Abstract
Pain is the most common reason patients with inflammatory arthritis see a rheumatologist. Patients consistently rate pain as one of their highest priorities, and pain is the single most important determinant of patient global assessment of disease activity. Although pain is commonly interpreted as a marker of inflammation, the correlation between pain intensity and measures of peripheral inflammation is imperfect. The prevalence of chronic, non-inflammatory pain syndromes such as fibromyalgia is higher among patients with inflammatory arthritis than in the general population. Inflammatory arthritis patients with fibromyalgia have higher measures of disease activity and lower quality of life than inflammatory patients who do not have fibromyalgia. This review article focuses on current literature involving the effects of pain on disease assessment and quality of life for patients with inflammatory arthritis. It also reviews non-pharmacologic and pharmacologic options for treatment of pain for patients with inflammatory arthritis, focusing on the implications of comorbidities and concurrent disease-modifying antirheumatic drug therapy. Although several studies have examined the effects of reducing inflammation for patients with inflammatory arthritis, very few clinical trials have examined the safety and efficacy of treatment directed specifically towards pain pathways. Most studies have been small, have focused on rheumatoid arthritis or mixed populations (e.g., rheumatoid arthritis plus osteoarthritis), and have been at high risk of bias. Larger, longitudinal studies are needed to examine the mechanisms of pain in inflammatory arthritis and to determine the safety and efficacy of analgesic medications in this specific patient population.
Keywords: Arthritis, Rheumatoid arthritis, Psoriatic arthritis, Fibromyalgia, Pain, Chronic pain, Quality of life, Comorbidity, Treatment, Non-steroidal anti-inflammatory agents, NSAIDs, Opioid analgesics, Neurotransmitter agents, Antidepressive agents
Introduction
Chronic pain affects 116 million people, more than the total affected by diabetes, heart disease, and cancer [1]. In the United States, the cost of pain, because of lost productivity and treatment costs, exceeds 635 billion dollars per year [1]. Pain is the primary reason patients with inflammatory arthritis seek rheumatologic care. Among rheumatoid arthritis (RA) patients, 68-88 % rate pain as one of their top three priorities, and 90 % rate pain as one of their top three priorities [2, 3]. The American College of Rheumatology Pain Management Task Force recently proclaimed that “pain is probably the most important patient-reported outcome in rheumatology” [4••].
Inflammatory, Peripheral Pain
Historically, pain among patients with inflammatory arthritis has been attributed to peripheral inflammation. Treatment with disease-modifying antirheumatic drugs (DMARDs) is effective in reducing inflammatory pain symptoms, with mean pain visual analog scores (VAS 0–100) decreasing approximately 20–40 points in clinical trials of new biologic agents, for example tocilizumab, and in clinical trials focusing on early inflammatory rheumatoid arthritis patients [5].
Non-inflammatory, Central Pain
Despite effective DMARD therapy, however, observational studies of inflammatory arthritis indicate that many patients continue to suffer from moderate pain [6]. In a recent study of RA patients in inflammatory disease remission (disease activity score in 28 joints (DAS28)<2.6), the prevalence of clinically significant pain (pain numeric rating score≥4) was 12% [7]. These findings are indicative of a non-inflammatory pain component, for example structural joint damage, pain from other etiologies, and/or dysregulation of central pain regulatory mechanisms, as occurs in chronic widespread pain states, for example fibromyalgia.
One of the most common sites for non-inflammatory pain is the back. Few studies have examined the prevalence of back pain in RA patients [8, 9]. In a study of 1076 RA patients and 1491 community controls, Neva et al. reported that the prevalence of chronic back pain was 19 % among RA patients and 25 % among controls [10]. These results suggest that, although chronic back pain is common among RA patients, it is not more prevalent among RA patients than healthy controls.
In contrast, the prevalence of widespread, non-inflammatory conditions, for example fibromyalgia, is significantly higher in RA than in the general population. Thirteen to 25 % of RA patients have fibromyalgia, and an additional 7–15 % have chronic widespread pain that does not meet criteria for fibromyalgia [11–13]. In a study of 1,487 early inflammatory arthritis patients in the Canadian early arthritis cohort (CATCH), the incidence of fibromyalgia was highest during the year after inflammatory arthritis diagnosis, with cumulative incidence of 6.77 per 100 person years [14]. Predictors of fibromyalgia included high pain severity, poor mental health, and the absence of cyclic citrullinated peptide antibodies. This study suggests that the development of chronic, non-inflammatory pain occurs early in the course of the disease, and moderate to severe pain may induce central nervous system sensitization, which has been associated with chronic widespread pain states [15–18].
