Using a computer simulation of human immunodeficiency virus infection we project that genotype resistance testing at first-line antiretroviral therapy failure is very cost-effective in South Africa. The cost-effectiveness will depend on prevalence of wild-type virus and timely response to genotype results.
Keywords: HIV, resistance testing, antiretroviral treatment failure, resource-limited settings
Abstract
Background. In resource-limited settings, genotype testing at virologic failure on first-line antiretroviral therapy (ART) may identify patients with wild-type (WT) virus. After adherence counseling, these patients may safely and effectively continue first-line ART, thereby delaying more expensive second-line ART.
Methods. We used the Cost-Effectiveness of Preventing AIDS Complications International model of human immunodeficiency virus (HIV) disease to simulate a South African cohort of HIV-infected adults at first-line ART failure. Two strategies were examined: no genotype vs genotype, assuming availability of protease inhibitor–based second-line ART. Model inputs at first-line ART failure were mean age 38 years, mean CD4 173/µL, and WT virus prevalence 20%; genotype cost was $300 per test and delay to results, 3 months. Outcomes included life expectancy, per-person costs (2010 US dollars), and incremental cost-effectiveness ratios (dollars per years of life saved [YLS]).
Results. No genotype had a projected life expectancy of 106.1 months, which with genotype increased to 108.3 months. Per-person discounted lifetime costs were $16 360 and $16 540, respectively. Compared to no genotype, genotype was very cost-effective, by international guidance, at $900/YLS. The cost-effectiveness of genotype was sensitive to prevalence of WT virus (very cost-effective when prevalence ≥12%), CD4 at first-line ART failure, and ART efficacy. Genotype-associated delays in care ≥5 months decreased survival and made no genotype the preferred strategy. When the test cost was <$100, genotype became cost-saving.
Conclusions. Genotype resistance testing at first-line ART failure is very cost-effective in South Africa. The cost-effectiveness of this strategy will depend on prevalence of WT virus and timely response to genotype results.
South Africa has the largest government-sponsored antiretroviral therapy (ART) program for human immunodeficiency virus (HIV). Limited resources require prudent management of healthcare investments. In South Africa, 2 sequential regimens or “lines” of ART are available, consistent with World Health Organization (WHO) guidelines [1].
After failure of first-line, nonnucleoside reverse transcriptase inhibitor (NNRTI)–based ART, guidelines recommend that individuals switch to protease inhibitor (PI)–based second-line ART. The relative effectiveness and cost determine this sequence; NNRTI-based ART is considerably less expensive than PI-based ART [2], although more likely to lead to viral resistance [3].
In the United States and Europe, genotype testing to distinguish resistant and nonresistant (wild-type [WT]) virus is the standard of care at ART initiation and failure [4]. In resource-limited settings, public health approaches to ART emphasize algorithms that exclude genotype testing, likely due to concerns for the complexity of healthcare delivery, upfront test costs, and the absence of ART options [1]. Without genotype testing, persistent observed HIV viremia or perceived virologic failure (based on CD4 count) prompts a switch to second-line ART. However, patients who fail ART with WT virus often “fail” due to medication nonadherence rather than drug resistance. Genotype may distinguish patients with resistant virus, who merit a switch to PI-based ART, from patients with WT virus, who with effective adherence counseling might succeed on a renewed trial of first-line ART [4, 5]. Such management of patients with WT virus would defer a switch to second-line ART. We used a computer model to project the clinical impact, cost, and cost-effectiveness of genotype resistance testing at first-line ART failure in South Africa.
