc expression is probably from a monocistronic mRNA, which would be potentially translatable by scanning. If part of the RLuc ORF is retained but most of the test 5′-UTR is eliminated in a way that places this RLuc mini-ORF in-frame with the FLuc ORF, the result would be a monocistronic mRNA encoding a fusion protein that would likely have at least some FLuc activity (van Eden et al. 2004). On the other hand, if the whole of the test 5′-UTR is retained together with the RLuc mini-ORF, the outcome would be a bicistronic mRNA which, in principle, could only express FLuc via an IRES-dependent mechanism that would be immune from siRNA-mediated knock-down. However, if IRES activity is manifest when the upstream cistron is a RLuc mini-ORF, a similar efficiency of IRES-dependent FLuc expression should also be showed by the anticipated full-length bicistronic mRNA (with a complete RLuc ORF), but in this case it would be subject to siRNA-mediated knock-down. This full-length bicistronic mRNA will certainly be present, and in most cases it will be the majority RNA species, according to typical Northern blot results.
With these various scenarios in mind, it is clear that if the siRNA causes no reduction whatsoever in FLuc expression, the most likely explanation is that it is all coming from a monocistronic mRNA rather than the intended bicistronic species. This outcome, and even siRNA-dependent stimulation of FLuc expression, has been observed with HIF-1α, VEGF, XIAP, c-myc, and EGR-1 5′-UTRs (van Eden et al. 2004; Bert et al. 2006). If the siRNA causes a reduction in FLuc expression that is statistically significant but distinctly less than the reduction in RLuc expression (or in FLuc expression when the EMCV IRES is tested), as has been reported for the Apaf-1 5′-UTR (Andreev et al. 2009), there are two alternative conclusions. One is that some FLuc expression is from the siRNA-sensitive bicistronic transcript and some from a monocistronic artefact. The alternative is that all FLuc expression is from bicistronic mRNAs, and therefore probably IRES-dependent, but some of these bicistronic mRNAs have a truncated RLuc ORF lacking the siRNA target site.
These various possibilities can only be distinguished by a thorough RNA analysis. At least in the first instance, this analysis would best be made on mRNA recovered from the siRNA-treated cells, because the reduced background of anticipated full-length transcripts should make it easier to detect the aberrant siRNA-resistant mRNA species, which could be present only at quite low abundance. One approach is to carry out RT-PCR with primers matching the very 5′-end of the anticipated transcript (upstream of the chimeric intron) and the 5′-end of the FLuc ORF (van Eden et al. 2004). However, this strategy presupposes that all aberrant transcripts originate from the intended (SV40) promoter, and so 5′-RACE would be a more open-ended and better method for RNA analysis (Lemp et al. 2012). In either case, the cDNA products should be cloned, and sufficient clones sequenced.
Direct RNA Transfections and In Vitro Translation Assays
In view of the problems arising from splicing and cryptic Pol II promoters, many investigators have turned to transfections of capped and polyadenylated bicistronic mRNAs, synthesized in vitro by a bacteriophage RNA polymerase and usually with the same RLuc and FLuc configuration. This avoids the potential artefacts associated with DNA transfections, although it is not without its own problems, because at least some transfection protocols result in much of the input RNA remaining sequestered in intracellular vesicles, unavailable for translation and also largely immune from mRNA degradation enzymes (Barreau et al. 2006). It has been suggested that introducing the RNA by electroporation, or even microinjection, might avoid this problem, but in the meantime the existence of two intracellular pools invalidates any attempt to relate protein expression to mRNA stability or abundance, because these measurements involve recovering total RNA, and not just the pool of translated mRNAs.
Nevertheless, these considerations do not invalidate assays of expression measured during the linear phase of translation, which has been shown to persist for at least ∼6 h posttransfection. Two striking results have emerged from such assays of bicistronic mRNAs. First, although the EMCV IRES elicits a 100- to 250-fold stimulation of the FLuc/RLuc expression ratio, the stimulation seen with putative cellular mRNA IRESs is seldom greater than 10-fold, even in the case of 5′-UTRs that stimulate >50-fold in DNA transfection assays. For example, in one such assay the greatest stimulation was 6.2-fold (Apaf-1 5′-UTR), almost fourfold for the β-globin 5′-UTR and only 1.9-fold for the c-myc 5′-UTR (Andreev et al. 2009). In other reports the increases seen with the HIF-1α, HIF-2α, VEGF, XIAP, PIM-1, and p27Kip1 5′-UTRs were all less than ∼6-fold (Liu et al. 2005; Wang et al. 2005; Bert et al. 2006; Young et al. 2008). These results raise the semantic question of whether such small stimulations, barely above that seen with the β-globin 5′-UTR and only a pale shadow of the EMCV IRES result, really warrant description as “IRES activity.”