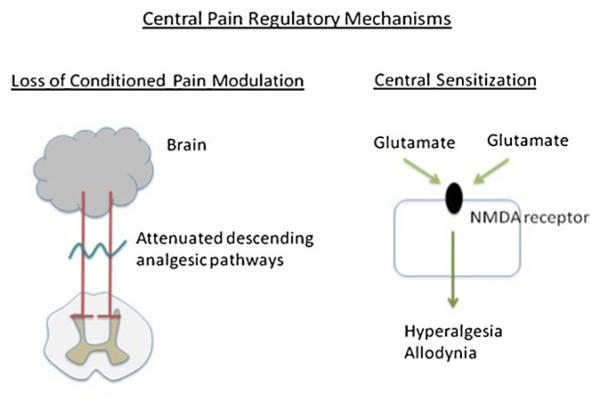
The mechanisms of development of chronic widespread pain in inflammatory arthritis patients are not well understood [19]. Studies of experimental pain sensitivity have shown that pressure pain thresholds (the pressures which elicit pain) are lower in RA patients than healthy controls [20, 21]. These thresholds are lower at joint and non-joint sites, suggesting that alterations in central pain regulatory mechanisms, for example loss of conditioned pain modulation and central sensitization, may mediate widespread pain in RA (Fig. 1). Loss of conditioned pain modulation is impairment of the descending opioidergic and/or serotonergic-noradrenergic pathways that normally induce analgesia [22, 23], whereas central sensitization is defined as heightened excitability of the central nervous system neurons transmitting pain. Loss of conditioned pain modulation is attenuated or absent in patients with chronic non-inflammatory pain conditios, for example fibromyalgia, irritable bowel syndrome and osteoarthritis, whereas central sensitization is enhanced. [18, 24, 25]. Studies are in progress to elucidate their involvement in inflammatory arthritis.
Fig. 1.
Central pain regulatory mechanisms may mediate pain sensitivity in inflammatory arthritis via loss of conditioned pain modulation and/or central sensitization. Loss of conditioned pain modulation occurs when the descending analgesic pathways are absent or attenuated. Central sensitization occurs as a result of enhanced glutamate sensitivity
Effect of Pain on Disease Assessment
Two recent manuscripts have reported that pain significantly affects patient assessment of RA disease activity. The first study included 7,028 RA patients from the Quantitative Standard Monitoring of Patients with RA (QUEST-RA) database [26]. These patients were from 83 sites across 30 countries, receiving “usual care” from their rheumatologists. In this cohort, pain was the single most important determinant of patient global assessment, with a partial R2>0.2 whereas the partial R2 for all other potential determinants was less than 0.05. The second study included 646 RA patients starting methotrexate treatment at an academic outpatient clinic. Similar to the QUEST-RA study, this study concluded that pain was the major determinant of patient global assessment scores, explaining 75.6 % of the variability in scores [27•].
Although pain contributes less to physician global assessment than patient global assessment, it remains one of the top predictors of physician global assessment. In the QUEST-RA study, pain followed swollen joint count, erythrocyte sedimentation rate, and tender joint count, as the fourth most important determinant of physician global assessment [26]. Among the 646 RA patients starting methotrexate treatment, pain was second only to swollen joint count as the most important predictor of physician global assessment [27•]. Pain was also one of the most significant predictors of discordance between patient and physician global assessment score in both studies, highlighting the importance of pain in patients’ “disease experience” and suggest that physicians should better assess the effect of pain on their patients’ lives.
Effect of Chronic Widespread Pain Conditions on Disease Assessment
Pain, however, does not necessarily equate with heightened inflammatory disease activity. Several cross-sectional studies have indicated that the presence of widespread pain conditions, for example fibromyalgia, significantly inflates assessment of RA disease activity. Using the 1990 ACR classification criteria for fibromyalgia to categorize 32 RA patients with fibromyalgia and 238 RA patients without fibromyalgia, Ranzolin et al. noted that the DAS28 score was significantly higher among patients with RA and fibromyalgia than among those with RA only (mean 5.36 vs. 4.03; P<0.001) [11]. These associations persisted after adjustment for inflammatory disease variables, for example ESR and swollen joint count. Measures of functional status and quality of life, for example the Health Assessment Questionnaire (HAQ) and the Medical Outcomes Study Short Form 36 (SF-36) were also significantly worse among RA patients with fibromyalgia than among those with RA only. On the basis of these findings, the authors concluded that disease activity measures may be artificially inflated among RA patients with concomitant fibromyalgia.
Using tender point counts to characterize RA patients with and without fibromyalgia, Pollard et al. obtained similar results [28]. RA patients with ≥11 tender points had higher DAS28 scores than other RA patients (mean 6.0 vs. 4.3). Although these groups had the same mean number of swollen joints, RA patients with ≥11 tender points had significantly higher tender joint counts (mean 17 vs. 6) and patient global assessment scores (mean 66 vs. 40). These results suggest that RA patients with fibromyalgia may have higher disease activity measurements because they report greater pain.
To more fully examine the range of tender point scores, Ton et al. categorized 200 RA patients into four groups: 0 tender points, 1–5 tender points, 6–10 tender points, and ≥11 tender points [29]. The distribution was skewed to the right, with 73 % of RA patients having ≤5 tender points. DAS28 scores increased as tender point count increased, even among RA patients who did not meet the ≥11 tender point count threshold for the 1990 ACR classification of fibromyalgia [30]. In analysis involving the individual components of the DAS28, the correlation between tender point count and specific disease activity measures was highest for subjective measures, specifically the tender joint count (r=0.37, P<0.0001) and the patient global assessment (r=0.29, P<0.0001). These results indicate that, even among patients who do not meet the 1990 ACR tender point criteria for RA, enhanced pain sensitivity is associated with elevated subjective assessment of disease activity.
