Population-based human immunodeficiency virus (HIV) RNA metrics can help estimate antiretroviral therapy effectiveness within a community. We developed a fingerprick-based viral load technique and measured population HIV RNA levels in a rural Ugandan community, providing the first report from a resource limited setting.
Keywords: population HIV-RNA levels, viral load, fingerprick, ART effectiveness, epidemiology
Abstract
Background. Population-based human immunodeficiency virus type 1 (HIV-1) RNA levels (viral load [VL]) are proposed metrics for antiretroviral therapy (ART) program effectiveness. We estimated population-based HIV RNA levels using a fingerprick-based approach in a rural Ugandan community implementing rapid ART scale-up.
Methods. A fingerprick-based HIV RNA measurement technique was validated against standard phlebotomy. This technique was deployed during a 5-day community-wide health campaign in a 6300-person community. Assessments included rapid HIV antibody testing, VL, and CD4+ T-cell count via fingerprick. We estimated population HIV RNA levels and the prevalence of undetectable RNA, assessed predictors of VL via linear regression, and mapped RNA levels within community geographic units.
Results. During the community-wide health campaign, 179 of 2282 adults (7.8%) and 10 of 1826 children (0.5%) tested seropositive for HIV. Fingerprick VL was determined in 174 of 189 HIV-positive persons (92%). The mean log(VL) was 3.67 log (95% confidence interval [CI], 3.50–3.83 log copies/mL), median VL was 2720 copies/mL (interquartile range, <486–38 120 copies/mL), and arithmetic mean VL was 64 064 copies/mL. Overall, 64 of 174 of individuals had undetectable RNA (37% [95% CI, 30%–44%]), 24% had VL 486–10 000; 25% had VL 10 001–100 000; and 15% had VL>100 000 copies/mL. Among participants taking ART, 83% had undetectable VL.
Conclusions. We developed and implemented a fingerprick VL testing method and provide the first report of population HIV RNA levels in Africa. In a rural Ugandan community experiencing ART scale-up, we found evidence of population-level ART effectiveness, but found a substantial population to be viremic, in need of ART, and at risk for transmission.
Efforts to stem the tide of the human immunodeficiency virus (HIV) epidemic are increasing, with acceleration of antiretroviral therapy (ART) scale-up [1] and implementation of combination prevention strategies [2, 3]. Epidemiologic measurements at a population or community level—both of the burden of HIV and of the effectiveness of HIV treatment strategies—are critical to guiding and monitoring national and local treatment and prevention strategies.
Population-level HIV type 1 (HIV-1) RNA levels (viral load [VL]) have been described among individuals in a geographic area or within specific demographic groups [4–7]. Population HIV RNA measurements have the potential to provide insight into the effectiveness of ART programs because increased ART penetration and treatment success are associated with lower average VLs and with a higher proportion of patients with an undetectable VL [4–6]. Because higher VL is correlated with greater infectivity [8, 9], and since ART-mediated virologic suppression has been shown to reduce the spread of HIV [10], population RNA levels may also provide insight into the risk of forward transmission of HIV, acknowledging its known limitations.
To date, reports assessing population HIV RNA levels have been conducted only in urban settings in the developed world such as Vancouver, San Francisco, and Washington, D.C. [4–6]. Population-level VLs have not yet been measured in sub-Saharan Africa, where >23 million persons live with HIV [1]. Several key barriers have precluded population HIV RNA estimation in resource-limited settings. The high cost of measuring HIV RNA levels is a major impediment to availability. Apart from South Africa and Botswana [11, 12], VL testing is thus not used in routine clinical care. Therefore, centralized data collection systems (eg, clinic databases) that could be used to estimate population RNA levels lack necessary data on VLs. Compounding the problem are estimates that up to one-half of HIV-infected individuals are unaware of their serostatus [1], and many diagnosed individuals have not successfully linked to HIV care and ART. Additionally, in rural settings, laboratory facilities are often far from clinics, making blood collection and cold-chain specimen transport highly challenging.