The other, even more striking outcome concerns comparison of the bicistronic mRNA with a monocistronic FLuc mRNA bearing the same test 5′-UTR, the comparison that regrettably is never performed with plasmid DNA transfections. In two independent reports, FLuc expression from the bicistronic mRNA with the Apaf-1, HIF-1α, c-myc, VEGF, and XIAP 5′-UTRs was <2% (range 0.125%–1.66%) of the expression from the corresponding monocistronic mRNA, although the EMCV IRES was 50%–125% as active in the bicistronic background compared with the monocistronic mRNA (Bert et al. 2006; Andreev et al. 2009).
These data imply that a switch from exclusive scanning-dependent initiation to exclusive IRES-dependent initiation would result in a >50-fold decrease in the translation efficiency of these mRNAs. This seriously undermines the idea that the persistence of translation and polysome association of a given mRNA under stress conditions is caused by such a switch, unless one invokes a massive stress-induced activation of such IRESs. The few cases in which the apparent IRES activity under stress conditions has been examined by RNA transfection have given contrary results: no increase in apparent VEGF, HIF-1α, and HIF-2α IRES activity was seen in hypoxia (Young et al. 2008), but a ∼5-fold increase in BCL-2 IRES activity was observed following treatment of cells with etoposide for 8 h (Sherrill et al. 2004). Proponents of the “nuclear experience” hypothesis will argue that the RNA transfection results are artefactually low because of the absence of this “experience,” but until we have more evidence as to what the “nuclear experience” entails (including evidence that it cannot be entirely or even partly explained by splicing or cryptic promoters), there is no logical justification for not accepting the RNA transfection results at face value.
In vitro translation of bicistronic mRNAs with putative IRESs in a Krebs-2 cell-free system (untreated with micrococcal nuclease) gave a hierarchy of apparent IRES activity similar to that seen in RNA transfections of HEK 293 cells, but (except for the EMCV IRES) the stimulations of the FLuc/RLuc expression ratio were even smaller than the low level observed in the RNA transfection assays (Andreev et al. 2009). It was suggested that the untreated Krebs-2 extract is a more appropriate system than the usual nuclease-treated rabbit reticulocyte lysate (RRL), because it shows both a greater stimulation by poly(A) tails and also synergy between 5′-caps and poly(A) tails, which may be partly ascribable to the presence of competing endogenous mRNAs. Nevertheless, several 5′-UTRs with putative IRES activity stimulated reporter expression when tested in a nuclease-treated HeLa cell-free extract as monocistronic polyadenylated Appp-capped mRNAs in which the test 5′-UTR was inserted between a 5′-proximal stable hairpin and the FLuc reporter (Thoma et al. 2004). The c-myc 5′-UTR gave the maximum stimulation of 12-fold (Hunsdorfer et al. 2005), which is much higher than was observed in the Krebs-2 system with a bicistronic mRNA (Andreev et al. 2009), but this higher activity may conceivably be attributable to the absence of any competing translation of other mRNAs. It would be better if assays in nuclease-treated extracts included at least an equimolar quantity of a heterologous m7Gppp-capped and polyadenylated mRNA (e.g., coding for RLuc if the reporter is FLuc), so as to reflect the competition between scanning-dependent and IRES-dependent mRNAs that will occur in intact cells.
The nuclease-treated RRL can translate EMCV RNA extremely efficiently but only if KCl is used rather than the customary KOAc (Jackson 1991), which is generally found to support higher translation of other mRNAs, including those translated by cap-dependent scanning. The RRL system is clearly deficient in ITAFs required for the Type I HRV IRES (Hunt and Jackson 1999; Hunt et al. 1999), and is also inefficient in the translation of capped polyadenylated monocistronic mRNAs with long 5′-UTRs, such as LINE-1, Apaf-1, and c-myc (Andreev et al. 2009), which is suggestive of poor processivity of scanning. Both of these defects are corrected by supplementing the system with a relatively small amount of HeLa cytoplasmic extract (Hunt and Jackson 1999; Dmitriev et al. 2007).
