SUMMARY
Background
The AST to platelet ratio index (APRI), a non-invasive marker of liver fibrosis, has not been well studied in HCV/HIV (hepatitis C virus/human immunodeficiency virus) co-infected patients with advanced HIV.
Aim
To compare the accuracy of APRI in HCV/HIV co-infected patients to that in HCV mono-infected patients and to determine the impact of CD4+ T-cell counts on its performance.
Methods
We identified 106 consecutive HCV/HIV co-infected patients and 105 matched HCV mono-infected patients who underwent liver biopsy at Harborview Medical Center over a 5-year period. Performance characteristics were calculated and receiver operating characteristic (ROC) analysis conducted.
Results
The area under the ROC curve (AUROC) of APRI for predicting significant fibrosis was similar when comparing those with and without HIV co-infection (0.77 vs. 0.86, P = 0.18), but was lower in HIV co-infected patients with CD4 counts <250 cells/mm3 (0.64 vs. 0.86, P = 0.05). In HIV co-infected patients with CD4 counts ≥250, APRI had higher negative predictive value (93% vs. 88%, P = 0.57), positive predictive value (63% vs. 40%, P = 0.43) and specificity (95% vs. 88%, P = 0.05) than in those with lower CD4 counts.
Conclusions
The AST to platelet ratio index (APRI) performance characteristics appear to be suboptimal in HCV/HIV co-infected patients with CD4 counts <250 and they require further study in this population at increased risk for advanced liver disease.
INTRODUCTION
Approximately 3% of the world’s population is infected with HCV, including roughly 3.2 million people in the United States.1, 2 It is the leading cause of decompensated cirrhosis, liver-related mortality and liver transplantation in the United States.3 Furthermore, liver-related morbidity and mortality is expected to continue to increase given the long period between HCV infection and the development of advanced fibrosis.4
As a result of shared routes of transmission, co-infection with HCV and HIV is common, particularly amongst individuals who have had blood exposure, such as injection drug users. It is estimated that approximately 25–30% of HIV positive patients in the United States are infected with HCV.5-7 HCV infection has emerged as a leading cause of morbidity and mortality in HIV-positive individuals, particularly with the widespread use of HAART and resultant improved HIV-related survival.8 The presence of HIV may be associated with a more accelerated progression of HCV-induced liver disease to cirrhosis, particularly in patients with low CD4+ T-cell counts.9-11
The HCV/HIV co-infected individuals can have significant reductions in liver-related complications with successful HCV treatment, and many patients undergo a liver biopsy to assess the degree of fibrosis prior to initiating treatment.12 Although liver biopsy is generally regarded as a safe procedure, it remains invasive, expensive and is associated with a small risk (0.2–0.6%) of major complications.13, 14 In addition, liver biopsy is not ideal given that inter-observer variability and sampling error can both lead to inaccurate staging.15, 16 In response to these limitations of liver biopsy, several non-invasive markers of liver fibrosis have been developed and validated in HCV-infected patients.17-24 Although non-invasive markers remain insufficiently accurate to replace liver biopsy in routine clinical practice,15 there have been significant advances in the past few years.
Of particular interest is the AST to platelet ratio index (APRI), an extensively studied model that is based on objective and readily available laboratory variables.22 Both components are performed regularly in clinical practice so APRI does not require additional specialised testing. Furthermore, APRI is a simple calculation that can be performed easily at the bedside. In contrast, most models require complex calculations or are based on laboratory parameters not routinely performed, thus limiting their overall clinical utility.
Whilst APRI has been widely examined in HCV mono-infected patients, there are fewer studies evaluating its utility in HCV/HIV co-infected patients.16, 25-30 The studies in co-infected patients have been limited in part by small sample size and have largely included patients with well-controlled HIV. In the largest study to date, a multicentre study of 357 co-infected patients, all patients had CD4 counts above 300 cells/mL and there was no HIV-negative comparison group.27 The aims of our study were (i) to compare the accuracy of APRI in a large and diverse cohort of patients with HCV/HIV co-infection to its accuracy in patients with HCV monoinfection, and (ii) to determine the impact of immune deficiency, as defined by the CD4+ T-cell count, on its performance in co-infected patients.
PATIENTS AND METHODS
Patient population and data
All patients who underwent percutaneous liver biopsy at Harborview Medical Center between July 2000 and May 2005 were identified using hospital billing records, and records were reviewed. All HCV/HIV co-infected patients who underwent liver biopsy during this time period and met inclusion criteria were included. A similar number of eligible HCV mono-infected patients were randomly selected from all (n = 547) who had undergone liver biopsy during the same time period and were hand-matched to the HCV/HIV co-infected patients. Matching was performed on the joint distribution by age (±5 years), gender, race and liver fibrosis stage (stages 0–2 vs. stages 3–4). The diagnosis of HCV was established by the presence of HCV RNA using either quantitative or qualitative polymerase chain reaction assays. HCV/HIV co-infection was established by a positive HIV Western blot assay in the setting of established HCV infection.
Exclusion criteria for the study included co-infection with hepatitis B, concurrent drug hepatotoxicity or other causes of acute aminotransferase elevation, immunosuppressive therapy, hepatocellular carcinoma, prior liver or bone marrow transplantation, and prior treatment for HCV. Patients with missing data, such as insufficient liver tissue for staging of fibrosis or unavailable laboratory data within 6 months of liver biopsy, were also excluded. If more than one set of laboratory test results was available, the results closest to the time of the biopsy were used. This study was approved by the Institutional Review Board of the University of Washington.
Laboratory testing
All laboratory testing was conducted at the University of Washington/Harborview Medical Center clinical laboratories. Serum AST concentration was assessed by the Beckman LX20 automated method (upper limit of normal 44 IU/mL) and platelet count was determined by impedance method (lower limit of normal 150 109/L). CD4+ T-cell counts were determined using a single platform method via flow cytometry. Serological assays for HIV and HCV were performed using the Genetic Systems HIV-1/HIV-2 Peptide EIA assay (Bio-Rad Laboratories, Redmond, WA, USA) and the Abbott HCV EIA 2.0 diagnostic kit (Chicago, IL, USA), respectively. Through July 2002, HCV RNA levels were determined using a third-generation branched DNA assay (Versant HCV RNA 3.0; Bayer Diagnostics, Tarrytown, New York, USA) (lower limit of detection, 600 IU/mL), after which time levels were determined using an in-house real-time reverse-transcriptase polymerase chain reaction (RT-PCR) assay (lower limit of detection, 50 IU/mL). Through 31 July 2004, HCV genotype was assigned using restriction fragment–length polymorphism analysis of the 5′-noncoding region, after which time genotype was determined using the Abott real-time method. Through July 2003, HIV-1 RNA quantification was done using a branched DNA assay (lower limit of detection, 50 copies/mL). Later determinations were performed using real-time RT PCR technology (lower limit of detection, 30 copies/mL). HIV viral loads below the assay’s detection threshold were assigned the threshold value when calculating descriptive statistics.
