Abstract
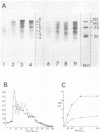
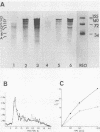
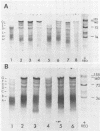
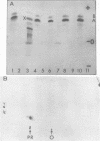
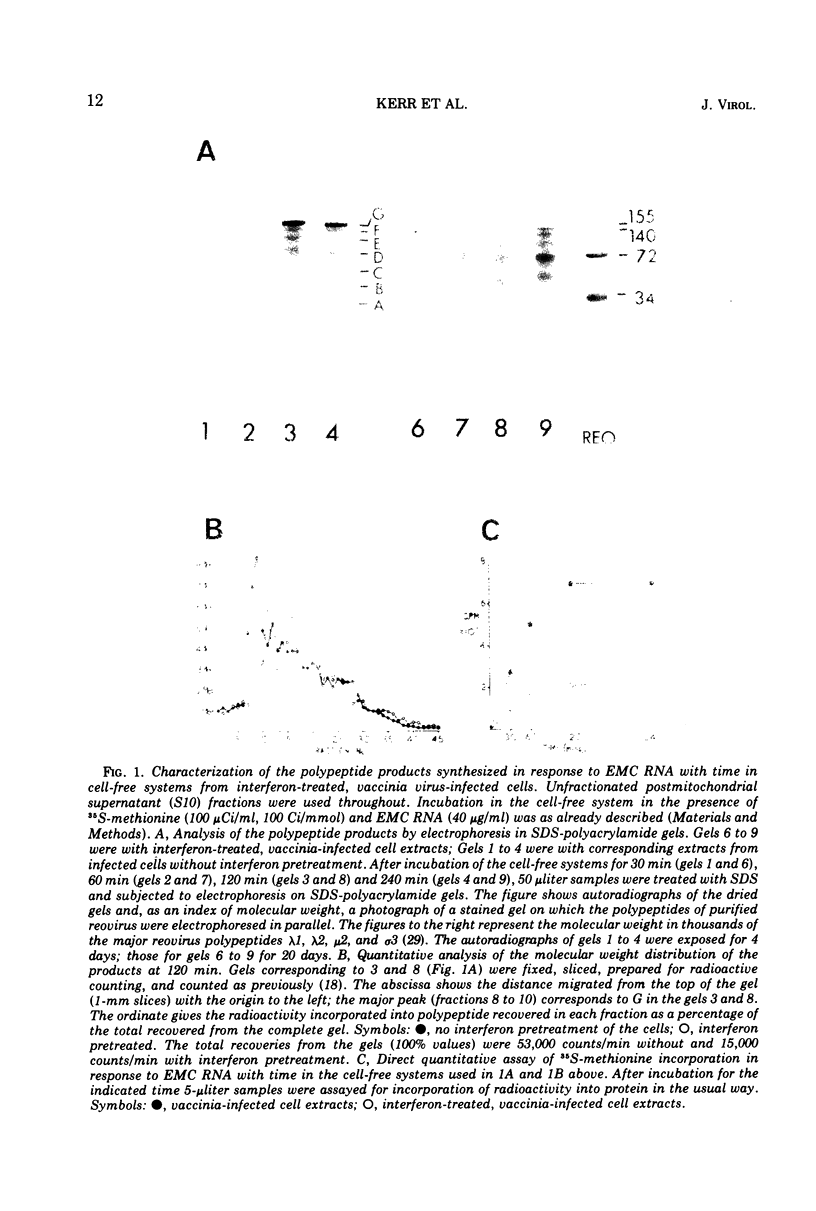
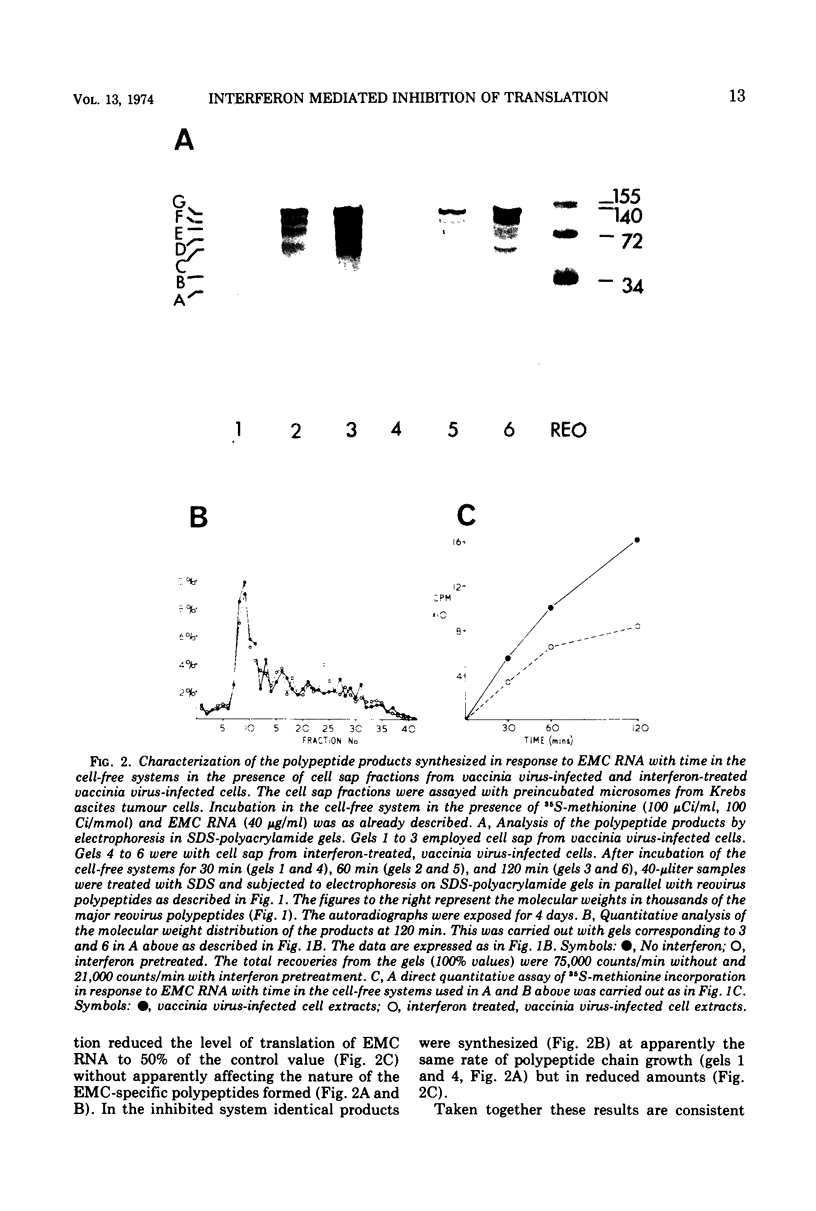
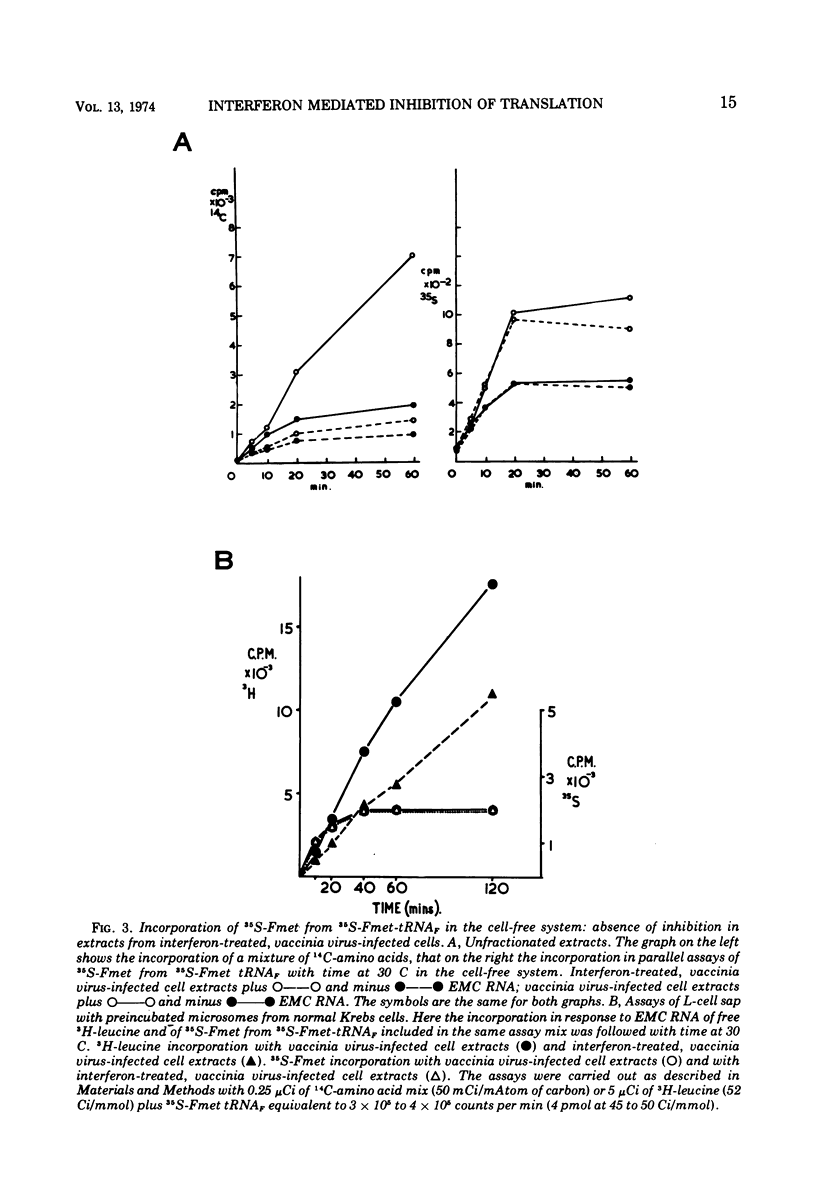
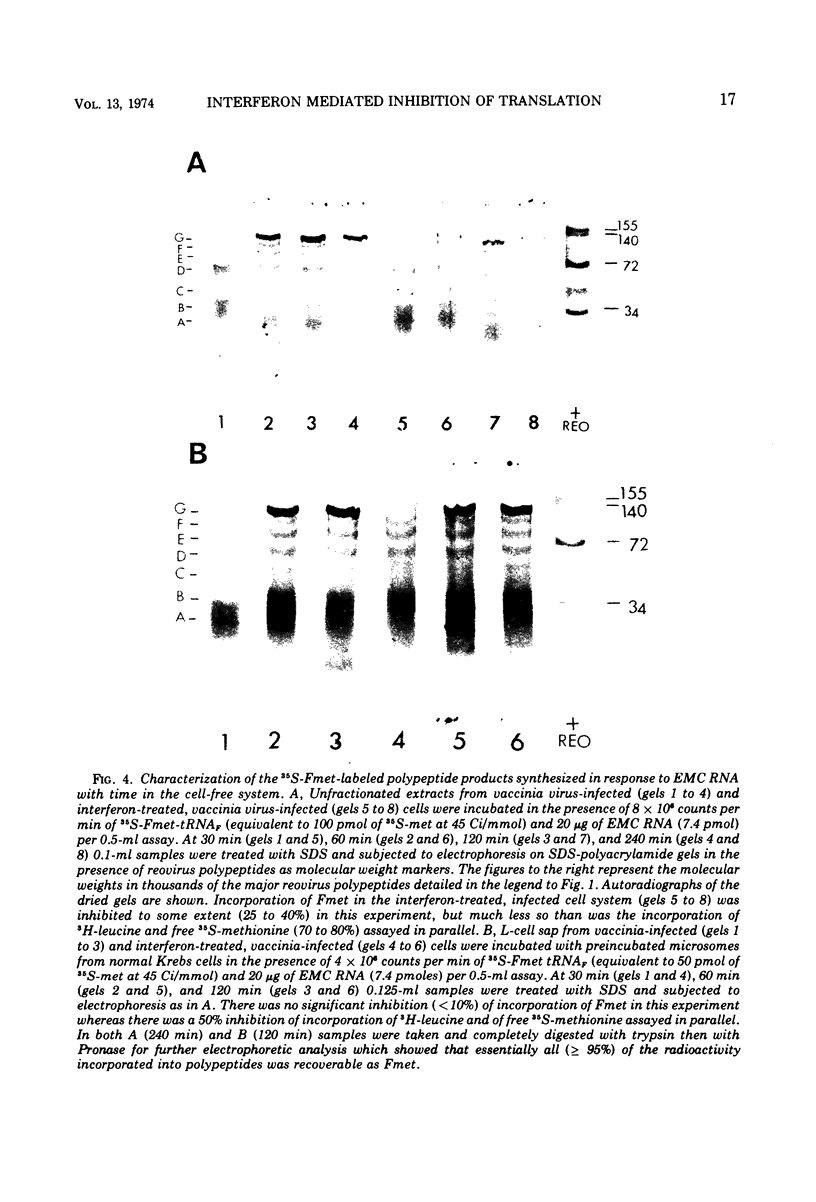
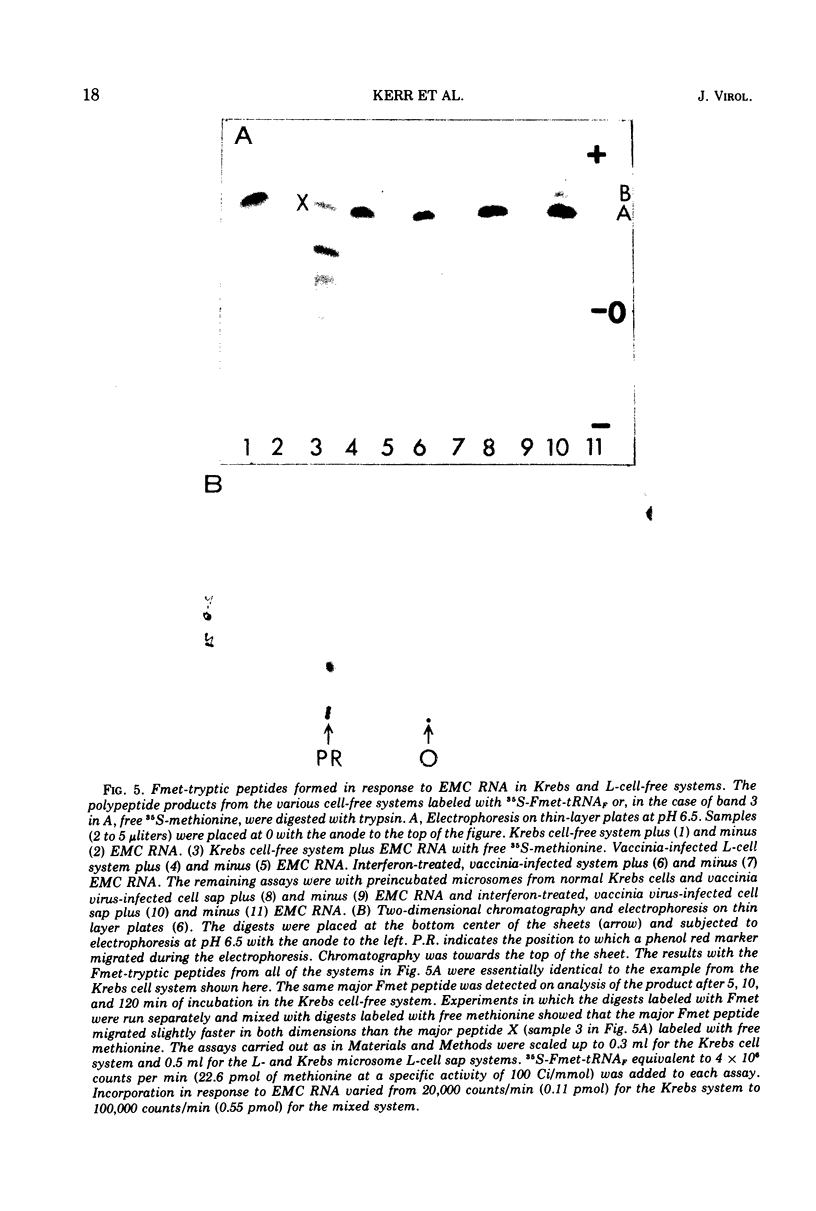
The translation of encephalomyocarditis virus (EMC) RNA is markedly inhibited in cell-free systems from interferon-treated, vaccinia virus-infected L-cells (10, 11). The polypeptide products synthesized in response to EMC RNA in cell-free systems from these and untreated infected cells have been analyzed by electrophoresis on polyacrylamide gels. Qualitatively, the same EMC-specific polypeptides were synthesized throughout. In experiments using preincubated microsomes from normal Krebs cells to assay cell sap from L-cells which had been exposed to interferon prior to infection, only the amount of the EMC-specific polypeptide products was reduced. This result suggests that there is an inhibition very early in translation in interferon-treated, infected cells. Initiation seems a priori the more attractive site for this inhibition, but an effect shortly after initiation cannot be excluded. With unfractionated cell-free systems from interferon-treated infected