Further Investigations into the Properties of Cellular mRNA IRESs
It is not possible to comment sensibly on the outcome of further studies of cellular mRNA IRESs, such as deletion mapping the IRES boundaries, mapping the putative ribosome entry site, and examining the influence of potential ITAFs on IRES activity, because most of these have been based on plasmid DNA transfection assays. Consequently, until it is definitively proven that there are no monocistronic FLuc mRNAs generated from the bicistronic DNA construct with a given IRES, we cannot be sure whether the mapping experiments, for example, are showing the boundaries of a genuine IRES, or the positions of the cryptic promoter(s) or the splice sites that are the origin of the monocistronic mRNA. An example that illustrates that this a real problem and not just hypothetical conjecture, is a mapping of the c-myc IRES to a 50 nt segment in the 5′-UTR, which was further narrowed down to two 14 nt motifs, one at each end of the 50 nt segment (Cencig et al. 2004). Recent work employing 5′-RACE has shown that one of the 14 nt motifs corresponds to a functional transcription start site (of a monocistronic FLuc mRNA) and the other to an associated promoter element (Lemp et al. 2012). This finding suggests that the explanation for the modular nature reported for many putative cellular IRESs (Stoneley and Willis 2004) may well be that the short modules are actually splice sites, cryptic promoters or transcription start sites.
In the case of potential ITAFs, which have been mainly explored by studying the influence of overexpression or siRNA-mediated knock-down on apparent IRES activity in plasmid DNA transfection assays, there are, admittedly, additional cell-free translation data showing weak stimulation (less than threefold) of apparent IRES activity when some putative ITAFs were added (Cobbold et al. 2008). However, it should be appreciated that the basal level of IRES activity in such in vitro systems is exceedingly low, and thus the absolute increase in FLuc expression will be very small indeed.
CONCLUSIONS AND PERSPECTIVES
To an outsider approaching this topic with as few preconceived notions as possible, the current state of the field seems extremely confusing, because many pre-2004 claims for cellular mRNA IRESs, which appeared to be well established on the basis of plasmid transfection results, have been seriously challenged by the more stringent controls developed in the past 8 years. Future historians of science are likely to be very surprised by the way in which the mRNA translation community embraced the idea of cellular mRNA IRESs so unreservedly that the warning signs of potential artefacts associated with plasmid DNA transfections (e.g., when insertion of the test 5′-UTR strongly reduces RLuc expression) were ignored for such a long time, and sometimes continue to be ignored. There still seems to be no general agreement as to which controls are the most decisive, and the importance of thorough RNA analysis remains greatly underappreciated. The most frequently used approaches are really best suited to showing that the anticipated bicistronic transcript is the major abundant mRNA species, whereas what is required is a strategy for detecting and characterizing low abundance monocistronic FLuc mRNAs, whatever their origin.
It is hard to avoid the conclusion that plasmid DNA transfection assays for IRES activity cannot be trusted unless they meet the criterion of near-equal percentage reductions in the FLuc and RLuc activities on coexpressing an siRNA against the upstream RLuc cistron. Thus far, only the Types I and II picornavirus IRESs (exemplified by EMCV and HRV) consistently come close to meeting this criterion (Bert et al. 2006; Araud et al. 2007; Andreev et al. 2009). There are also single reports in which the Unr 5′-UTR (albeit tested with FLuc as the upstream cistron) and DAP5 5′-UTR met this criterion (Araud et al. 2007; Schepens et al. 2007), but in view of the EST evidence for 3′-splice sites in both cases (Baranick et al. 2008), independent confirmation of these findings is desirable. At the other extreme, the HIF-1α, VEGF, XIAP, and EGR-1 5′-UTRs comprehensively failed the siRNA test (Bert et al. 2006). In addition, the putative IRESs in the eIF4GI 5′-UTR of Gan and Rhoads (1996), and in the c-myc, HIF-2α, VEGF receptor1, Glut-1, TPI, NRF, PIM-1, p57Kip2, and p27Kip1 5′-UTRs all failed in at least one of the other recently developed sensitive controls for cryptic promoters and/or splicing artefacts. Failure in these tests suggests that the massive stimulations of the FLuc/RLuc expression ratio elicited by some of these 5′-UTRs in plasmid transfections are most unlikely to be a measure of true IRES activity. However, because the siRNA test and these other controls are essentially methods of screening for probable artefacts, rather than strictly quantitative assays, the possibility remains open that a 5′-UTR that fails these tests could nevertheless have some IRES activity, albeit an extremely weak activity probably in the same range as generally observed in RNA transfection or in vitro translation assays.
The literature has claims for ∼100 cellular mRNA IRESs (see Mokrejs et al. 2010 for the most recent, but not quite complete list), the great majority of them based primarily on plasmid DNA transfection assays, usually with only Nothern blot or RT-qPCR controls. Although a number have been examined by in vitro translation, and many, but not all, show modest IRES activity in these tests, it is difficult to evaluate these results because such a wide variety of conditions and cell-free systems have been used. Few of them (apart from those listed above) have been subjected to the siRNA test or any of the other stringent controls that have been recently developed, and comparatively few have been thoroughly tested in RNA transfections. The current status of a great many of these ∼100 putative IRESs (apart from those listed in the preceding paragraph) must therefore be regarded as “uncertain” or “not proven,” until these gaps in the data are plugged.