We sought to address the above challenges and estimate population-level HIV RNA levels in a rural community in southwestern Uganda by measuring VL using a fingerprick blood collection method during a 5-day community-wide health campaign offering diagnostic, prevention, and treatment services for communicable and noncommunicable diseases [13].
This approach may hold potential for assessing the rapid ongoing ART scale-up in resource-limited settings that do not offer VL testing during HIV care.
METHODS
Development of Fingerprick-Based Method for HIV-1 Plasma RNA Measurement
Paired plasma samples were collected from women initiating ART in Tororo, Uganda, using standard lancet fingerprick and venipuncture methods. Healthcare workers received in-person training in these procedures by a laboratory technician, viewed videos illustrating the procedures, and reviewed written operating procedures. For standard phlebotomy, 2 mL of EDTA blood was collected via venipuncture, and plasma was isolated after centrifugation and stored at −20°C. In parallel, fingerprick was used to obtain 200 µL of blood via capillary-Eppendorf hybrid tubes (Safe-T-Fill, Ram Scientific) and plasma was isolated by centrifugation at 8000g for 10 minutes at room temperature. Seventy microliters of plasma was removed, added to 70 µL of phosphate-buffered saline and 560 µL RNA lysis buffer (AVL buffer, Qiagen), vortexed, then stored at −20°C. Prior to testing the fingerprick plasma for VL (Abbott RealTime HIV-1, 0.6-mL protocol), an additional 150 µL of lysis buffer was added for a final volume of 850 µL. RNA was extracted using the automated m2000 system (Abbott) following the manufacturer's instructions. RNA values and the lower limit of detection (LLOD; 40 copies/mL) were multiplied by a factor of 12.14 to account for dilution of the fingerprick samples. Samples with undetectable VL were considered to have 486 copies/mL (40 copies/mL × 12.14, the assay limit of detection).
We assessed the pairwise agreement of fingerprick vs phlebotomy-derived VL values by Pearson correlation coefficient, as well as by Bland-Altman analysis.
Community Health Screening and Treatment Campaign
We conducted a 5-day community-wide health campaign 16–21 May 2011 in Kakyerere Parish (Mbarara District, southwestern Uganda), described in detail elsewhere [13]. This health campaign was approved by the University of California, San Francisco Committee on Human Research, the Makerere University School of Medicine Research Ethics Council, and the Uganda National Council for Science and Technology.
All participants underwent a health questionnaire that asked about prior knowledge of HIV diagnosis and ART use, had pretest counseling, and then underwent HIV testing via a fingerprick-derived blood sample (Determine HIV 1/2 antibody test, Inverness). Persons testing positive who did not report a history of HIV had immediate confirmatory testing with the HIV 1/2 STAT-PAK test (Chembio Diagnostic Systems). If a STAT-PAK result was equivocal or negative, a UniGold RecombiGen HIV test (Trinity Biotech) was performed, and a final HIV diagnosis was determined using all 3 test results according to Uganda's national HIV screening algorithm [14].
HIV-1 Plasma RNA and CD4+ T-Cell Count Measurements
Patients who self-reported a diagnosis of HIV and had a positive antibody test did not undergo confirmatory testing. In all participants diagnosed with HIV, a second fingerprick-derived 200-µL blood sample was obtained as described above. Every 2 hours during each campaign day, blood samples were transported to a central laboratory located in Mbarara (approximately 25 km from the 3 study sites located in Kakyerere Parish) and processed for reverse transcription polymerase chain reaction (RT-PCR) as described above. Samples did not exceed 4 hours at ambient temperature.
Fingerprick-derived blood was also used to perform a point-of-care CD4+ T-cell count measurement (PIMA, Alere) which yields a CD4+ result in 20 minutes and produces results concordant with phlebotomy-derived CD4+ counts [15], particularly at CD4+ counts <500 cells/μL [16].