Similar results were noted in a study by Pollard et al. which used pressure pain thresholds to characterize pain sensitivity in 105 RA patients. The authors reported significant inverse associations between pressure pain thresholds and tender joint count and patient global assessment, but not swollen joint count, in unadjusted analyses [31]. Because of strong collinearity with tender point count, tender joint count and patient global assessment were not included in multivariable analyses. Thus, no conclusions could be made regarding the independent association between these variables and pressure pain thresholds.
Despite differences in the characterization of fibromyalgia, these studies all yielded similar results, showing that the presence of enhanced pain sensitivity and/or concomitant fibromyalgia inflates RA disease assessment, particularly when using subjective measures (e.g., tender joint count, patient global assessment) or composite measures that include subjective components (e.g., DAS28). Collectively, these results suggest that physicians should carefully consider the effect of fibromyalgia on disease assessment measures when evaluating inflammatory arthritis disease activity.
Effect of Pain on Patient-Reported Outcomes and Quality of Life
Beyond its effect on measures of disease activity, pain is also strongly associated with other patient-reported outcomes and quality of life. In a Finnish study of 1,076 adult RA patients, Neva et al. reported that RA patients with chronic low back pain had significantly lower scores on the HAQ, a self-report measure of disability [10]. Similar results have been noted in children with juvenile inflammatory arthritis. In a cross-sectional analysis of 152 juvenile inflammatory arthritis patients in The Netherlands, pain was significantly associated with overall health-related quality of life, and with specific measures of physical and emotional functioning in models adjusted for age, disease activity, medication use, body mass index, parental education, and school attendance [32]. These studies reveal the overall effect of pain on patients’ daily lives, irrespective of age and other sociodemographic and disease-related factors.
Several studies have more specifically examined the effects of pain on patients’ emotional health. In a large, combined analysis of 11,223 RA patients from the National Data Bank for Rheumatic Diseases and 4,059 RA patients from the Swiss Quality Management Program for Rheumatoid Arthritis, Courvoisier et al. used a latent state-trait model to assess the contribution of pain to overall emotional health and emotional health divided into a stable component (state) and a variable component (trait), reflecting emotional fluctuations at the time of measurement [33]. In this model, pain was the most significant contributor to overall emotional health, accounting for 44 % of the observed variance in total mental component summary scores of the SF-36. When the state and trait components were assessed separately, pain accounted for 60 % of the observed variance in the stable component of mental component summary scores and 5 % of the observed variance in the variable component of mental component summary scores. These results suggest that pain has a significant effect on long-term emotional health, beyond day-to-day fluctuations.
In a longitudinal analysis examining the directionality of associations between pain and depression, Husted et al. followed 394 psoriatic arthritis patients annually over a mean of 7.5 years [34]. A linear mixed effects modeling approach revealed that the most significant predictors of pain and depression were the corresponding scores at the previous visit. However, even after controlling for these associations, there were statistically significant cross-variable associations between pain and depression, indicating that pain predicts depression and vice versa. These results suggest that effective management strategies should be directed at both symptoms when they co-exist in inflammatory arthritis patients.
Similar to the relationship between pain and depression, pain and sleep disturbance are also intricately intertwined. We have previously shown that pressure pain thresholds at joint and non-joint sites are associated with sleep problems among RA patients [35]. In a cross-sectional study involving surveys mailed to 115 juvenile inflammatory arthritis patients and 40 juvenile dermatomyositis patients, Butbul Aviel et al. also noted that pain intensity within the last week was significantly correlated with sleep disturbance [36]. However, because of the cross-sectional design, causality could not be assessed. By use of mediation analysis, Nicassio et al. identified pathways between pain and sleep disturbance in a cross-sectional cohort of 106 RA patients [37]. In one pathway, depression mediated the relationship between pain and sleep disturbance. In a separate, independent pathway, pain was directly associated with sleep problems. However, this study was also limited by the cross-sectional design, and no conclusions could be made regarding the directionality of effects. In a follow-up study, these investigators examined the effects of sleep deprivation on pain, depression, and anxiety in RA patients compared with healthy controls [38]. After partial night-sleep deprivation, RA patients had greater increases in the severity of pain, depression, and anxiety than controls, indicating that sleep disturbance increased pain and emotional distress. Together, these results suggest that, similar to the relationship between pain and depression, the relationship between pain and sleep is bidirectional and complex.