Histological analysis
A single, experienced hepatopathologist (L.V.T.) who was blinded to all clinical data including HIV status reviewed all liver biopsies. Liver fibrosis stage was assessed using the Batts-Ludwig system: F0, no fibrosis; F1, portal fibrosis; F2, periportal fibrosis with rare bridges; F3, bridging/septal fibrosis or F4, cirrhosis.31 All samples included were deemed adequate for staging. We a priori defined significant fibrosis as stage 3 or 4 for the following reasons: (i) The multiple scoring systems utilised for staging HCV liver disease differ in their description of periportal fibrosis with or without bridging fibrosis, whilst stages encompassing definite bridging fibrosis/cirrhosis are comparable across scoring systems; (ii) Treatment is indicated for bridging fibrosis/cirrhosis, whereas it is optional in periportal fibrosis,32 especially as more effective treatments are expected; and (iii) The diagnosis of bridging fibrosis/cirrhosis alters clinical management beyond HCV treatment (e.g. HCC surveillance is indicated).
Statistical analysis
The APRI was calculated using AST, expressed as a ratio to the upper limit of normal, divided by the platelet count.22 Values of 0.5 and 1.5 were defined as cut-offs to predict the absence or presence of significant fibrosis, respectively.
All quantitative data are expressed as mean ± s.d. unless otherwise stated. Differences between groups were determined using the Student t-test for continuous variables and the chi-square test or Fisher exact test for categorical variables. Linear regression or ordered logistic regression was used where appropriate to evaluate the association between predictors and continuous or ordered categorical outcomes, respectively.
We calculated the percentage of patients who were able to be classified by APRI using the cut-offs of less than 0.5 and greater than 1.5 to predict the absence or presence of significant fibrosis, respectively.22 With these cut-offs, APRI values of 0.5–1.5 are unable to classify patients as likely or unlikely of having significant fibrosis. The percentage of patients correctly classified was defined as the number of patients correctly classified divided by the total number of patients who were classified.
The sensitivity, specificity, positive predictive value and negative predictive value of APRI for the detection of significant fibrosis were calculated for HCV patients and HCV/HIV co-infected patients. The diagnostic value of the index was also assessed using the area under the receiver operating characteristic (AUROC) curves in both groups.33 The AUROC for APRI was compared between groups using the Delong method.34 All statistical analysis was performed using STATA 8.0 (College Station, TX, USA).
RESULTS
Patient characteristics
One hundred twenty-four HCV/HIV co-infected patients had percutaneous liver biopsies between July 2000 and May 2005. Nineteen patients were excluded for the following reasons: seven had highly fragmented or small biopsies limiting the ability to stage disease, six had incomplete laboratory data, three had suspected concurrent drug hepatotoxicity, two had previously received treatment for HCV, and one had a prior bone marrow transplant. The excluded patients otherwise did not differ from those who remained eligible for further study (data not shown).
Characteristics of all included patients, according to HIV status, are detailed in Table 1. The groups were of similar age, gender and racial distribution. AST levels, platelet counts and calculated APRI were similar between the two groups, although co-infected patients were noted to have lower ALT (P = 0.02), bilirubin (P = 0.002) and albumin levels (P = 0.01). The majority of the patients in both groups had genotype 1 HCV disease. HCV RNA levels were higher in the co-infected patients than in the HIV-negative patients (P = 0.01). Liver biopsy samples submitted to histopathology were longer in HIV-negative patients (P = 0.0001). This difference was an artefact of the inclusion of more HIV-positive than HIV-negative patients in translational studies (70% vs. 20%, P < 0.0001). In these studies, liver tissue in excess of 20–25 mm was utilised for research assays examining HCV liver disease pathogenesis.
Table 1.
Patient characteristics according to HIV status
| HCV (n = 106) | HCV/HIV (n = 105) | P-value | |
|---|---|---|---|
| Age (years) | 42 ± 7 | 41 ± 7 | 0.48 |
| Male gender, n (%) | 90 (84.9) | 89 (84.7) | 0.98 |
| Race, n (%) | |||
| White | 74 (69.8) | 76 (72.4) | 0.19 |
| Black | 18 (17) | 20 (19) | |
| Hispanic | 7 (6.6) | 6 (5.7) | |
| Other | 7 (6.6) | 3 (2.9) | |
| HCV genotype (%)* | |||
| Genotype 1 | 77 (77) | 78 (77.2) | 0.86 |
| Genotype 2 | 12 (12) | 14 (13.9) | |
| Genotype 3 | 10 (10) | 8 (7.9) | |
| Other | 1 (1) | 1 (1) | |
| AST (IU/mL) | 72 ± 54 | 62 ± 35 | 0.12 |
| ALT (IU/mL) | 123 ± 120 | 90 ± 75 | 0.02 |
| Bilirubin (mg/dL) | 1.0 ± 0.3 | 0.9 ± 0.4 | 0.002 |
| Albumin (g/dL) | 3.9 ± 0.4 | 3.8 ± 0.4 | 0.01 |
| Platelet count (109/L) | 208 ± 58 | 206 ± 64 | 0.8 |
| INR | 0.97 ± 0.1 | 0.99 ± 0.1 | 0.24 |
| APRI | 0.92 ± 0.87 | 0.79 ± 0.62 | 0.24 |
| HCV RNA (log10) | 5.9 ± 0.9 | 6.3 ± 0.8 | 0.01 |
| HAART, n (%) | NA | 73 (69.5) | – |
| HIV RNA (log10) | N/A | 2.9 ± 1.3 | – |
| Undetectable HIV RNA, n (%) | N/A | 42 (40) | – |
| CD4+ T-cell count (cells/mm3) | N/A | 430 ± 267 | – |
| CD4 > 250 cells/mm3, n (%) | N/A | 72 (68.5) | – |
| Liver biopsy length (mm) | 25 ± 12 | 20 ± 9 | 0.0001 |
| Stage, n (%) | |||
| Stage 0 | 0 | 1 (1.0) | 0.49 |
| Stage 1 | 14 (13.2) | 19 (18.1) | |
| Stage 2 | 67 (63.2) | 61 (58.1) | |
| Stage 3 | 17 (16.0) | 16 (15.2) | |
| Stage 4 | 8 (7.6) | 8 (7.6) |
Data presented as mean ± s.d. or n (%).