Antiretroviral Medication Measurement From Dried Blood Spots
During the campaign, Whatman #3 filter paper (GE Healthcare Life Sciences) was used to collect 4 fingerprick samples of approximately 50 µL of blood (spotted onto filter paper as dried blood spots (DBSs) and stored in plastic bags with desiccant at ambient temperature). In individuals with undetectable VL or low-level detectable VL (<486 copies/mL), presence of nevirapine (NVP) and efavirenz (EFV) was tested through qualitative measurements from DBSs. DBSs were cut from filter paper and analytes extracted with 80% acetonitrile containing 1% formic acid and an internal standard. After centrifugation the solution was analyzed simultaneously on 2 systems. NVP levels were measured qualitatively by liquid chromatography–tandem mass spectrometry (LLOD, 150 ng/mL). EFV levels were measured qualitatively by reverse-phase high-performance liquid chromatography (LLOD, 175 ng/mL).
Classification of ART Use
We classified participants’ ART status by self-report, presence or absence of ART (EFV or NVP) on DBS testing, and targeted chart reviews. Individuals who self-reported not being on ART were reclassified as being on ART if they either (1) had an undetectable plasma RNA level and either NVP or EFV was detected, or (2) if chart review documented receipt of ART.
Estimation of Population RNA Metrics
We computed the median VL with interquartile range (IQR), the mean log(VL), and the arithmetic mean VL among all HIV-positive participants. We also calculated the median VL/IQR among persons with detectable viremia. Using an assay LLOD of 486 copies/mL, we also computed the proportion of patients with undetectable VL overall, and within demographic and clinical subgroups.
Geographic Mapping of RNA Levels
Maps from the Ugandan Bureau of Statistics were used to plot the boundaries of the 7 villages comprising Kakyerere Parish (Mbarara district, Uganda). Mapping functions were carried out in ArcGIS. Mean VL within each village was computed and mapped according to strata of VL. Numbers of HIV-positive individuals within each village were depicted graphically. The distance between the approximate centroid of each village to the district HIV treatment center was calculated.
Statistical Analyses
Individual level associations between log10-transformed VL and demographic/clinical predictors among HIV-positive persons were assessed with multivariable linear regression. Using logistic regression, associations were also assessed between the distance from each village center to the district health center and whether individual participants’ VLs were undetectable. All analyses were performed in Stata 12/SE (StataCorp).
RESULTS
Fingerprick Blood Collection Method
Figure 1A shows a scatterplot of log-transformed VLs from standard venipuncture/phlebotomy vs fingerprick-derived specimens. Correlation was strong (Pearson r coefficient = 0.986, P < .0001). Figure 1B is a Bland-Altman mean-difference plot depicting the pairwise difference in log(VL) between the phlebotomy and fingerprick methods on the y-axis vs the mean of the 2 values on the x-axis. VLs from fingerprick and standard phlebotomy showed good pairwise agreement in the detectable range (486–10 000 000 copies/mL) normalized for original sample dilution (median difference in log10 copies/mL was 0.039 [5th, 95th percentile, −0.24, 0.32]). As shown in Figure 1B, VL values were in agreement across a wide dynamic range ranging from 100 copies/mL to 1 000 000 copies/mL.
Figure 1.
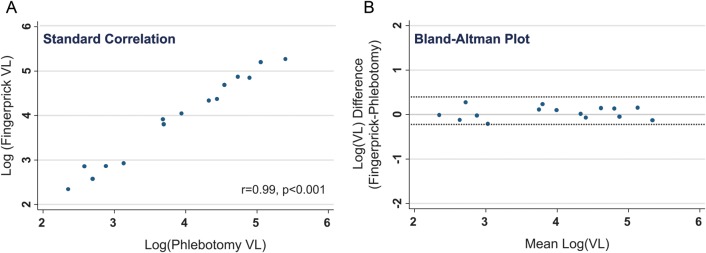
Standard correlational and Bland-Altman plots assessing relationship of human immunodeficiency virus (HIV)–1 plasma RNA values from phlebotomy-derived samples with fingerprick-derived samples among 15 HIV-positive persons. A, Standard correlation of log-transformed fingerprick–derived plasma HIV-1 RNA (ie, viral load) levels (y-axis) vs phlebotomy-derived plasma HIV-1 RNA levels (x-axis). The y-axis is truncated at 6 logs, though the assay limit of detection is 7 logs. B, Bland-Altman plot. The y-axis displays the difference between the 2 measurements (phlebotomy vs fingerprick) and the x-axis displays the mean of the 2 measurements. Dashed lines indicate 95% limits of agreement for all pairwise comparisons. Abbreviation: VL, viral load.