Non-pharmacologic Treatment of Pain
Although the development of immunosuppressive medications targeted at specific inflammatory pathways has increased substantially in the past two decades, relatively little research has focused on the treatment of pain in in-flammatory arthritis. Given the associations between pain, sleep disturbance, and emotional health, it is plausible that improving emotional health may improve pain symptoms. In a meta-analysis of 27 randomized controlled trials of face-to-face psychological intervention (e.g., cognitive behavioral therapy, patient education, stress management), Knittle et al. determined that psychological intervention was associated with significant improvements in pain, depression, and physical activity among RA patients [39]. These authors subsequently reported that the effects of self-efficacy on reducing pain were mediated by achievement of physical activity objectives [40]. Together, these studies indicate that physicians should consider cognitive behavioral therapy and self-management programs to help inflammatory arthritis patients improve self-efficacy, enhance physical activity, and reduce pain.
Pharmacologic Treatment of Pain
The 3e (evidence, expertise, exchange) initiative, a 17-nation collaboration that promotes evidence-based practice in rheumatology, recently published recommendations for pharmacologic pain management for inflammatory arthritis patients [41••]. The authors highlighted six factors to consider when making a decision regarding pharmacologic pain management:
type of pain
type of inflammatory arthritis
the presence of residual inflammation
comorbidities
patient preference; and
the addictive potential of the medication and the patient.
The main treatment options included:
non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen as first line therapy;
NSAIDs+acetaminophen or an alternative NSAID second line therapy; and
weak opioids when NSAIDs and acetaminophen have failed or are contraindicated (Table 1).
Table 1.
Medications for treatment of pain in inflammatory arthritis
| Treatment | Description |
|---|---|
| Acetaminophen | First line therapy; may be used in combination with NSAIDs if monotherapy does not work |
| Non-steroidal anti- inflammatory drugs (NSAIDs) |
First line therapy; may be used in combination with acetaminophen if monotherapy does not work |
| Opioids | Weak evidence for use when acetaminophen and NSAIDs are contraindicated or inefficacious. Long-term use should be minimized because of potential side effects |
| Antidepressants | Inconclusive data, but expert opinion supports use of tricyclic antidepressants as adjuvant treatment in select cases |
| Neuromodulators | Weak evidence for some agents (nefopam and capsaicin) as adjuvant treatment. Expert opinion endorses gabapentin and pregabalin in some cases |
The authors also advocated the use of adjuvant therapy, for example tricyclic antidepressants and neuro-modulators, when appropriate. Of note, the authors specifically advised against the use of systemic glucocorticoids to treat pain in inflammatory arthritis patients who did not have signs or symptoms of active inflammation. No data exist regarding the use of systemic glucocorticoids for pain, whereas substantial data exist regarding the adverse effects of glucocorticoids [42].
Many of the 3e recommendations were based upon a series of Cochrane Database Systematic Reviews, published in 2011–2012, regarding pain management in inflammatory arthritis and, more specifically, RA. A systematic review of 11 randomized or quasi-randomized controlled trials comparing opioids with placebo or an active analgesic agent concluded there was weak evidence for use of weak opioids (codeine, dextropropoxyphene, pentazosine, tilidine, tramadol) for RA patients [43]. However, side effects, such as constipation, dizziness, nausea, and vomiting, were common. The risk ratio for study withdrawal because of side effects was 2.7 among participants taking opioids compared with those on placebo. In addition to these adverse effects, opioid use may also lead to opioid-induced hyperalgesia, which is associated with heightened pain sensitivity and increased clinical pain intensity. Given these effects, it is generally recommended that long-term opioid prescriptions be minimized and, when opioids are necessary, use should be regularly and judiciously monitored [44].
A Cochrane systematic review also found weak evidence for the use of neuromodulators in the treatment of pain in RA [45]. This review, however, included only four small, randomized controlled trials. Two trials compared nefopam, a centrally-acting analgesic used in Europe (but not the United States), with placebo. One trial compared topical capsaicin with placebo, and one study compared oromucosal cannabis with placebo. The authors concluded that topical capsaicin could be regarded as adjuvant therapy for pain for RA patients. However, because of the side effect profiles of nefopam (nausea, sweating) and cannabis (dizziness, dry mouth, light headedness), the authors did not recommend use of these agents. Of note, this review did not identify any randomized controlled trials of gabapentin or pregabalin, neuromodulators commonly used to treat pain in the United States, for pain in RA. On the basis of the 3e recommendations, gabapentin and pregabalin are included as potential adjuvant treatment options for pain in inflammatory arthritis. These recommendations are mainly based on their efficacy in studies of pain in fibromyalgia, a non-inflammatory, chronic widespread pain condition [41••, 46–48].
The use of antidepressants as analgesic therapy in inflammatory arthritis remains controversial. A Cochrane systematic review of eight randomized controlled trials comparing antidepressant therapy with placebo or an active intervention found insufficient evidence to advocate the use of antidepressants to treat pain in RA [49], whereas the 3e recommendations include antidepressants, specifically tricyclic antidepressants, as a potential adjuvant treatment for pain patients with inflammatory arthritis [41••]. However, the 3e investigators specifically note that these medications should only be considered for a subset of patients (not all patients with pain), stating very clearly that the data remain unclear.