METHODS
Analytic Overview
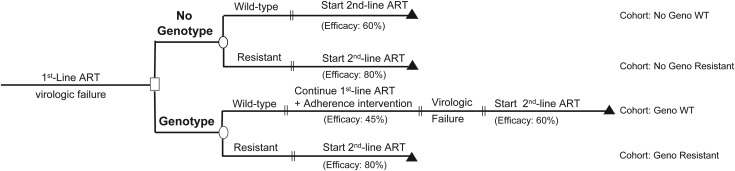
Using a computer simulation model of HIV disease, we assessed clinical and cost-effectiveness outcomes of genotype testing among HIV-infected patients failing first-line NNRTI-based ART. We first simulated the period between ART initiation and first-line ART failure (“initialization cohort”) to determine cohort characteristics at first-line ART failure. Next, in the “main analysis,” we investigated 2 strategies: the current standard of care [6] (no genotype) and genotype testing at first-line ART failure (genotype). Two cohorts of patients were simulated for each strategy: those who failed first-line ART with WT virus (“No Geno WT” and “Geno WT”) and those who failed with NNRTI-resistant virus (“No Geno Resistant” and “Geno Resistant”; Figure 1). Projected outcomes were time on PI-based second-line ART, life expectancy, and mean per-person lifetime costs, all from the time of first-line ART failure. Total cohort outcomes for genotype and no genotype strategies were calculated as the weighted average of No Geno WT and No Geno Resistant for no genotype and Geno WT and Geno Resistant for genotype, weighted in each case by the prevalence of resistant and WT virus at first-line ART failure.
Figure 1.
Diagram for evaluation of genotype testing at first-line antiretroviral therapy (ART) failure. Two strategies for management of confirmed virologic failure after first-line ART failure are compared. The genotype strategy represents the implementation of drug resistance genotype testing at the time of first-line ART failure. In this strategy, the switch to protease inhibitor–based second-line ART is dependent on whether the test result indicates the presence of wild-type or resistant virus. The percentages reflect the proportion of the cohort with virologic suppression to <400 copies/mL at 24 weeks, or ART efficacy, on the given regimen. Abbreviations: ART, antiretroviral therapy; WT, wild-type.
In no genotype, at confirmed virologic failure all individuals switched immediately to PI-based second-line ART with lopinavir/ritonavir and 2 nucleoside reverse transcriptase inhibitors (NRTIs). In genotype, a genotype test was performed. Results informed clinical decisions as follows: (1) if the test result indicated WT virus (Geno WT), we modeled a continuation of NNRTI-based ART following a routine adherence intervention. If subsequent failure occurred, PI-based second-line ART was initiated; (2) if the test result indicated resistant virus (Geno Resistant), we modeled a switch to PI-based second-line ART. The efficacy of the PI-based regimen for each of the 4 modeled cohorts depended on the presence of WT or resistant virus (Figure 1). Because the acquisition of viral resistance to ART is often time-dependent [7], we conservatively assumed a 5% decrement in the efficacy of ART for every 3 months of a genotype-associated delay in care (base case delay = 3 months; Supplementary Appendix).
Genotype was compared with no genotype using an incremental cost-effectiveness ratio (ICER) in 2010 US dollars (USD) per year of life saved ($/YLS). We adopted a modified societal perspective considering only HIV-associated direct costs. Future costs and life expectancy were discounted at 3% per year [8]. Following the general guidance of the WHO Commission on Macroeconomics and Health, we considered a strategy “very cost-effective” if its ICER was <1 times the per capita gross domestic product (GDP = US$7100 for South Africa in 2010), and “cost effective” if <3 times the GDP [9, 10]. A strategy was “dominated” if it was less effective and costlier than the comparator strategy [11].
Model Structure
The Cost-Effectiveness of Preventing AIDS Complications (CEPAC)–International Model is a state-transition model of HIV infection that simulates disease progression and clinical care in resource-limited settings using country-specific data [12] (Supplementary Appendix). In the model, HIV-infected individuals are simulated individually from the beginning of HIV care until death. In each month, hypothetical individuals can move between health states including chronic HIV infection, acute clinical events (eg, opportunistic diseases or medication toxicities), and death from both HIV-related and HIV-unrelated causes. CD4 count, prophylaxis against opportunistic infection, and history of opportunistic infections determine the risk of these clinical events [13].
In the model, effective ART leads to suppression of HIV RNA, an increase in CD4 count, and decreased risks for clinical events, as well as an additional, CD4-independent reduction in risk of opportunistic diseases and chronic AIDS death [14, 15]. ART efficacy represents virologic suppression to <400 copies/mL at 24 weeks. Modeled virologic failure can occur either “early” (≤24 weeks) or “late” (>24 weeks) after ART initiation. When virologic failure occurs, HIV RNA rises and CD4 count declines [16]. Consistent with ART guidelines [1, 6], we modeled individual clinic visits every 3 months, with CD4 count and HIV RNA measured every 6 months. In the model, we simulated 2-lines of sequential ART, NNRTI-based first-line ART and PI-based second-line ART; individuals who fail second-line ART continue on this regimen [6]. ART switching relies on the observation of confirmed and persistent virologic failure (2 consecutive clinic visits with >1 log increase in HIV RNA) in both strategies.