Community Health Campaign Study Population
A total of 4343 individuals out of the Kakyerere Parish's estimated population of 6300 persons (Ugandan Bureau of Statistics data) participated in the community health campaign (69% coverage of community). In total, 2282 of 2323 (95%) adults and 1826 of 2020 children (aged 18 months to 17 years, 90%) were tested for HIV. Overall, 179 of 2282 adults (7.8%), and 10 of 1826 (0.5%) children were HIV seropositive. Of the 189 individuals who tested positive for HIV, 47% were newly diagnosed while 53% had been previously diagnosed. Fingerprick VL was determined in 174 of 189 HIV-positive persons (92%). Demographics of this population are summarized in Table 1. In brief, HIV-positive participants were young, with a median age of 30 years (IQR, 24–38), and were predominantly female (73%) and married (55%), though many were widowed, divorced, or separated (31%). Commercial farmers comprised 60% of our population. Median CD4+ T-cell count of adults was 415 cells/μL (IQR, 281–568 cells/μL), and 60% of participants were above the current Ugandan threshold for ART initiation of 350 cells/μL. Of 165 adults, 66 were classified as being on ART.
Table 1.
Baseline Characteristics of Participants Diagnosed With HIV and Assessed for Plasma HIV-1 RNA Levels
| Variable | CHC Participants (n = 174) |
|---|---|
| Age, y, median (IQR) | 30 (24–38) |
| Female sex | 73% |
| Marital status | |
| Single | 15 (9) |
| Married | 95 (55) |
| Widowed/divorced/separated | 55 (31) |
| Unknown status | 9 (5) |
| No. of children | |
| 0 | 39 (22) |
| 1 | 43 (25) |
| 2 | 35 (20) |
| ≥3 | 58 (33) |
| Occupation | |
| Peasant farmer | 105 (60) |
| Commercial farmer | 2 (1) |
| Shopkeeper/market vendor | 13 (7) |
| Transport/driver (truck/taxi) | 5 (3) |
| Teacher | 4 (2) |
| Other | 36 (21) |
| Not provided | 9 (5) |
| HIV not previously diagnoseda | 72 (44) |
| Plasma HIV-1 RNA level | |
| Undetectable (≤486 copies/mL) | 64 (36.8) |
| 486–10 000 copies/mL | 41 (23.6) |
| 10 001–100 000 copies/mL | 43 (24.7) |
| >100 000 copies/mL | 26 (14.9) |
| CD4+ T-cell count,a median (IQR) | 415 (281–568) |
| <200 cells/μL | 30 (17) |
| 201–350 cells/μL | 42 (24) |
| >350 cells/μL | 102 (59) |
Data are presented as No. (%) unless otherwise specified.
Abbreviations: CHC, community health campaign; HIV-1, human immunodeficiency virus type 1; IQR, interquartile range.
a Based on 165 adults.
Population-Level HIV RNA Metrics
The median VL was 2720 copies/mL (IQR, <486–38 120 copies/mL), and the mean log(VL) was 3.67 log copies/mL (95% CI, 3.50–3.83 log). The arithmetic mean VL was 64 064 copies/mL, and the median VL among patients with detectable viremia was 20 031 copies/mL. Overall, 24% of participants had VL 486–10 000 copies/mL, 25% had VL 10 001–100 000 copies/mL, and 15% had VL >100 000 copies/mL. 37% of participants had undetectable VL (95% CI, 30%–44%). Among participants on ART, the overall virologic suppression rate was 83%. Table 2 summarizes 4 population-aggregated VL metrics within subgroups of the population: proportion of participants with an undetectable VL, median VL, mean log(VL), and the median VL among subjects with detectable viremia.
Table 2.