Because the results of plasmid DNA transfections without really stringent controls cannot be trusted, increasing reliance may have to be placed on the outcome of RNA transfections. For a comprehensive test, the following polyadenylated mRNA species should be assayed: m7Gppp-capped bicistronic mRNA (1) with the test 5′-UTR, and (2) with no insert and/or the reverse complement of the test 5′-UTR; Appp-capped monocistronic mRNA with a 5′-proximal stable hairpin, and (3) the test 5′-UTR, or (4) either no test sequence or the reverse complement of the test 5′-UTR; (5) m7Gppp-capped, and (6) ApppG-capped monocistronic mRNAs with the test 5′-UTR but no hairpin. The comparisons of (1) with (2), and (3) with (4) give two independent measures of IRES activity, the latter avoiding the potential complication of cross-interference between translation of two cistrons in close mutual proximity; and the comparisons of (1) with (5), and (3) with (5) give the exceedingly important ratio of IRES-dependent versus scanning-dependent initiation efficiency. The (5) versus (6) comparison provides a measure of the “cap-dependency” of initiation via the scanning mechanism, although it should be appreciated that IRES-dependent translation could also be occurring in parallel with scanning on such mRNAs. It would be worth considering transfecting the mRNAs by electroporation rather than with chemical reagents, and in due course it could be instructive to also test the outcome of appending the natural 3′-UTR to the monocistronic reporters, rather than the 3′-UTR supplied by the expression vector. Putative IRESs that are thought to play an essential role in the persistence of translation under stress conditions should also be tested under these conditions, to reveal any stress-dependent activation.
Although it is early days, current indications suggest that the cellular mRNA IRES activity observed in such assays will usually turn out to be rather weak. So far, cellular IRES-dependent FLuc expression from a bicistronic mRNA has never exceeded 2% of the expression from a m7Gppp-capped monocistronic mRNA with the same test 5′-UTR; and the stimulation of the FLuc/RLuc expression ratio following insertion of the test 5′-UTR into the bicistronic mRNA is usually in the range 5- ± 2-fold (maximum ∼10-fold) over the no IRES control, which should in principle be zero, and in practice is likely to be very small. Although there may be situations in which even such weak IRES activity is nevertheless biologically relevant, there are other situations, such as the persistence of translation of certain mRNAs under stress conditions, in which it is difficult to see how IRES activity as weak as this could possibly account for the observed outcome. Rather than jump to the presumption that IRES-dependent translation must be the explanation, it would be preferable to keep an open mind as to the other possibilities, namely: persistence of scanning-dependent translation (perhaps because the particular mRNA has an unusually high affinity for the residual eIF4F activity), ribosome shunting, or a mechanism dependent on a TE-like element in the 5′-UTR.
ACKNOWLEDGMENTS
I thank Rick Lloyd and Chris Logg for helpful comments on a draft version of this article, Chris Smith for enlightenment on the intricacies of alternative splicing, and Christopher Hellen for help with the figures.
Footnotes
Editors: John W.B. Hershey, Nahum Sonenberg, and Michael B. Mathews
Additional Perspectives on Protein Synthesis and Translational Control available at www.cshperspectives.org
REFERENCES
- Alexander L, Lu HH, Wimmer E 1994. Polioviruses containing picornavirus type 1 and/or type 2 internal ribosomal entry site elements: Genetic hybrids and the expression of a foreign gene. Proc Natl Acad Sci 91: 1406–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali IK, Jackson RJ 2001. The translation of capped mRNAs has an absolute requirement for the central domain of eIF4G but not for the cap-binding initiation factor eIF4E. Cold Spring Harb Symp Quant Biol 66: 377–387 [DOI] [PubMed] [Google Scholar]