APRI, AST to platelet ratio index; HAART, highly active antiretroviral therapy.
Available in 100 HCV-infected and 101 HCV/HIV co-infected patients.
There was no significant difference in stage of fibrosis between the two groups (P = 0.49). Fewer than 20% of the patients overall had F0-F1 fibrosis. In HCV mono-infected patients, significant fibrosis (F3 or F4) was seen in 25 (24%) patients and cirrhosis (F4) was seen in eight (8%) patients. Significant fibrosis was found in 24 (23%) HCV/HIV co-infected patients, whilst eight (8%) patients had cirrhosis.
In the co-infected patients, the mean CD4+ T-cell count was 430 ± 267 cells/μL (median 340, range 0–1140). CD4 counts were greater than 250 cells/mm3 in 72 (69%) patients. The median HIV viral level was 308 (range <30–1 000 000) copies/mL. Forty-two patients (40%) had HIV levels below the assay’s detection limit.
Characteristics of the HCV/HIV co-infected patients, according to CD4 count, are detailed in Table 2. The two groups were similar in age, gender and race. There were no significant differences in AST, ALT, bilirubin and platelet counts. There was a higher percentage of genotype 1 HCV disease in the group with CD4 counts ≤250 cells/mm3 (P = 0.08). There was no significant difference in stage of fibrosis between the two groups (P = 0.69). In patients with CD4 counts ≤250 cells/mm3, significant fibrosis was found in eight (24%) patients and cirrhosis in four (12%) patients. Significant fibrosis was found in 16 (22%) patients with CD4 counts >250 cells/mm3, whereas four (6%) patients had cirrhosis.
Table 2.
Characteristics of HCV/HIV co-infected patients
| CD4 ≤ 250 (n = 33) | CD4 > 250 (n = 72) | P-value | |
|---|---|---|---|
| Age (years) | 41 ± 6 | 41 ± 8 | 0.98 |
| Male gender, n (%) | 30 (90.9) | 59 (81.9) | 0.24 |
| Race, n (%) | |||
| White | 23 (69.7) | 53 (73.6) | 0.75 |
| Black | 6 (18.2) | 14 (19.4) | |
| Hispanic | 4 (12.1) | 2 (2.8) | |
| Other | 0 | 3 (4.2) | |
| HCV genotype (%) | |||
| Genotype 1 | 28 (84.9) | 50 (69.5) | 0.08 |
| Genotype 2 | 3 (9.1) | 11 (15.3) | |
| Genotype 3 | 1 (3.0) | 7 (9.7) | |
| Other | 1 (3.0) | 4 (5.5) | |
| AST (IU/mL) | 63.91 ± 32.21 | 60.75 ± 36.72 | 0.67 |
| ALT (IU/mL) | 100.94 ± 105.08 | 84.53 ± 56.22 | 0.30 |
| Bilirubin (mg/dL) | 0.93 ± 0.58 | 0.84 ± 0.36 | 0.32 |
| Albumin (g/dL) | 3.86 ± 0.43 | 3.75 ± 0.32 | 0.16 |
| Platelet count (109/L) | 197.12 ± 59.49 | 210.04 ± 66.37 | 0.34 |
| INR | 0.99 ± 0.09 | 0.99 ± 0.08 | 0.79 |
| APRI | 0.84 ± 0.56 | 0.78 ± 0.65 | 0.65 |
| HCV RNA (log10) | 6.3 ± 0.8 | 6.2 ± 0.8 | 0.59 |
| HAART, n (%) | 25 (75.8) | 48 (66.7) | 0.35 |
| HIV RNA (log10) | 4.83 ± 5.29 | 4.18 + 4.58 | 0.03 |
| Undetectable HIV RNA, n (%) | 12 (36.4) | 30 (41.7) | 0.76 |
| CD4+ T-cell count (cells/mm3) | 170 ± 70 | 550 ± 240 | <0.001 |
| Liver biopsy length (mm) | 21 ± 9 | 19 ± 9 | 0.35 |
| Stage, n (%) | |||
| Stage 0 | 0 | 1 (1.4) | 0.69 |
| Stage 1 | 7 (21.2) | 12 (16.7) | |
| Stage 2 | 18 (54.6) | 43 (59.7) | |
| Stage 3 | 4 (12.1) | 12 (16.7) | |
| Stage 4 | 4 (12.1) | 4 (5.5) | |
Data presented as mean ± s.d. or n (%).
APRI, AST to platelet ratio index; HAART, highly active anti-retroviral therapy.
Performance of the APRI in the prediction of significant fibrosis in HIV-positive and HIV-negative patients
There was a strong positive correlation between APRI and stage of fibrosis in both HCV mono-infected patients (P < 0.001) and HCV/HIV co-infected patients (P = 0.002). However, there was substantially more overlap in APRI values amongst stages of fibrosis in HCV/HIV co-infected patients (Figure 1).
Figure 1.
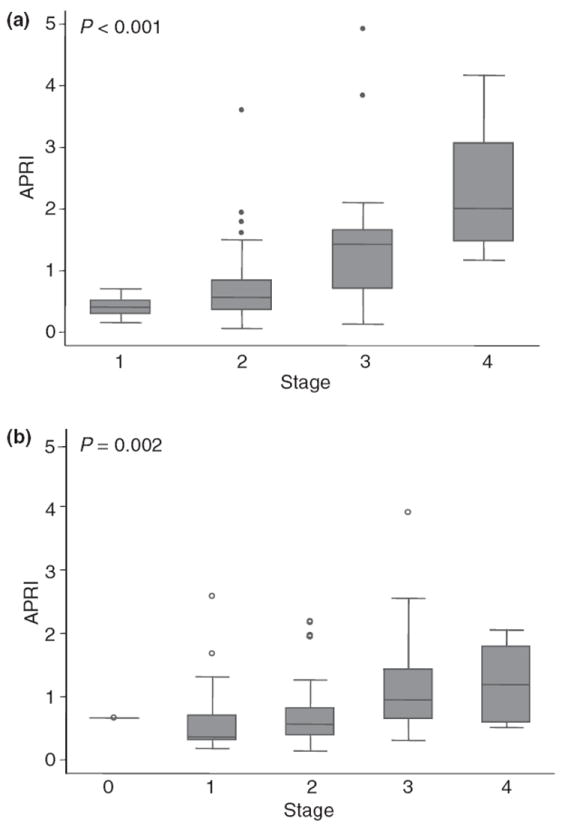
Box plot of APRI values by stage of fibrosis in HCV mono-infected patients (a) and in HCV/HIV co-infected patients (b). Horizontal lines within the boxes indicate median values. Lower and upper box limits indicate the 25th and 75th percentiles, and the vertical bars indicate lower and upper adjacent values. Dots indicate outside values.