L-cells, however, there appeared to be an additional minor inhibitory effect on polypeptide chain elongation, in that the EMC-specific polypeptides synthesized showed not only a reduction in amount but also a bias towards lower molecular weight. The formylated methionyl initiator tRNA (Fmet-tRNAF) was used as a further probe into the apparent effect on intiation. With this reagent we have confirmed that there is one major initiation site for the translation of EMC RNA in these cell-free systems. In addition, the results have shown that EMC-specific polypeptide chains initiated with Fmet escape the major interferon-mediated inhibition at or shortly after initiation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boime I., Aviv H., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA. II. The in vitro synthesis of high molecular weight proteins and elements of the viral capsid. Biochem Biophys Res Commun. 1971 Nov 5;45(3):788–795. doi: 10.1016/0006-291x(71)90486-4. [DOI] [PubMed] [Google Scholar]
- Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus mRNA. 3. Discrete polypeptides translated from a monocistronic messenger in vitro. Arch Biochem Biophys. 1972 Dec;153(2):706–713. doi: 10.1016/0003-9861(72)90389-x. [DOI] [PubMed] [Google Scholar]
- Brown J. C., Smith A. E. Initiator codons in eukaryotes. Nature. 1970 May 16;226(5246):610–612. doi: 10.1038/226610a0. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Anderson W. F. Initiation of hemoglobin synthesis: comparison of model reactions that use artificial templates with those using natural messenger RNA. Proc Natl Acad Sci U S A. 1972 Mar;69(3):706–711. doi: 10.1073/pnas.69.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., Nienhuis A. W., Prichard P. M., Picciano D., Elson N. A., Merrick W. C., Graf H., Shafritz D. A., Laycock D. G., Last J. A. Initiation of globin synthesis. FEBS Lett. 1972 Aug 15;24(3):310–314. doi: 10.1016/0014-5793(72)80379-x. [DOI] [PubMed] [Google Scholar]
- Dobos P., Kerr I. M., Martin E. M. Synthesis of capsid and noncapsid viral proteins in response to encephalomyocarditis virus ribonucleic acid in animal cell-free systems. J Virol. 1971 Oct;8(4):491–499. doi: 10.1128/jvi.8.4.491-499.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drews J., Högenauer G., Unger F., Weil R. Incorporation of methionine from met-tRNA-Met-F into internal positions of polypeptides by mouse liver polysomes. Biochem Biophys Res Commun. 1971 May 21;43(4):905–912. doi: 10.1016/0006-291x(71)90703-0. [DOI] [PubMed] [Google Scholar]