Neither expert opinion from the 3e initiative nor combined data from a Cochrane systematic review supported the use of muscle relaxants to treat the pain of inflammatory arthritis patients [41••, 50]. Most studies included in the review were small, short-term (≤2 week) studies of benzodiazepines. Compared with placebo, muscle relaxants were significantly associated with an increased risk of adverse effects, particularly drowsiness and dizziness. The number needed to harm was 3 (95 % confidence interval 2–7).
The use of combination therapy to treat pain in inflammatory arthritis has not been well-studied. A Cochrane systematic review recently summarized the existing literature, but a meta-analysis was not performed because of substantial heterogeneity among studies [51]. Eighteen of 23 studies reported no significant difference in a standardized pain outcome between monotherapy and combination therapy groups. However, the generalizability of these results was unclear. Almost all studies were performed before 1990. Most of the subjects were not taking any disease-modifying antirheumatic drugs (DMARDs), and no subjects were taking biologic DMARDs. Based largely on expert opinion, the 3e initiative supported the use of combination therapy to treat pain among inflammatory arthritis patients who did not achieve adequate response to monotherapy [41••]. However, because of increased risk of side effects, the 3e investigators warned against the concomitant use of two drugs with the same mechanism of action. New studies are needed to investigate the use of combination analgesic therapy to treat pain in patients with inflammatory arthritis.
Few studies have examined the use of pain medications among inflammatory arthritis patients with medical comorbidities. A Cochrane systematic review found no studies that met inclusion criteria to assess the safety and efficacy of analgesic medications among RA patients with renal or cardiovascular comorbidities [52]. A Cochrane review examining the safety and efficacy of analgesic medications among inflammatory arthritis patients with gastrointestinal and/or hepatic comorbidities identified only one study that fulfilled inclusion criteria [53]. This small study (N=58), published in 1975, concluded that naproxen, at doses of 500 to 750 mg daily, was safe for patients with gastrointestinal and hepatic comorbidities [54]. However, none of the study participants were using modern DMARDs. Results from more recent studies of other patient populations (e.g., osteoarthritis, combination of rheumatic conditions) were indicative of increased risk of gastrointestinal side effects among patients with a history of gastrointestinal comorbidity, treated with NSAIDs [55, 56]. Ultimately, the Cochrane review concluded there was “scant evidence” of appropriate use of pain medications by patients with inflammatory arthritis and gastrointestinal and hepatic comorbidities.
Studies to examine the safety of analgesics among patients taking DMARDs are particularly needed, because many common DMARDs have adverse effects that may be potentiated by specific analgesic medications. A Cochrane systematic review examining the safety of NSAIDs among inflammatory arthritis patients concluded that the use of NSAIDs with methotrexate was generally safe when appropriate monitoring was performed [57]. However, the use of high-dose aspirin (e.g., 2 g daily) was associated with increased risk of kidney and liver toxicity.
Conclusions
Pain is a significant problem among patients with inflammatory arthritis. It affects disease assessment measures and quality of life. Peripheral inflammation is one common cause of pain in inflammatory arthritis, but other factors, including non-inflammatory central pain mechanisms, may also contribute to the pain experience. Larger, longitudinal studies, involving quantitative sensory testing and functional neuroimaging, are needed to elucidate the involvement of central pain mechanisms in inflammatory arthritis. It will also be important to determine whether these mechanisms differ depending on the type of inflammatory arthritis. Thus far, most studies have focused on RA and very few studies have examined the effects of pain in other types of inflammatory arthritis.
Although the treatment of pain in inflammatory arthritis has historically focused on reducing inflammation via DMARD therapy, more attention has recently been directed towards treating pain itself. Few studies have examined non-pharmacologic intervention for pain in inflammatory arthritis, although studies in non-inflammatory pain conditions, for example fibromyalgia, have produced impressive results. Several systematic reviews of pharmacologic treatments for pain in inflammatory arthritis have been attempted. However, these reviews only identified a small number of studies that examined the safety and efficacy of analgesic pain medications in this patient population. Many of these studies were small and at high risk of bias. Many included data from studies that were performed before modern DMARDs were available. Randomized clinical trials and/or large, well-controlled observational cohort studies are critically needed to determine the effects of both non-pharmacologic arthritis and adjuvant pharmacologic treatment for pain in inflammatory arthritis.
Footnotes
Disclosure Dr Lee has received grant support from Forest Laboratories and has owned stock/stock options in Merck and Co., Novartis, and Elan Corp.