The diagnostic utility of the APRI is summarised in Table 3. APRI ≤ 0.5 had a somewhat higher negative predictive value (97% vs. 92%, P = 0.32) and sensitivity (96% vs. 88%, P = 0.28) in HCV mono-infected patients compared with HCV/HIV co-infected patients. APRI > 1.5 also had a somewhat higher positive predictive value (71% vs. 54%, P = 0.35) although similar specificity (94% vs. 93%, P = 0.76) when comparing mono-infected and co-infected patients.
Table 3.
Accuracy of APRI in predicting liver fibrosis
| APRI | All patients | Stage 0–2 | Stage 3–4 | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| HIV negative | ≤0.5 | 38 | 37 | 1 | 0.96 | 0.46 | 0.35 | 0.97 |
| >0.5 | 68 | 44 | 24 | |||||
| ≤1.5 | 89 | 76 | 13 | 0.48 | 0.94 | 0.71 | 0.85 | |
| >1.5 | 17 | 5 | 12 | |||||
| HIV positive | ≤0.5 | 39 | 36 | 3 | 0.88 | 0.44 | 0.32 | 0.92 |
| >0.5 | 66 | 45 | 21 | |||||
| ≤1.5 | 92 | 75 | 17 | 0.29 | 0.93 | 0.54 | 0.82 | |
| >1.5 | 13 | 6 | 7 | |||||
| CD4 ≤ 250 | ≤0.5 | 8 | 7 | 1 | 0.88 | 0.28 | 0.28 | 0.88 |
| >0.5 | 25 | 18 | 7 | |||||
| ≤1.5 | 28 | 21 | 6 | 0.25 | 0.88* | 0.40 | 0.75 | |
| >1.5 | 5 | 3 | 2 | |||||
| CD4 > 250 | ≤0.5 | 31 | 29 | 2 | 0.88 | 0.52 | 0.34 | 0.93 |
| >0.5 | 41 | 27 | 14 | |||||
| ≤1.5 | 64 | 53 | 11 | 0.31 | 0.95* | 0.63 | 0.83 | |
| >1.5 | 8 | 3 | 5 |
PPV, positive predictive value; NPV, negative predictive value.
Specificity in CD4 ≤ 250 cells/mm3 vs. CD4 > 250 cells/mm3, P = 0.05.
The number of patients who could be classified and the percentage correctly classified were calculated according to HIV status (Table 4). APRI was able to classify nearly 50% of patients in both groups, although a somewhat higher percentage of HIV-negative patients than HIV-positive patients were correctly classified (89% vs. 83%, P = 0.34). Amongst the 24 co-infected patients who had significant fibrosis, three patients (all with F3 fibrosis) were misclassified with an APRI of 0.5 or less, whilst seven (three with F3 fibrosis and four with cirrhosis) were correctly classified. In the 81 co-infected patients without significant fibrosis, 36 patients had APRI levels of 0.5 or less whilst six were misclassified with APRI levels of 1.5 or greater (three with F1 fibrosis and four with F2 fibrosis). Thus, for co-infected patients with APRI of 0.5 or less, 36 of 39 did not have significant fibrosis on liver biopsy and were correctly classified. In HCV/HIV co-infected patients with APRI of 1.5 or greater, seven of 13 had significant fibrosis on liver biopsy and were correctly classified. In the HCV mono-infected group, only one patient with F3 fibrosis, and five with F2 fibrosis were misclassified.
Table 4.
Proportion of patients classified and correctly classified by APRI
| Classified | Correctly classified* | |
|---|---|---|
| HIV negative | ||
| Stage 0 | N/A | N/A |
| Stage 1 | 10/14 (71.4%) | 10/10 (100%) |
| Stage 2 | 32/67 (47.8%) | 27/32 (84.4%) |
| Stage 3 | 7/17 (41.2%) | 6/7 (85.7%) |
| Stage 4 | 6/8 (75%) | 6/6 (100%) |
| All patients | 55/106 (51.9%) | 49/55 (89.1%) |
| HIV positive | ||
| Stage 0 | 0/1 (0%) | N/A |
| Stage 1 | 15/19 (78.9%) | 13/15 (86.7%) |
| Stage 2 | 27/61 (44.3%) | 23/27 (85.2%) |
| Stage 3 | 7/16 (43.8%) | 4/7 (57.1%) |
| Stage 4 | 3/8 (37.5%) | 3/3 (100%) |
| All patients | 52/105 (49.5%) | 43/52 (82.7%) |
| CD4 ≤ 250 cells/mm3 | ||
| Stage 0 | N/A | N/A |
| Stage 1 | 4/7 (57.1%) | 2/4 (50%) |
| Stage 2 | 6/18 (33.3%) | 5/6 (83.3%) |
| Stage 3 | 1/4 (25%) | 1/1 (100%) |
| Stage 4 | 2/4 (50%) | 2/2 (100%) |
| All patients | 13/33 (39.4%) | 10/13 (76.9%) |
| CD4 > 250 cells/mm3 | ||
| Stage 0 | 0/1 (0%) | N/A |
| Stage 1 | 11/12 (91.7%) | 11/11 (100%) |
| Stage 2 | 21/43 (48.8%) | 18/21 (85.7%) |
| Stage 3 | 6/12 (50%) | 4/6 (66.7%) |
| Stage 4 | 1/4 (25%) | 1/1 (100%) |
| All patients | 39/72 (54.2%) | 34/39 (87.2%) |
Correctly classified (%) is defined as the number of patients correctly classified divided by the total number of patients who were classified.
Impact of the CD4+ T-cell count on the performance of APRI
The AST to platelet ratio index (APRI) expressed as a continuous variable was significantly associated with liver disease stage in HIV-positive patients with CD4+ T-cell counts >250 cells/μL (ordered logistic regression, P < 0.001), but not in those with lower CD4 counts (P = 0.82). Neither AST nor platelet count predicted disease stage in those with CD4 counts ≤250 (ordered logistic regression, P = 0.66 and 0.96, respectively,) but both were significantly associated with stage in patients with higher CD4 counts (P = 0.001 and 0.002, respectively). There was no difference in AST or platelet count between the two groups (Table 2).