- Eggen K. L., Shatkin A. J. In vitro translation of cardiovirus ribonucleic acid by mammalian cell-free extracts. J Virol. 1972 Apr;9(4):636–645. doi: 10.1128/jvi.9.4.636-645.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcoff E., Falcoff R., Lebleu B., Revel M. Interferon treatment inhibits Mengo RNA and haemoglobin mRNA translation in cell-free extracts of L cells. Nat New Biol. 1972 Nov 29;240(100):145–147. doi: 10.1038/newbio240145a0. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Esteban R. M., Metz D. H., Tovell D. R., Kerr I. M., Williamson R. Translation of RNA by L cell extracts: Effect of interferon. FEBS Lett. 1972 Aug 15;24(3):273–277. doi: 10.1016/0014-5793(72)80371-5. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Metz D. H., Esteban R. M., Tovell D. R., Ball L. A., Kerr I. M. Mechanism of interferon action: inhibition of viral messenger ribonucleic acid translation in L-cell extracts. J Virol. 1972 Dec;10(6):1184–1198. doi: 10.1128/jvi.10.6.1184-1198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N. K., Chatterjee N. K., Bose K. K., Bhaduri S., Chung A. Roles of methionine transfer RNA's in protein synthesis in rabbit reticulocytes. J Mol Biol. 1970 Nov 28;54(1):145–154. doi: 10.1016/0022-2836(70)90452-3. [DOI] [PubMed] [Google Scholar]
- Gupta N., Chatterjee N. K., Woodley C. L., Bose K. K. Protein synthesis in rabbit reticulocytes. Factors controlling internal and terminal methionine codon recognition by the methionyl transfer ribonucleic acid species. J Biol Chem. 1971 Dec 25;246(24):7460–7469. [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