This article is part of the Topical Collection on Chronic Pain
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Institute of Medicine . Relieving pain in America: a blueprint for transforming prevention, care, education and research. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 2.Heiberg T, Finset A, Uhlig T, Kvien TK. Seven year changes in health status and priorities for improvement of health in patients with rheumatoid arthritis. Ann Rheum Dis. 2005;64(2):191–5. doi: 10.1136/ard.2004.022699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ten Klooster PM, Veehof MM, Taal E, van Riel PL, van de Laar MA. Changes in priorities for improvement in patients with rheumatoid arthritis during 1 year of anti-tumour necrosis factor treatment. Ann Rheum Dis. 2007;66(11):1485–90. doi: 10.1136/ard.2007.069765. Epub 2007 May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4 ••.Borenstein D, Altman R, Bello A, Chatham W, Clauw DJ, Crofford LJ, et al. Report of the American college of rheumatology pain management task force. Arthritis Care Res. 2010;62(5):590–9. doi: 10.1002/acr.20005. [DOI] [PubMed] [Google Scholar]
- 5.Strand V, Burmester GR, Ogale S, Devenport J, John A, Emery P. Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24-week randomized controlled RADIATE study. Rheumatology (Oxford) 2012;51(10):1860–9. doi: 10.1093/rheumatology/kes131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolfe F, Michaud K. Assessment of pain in rheumatoid arthritis: minimal clinically significant difference, predictors, and the effect of anti-tumor necrosis factor therapy. J Rheumatol. 2007;34(8):1674–83. [PubMed] [Google Scholar]
- 7.Lee YC, Cui J, Lu B, Frits ML, Iannaccone CK, Shadick NA, et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther. 2011;13(3):R83. doi: 10.1186/ar3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawaguchi Y, Matsuno H, Kanamori M, Ishihara H, Ohmori K, Kimura T. Radiologic findings of the lumbar spine in patients with rheumatoid arthritis, and a review of pathologic mechanisms. J Spinal Disord Tech. 2003;16(1):38–43. doi: 10.1097/00024720-200302000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Helliwell PS, Zebouni LN, Porter G, Wright V. A clinical and radiological study of back pain in rheumatoid arthritis. Br J Rheumatol. 1993;32(3):216–21. doi: 10.1093/rheumatology/32.3.216. [DOI] [PubMed] [Google Scholar]
- 10.Neva MH, Hakkinen A, Isomaki P, Sokka T. Chronic back pain in patients with rheumatoid arthritis and in a control population: prevalence and disability—a 5-year follow-up. Rheumatology (Oxford) 2011;50(9):1635–9. doi: 10.1093/rheumatology/ker173. [DOI] [PubMed] [Google Scholar]
- 11.Ranzolin A, Brenol JC, Bredemeier M, Guarienti J, Rizzatti M, Feldman D, et al. Association of concomitant fibromyalgia with worse disease activity score in 28 joints, health assessment questionnaire, and short form 36 scores in patients with rheumatoid arthritis. Arthritis Rheum. 2009;61(6):794–800. doi: 10.1002/art.24430. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F, Cathey MA, Kleinheksel SM. Fibrositis (Fibromyalgia) in rheumatoid arthritis. J Rheumatol. 1984;11(6):814–8. [PubMed] [Google Scholar]
- 13.Wolfe F, Hauser W, Hassett AL, Katz RS, Walitt BT. The development of fibromyalgia—I: examination of rates and predictors in patients with Rheumatoid Arthritis (RA) Pain. 2011;152(2):291–9. doi: 10.1016/j.pain.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Lee YC, Lu B, Boire G, Haraoui BP, Hitchon CA, Pope JE, et al. Incidence and predictors of secondary fibromyalgia in an early arthritis cohort. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-201506. doi:10.1136/annrheumdis-2012-201506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res. 2011;63(3):320–7. doi: 10.1002/acr.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy SL, Lyden AK, Phillips K, Clauw DJ, Williams DA. Subgroups of older adults with osteoarthritis based upon differing comorbid symptom presentations and potential underlying pain mechanisms. Arthritis Res Ther. 2011;13(4):R135. doi: 10.1186/ar3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy SL, Lyden AK, Phillips K, Clauw DJ, Williams DA. Association between pain, radiographic severity, and centrallymediated symptoms in women with knee osteoarthritis. Arthritis Care Res. 2011;63(11):1543–9. doi: 10.1002/acr.20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010 Apr 23; doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Lee YC, Nassikas NJ, Clauw DJ. The role of the central nervous system in the generation and maintenance of chronic pain in rheumatoid arthritis, osteoarthritis and fibromyalgia. Arthritis Res Ther. 2011;13(2):211. doi: 10.1186/ar3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huskisson EC, Hart FD. Pain threshold and arthritis. Br Med J. 1972;4(5834):193–5. doi: 10.1136/bmj.4.5834.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerecz-Simon EM, Tunks ER, Heale JA, Kean WF, Buchanan WW. Measurement of pain threshold in patients with rheumatoid arthritis, osteoarthritis, ankylosing spondylitis, and healthy controls. Clin Rheumatol. 1989;8(4):467–74. doi: 10.1007/BF02032098. [DOI] [PubMed] [Google Scholar]