The accuracy of the APRI according to CD4+ T-cell count is summarised in Table 3. In HIV-positive patients with CD4 counts >250, the negative predictive value of APRI ≤ 0.5 was somewhat higher than in those with lower CD4 counts (93% vs. 88%, P = 0.57), whilst sensitivity was similar (P = 0.75). Furthermore, APRI > 1.5 had a higher positive predictive value (63% vs. 40%, P = 0.43) and specificity (95% vs. 88%, P = 0.05) when compared with those with higher and lower CD4 counts, although only the latter comparison bordered on statistical significance.
The AST to platelet ratio index (APRI) was able to classify 54% of patients with CD4 counts >250 cells/mm3, but only 39% of those with CD4 counts ≤250 cells/mm3 (P = 0.16) (Table 4). Additionally, a somewhat higher proportion of those with CD4 counts >250 cells/mm3 were correctly classified (87% vs. 77%, P = 0.38). Of note, the proportions classified and correctly classified in those with higher CD4 counts were similar to the results in the HIV-negative patients.
Potential mechanisms explaining the decreased accuracy of APRI in co-infected patients with low CD4 counts were sought. A similar proportion of patients in each group was on HAART (Table 2), and the presence or absence of HAART was not associated with AST (P = 0.71) or platelet count (P = 0.97). Daily alcohol use in the 6 months preceding liver biopsy was available in 73 (70%) of the co-infected patients who completed detailed questionnaires in the context of another study. There was no difference in alcohol use between those with CD4 counts ≤250 and those with counts >250 (5.5 vs. 3.2 gm/day, P = 0.41), and no association between alcohol use and AST (P = 0.71) or platelet count (P = 0.28).
Whilst there was no difference in AST between the groups stratified by a CD4 count threshold of 250 cells/mm3 (Table 2), there was a significant inverse relationship between CD4 count and AST (P = 0.022). This finding did not appear to be because of higher liver disease stage in those with lower CD4 counts; adjusting for stage in the analysis did not substantially lessen the association between CD4 count and AST (coefficient for CD4 count changed by <10%, P = 0.034). Similarly, there was a positive association between CD4 count and platelet count (P = 0.055). This association was lessened, but not negated, when adjusting for stage in the analysis (coefficient for CD4 count changed by 12%, P = 0.082). Although alcohol use and HAART were not true confounders in our dataset, we adjusted for these variables for completeness and confirmed that they did not impact the observed relationships between CD4 count and AST or platelet count (data not shown). As expected, there was a significant, inverse relationship between CD4 count and APRI (P = 0.008) that persisted after adjusting for liver disease stage (coefficient for CD4 changed by <10%, P = 0.013).
ROC curve analysis
The ROC curve analysis was also conducted to compare the diagnostic performance of APRI in HCV/HIV co-infected patients and HCV mono-infected patients (Figure 2). The AUROC for predicting significant fibrosis was lower in the HCV/HIV co-infected group (0.77; 95% CI, 0.66–0.87) compared with the HCV mono-infected group (0.86; 95% CI, 0.77–0.96), but this difference did not reach statistical significance (P = 0.18) (Figure 2). When biopsies <15 mm in length were excluded (n = 47), the AUROC for predicting significant fibrosis did not change [0.72 (95% CI, 0.59–0.85) vs. 0.84 (95% CI, 0.71–0.96) for the co-infected and monoinfected groups, respectively]. Subgroup analysis revealed that the APRI predicted liver fibrosis stage less well in co-infected patients with CD4 counts ≤250 cells/mm3 than in patients with higher CD4 counts (AUROC 0.64, 95%CI 0.42–0.85, vs. AUROC 0.83, CI 0.71–0.95, P = 0.12) (Figure 3). Of note, the APRI AUROC in co-infected patients with low CD4 cell counts was substantially lower than that in the HIV negative patients, and this difference bordered on statistical significance (P = 0.054). A similar proportion in each group, 83% of HIV-negative patients and 82% of HIV-positive patients with low CD4 cell counts, had liver biopsies ≥15 mm in length (P = 0.9). When biopsies <15 mm in length were excluded, the AUROC for predicting significant fibrosis in co-infected patients with low CD4 cell counts did not change (AUROC 0.63; 95% CI, 0.39–0.87).
Figure 2.
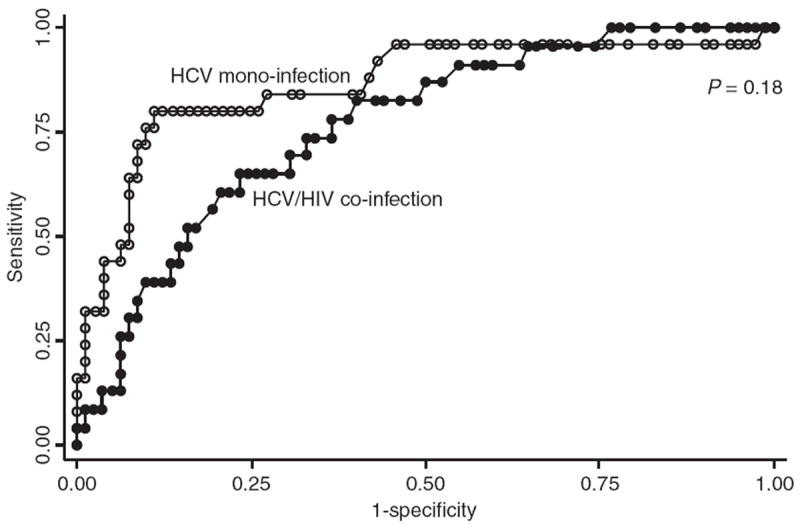
Receiver operating characteristic curve of APRI for significant fibrosis in HCV/HIV co-infected patients and HCV mono-infected patients.
Figure 3.