Population-Level Plasma HIV RNA Metrics Among Groups of HIV-Positive Persons Attending a Community-Wide Health Assessment
| Variable | Category | No. | Proportion Undetectable, % | Median VL, copies/mL | Mean log(VL), copies/mL | Arithmetic Mean VL, copies/mL | Median VL Among Viremic Individuals, copies/mL |
|---|---|---|---|---|---|---|---|
| Overall | 174 | 37 | 2720 | 3.67 | 64 064 | 20 031 | |
| Age | <15 | 9 | 22 | 114 869 | 4.51 | 223 158 | 148 788 |
| 15–30 | 69 | 19 | 15 515 | 4.06 | 85 541 | 32 050 | |
| 31–45 | 23 | 48 | <486 | 3.32 | 39 799 | 10 076 | |
| >45 | 73 | 61 | <486 | 3.22 | 14 397 | 20 237 | |
| Sex | Female | 127 | 39 | 2586 | 3.65 | 52 947 | 18 459 |
| Male | 47 | 32 | 3472 | 3.72 | 94 104 | 46 551 | |
| Marital statusa | Single | 15 | 13 | 19 825 | 4.19 | 103 115 | 25 506 |
| Married | 95 | 38 | 923 | 3.52 | 52 742 | 11 982 | |
| Otherb | 55 | 44 | 2185 | 3.64 | 46 938 | 23 989 | |
| No. of children | 0 | 39 | 36 | 4528 | 3.65 | 57 816 | 16 061 |
| 1–2 | 78 | 40 | 1087 | 3.56 | 44 843 | 19 825 | |
| ≥3 | 57 | 33 | 4650 | 3.82 | 94 642 | 29 482 | |
| Occupation | Farmer | 107 | 45 | 607 | 3.49 | 52 702 | 13 548 |
| Otherc | 67 | 24 | 16 601 | 3.95 | 82 210 | 33 045 | |
| HIV ART statusa | No ART | 99 | 10 | 19 048 | 4.23 | 100 319 | 25 506 |
| Receiving ART | 66 | 83 | <486 | 2.71 | 990 | 243 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; VL, viral load.
a Assessed in 165 adults.
b Other marital status defined as participants who were single, widowed, divorced, or unspecified.
c Other occupations include those detailed in Table 1.
Predictors of Virologic Suppression
In individual-level multivariable regression models, receipt of ART was strongly associated with lower mean VL (−1.45 log, P < .0001; 990 copies/mL vs 91 651 copies/mL, respectively, Table 3). Married status was also associated with lower mean VL (−0.27 log, P = .017). However, although differences in VL by age, sex, and occupation were evident (Table 2), none of these was statistically significant in adjusted analyses.
Table 3.
Predictors of HIV-1 Plasma RNA Levels in Patients Attending a Community-Wide Health Assessment
| Variable | Univariate | Multivariate |
|---|---|---|
| Age (+10 y) | −0.27 log, P < .001 | −0.05 log, P = .29 |
| Female | −0.07 log, P = .7 | … |
| Marital status: married | −0.32 log, P = .047 | −0.27 log, P = .017 |
| No. of children | ||
| 1–2a | −0.09 log, P = .67 | … |
| ≥3a | −0.17 log, P = .45 | … |
| Occupation: farmerb | −0.46 log, P = .005 | −0.23 log, P = .06 |
| Receiving ART | −1.52 log, P < .001 | −1.45 log, P < .001 |
Patients with undetectable plasma RNA levels were assigned a value of 486 copies/mL, the limit of detection of the assay.
Abbreviations: ART, antiretroviral therapy; HIV-1, human immunodeficiency virus type 1.
a Comparison reference is to patients with no children.
b Comparison is of patients self-identifying as peasant or commercial farmer (n = 105) vs other occupations (n = 69).