- Hunter A. R., Jackson R. J. The origin and nature of the methionine residue initiating the synthesis of haemoglobin in vivo and in vitro. Eur J Biochem. 1971 Apr;19(3):316–322. doi: 10.1111/j.1432-1033.1971.tb01321.x. [DOI] [PubMed] [Google Scholar]
- Joklik W. K., Merigan T. C. Concerning the mechanism of action of interferon. Proc Natl Acad Sci U S A. 1966 Aug;56(2):558–565. doi: 10.1073/pnas.56.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Tovell D. R. Characterization of the polypeptides formed in response to encephalomyocarditis virus ribonucleic acid in a cell-free system from mouse ascites tumor cells. J Virol. 1972 Jul;10(1):73–81. doi: 10.1128/jvi.10.1.73-81.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Cohen N., Work T. S. Factors controlling amino acid incorporation by ribosomes from krebs II mouse ascites-tumour cells. Biochem J. 1966 Mar;98(3):826–835. doi: 10.1042/bj0980826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Simple method for the isolation of encephalomyocarditis virus ribonucleic acid. J Virol. 1972 Mar;9(3):559–561. doi: 10.1128/jvi.9.3.559-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M. Protein synthesis in cell-free systems: an effect of interferon. J Virol. 1971 Apr;7(4):448–459. doi: 10.1128/jvi.7.4.448-459.