- 22.Le Bars D, Dickenson AH, Besson JM. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurones in the rat. Pain. 1979;6(3):283–304. doi: 10.1016/0304-3959(79)90049-6. [DOI] [PubMed] [Google Scholar]
- 23.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306(5944):686–8. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 24.Wilder-Smith CH, Robert-Yap J. Abnormal endogenous pain modulation and somatic and visceral hypersensitivity in female patients with irritable bowel syndrome. World J Gastroenterol. 2007;13(27):3699–704. doi: 10.3748/wjg.v13.i27.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosek E, Ordeberg G. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain. 2000;88(1):69–78. doi: 10.1016/S0304-3959(00)00310-9. [DOI] [PubMed] [Google Scholar]
- 26.Khan NA, Spencer HJ, Abda E, Aggarwal A, Alten R, Ancuta C, et al. Determinants of discordance in patients’ and physicians’ rating of rheumatoid arthritis disease activity. Arthritis Care Res. 2012;64(2):206–14. doi: 10.1002/acr.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27 •.Studenic P, Radner H, Smolen JS, Aletaha D. Discrepancies between patients and physicians in the perception of rheumatoid arthritis disease activity. Arthritis Rheum. 2012;64(9):2814–23. doi: 10.1002/art.34543. [DOI] [PubMed] [Google Scholar]
- 28.Pollard LC, Kingsley GH, Choy EH, Scott DL. Fibromyalgic rheumatoid arthritis and disease assessment. Rheumatology (Oxford) 2010;49(5):924–8. doi: 10.1093/rheumatology/kep458. [DOI] [PubMed] [Google Scholar]
- 29.Ton E, Bakker MF, Verstappen SM, Ter Borg EJ, van Albada-Kuipers IA, Schenk Y, et al. Look beyond the Disease Activity Score of 28 joints (DAS28): tender points influence the DAS28 in patients with rheumatoid arthritis. J Rheumatol. 2012;39(1):22–7. doi: 10.3899/jrheum.110072. [DOI] [PubMed] [Google Scholar]
- 30.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American college of rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33(2):160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 31.Pollard LC, Ibrahim F, Choy EH, Scott DL. Pain thresholds in rheumatoid arthritis: the effect of tender point counts and disease duration. J Rheumatol. 2012;39(1):28–31. doi: 10.3899/jrheum.110668. [DOI] [PubMed] [Google Scholar]
- 32.Haverman L, Grootenhuis MA, van den Berg JM, van Veenendaal M, Dolman KM, Swart JF, et al. Predictors of health-related quality of life in children and adolescents with juvenile idiopathic arthritis: results from a web-based survey. Arthritis Care Res. 2012;64(5):694–703. doi: 10.1002/acr.21609. [DOI] [PubMed] [Google Scholar]
- 33.Courvoisier DS, Agoritsas T, Glauser J, Michaud K, Wolfe F, Cantoni E, et al. Pain as an important predictor of psychosocial health in patients with rheumatoid arthritis. Arthritis Care Res. 2012;64(2):190–6. doi: 10.1002/acr.20652. [DOI] [PubMed] [Google Scholar]
- 34.Husted JA, Tom BD, Farewell VT, Gladman DD. Longitudinal study of the bidirectional association between pain and depressive symptoms in patients with psoriatic arthritis. Arthritis Care Res. 2012;64(5):758–65. doi: 10.1002/acr.21602. [DOI] [PubMed] [Google Scholar]
- 35.Lee YC, Chibnik LB, Lu B, Wasan AD, Edwards RR, Fossel AH, et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a crosssectional study. Arthritis Res Ther. 2009;11(5):R160. doi: 10.1186/ar2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butbul Aviel Y, Stremler R, Benseler SM, Cameron B, Laxer RM, Ota S, et al. Sleep and fatigue and the relationship to pain, disease activity and quality of life in juvenile idiopathic arthritis and juvenile dermatomyositis. Rheumatology (Oxford) 2011;50(11):2051–60. doi: 10.1093/rheumatology/ker256. [DOI] [PubMed] [Google Scholar]
- 37.Nicassio PM, Ormseth SR, Kay M, Custodio M, Irwin MR, Olmstead R, et al. The contribution of pain and depression to self-reported sleep disturbance in patients with rheumatoid arthritis. Pain. 2012;153(1):107–12. doi: 10.1016/j.pain.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irwin MR, Olmstead R, Carrillo C, Sadeghi N, Fitzgerald JD, Ranganath VK, et al. Sleep loss exacerbates fatigue, depression, and pain in rheumatoid arthritis. Sleep. 2012;35(4):537–43. doi: 10.5665/sleep.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knittle K, Maes S, de Gucht V. Psychological interventions for rheumatoid arthritis: examining the role of self-regulation with a systematic review and meta-analysis of randomized controlled trials. Arthritis Care Res. 2010;62(10):1460–72. doi: 10.1002/acr.20251. [DOI] [PubMed] [Google Scholar]