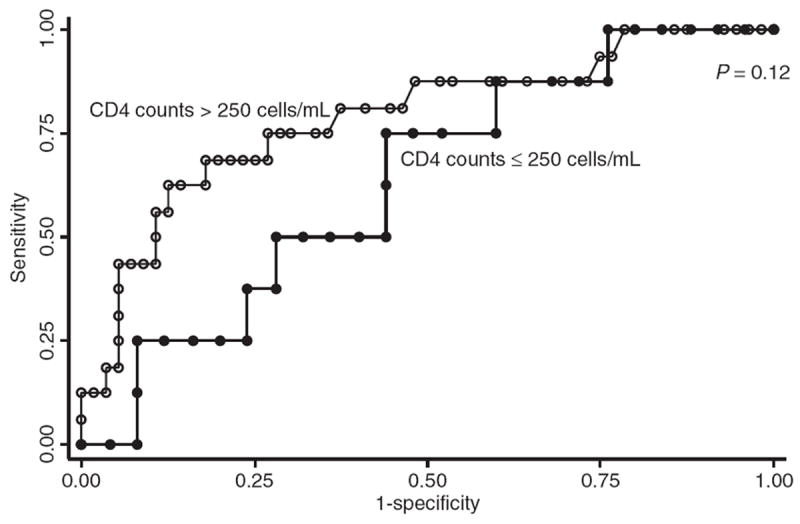
Receiver operating characteristic curve of APRI for significant fibrosis in HCV/HIV co-infected patients with CD4 counts ≤250 cells/mm3 and with CD4 counts >250 cells/mm3.
For comparison, ROC curve analysis also was performed where significant fibrosis was defined as Batts-Ludwig stages 2–4. The AUROC was again similar when comparing HCV monoinfected and HCV/HIV co-infected patients (AUROC 0.76, 95% CI 0.66–0.86, vs. AUROC 0.67, CI 0.52–0.82, P = 0.33), but overall performance was lower than when significant fibrosis was defined as stage 3–4. The AUROC differed according to CD4 cell count: AUROC 0.41 (95% CI 0.14–0.68) for CD4 cell counts ≤250, and 0.81 (CI 0.67–0.95) for CD4 counts >250 (P = 0.01). Finally, AUROC remained significantly different when comparing HIV-negative patients to HIV-positive patients with low CD4 counts (P = 0.02).
FIB-4 ROC curve analysis
As a result of the suboptimal performance of APRI in HIV-positive patients with CD4 counts ≤250, we assessed the predictive ability of a second non-invasive marker, FIB-4. FIB-4 was chosen because it was initially developed in HCV/HIV co-infected patients and also utilises readily available laboratory data.35 FIB-4 was calculated as follows: (age × AST)/(platelets × ALT1/2).35 Using values of 1.45 and 3.25 as predefined cut-offs to predict the absence or presence of significant fibrosis, respectively, FIB-4 was able to classify 73% of all patients. The FIB-4 AUROCs for predicting significant fibrosis in the HCV/HIV co-infected group (0.77; 95% CI, 0.65–0.90) and the HCV mono-infected group (0.85; 95% CI, 0.75–0.95) were similar to those of the APRI. In contrast, subgroup analysis revealed that the FIB-4 predicted significant fibrosis equally well in co-infected patients with CD4 counts ≤250 cells/mm3 as in patients with higher CD4 counts (AUROC 0.74, 95% CI 0.57–0.91, vs. AUROC 0.76, CI 0.54–0.97, P = 0.91). The negative predictive value of FIB-4 was comparable to that of APRI across groups (data not shown). However, no patients with low CD4 counts had FIB-4 values >3.25, limiting our ability to compare other FIB-4 performance measures across groups and with the APRI.
DISCUSSION
Our study is only the second, and the larger, to date to compare the performance of APRI in HCV/HIV co-infected patients to that in patients infected with HCV alone.28 In addition, we are the first to find a difference in the performance of the APRI according to CD4+ T-cell count. By including a diverse group of co-infected patients with respect to CD4+ T-cell count, we were able to assess the effect of HIV-related factors on the performance of APRI. Evaluation of the diagnostic performance of APRI by ROC curve analysis revealed a lower AUROC in the co-infected group (0.77 vs. 0.86, P = 0.18). This difference appeared to be largely driven by relatively poor performance of the APRI in HCV/HIV co-infected patients with lower CD4 counts ≤250 cells/mm3: in those with higher CD4 cell counts the AUROC was similar to that found in the HIV-negative patients (0.83 vs. 0.86), whilst the AUROC was substantially lower in those with CD4 counts ≤250 (0.64 vs. 0.86, P = 0.05).
Prior studies evaluating the accuracy of APRI in HCV/HIV co-infection have been limited by small sample size and/or proportionately few patients with low CD4 counts.16, 25, 27-29 In addition, many of these other studies had a larger proportion of patients who were on HAART (83% to 100% in four out of five studies reporting data)25-28 and with undetectable HIV (44–60%).16, 25-29 In the largest study to date, where all patients had CD4 counts >300, the AUROC for APRI ranged from 0.73 to 0.80, but there was no effect of CD4 count (≤500 vs. >500) on the performance of APRI.27 No other study has evaluated the impact of CD4 count on the accuracy of APRI in predicting liver fibrosis.
We found that APRI was able to classify, and correctly classify, a higher proportion of patients in the HCV/HIV co-infected group with higher CD4 counts when compared with patients with lower CD4 counts (Table 4). APRI was able to classify 54% of patients with CD4 counts >250 cells/mm3, but only 39% of those with lower CD4 counts (P = 0.16). In addition, these APRI performance measures were similar when comparing HCV/HIV co-infected patients with higher CD4 counts to HCV monoinfected patients. Liver biopsies could have been avoided in approximately 45% of both HIV-negative and HIV-positive patients with higher CD4 counts, but only 30% of HIV-positive patients with lower CD4 counts. In HIV-negative patients and in co-infected patients with CD4 counts >250, the majority who were misclassified had stage 2 fibrosis on liver biopsy. Thus, this discordance between APRI and the biopsy may have been a result of biopsy sampling error. However, in co-infected patients with CD4 counts ≤250, two of the three patients who were misclassified had stage 1 fibrosis on biopsy, suggesting a lower likelihood of sampling error in this group.
When compared with the HCV monoinfected patients, APRI ≤ 0.5 in those with HCV/HIV coinfection had consistently lower sensitivity (Table 3). Conversely, the specificity of APRI > 1.5 was comparable across groups with the exception of lower specificity in the HIV-positive group with CD4 counts ≤250. It should be noted that the primary strength of APRI in these patients appears to be its ability to exclude significant fibrosis when APRI < 0.5, similar to what has been previously reported in HCV mono-infection.36 A recent meta-analysis found that APRI < 0.5 has an acceptable negative predictive value in a population with average prevalence of signficant fibrosis, whereas the positive predictive value of APRI > 1.5 remains unacceptable.37 Only three of the sixteen included studies had HCV/HIV co-infected patients, and these three studies had limited numbers of patients with low CD4 counts. We found that the negative predictive value of APRI ≤ 0.5 in co-infected patients with CD4 counts ≤250 was less than 90%, suggesting more limited utility in this subgroup when compared with those with higher CD4 counts and to HIV-negative patients.