- Ali IK, McKendrick L, Morley SJ, Jackson RJ 2001. Activity of the hepatitis A virus IRES requires association between the cap-binding translation initiation factor (eIF4E) and eIF4G. J Virol 75: 7854–7863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev DE, Dmitriev SE, Terenin IM, Prassolov VS, Merrick WC, Shatsky IN 2009. Differential contribution of the m7G-cap to the 5′ end dependent translation initiation of mammalian mRNAs. Nucleic Acids Res 37: 6135–6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araud T, Genolet R, Jaquier-Gubler P, Curran J 2007. Alternatively spliced isoforms of the human elk-1 mRNA within the 5′-UTR: Implications for ELK-1 expression. Nucleic Acids Res 35: 4649–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird SD, Turcotte M, Korneluk RG, Holcik M 2006. Searching for IRES. RNA 12: 1755–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranick BT, Lemp NA, Nagashima J, Hiraoka K, Kasahara N, Logg CR 2008. Splicing mediates the activity of four putative cellular internal ribosome entry sites. Proc Natl Acad Sci 105: 4733–4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau C, Dutertre S, Paillard L, Osborne HB 2006. Liposome-mediated RNA transfection should be used with caution. RNA 12: 1790–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard KM, Daijogo S, Semler BL 2007. A nucleo-cytoplasmic SR protein functions in viral IRES-mediated translation initiation. EMBO J 26: 459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bert AG, Grépin R, Vadas MJ, Goodall GJ 2006. Assessing IRES activity in the HIF-1α and other cellular 5′-UTRs. RNA 12: 1074–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman AM, Michel YM, Kean KM 2001. Detailed analysis of the requirements of hepatitis A virus internal ribosome entry segment for the eukaryotic initiation factor complex eIF4F. J Virol 75: 7864–7871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussadia O, Niepmann M, Créancier L, Prats AC, Dautry F, Jacquemin-Sablon H 2003. Unr is required in vivo for efficient initiation of translation from the internal ribosome entry sites of both rhinovirus and poliovirus. J Virol 77: 3353–3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein S, Karpisheva K, Pola C, Goldberg J, Hochman T, Yee H, Cangiarella J, Arju R, Formenti SC, Schneider RJ 2007. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol Cell 28: 501–512 [DOI] [PubMed] [Google Scholar]
- Bushell M, Stoneley M, Kong YW, Hamilton TL, Spriggs KA, Dobbyn HC, Qin X, Sarnow P, Willis AE 2006. Polypyrimidine tract binding proteins regulates IRES-mediated gene expression during apoptosis. Mol Cell 23: 401–412 [DOI] [PubMed] [Google Scholar]
- Byrd MP, Zamora M, Lloyd RE 2005. Translation of eukaryotic translation initiation factor 4GI (eIF4GI) proceeds from multiple mRNAs containing a novel cap-dependent internal ribosome entry site (IRES) that is active during poliovirus infection. J Biol Chem 280: 18610–18622 [DOI] [PubMed] [Google Scholar]
- Cencig S, Nanbru C, Le SY, Gueydan C, Huez G, Kruys V 2004. Mapping and characterization of the minimal internal ribosome entry segment in the human c-myc mRNA 5′ untranslated region. Oncogene 23: 267–277 [DOI] [PubMed] [Google Scholar]
- Cobbold LC, Spriggs KA, Haines SJ, Dobbyn HC, Hayes C, de Moor CH, Lilley KS, Bushell M, Willis AE 2008. Identification of internal ribosome entry segment (IRES)-trans-acting factors for the Myc family of IRESs. Mol Cell Biol 28: 40–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Svitkin Y, Sonenberg N 2004. La autoantigen is necessary for optimal function of the poliovirus and hepatitis C virus internal ribosome entry site in vivo and in vitro. Mol Cell Biol 24: 6861–6870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Breyne S, Yu Y, Pestova TV, Hellen CU 2008. Factor requirements for translation initiation on the Simian picornavirus internal ribosomal entry site. RNA 14: 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Breyne S, Yu Y, Unbehaun A, Pestova TV, Hellen CUT 2009. Direct functional interaction of initiation factor eIF4G with type 1 internal ribosomal entry sites. Proc Natl Acad Sci 106: 9197–91202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev SE, Andreev DE, Terenin IM, Olovnikov IA, Prassolov VS, Merrick WC, Shatsky IN 2007. Efficient translation initiation directed by the 900-nucleotide-long and GC-rich 5′-untranslated region of the human retrotransposon LINE-1 mRNA is strictly cap-dependent rather than internal ribosome entry site mediated. Mol Cell Biol 27: 4685–4697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan W, Rhoads RE 1996. Internal initiation of translation directed by the 5′-untranslated region of the mRNA for eIF4G, a factor involved in the picornavirus-induced switch from cap-dependent to internal initiation. J Biol Chem 271: 623–626 [DOI] [PubMed] [Google Scholar]