Model Input Parameters
Initialization Cohort: ART Initiation to Failure of First-line ART
Characteristics of the ART-naive population were drawn from published reports from South Africa [13, 17, 18]; mean age was 33 years, 55% were male, mean CD4 count was 73/μL, and median HIV RNA was 4.9 log copies/mL. First-line ART efficacy was 75% [19] (Supplementary Appendix).
Main Analysis: After Failure of First-line ART
Cohort Characteristics
At the conclusion of the initialization analyses (when patients failed first-line ART) the cohort mean age was 38.1 years, consistent with prior reports [20]. In the base case, mean CD4 count was 173/μL, and 20% had WT virus [20, 21] (Table 1).
Table 1.
Model Input Parameters for Analysis of Genotype Drug Resistance Testing at First-line Antiretroviral Therapy Failure in South Africa
| Variable | Estimate (Range Examined) | Data Sources |
|---|---|---|
| First-line ART failure cohort characteristics | ||
| Age, y, mean ± SD | 38.1 ± 4.6 | Initialization simulation |
| Male (%) | 55 | [13] |
| Distribution of initial CD4, cells/µL, mean ± SD | 173 ± 25 | [20, 21] |
| Median HIV RNA, log10 copies/mL | 4.9 | [18] |
| Prevalence of WT virus at first-line ART failure | 20% (1%–30%) | [21] |
| Natural history of disease | ||
| Mean monthly CD4 decline, cells/µL, by HIV RNA stratum | [40] | |
| >30 000 copies/mL | 6.4 | |
| 10 001–30 000 copies/mL | 5.4 | |
| 3001–10 000 copies/mL | 4.6 | |
| 501–3000 copies/mL | 3.7 | |
| 0–500 copies/mL | 3.0 | |
| Monthly risk of severe opportunistic diseasesa, range by CD4, % | [13] | |
| Active tuberculosis | 0.16–1.96 | |
| Other severe bacterial infection | 0.04–0.71 | |
| Other WHO stage III–IV event (mucocutaneous) | 0.03–2.26 | |
| Other WHO stage III–IV, nonspecific | 0.03–0.71 | |
| Non-WHO stage III–IV event | 0.25–1.67 | |
| Monthly risk of mild opportunistic diseasesa, range by CD4, % | [13] | |
| Fungal | 1.76–3.14 | |
| Other WHO stage II | 2.33–2.67 | |
| Monthly risk of HIV-related deatha, range by CD4, % | [13] | |
| No history of opportunistic infection | 0.11–4.0 | |
| History of opportunistic infection | 7.9–9.5 | |
| Antiretroviral therapy | ||
| Continued NNRTI-based ART after first-line ART failure (Geno WT cohort only) | ||
| Efficacyb | 45%c (10%–100%) | [20] |
| Second-line ART: PI-based (nucleoside-resistant virus) | ||
| Efficacyb | 80%c (10%–100%) | [22] |
| Second-line ART: PI-based (WT virus) | ||
| Efficacyb | 60% (10%–100%) | Assumption |
| Second-line ART: PI-based after failure of continued NNRTI-based ART (Geno WT cohort only) | ||
| Efficacyb | 60% (10%–100%) | Assumption |
| CD4 count increase at 24 wk (all strategies/cohorts) | 148 cells/µL | [23] |
| Probability of late failure, monthly, after 24 wk (all strategies/cohorts) | 1.3% (0%–30%) | [24–26] |
| Genotype-associated delays in ART switching, mo | 3 (0–12) | Assumption |
| Costs (2010 USD) | ||
| NNRTI-based ART, monthly | 10.33 | [2] |
| Lopinavir/ritonavir-based second-line ART, monthly | 51.07 (10–70) | [2] |
| Darunavir/etravirine/tenofovir-based third-line ART, monthlyd | 254.00 (25–300) | [2] |
| CD4 count test | 12.31 (6–23) | [41] |
| HIV RNA test | 61.55 (29–116) | [41] |
| Genotype test | 300 (50–600) | (Personal communication) |
| Discount rate | 3% (0%–5%) | [8] |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; SD, standard deviation; USD, US dollars; WHO, World Health Organization; WT, wild-type.