Geographic Mapping of HIV RNA Levels
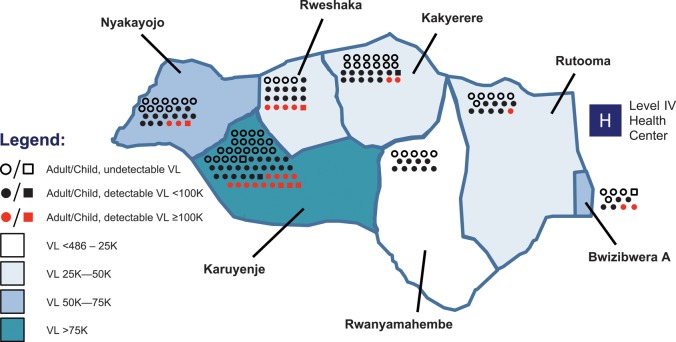
Figure 2 depicts a map of Kakyerere Parish, with the mean VL within each of 7 villages categorized by stratum. The number of participants from each village is depicted graphically, along with each person's VL. We assessed whether the distance from the approximate geographic center of each village to the district health center was associated with individuals’ probability of having an undetectable VL, but did not find any association.
Figure 2.
Geographic mapping of mean human immunodeficiency virus (HIV) RNA levels among 165 individuals with undetectable, midrange, and high levels of HIV-1 plasma RNA within villages of a rural parish of southwestern Uganda. Mean viral load (VL) for each of 7 villages (names given) within the parish are displayed in 4 categories. Individual adults (circles, n = 156) and children aged <15 y (squares, n = 9) are depicted in each region according to VL as follows: undetectable (486 copies/mL) VL (open circles/squares), detectable VL <100 000 copies/mL (black circles/squares), detectable VL ≥100 000 copies/mL (red circles/squares). The location of the parish's level IV government health center is marked at the eastern boundary of the region (white letter “H” in blue square). Abbreviation: VL, viral load.
DISCUSSION
We report here the estimation of population-based HIV RNA levels in a rural southwestern Ugandan community of 6300 persons using a fingerprick-based blood collection method for VL determination. To our knowledge, our report is the first to estimate population HIV RNA levels in a resource-limited setting where HIV RNA testing is not part of routine clinical care. This approach to aggregated population HIV RNA metrics can help assess the overall impact of ART at a community level.
As expected, the geometric and arithmetic mean VL values we found were higher than estimates for San Francisco, Vancouver, and Washington, D.C., and the median value was detectable in our study whereas the median VL was below detection in these prior studies, indicating that >50% of the population had virologic suppression. Guidelines in these well-resourced settings recommend ART initiation at higher CD4+ T-cell count thresholds, and ART has been available far longer than in resource-limited settings. In Mbarara, for example, ART became widely available in 2003–2004; the CD4+ T-cell count ART threshold had been 200 cells/μL from that time [17] until it was raised to 250 cells/μL in 2009 [18], and to 350 cells/μL in 2011 in parallel with shifts in WHO guidelines [19]. By contrast, in the United States and Canada, the recommended ART threshold was 350 cells/μL throughout most of this period, increasing to 500 cells/μL in late 2009 in the United States [20, 21]. Furthermore, fewer antiretroviral medications have been available in Uganda, and the lack of VL testing has made the detection and management of virologic failure due to drug resistance more challenging. All of these factors could account for the higher population HIV RNA levels we observed.
Nevertheless, in our study, 37% of HIV-positive individuals had an undetectable VL. Viewed against the backdrop of a recent report from the US Centers for Disease Control and Prevention estimating that in the United States, only 28% of all HIV-positive individuals have an undetectable VL [22], these data from rural Uganda could be viewed as encouraging, and demonstrate a measure of success of HIV treatment programs in the region.
Our approach to estimating population HIV RNA levels differed from those previously reported. In prior estimates, an aggregate database-driven approach was used to analyze VL levels captured from large numbers of patients, primarily from HIV care sites [4–6]. This approach was possible because VL is routinely measured in clinical practice in these areas and deposited into a central surveillance database that can be exploited for VL calculations. Missing data is always a limitation for any approach, and in San Francisco, investigators used multiple imputation to generate representative VL values for persons with missing data [5]. We took a more direct population-based approach to VL metrics by offering HIV testing to all community members (and VL measurement in HIV-positive persons) during a large-scale health campaign. By performing HIV and VL testing at the same visit, we were able to capture individuals who might be missing from a clinic-based analysis because they failed to link to HIV care following diagnosis.