There are several reasons why APRI may have poor diagnostic performance in HCV/HIV patients with low CD4 counts. First, HIV infection can cause thrombocytopenia. Additionally, HAART is not uncommonly associated with idiosyncratic hepatotoxicity, and hepatic steatosis may develop as a consequence of HAART-associated mitochondrial toxicity or metabolic dysfunction (e.g. lipodystrophy); both processes can contribute to increased AST levels. Finally, antiretroviral therapy may contribute to fibrosis progression through steatosis/other mechanisms, which may not be reflected by non-invasive markers that were validated in groups of patients with fewer coexisting risk factors for disease severity. For example, Lieber et al. found that APRI was a poor predictor of significant fibrosis in patients with alcoholic liver fibrosis, even amongst those with HCV infection.38 We found associations between the CD4 T-cell count (expressed as a linear variable) and both AST and platelet count that were independent of liver disease stage. HIV-associated thrombocytopenia may explain the observed inverse relationship between the CD4 count and the platelet count. However, the poorer performance of APRI in those with low CD4 counts could not be explained by HAART or other possible factors influencing AST and/or platelet count, such as alcohol use. In addition, biopsy length did not appear to impact the accuracy of APRI in this group of patients. Further research is needed to elucidate the mechanisms behind the poor overall performance of APRI in patients with low CD4 T-cell counts.
Whilst we did not originally intend to conduct a detailed comparison of APRI and FIB-4, the latter model appeared to perform equally well in those with low and higher CD4 counts based on the AUROC. Unfortunately, the lack of subjects with significant fibrosis and FIB-4 values >3.25 in the former group limited our ability to assess the impact of low CD4 counts on other FIB-4 performance measures. It is possible that the inclusion of ALT in the latter model ‘corrects’ for HAART-related effects on AST: whilst AST did not predict stage in our patients with low CD4 counts (P = 0.11), it was significantly associated with stage after adjusting for ALT (P = 0.007). In addition, including age as a surrogate for disease duration, a well-known predictor of fibrosis stage, may enhance the predictive ability of this model in patients with low CD4 counts and HIV-related thrombocytopenia. However, even after adjusting for age, platelet count remained a poor predictor of stage in our patients with CD4 counts <250 (data not shown). Previous studies comparing APRI and FIB-4, largely in patients with higher CD4 counts, found no significant difference between these two models.26, 30 Shire and colleagues recently reported that the FIB-4 performed best amongst four models, but comparisons were not made according to CD4 count. Future studies should aim to compare FIB-4 and APRI in a larger number of HCV/HIV co-infected patients with low CD4 T-cell counts.
We acknowledge that our study had several limitations. It was performed in a single academic medical centre and thus one may not be able to generalise our results to HCV/HIV co-infected patients seen in community practice settings. Second, we included a relatively small number of patients who had HIV infection and low CD4+ T-cell counts when compared with the size of the overall cohort. The difference in ROC curves between patients with low CD4 counts and HIV negative patients achieves borderline statistical significance, although would likely reach greater statistical significance with a larger number of patients in the former group. Similarly, clinically important differences in performance measures between groups do not reach statistical significance, and this is likely because of the small number of observations. We also had an insufficient number of patients with cirrhosis to assess the accuracy of APRI in predicting that outcome. Finally, there is the inherent problem of intra-observer variability and sampling error when using the liver biopsy as a gold standard. In fact, Poynard et al. found that divergent results may be more often a result of inadequate biopsies than inaccurate non-invasive markers.39 We did not restrict our study to biopsies greater than 15 or 20 mm in size so as not to overly limit our sample size, and instead relied on the expertise of our study pathologist to determine the adequacy of each biopsy for staging purposes. The vast majority of liver biopsy samples were at least 15 mm in length, and eliminating smaller biopsies did not affect the performance of the APRI. In the largest study to evaluate the accuracy of APRI in HCV/HIV co-infected patients, there was no difference in the AUROC when comparing biopsies ≥15 mm to those ≥20 mm in length.27 In addition, APRI accuracy was not affected by adequacy of biopsy specimens in a recent meta-analysis.37 Overall, we believe that this study’s limitations are outweighed by its notable strengths. These include its relatively large sample size compared with previously published studies, the inclusion of matched HIV-negative and -positive patients, the diverse HIV-infected population including patients with lower CD4 counts than previously studied, and the interpretation of all biopsies by a single, experienced hepatopathologist.
In conclusion, the APRI is a simple model that may be utilised to predict significant fibrosis in some patients with HCV/HIV coinfection, although it appears to be less accurate in co-infected patients with low CD4 counts. Whereas liver biopsies could be avoided in nearly one-half of patients with well-controlled HIV, this would be possible in only 30% of patients with CD4 counts <250 cells/mm3. In contrast, ROC curve analysis suggests that FIB-4 performs well in co-infected patients regardless of CD4 count. If these results are confirmed, APRI can be considered for use in HCV mono-infected patients and HCV/HIV co-infected patients with high CD4 counts but should be avoided in co-infected patients with CD4 counts <250 cells/mm3.
Acknowledgments
Declaration of personal interests: None. Declaration of funding interests: This study was funded in part by NIH grant number R01 AI49168.