a Risk of opportunistic infection varies by CD4 stratum, classified as <50 cells/µL, 50–99 cells/µL, 100–199 cells/µL, 200–349 cells/µL, 350–499 cells/µL, or ≥500 cells/µL.
b Efficacy is modeled as the proportion with HIV RNA <400 copies/mL at week 24.
c In the base case, there is a 3-month genotype-associated delay in ART switching and a 5% absolute decrease in ART efficacy per 3-month delay (or 1.67% decrease per month) while remaining on a failing regimen. Therefore in the Geno WT cohort, the efficacy of continued NNRTI-based ART in the base case is 40% at 3 months, and in the Geno Resistant cohort, the efficacy of PI-based ART is 75% at 3 months.
d Third-line ART: modeled to be available only in sensitivity analyses.
ART Efficacy
The type and efficacies of ART regimens modeled after first-line ART failure differed among the 4 modeled cohorts (Figure 1):
No genotype (PI-based ART only): For the No Geno WT cohort, PI-based ART efficacy was modeled as 60%. For the No Geno Resistant cohort, PI-based ART efficacy was 80% [22]. Modeled PI efficacy was lower among the No Geno WT cohort (60%) than the No Geno Resistant cohort (80%), because we assumed nonadherence as the cause of ART failure in those with WT virus.
Genotype (NNRTI- or PI-based ART): In the Geno WT cohort, following a routine adherence intervention, patients continued NNRTI-based ART with an efficacy of 45% [20]. We modeled this efficacy as lower than that of NNRTI-based ART among treatment-naive patients (75%, see “initialization cohort” above) assuming prior ART nonadherence. In the Geno WT cohort, persistent virologic failure on continued NNRTI-based ART led to a switch to PI-based second-line ART. We assigned an efficacy of 60% to PI-based ART in the Geno WT strategy, lower than the efficacy of PI-based ART in the Resistant cohorts. This was to account for time-dependent selection of NRTI resistance on the “second-chance” on first-line ART. In the Geno Resistant cohort, patients switched to PI-based second-line ART with an efficacy of 80% (equal to the efficacy of PI-based second-line ART in the No Geno Resistant cohort) [22].
For all regimens and cohorts, individuals on ART with virologic suppression had a modeled increase in CD4 cells of 148/μL at week 24 [23] and 1.3% monthly probability of “late” failure [24–26].
Costs
Costs of HIV-related care were derived from HIV-infected cohorts from South Africa (Table 1 and Supplementary Appendix Table 1). We converted South African rand (R) to 2010 USD using the South African 2010 mean exchange rate (7.33R = 1USD) and South African GDP deflators [9, 27]. Since adherence counseling is generally part of routine HIV care, the costs of counseling for individuals with detected WT virus in the genotype cohorts were considered part of routine care costs. Costs of ART were derived from public sector sources (Table 1) [2]. Genotype drug resistance testing was $300 per test (Toga Laboratories, personal communication, 5 August 2011).
Sensitivity Analyses
We performed broad univariate analyses and multiway sensitivity analyses, guided by national recommendations, which examined the impact of simultaneous variations in the parameters with the greatest effect on results [8]. Although not currently available in the South African ART rollout, we modeled available third-line ART as etravirine, darunavir/ritonavir, and raltegravir, with a week 48 efficacy of 86% at $254 per month [28, 29].
RESULTS
Base Case
Cohort-Based Outcomes
In no genotype, cohort No Geno WT, life expectancy after first-line ART failure was projected as 93.9 months (115.4 undiscounted months; Table 2). For the No Geno Resistant cohort, life expectancy was 109.1 months (136.5 undiscounted months). In genotype, cohort Geno WT, life expectancy was 116.5 months (149.6 undiscounted months). For cohort Geno Resistant, life expectancy was 106.2 months (132.5 undiscounted months), shorter than No Geno Resistant due to the modeled genotype-associated delay in switching to second-line ART (Table 2).
Table 2.