- Hellen CUT, de Breyne S 2007. A distinct group of hepacivirus/pestivirus-like internal ribosomal entry sites in members of diverse picornavirus genera: Evidence for modular exchange of functional noncoding RNA elements by recombination. J Virol 81: 5850–5863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcík M, Gordon BW, Korneluk RG 2003. The internal ribosome entry site-mediated translation of antiapoptotic protein XIAP is modulated by the heterogeneous nuclear ribonucleoproteins C1 and C2. Mol Cell Biol 23: 280–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundsdoerfer P, Thoma C, Hentze MW 2005. Eukaryotic translation initiation factor 4GI and p97 promote cellular internal ribosome entry sequence-driven translation. Proc Natl Acad Sci 102: 13421–13426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SL, Jackson RJ 1999. Polypyrimidine–tract binding protein (PTB) is necessary, but not sufficient, for efficient internal initiation of translation of human rhinovirus-2 RNA. RNA 5: 344–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SL, Hsuan JJ, Totty N, Jackson RJ 1999. Unr, a cellular cytoplasmic RNA-binding protein with five cold shock domains, is required for internal initiation of translation of human rhinovirus RNA. Genes Dev 13: 437–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizuka N, Kohara M, Hagino-Yamagishi K, Abe S, Komatsu T, Tago K, Arita M, Nomoto A 1989. Construction of less neurovirulent polioviruses by introducing deletions into the 5′ noncoding sequence of the genome. J Virol 63: 5354–5363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RJ 1991. Potassium salts influence the fidelity of mRNA translation initiation in rabbit reticulocyte lysates: Unique features of encephalomyocarditis virus RNA translation. Biochim Biophys Acta 1088: 345–358 [DOI] [PubMed] [Google Scholar]
- Jackson RJ, Kaminski A 1995. Internal initiation of translation in eukaryotes: The picornavirus paradigm and beyond. RNA 1: 985–1000 [PMC free article] [PubMed] [Google Scholar]
- Jang SK, Wimmer E 1990. Cap-independent translation of encephalomyocarditis virus RNA: Structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev 4: 1560–1572 [DOI] [PubMed] [Google Scholar]
- Johannes G, Sarnow P 1998. Cap-independent polysomal association of natural mRNAs encoding c-myc, BiP, and eIF4G conferred by internal ribosome entry site. RNA 4: 1500–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafasla P, Morgner N, Pöyry TA, Curry S, Robinson CV, Jackson RJ 2009. Polypyrimidine tract binding protein stabilizes the encephalomyocarditis virus IRES structure via binding multiple sites in a unique orientation. Mol Cell 34: 556–568 [DOI] [PubMed] [Google Scholar]
- Kafasla P, Morgner N, Robinson CV, Jackson RJ 2010. Polypyrimidine tract binding protein stimulates the poliovirus IRES by modulating eIF4G binding. EMBO J 29: 3710–3722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski A, Jackson RJ 1998. The polypyrimidine tract binding protein (PTB) requirement for internal initiation of translation of cardiovirus RNAs is conditional rather than absolute. RNA 4: 626–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski A, Howell MT, Jackson RJ 1990. Initiation of encephalomyocarditis virus RNA translation: The authentic initiation site is not selected by a scanning mechanism. EMBO J 9: 3753–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski A, Belsham GJ, Jackson RJ 1994. Translation of encephalomyocarditis virus RNA: Parameters influencing the selection of the internal initiation site. EMBO J 13: 1673–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski A, Pöyry TAA, Skene PJ, Jackson RJ 2010. Mechanism of initiation site selection promoted by the human rhinovirus 2 internal ribosome entry site. J Virol 84: 6578–6589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King HA, Cobbold LC, Willis AE 2010. The role of IRES trans-acting factors in regulating translation initiation. Biochem Soc Trans 38: 1581–1586 [DOI] [PubMed] [Google Scholar]
- Koeller DM, Casey JL, Hentze MW, Gerhardt EM, Chan LN, Klausner RD, Harford JB 1989. A cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mRNA. Proc Natl Acad Sci 86: 3574–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolupaeva VG, Pestova TV, Hellen CU, Shatsky IN 1998. Translation eukaryotic initiation factor 4G recognizes a specific structural element within the internal ribosome entry site of encephalomyocarditis virus RNA. J Biol Chem 273: 18599–18604 [DOI] [PubMed] [Google Scholar]
- Kolupaeva VG, Lomakin IB, Pestova TV, Hellen CUT 2003. Eukaryotic initiation factors 4G and 4A mediate conformational changes downstream of the initiation codon of the encephalomyocarditis virus internal ribosomal entry site. Mol Cell Biol 23: 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M 2003. Alternative ways to think about mRNA sequences and proteins that appear to promote internal initiation of translation. Gene 318: 1–23 [DOI] [PubMed] [Google Scholar]
- Kozak M 2005. A second look at cellular mRNA sequences said to function as internal ribosome entry sites. Nucleic Acids Res 33: 6593–6602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Hir H, Nott A, Moore MJ 2003. How introns influence and enhance eukaryotic gene expression. Trends Biochem Sci 28: 215–220 [DOI] [PubMed] [Google Scholar]