- 40.Knittle KP, De Gucht V, Hurkmans EJ, Vlieland TP, Peeters AJ, Ronday HK, et al. Effect of self-efficacy and physical activity goal achievement on arthritis pain and quality of life in patients with rheumatoid arthritis. Arthritis Care Res. 2011;63(11):1613–9. doi: 10.1002/acr.20587. [DOI] [PubMed] [Google Scholar]
- 41 ••.Whittle SL, Colebatch AN, Buchbinder R, Edwards CJ, Adams K, Englbrecht M, et al. Multinational evidence-based recommendations for pain management by pharmacotherapy in inflammatory arthritis: integrating systematic literature research and expert opinion of a broad panel of rheumatologists in the 3e initiative. Rheumatology (Oxford) 2012;51(8):1416–25. doi: 10.1093/rheumatology/kes032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Goes MC, Jacobs JW, Boers M, Andrews T, Blom-Bakkers MA, Buttgereit F, et al. Monitoring adverse events of low-dose glucocorticoid therapy: EULAR recommendations for clinical trials and daily practice. Ann Rheum Dis. 2010;69(11):1913–9. doi: 10.1136/ard.2009.124958. [DOI] [PubMed] [Google Scholar]
- 43.Whittle SL, Richards BL, Husni E, Buchbinder R. Opioid therapy for treating rheumatoid arthritis pain. Cochrane Database Syst Rev. 2011;(11):CD003113. doi: 10.1002/14651858.CD003113.pub3. [DOI] [PubMed] [Google Scholar]
- 44.Crofford LJ. Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat Rev. 2010;6(4):191–7. doi: 10.1038/nrrheum.2010.24. [DOI] [PubMed] [Google Scholar]
- 45.Richards BL, Whittle SL, Buchbinder R. Neuromodulators for pain management in rheumatoid arthritis. Cochrane Database Syst Rev. 2012;1:CD008921. doi: 10.1002/14651858.CD008921.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arnold LM, Goldenberg DL, Stanford SB, Lalonde JK, Sandhu HS, Keck PE, Jr, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56(4):1336–44. doi: 10.1002/art.22457. [DOI] [PubMed] [Google Scholar]
- 47.Crofford LJ, Mease PJ, Simpson SL, Young JP, Jr, Martin SA, Haig GM, et al. Fibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM): a 6-month, double-blind, placebo-controlled trial with pregabalin. Pain. 2008;136(3):419–31. doi: 10.1016/j.pain.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 48.Mease PJ, Russell IJ, Arnold LM, Florian H, Young JP, Jr, Martin SA, et al. A randomized, double-blind, placebo-controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. J Rheumatol. 2008;35(3):502–14. [PubMed] [Google Scholar]
- 49.Richards BL, Whittle SL, Buchbinder R. Antidepressants for pain management in rheumatoid arthritis. Cochrane Database Syst Rev. 2011;(11):CD008920. doi: 10.1002/14651858.CD008920.pub2. [DOI] [PubMed] [Google Scholar]
- 50.Richards BL, Whittle SL, Buchbinder R. Muscle relaxants for pain management in rheumatoid arthritis. Cochrane Database Syst Rev. 2012;1:CD008922. doi: 10.1002/14651858.CD008922.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramiro S, Radner H, van der Heijde D, van Tubergen A, Buchbinder R, Aletaha D, et al. Combination therapy for pain management in inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis) Cochrane Database Syst Rev. 2011;(10):CD008886. doi: 10.1002/14651858.CD008886.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Marks JL, Colebatch AN, Buchbinder R, Edwards CJ. Pain management for rheumatoid arthritis and cardiovascular or renal comorbidity. Cochrane Database Syst Rev. 2011;(10):CD008952. doi: 10.1002/14651858.CD008952.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Radner H, Ramiro S, Buchbinder R, Landewe RB, van der Heijde D, Aletaha D. Pain management for inflammatory arthritis (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and other spondylarthritis) and gastrointestinal or liver comorbidity. Cochrane Database Syst Rev. 2012;1:CD008951. doi: 10.1002/14651858.CD008951.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roth SH, Boost G. An open trial of naproxen in rheumatoid arthritis patients with significant esophageal, gastric, and duodenal lesions. J Clin Pharmacol. 1975;15(4 Pt. 2):378–84. doi: 10.1002/j.1552-4604.1975.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 55.Laine L, Curtis SP, Langman M, Jensen DM, Cryer B, Kaur A, et al. Lower gastrointestinal events in a double-blind trial of the cyclo-oxygenase-2 selective inhibitor etoricoxib and the traditional nonsteroidal anti-inflammatory drug diclofenac. Gastroenterology. 2008;135(5):1517–25. doi: 10.1053/j.gastro.2008.07.067. [DOI] [PubMed] [Google Scholar]
- 56.Chan FK, Lanas A, Scheiman J, Berger MF, Nguyen H, Goldstein JL. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet. 2010;376(9736):173–9. doi: 10.1016/S0140-6736(10)60673-3. [DOI] [PubMed] [Google Scholar]
- 57.Colebatch AN, Marks JL, Edwards CJ. Safety of non-steroidal anti-inflammatory drugs, including aspirin and paracetamol (acetaminophen) in people receiving methotrexate for inflammatory arthritis (rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, other spondyloarthritis) Cochrane Database Syst Rev. 2011;(11):CD008872. doi: 10.1002/14651858.CD008872.pub2. [DOI] [PubMed] [Google Scholar]