Our report illustrates that fingerprick collection can be deployed in a rapid, high-throughput fashion in a community campaign run by locally trained staff and yield useful measurements including reliable HIV RNA levels with an acceptable LLOD, CD4+ T-cell counts using the PIMA system, and antiretroviral medication levels from DBSs. However, the actual assays to measure HIV RNA and antiretroviral drug levels required transport of fingerprick specimens to a central laboratory. Second, our technique relied on standard RT-PCR methods and thus does not harness emerging lower-cost point-of-care technologies for VL measurement.
Our findings may have implications for the study of HIV transmission dynamics, as assessing population HIV RNA levels can help identify groups with higher VLs who are at increased risk for transmission. Measuring population RNA levels over time may also be a rapid way to measure the impact of interventions targeting at-risk groups, using reductions in VL metrics as key outcome variables. Additionally, our geographic mapping of individuals’ VLs, if combined with behavioral surveys, might illuminate “hot spots” of transmission within communities, providing additional targets for intervention. Our group is actively engaged in making longitudinal measurements of population RNA levels in southwestern Uganda within the study community in order to assess how these are changing over time.
Our findings represent a novel approach to monitoring ART program performance and ART effectiveness. If effectiveness represents the result of a successful “cascade of care” [22], from HIV diagnosis to linkage to care, followed by ART initiation, retention in care, and virologic suppression, population HIV RNA levels are a downstream metric that integrates these drivers and other factors that are more difficult to measure. These include the proportion of a population on ART, the durability of ART regimens, adherence levels, and drug resistance causing ART failure. In this manner, population RNA levels may be a valuable summary metric that can inform policy makers on the impact of interventions in a region or population.
Our study was unable to detect persons with acute HIV infection, a limitation that should be addressable in the future by point-of-care tests that can diagnose some individuals who are viremic but who have not yet seroconverted. Another limitation was that only 52% of community men were tested, compared with 95% of women [13]. This may have been due to competing demands of work, testing stigma [23], the phenomenon of men “testing by proxy” (ie, inferring HIV status via their partners’ results) [24], or suboptimal mobilization of men [25]. Failure to capture a larger proportion of men may lead to biases—either upward or downward—in the estimated RNA metrics in this report. Downward bias may have occurred, for example, if missing individuals were less likely to be engaged in care, on ART, and virologically suppressed, whereas upward bias may have occurred if missing individuals were more likely to be in care and on ART and did not attend the campaign because they already knew their HIV status. Subsequent campaigns have successfully addressed several of these barriers in recruiting men.
In summary, by offering HIV testing at a community health campaign in a defined geographic population and measuring VL on all HIV-infected persons using a fingerprick blood collection technique, we were able to estimate population HIV RNA levels in a resource-limited setting that does not have routine VL testing in its HIV clinics. This strategy may allow for measurements of HIV epidemiology and HIV transmission dynamics at the population level in areas of the world with the highest burden of HIV, and may be a valuable tool in evaluating the reach, scope, and effectiveness of ART delivery programs at the community level.