References
- 1.Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47:1–39. [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Kim WR. The burden of hepatitis C in the United States. Hepatology. 2002;36(5 Suppl. 1):S30–4. doi: 10.1053/jhep.2002.36791. [DOI] [PubMed] [Google Scholar]
- 4.Deuffic-Burban S, Poynard T, Sulkowski MS, Wong JB. Estimating the future health burden of chronic hepatitis C and human immunodeficiency virus infections in the United States. J Viral Hepat. 2007;14:107–15. doi: 10.1111/j.1365-2893.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 5.Tedeschi R, Pivetta E, Zanussi S, et al. Quantification of hepatitis C virus (HCV) in liver specimens and sera from patients with human immunodeficiency virus coinfection by using the Versant HCV RNA 3.0 (branched DNA-based) DNA assay. J Clin Microbiol. 2003;41:3046–50. doi: 10.1128/JCM.41.7.3046-3050.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staples CT, Jr, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29:150–4. doi: 10.1086/520144. [DOI] [PubMed] [Google Scholar]
- 7.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34:831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 8.Weber R, Friis-Moller N, Sabin C. HIV and non-HIV Related Deaths and Their Relationship to Immunodeficiency: The D:A:D Study; Proceedings of the 12th Conference on Retroviruses and Opportunistic Infections; Boston, MA. Feb, 2005. Abstract 595. [Google Scholar]
- 9.Mohsen AH, Easterbrook PJ, Taylor C, et al. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut. 2003;52:1035–40. doi: 10.1136/gut.52.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–9. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 11.Puoti M, Bonacini M, Spinetti A, et al. Liver fibrosis progression is related to CD4 cell depletion in patients coinfected with hepatitis C virus and human immunodeficiency virus. J Infect Dis. 2001;183:134–7. doi: 10.1086/317644. [DOI] [PubMed] [Google Scholar]
- 12.Berenguer J, Alvarez-Pellicer J, Martin PM, et al. Sustained virological response to interferon plus ribavirin reduces liver-related complications and mortality in patients coinfected with human immunodeficiency virus and hepatitis C virus. Hepatology (Baltimore, Md) 2009;50:407–13. doi: 10.1002/hep.23020. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Tsao G, Boyer JL. Outpatient liver biopsy: how safe is it? Ann Intern Med. 1993;118:150–3. doi: 10.7326/0003-4819-118-2-199301150-00013. [DOI] [PubMed] [Google Scholar]
- 14.Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165–73. doi: 10.1016/s0168-8278(86)80075-7. [DOI] [PubMed] [Google Scholar]
- 15.Strader DB, Wright T, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C. Hepatology (Baltimore, Md) 2004;39:1147–71. doi: 10.1002/hep.20119. [DOI] [PubMed] [Google Scholar]
- 16.Al-Mohri H, Cooper C, Murphy T, Klein MB. Validation of a simple model for predicting liver fibrosis in HIV/hepatitis C virus-coinfected patients. HIV Med. 2005;6:375–8. doi: 10.1111/j.1468-1293.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 17.Fontana RJ, Lok AS. Noninvasive monitoring of patients with chronic hepatitis C. Hepatology (Baltimore, Md) 2002;36(5 Suppl. 1):S57–64. doi: 10.1053/jhep.2002.36800. [DOI] [PubMed] [Google Scholar]
- 18.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–75. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 19.Forns X, Ampurdanes S, Llovet JM, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology (Baltimore, Md) 2002;36(4 Pt 1):986–92. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 20.Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol. 2001;96:3142–6. doi: 10.1111/j.1572-0241.2001.05268.x. [DOI] [PubMed] [Google Scholar]
- 21.Poynard T, Bedossa P. Age and platelet count: a simple index for predicting the presence of histological lesions in patients with antibodies to hepatitis C virus. METAVIR and CLINIVIR Cooperative Study Groups. J Viral Hepat. 1997;4:199–208. doi: 10.1046/j.1365-2893.1997.00141.x. [DOI] [PubMed] [Google Scholar]
- 22.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology (Baltimore, Md) 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 23.Lok AS, Ghany MG, Goodman ZD, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology (Baltimore, Md) 2005;42:282–92. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]
- 24.Myers RP, Benhamou Y, Imbert-Bismut F, et al. Serum biochemical markers accurately predict liver fibrosis in HIV and hepatitis C virus co-infected patients. AIDS. 2003;17:721–5. doi: 10.1097/00002030-200303280-00010. [DOI] [PubMed] [Google Scholar]
- 25.Kelleher TB, Mehta SH, Bhaskar R, et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol. 2005;43:78–84. doi: 10.1016/j.jhep.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Loko MA, Castera L, Dabis F, et al. Validation and comparison of simple non-invasive indexes for predicting liver fibrosis in HIV-HCV-coinfected patients: ANRS CO3 Aquitaine cohort. Am J Gastroenterol. 2008;103:1973–80. doi: 10.1111/j.1572-0241.2008.01954.x. [DOI] [PubMed] [Google Scholar]
- 27.Macias J, Giron-Gonzalez JA, Gonzalez-Serrano M, et al. Prediction of liver fibrosis in human immunodeficiency virus/hepatitis C virus coinfected patients by simple non-invasive indexes. Gut. 2006;55:409–14. doi: 10.1136/gut.2005.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nunes D, Fleming C, Offner G, et al. HIV infection does not affect the performance of noninvasive markers of fibrosis for the diagnosis of hepatitis C virus-related liver disease. J Acquir Immune Defic Syndr. 2005;40:538–44. doi: 10.1097/01.qai.0000184856.31695.bf. [DOI] [PubMed] [Google Scholar]
- 29.Shire NJ, Rao MB, Succop P, et al. Improving noninvasive methods of assessing liver fibrosis in patients with hepatitis C virus/human immunodeficiency virus co-infection. Clin Gastroenterol Hepatol. 2009;7:471–80, e1-2. doi: 10.1016/j.cgh.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trang T, Petersen JR, Snyder N. Noninvasive markers of hepatic fibrosis in patients co-infected with HCV and HIV: comparison of the APRI and FIB-4 index. Clin Chim Acta. 2008;397:51–4. doi: 10.1016/j.cca.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 31.Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–17. doi: 10.1097/00000478-199512000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology (Baltimore, Md) 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zou KH, O’Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–7. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 34.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 35.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology (Baltimore, Md) 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 36.Smith JO, Sterling RK. Systematic review: non-invasive methods of fibrosis analysis in chronic hepatitis C. Aliment Pharmacol Ther. 2009;30:557–76. doi: 10.1111/j.1365-2036.2009.04062.x. [DOI] [PubMed] [Google Scholar]
- 37.Shaheen AA, Myers RP. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis C-related fibrosis: a systematic review. Hepatology (Baltimore, Md) 2007;46:912–21. doi: 10.1002/hep.21835. [DOI] [PubMed] [Google Scholar]
- 38.Lieber CS, Weiss DG, Morgan TR, Paronetto F. Aspartate aminotransferase to platelet ratio index in patients with alcoholic liver fibrosis. Am J Gastroenterol. 2006;101:1500–8. doi: 10.1111/j.1572-0241.2006.00610.x. [DOI] [PubMed] [Google Scholar]
- 39.Poynard T, Munteanu M, Imbert-Bismut F, et al. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344–55. doi: 10.1373/clinchem.2004.032227. [DOI] [PubMed] [Google Scholar]