- Lemp NA, Hiraoka K, Kasahara N, Logg CR 2012. Cryptic transcripts from the ubiquitous pMB1 origin of replication confound functional tests for cis-regulatory elements. Nucleic Acids Res 10.1093/nar/gks451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Dong Z, Han B, Yang Y, Liu Y, Zhang JT 2005. Regulation of expression by promoters versus internal ribosome entry site in the 5′-untranslated sequence of the human cyclin-dependent kinase inhibitor 27Kip1. Nucleic Acids Res 33: 3763–3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakin IV, Hellen CUT, Pestova TV 2000. Physical association of eukaryotic initiation factor 4G (eIF4G) with eIF4A strongly enhances binding of eIF4G to the internal ribosomal entry site of encephalomyocarditis virus is required for internal initiation of translation. Mol Cell Biol 20: 6019–6029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerovitch K, Nicholson R, Sonenberg N 1991. In vitro mutational analysis of cis-acting RNA translational elements within the poliovirus type 2 5′ untranslated region. J Virol 65: 5895–5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WA, Wang Z, Treder K 2007. The amazing diversity of cap-independent translation elements in the 3′-untranslated regions of plant viral RNAs. Biochem Soc Trans 35: 1629–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokrejs M, Masek T, Vopalensky A, Hlubucek P, Delbos P, Pospisek M 2010. IRESite—a tool for the examination of viral and cellular internal ribosome entry sites. Nucleic Acids Res 38: D131–D136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy E, Maquat LE 1998. A rule for termination-codon position within intron-containing genes: When nonsense affects RNA abundance. Trends Biochem Sci 23: 198–199 [DOI] [PubMed] [Google Scholar]
- Nicholson R, Pelletier J, Le S-Y, Sonenberg N 1991. Structural and functional analysis of the ribosome landing pad of poliovirus type 2: In vivo translation studies. J Virol 65: 5886–5894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberstrass FC, Auweter SD, Erat M, Hargous Y, Henning A, Wenter P, Reymond L, Amir-Ahmady B, Pitsch S, Black DL, et al. 2005. Structure of PTB bound to RNA: Specific binding and implications for splicing regulation. Science 309: 2054–2057 [DOI] [PubMed] [Google Scholar]
- Özeş AR, Feoktistova K, Avanzino BC, Fraser CS 2011. Duplex unwinding and ATPase activities of the DEAD-box helicase eIF4A are coupled by eIF4G and eIF4B. J Mol Biol 412: 674–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J, Sonenberg N 1988. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature 334: 320–325 [DOI] [PubMed] [Google Scholar]
- Pelletier J, Flynn ME, Kaplan G, Racaniello V, Sonenberg N 1988. Mutational analysis of upstream AUG codons of poliovirus RNA. J Virol 62: 4486–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, Hellen CU, Wimmer E 1994. A conserved AUG triplet in the 5′ nontranslated region of poliovirus can function as an initiation codon in vitro and in vivo. Virology 204: 729–737 [DOI] [PubMed] [Google Scholar]
- Pestova TV, Hellen CUT, Shatsky IN 1996. Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Mol Cell Biol 16: 6859–6869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, Shatsky IN, Fletcher SP, Jackson RJ, Hellen CUT 1998. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C and classical swine fever virus RNAs. Genes Dev 12: 67–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, de Breyne S, Pisarev AV, Abaeva IS, Hellen CU 2008. eIF2-dependent and eIF2-independent modes of initiation on the CSFV IRES: A common role of domain II. EMBO J 27: 1060–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko EV, Pestova TV, Kolupaeva VG, Khitrina EV, Poperechnaya AN, Agol VI, Hellen CUT 2000. A cell cycle-dependent protein serves as a template-specific translation initiation factor. Genes Dev 14: 2028–2045 [PMC free article] [PubMed] [Google Scholar]
- Pöyry T, Kinnunen L, Hovi T 1992. Genetic variation in vivo and proposed functional domains of the 5′ noncoding region of poliovirus RNA. J Virol 66: 5313–5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévôt D, Décimo D, Herbreteau CH, Roux F, Garin J, Darlix JL, Ohlmann T 2003. Characterization of a novel RNA-binding region of eIF4GI critical for ribosomal scanning. EMBO J 22: 1909–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X, Sarnow P 2004. Preferential translation of internal ribosome entry site-containing mRNAs during the mitotic cycle in mammalian cells. J Biol Chem 279: 13721–13728 [DOI] [PubMed] [Google Scholar]