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz D. H., Esteban M. Interferon inhibits viral protein synthesis in L cells infected with vaccinia virus. Nature. 1972 Aug 18;238(5364):385–388. doi: 10.1038/238385a0. [DOI] [PubMed] [Google Scholar]
- Oberg B. F., Shatkin A. J. Initiation of picornavirus protein synthesis in ascites cell extracts. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3589–3593. doi: 10.1073/pnas.69.12.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paucker K., Berman B. J., Golgher R. R., Stancek D. Purification, characterization, and attempts at isotopic labeling of mouse interferon. J Virol. 1970 Feb;5(2):145–152. doi: 10.1128/jvi.5.2.145-152.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. Cytoplasmic methionine transfer RNAs from eukaryotes. Nature. 1970 May 16;226(5246):607–610. doi: 10.1038/226607a0. [DOI] [PubMed] [Google Scholar]
- Smith A. E. The initiation of protein synthesis directed by the RNA from encephalomyocarditis virus. Eur J Biochem. 1973 Mar 1;33(2):301–313. doi: 10.1111/j.1432-1033.1973.tb02684.x. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Sundararajan T. A., Thach R. E. Role of the formylmethionine codon AUG in phasing translation of synthetic messenger RNA. J Mol Biol. 1966 Aug;19(1):74–90. doi: 10.1016/s0022-2836(66)80051-7. [DOI] [PubMed] [Google Scholar]