Notes
Acknowledgments. We are grateful to the residents of Kakyerere Parish, Mbarara district, Uganda, for their generous participation in this research study. We also thank the staff of the Bwizibwera Level IV Health Center and the Mulago-Mbarara Joint AIDS Program.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (U01 AI069502 to D. V. H. and P30 AI027763 to P. Volberding, UCSF).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Joint United Nations Programme on HIV/AIDS. UNAIDS World AIDS Day Report: 2011:1–52. Available at http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2011/JC2216_WorldAIDSday_report_2011_en.pdf. Accessed 9 June 2012. [Google Scholar]
- 2.Burns DN, Dieffenbach CW, Vermund SH. Rethinking prevention of HIV type 1 infection. Clin Infect Dis. 2010;51:725–31. doi: 10.1086/655889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millett GA, Crowley JS, Koh H, et al. A way forward: the national HIV/AIDS strategy and reducing HIV incidence in the United States. J Acquir Immune Defic Syndr. 2010;55:S144–7. doi: 10.1097/QAI.0b013e3181fbcb04. [DOI] [PubMed] [Google Scholar]
- 4.Castel AD, Befus M, Willis S, et al. Use of the community viral load as a population-based biomarker of HIV burden. AIDS. 2012;26:345–53. doi: 10.1097/QAD.0b013e32834de5fe. [DOI] [PubMed] [Google Scholar]
- 5.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010;5:e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376:532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood E, Kerr T, Marshall BD, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 9.Hughes JP, Baeten JM, Lingappa JR, et al. Determinants of per-coital-act HIV-1 infectivity among African HIV-1-serodiscordant couples. J Infect Dis. 2012;205:358–65. doi: 10.1093/infdis/jir747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornell M, Technau K, Fairall L, et al. Monitoring the South African National Antiretroviral Treatment Programme, 2003–2007: the IeDEA Southern Africa collaboration. S Afr Med J. 2009;99:653–60. [PMC free article] [PubMed] [Google Scholar]
- 12.Filler SJ, Berruti AA, Menzies N, et al. Characteristics of HIV care and treatment in PEPFAR-supported sites. J Acquir Immune Defic Syndr. 2011;57:e1–6. doi: 10.1097/QAI.0b013e3182158980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamie G, Kwarisiima D, Clark TD, et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PLoS One. 2012;7:e43400. doi: 10.1371/journal.pone.0043400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ministry of Health Uganda. Uganda national policy guidelines for HIV voluntary counseling and testing. 2005. Kampala, Uganda: Director General Health Services. [Google Scholar]
- 15.Mtapuri-Zinyowera S, Chideme M, Mangwanya D, et al. Evaluation of the PIMA point-of-care CD4 analyzer in VCT clinics in Zimbabwe. J Acquir Immune Defic Syndr. 2010;55:1–7. doi: 10.1097/QAI.0b013e3181e93071. [DOI] [PubMed] [Google Scholar]
- 16.Diaw PA, Daneau G, Coly AA, et al. Multisite evaluation of a point-of-care instrument for CD4(+) T-cell enumeration using venous and finger-prick blood: the PIMA CD4. J Acquir Immune Defic Syndr. 2011;58:e103–11. doi: 10.1097/QAI.0b013e318235b378. [DOI] [PubMed] [Google Scholar]
- 17.Ministry of Health Uganda. National antiretroviral treatment guidelines for adults and children. Kampala, Uganda: Director General Health Services, 1st ed. 2003:1–66. [Google Scholar]
- 18.Ministry of Health Uganda. National antiretroviral treatment guidelines for adults, adolescents, and children. Kampala, Uganda: Director General Health Services, 3rd ed. 2009:1–80. [Google Scholar]
- 19.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach—2010 revision. 2010:1–156. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 20.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; 2011. pp. 1–167. Washgton, DC: [Google Scholar]
- 21.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society–USA panel. JAMA. 2010;304:321–33. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Vital signs: HIV prevention through care and treatment—United States. MMWR. 2011;60:1618–23. Morbid Mortal Wkly Rep. [PubMed] [Google Scholar]
- 23.Young SD, Hlavka Z, Modiba P, et al. HIV-related stigma, social norms, and HIV testing in Soweto and Vulindlela, South Africa: National Institutes of Mental Health Project Accept (HPTN 043) J Acquir Immune Defic Syndr. 2010;55:620–4. doi: 10.1097/QAI.0b013e3181fc6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrill AC, Noland C. Interpersonal issues surrounding HIV counseling and testing, and the phenomenon of “testing by proxy.”. J Health Commun. 2006;11:183–98. doi: 10.1080/10810730500526745. [DOI] [PubMed] [Google Scholar]
- 25.Mills EJ, Beyrer C, Birungi J, Dybul MR. Engaging men in prevention and care for HIV/AIDS in Africa. PLoS Med. 2012;9:e1001167. doi: 10.1371/journal.pmed.1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]