- Ramírez-Valle F, Braunstein S, Zavadil J, Formenti SC, Schneider RJ 2008. eIF4GI links nutrient sensing by mTOR to cell proliferation and inhibition of autophagy. J Cell Biol 181: 293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffran HA, Smiley JR 2009. The XIAP IRES activates 3′ cistron expression by inducing production of monocistronic mRNA in the βgal/CAT bicistronic reporter system. RNA 15: 1980–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki J, Nakashima N 2000. Methionine-independent initiation of translation in the capsid protein of an insect RNA virus. Proc Natl Acad Sci 97: 1512–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens B, Tinton SA, Bruynooghe Y, Parthoens E, Haegman M, Beyaert R, Cornelis S 2007. A role for hnRNP C1/C2 and Unr in internal initiation of translation during mitosis. EMBO J 26: 158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatsky IN, Dmitriev SE, Terenin IM, Andreev DE 2010. Cap- and IRES-independent scanning mechanism of translation initiation as an alternative to the concept of cellular IRESs. Mol Cells 30: 285–293 [DOI] [PubMed] [Google Scholar]
- Sherrill KW, Lloyd RE 2008. Translation of cIAP2 mRNA is mediated exclusively by a stress-modulated ribosome shunt. Mol Cell Biol 28: 2011–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill KW, Byrd MP, Van Eden ME, Lloyd RE 2004. BCL-2 translation is mediated via internal ribosome entry during cell stress. J Biol Chem 279: 29066–29074 [DOI] [PubMed] [Google Scholar]
- Shiroki K, Ohsawa C, Sugi N, Wakiyama M, Miura K, Watanabe M, Suzuki Y, Sugano S 2002. Internal ribosome entry site-mediated translation of Smad5 in vivo: Requirement for a nuclear event. Nucleic Acids Res 30: 2851–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvera D, Arju R, Darvishian F, Levine PH, Zolfaghari L, Goldberg J, Hochman T, Formenti SC, Schneider RJ 2009. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat Cell Biol 11: 903–908 [DOI] [PubMed] [Google Scholar]
- Stoneley M, Willis AE 2004. Cellular internal ribosome entry segments: Structures, trans-acting factors and regulation of gene expression. Oncogene 23: 3200–3207 [DOI] [PubMed] [Google Scholar]
- Stoneley M, Subkhankulova T, Le Quesne JP, Coldwell MJ, Jopling CL, Belsham GJ, Willis AE 2000. Analysis of the c-myc IRES; a potential role for cell-type specific trans-acting factors and the nuclear compartment. Nucleic Acids Res 28: 687–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkin YV, Meerovitch K, Lee HS, Dholakia JN, Kenan DJ, Agol VI, Sonenberg N 1994. Internal translation initiation on poliovirus RNA: Further characterization of La function in poliovirus translation in vitro. J Virol 68: 1544–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma C, Bergamini G, Galy B, Hundsdoerfer P, Hentze MW 2004. Enhancement of IRES-mediated translation of the c-myc and BiP mRNAs by the poly(A) tail is independent of intact eIF4G and PABP. Mol Cell 15: 925–935 [DOI] [PubMed] [Google Scholar]
- van Eden ME, Byrd MP, Sherrill KW, Lloyd RE 2004. Demonstrating internal ribosome entry sites in eukaryotic mRNAs using stringent RNA test procedures. RNA 10: 720–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vopálenský V, Masek T, Horváth O, Vicenová B, Mokrejs M, Pospísek M 2008. Firefly luciferase gene contains a cryptic promoter. RNA 14: 1720–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter BL, Nguyen JH, Ehrenfeld E, Semler BL 1999. Differential utilization of poly(rC) binding protein 2 in translation directed by picornavirus IRES elements. RNA 5: 1570–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Weaver M, Magnuson N 2005. Cryptic promoter activity in the DNA sequence corresponding to the pim-1 5′-UTR. Nucleic Acids Res 33: 2248–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JE, Pestova TV, Hellen CU, Sarnow P 2000. Initiation of protein synthesis from the A site of the ribosome. Cell 102: 511–520 [DOI] [PubMed] [Google Scholar]
- Young RM, Wang S-J, Gordan JD, Ji X, Liebhaber SA, Simon MC 2008. Hypoxia-mediated selective mRNA translation by an internal ribosome entry site-independent mechanism. J Biol Chem 283: 16309–16319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Sweeney TR, Kafasla P, Jackson RJ, Pestova TV, Hellen CUT 2011. The mechanism of translation initiation on Aichivirus RNA mediated by a novel type of picornavirus IRES. EMBO J 30: 4423–4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh A, Schneider RJ 1996. Selective translation initiation by ribosome jumping in adenovirus-infected and heat-shocked cells. Genes Dev 10: 1557–1567 [DOI] [PubMed] [Google Scholar]
- Yueh A, Schneider RJ 2000. Translation by ribosome shunting on adenovirus and hsp70 mRNAs facilitated by complementarity to 18S rRNA. Genes Dev 14: 414–421 [PMC free article] [PubMed] [Google Scholar]