Base Case Results Assuming 20% Wild-Type Virus at Confirmed First-line Antiretroviral Therapy Failure
| Base Case Result | Time on Second-line ARTa (mo) | Undiscounted Life Expectancy (mo) | Discounted Life Expectancy (mo) | Discounted Cost ($) | Cost-effectiveness ($/YLS) |
|---|---|---|---|---|---|
| Cohort-based outcomes | |||||
| No genotype WT virus | 93.9 | 115.4 | 93.9 | 15 350 | |
| No genotype resistant virus | 109.1 | 136.5 | 109.1 | 16 610 | |
| Genotype WT virus | 80.1 | 149.6 | 116.5 | 16 220 | |
| Genotype resistant virus | 106.2 | 132.5 | 106.2 | 16 620 | |
| Strategy-based outcomes, weighted average, by prevalence of resistance | |||||
| No genotype | 106.1 | 132.3 | 106.1 | 16 360 | … |
| Genotype | 101.0 | 135.9 | 108.3 | 16 540 | 900 |
Abbreviation: ART, antiretroviral therapy; WT, wild-type; YLS, year of life saved.
a Less time on protease inhibitor–based second-line ART confers decreased costs because second-line ART is more expensive than nonnucleoside reverse transcriptase inhibitor–based first-line ART and is the last available regimen.
Strategy-Based Outcomes
In no genotype, life expectancy was projected as 106.1 months (132.3 undiscounted months; Table 2). In genotype, projected life expectancy was higher, at 108.3 months (135.9 undiscounted months). Per-person discounted lifetime costs were $16 360 in no genotype and $16 540 in genotype. Genotype compared with no genotype yielded an ICER of $900 per YLS, considered very cost-effective for South Africa [10]. Time on PI-based second-line ART was shorter in genotype at 101.0 months due to continued NNRTI-based ART in the WT cohort compared with 106.1 months in no genotype.
One-Way Sensitivity Analyses
Clinical Outcomes
Projected life expectancy for genotype and no genotype was most influenced by 6 parameters, holding all others equal to the base case: (1) prevalence of WT virus: genotype increased life expectancy compared with no genotype when the prevalence of WT virus was >11% (Table 3, Supplementary Appendix Table 2); (2) CD4 count at first-line ART failure: genotype improved life expectancy in individuals whose CD4 count was >80/μL; (3) genotype-associated delays in ART switching: delays <5 months improved survival in genotype compared with no genotype; (4) efficacy of continued NNRTI-based ART (cohort Geno WT): genotype increased life expectancy compared with no genotype when the efficacy of continued NNRTI-based ART was >10%; (5) efficacy of PI-based second-line ART after continued NNRTI-based ART (cohort Geno WT): genotype increased life expectancy compared with no genotype when the efficacy of PI-based ART was >38%; (6) monthly probability of “late” ART failure: when the probability was ≥0.25%, genotype increased life expectancy.
Table 3.
Selected 1-Way Sensitivity Analyses of Genotype vs No Genotype at First-line Antiretroviral Therapy Failure in South Africa
| Undiscounted Life Expectancy (mo) | Discounted Life Expectancy (mo) | Discounted Cost ($) | Cost-effectiveness ($/YLS) | Clinical Thresholda | Cost-effectiveness Thresholdb | |
|---|---|---|---|---|---|---|
| Prevalence of WT virus at first-line ART failurec (base case = 20%) | WT virus >11% | WT virus ≥12% | ||||
| Prevalence = 5% WT virus | ||||||
| No genotype | 135.4 | 108.4 | 16 550 | … | ||
| Genotype | 133.3 | 106.7 | 16 600 | Dominatedd | ||
| Prevalence = 30% WT virus | ||||||
| No genotype | 130.1 | 104.6 | 16 230 | … | ||
| Genotype | 137.6 | 109.3 | 16 500 | 700 | ||
| CD4 count at first-line ART failure (base case = 173 cells/µL) | >80 cells/µL | >80 cells/µL | ||||
| CD4 count = 25 cells/µL | ||||||
| Genotype | 91.9 | 74.0 | 13 450 | … | ||
| No genotype | 100.5 | 81.4 | 14 440 | 1600 | ||
| CD4 count = 500 cells/µL | ||||||
| No genotype | 151.2 | 118.6 | 15 770 | … | ||
| Genotype | 154.7 | 120.8 | 15 800 | 200 | ||
| Genotype-associated delays in ART switching (base case = 3 mo) | <5 mo | <5 mo | ||||
| Delay = 1 mo | ||||||
| No genotype | 132.3 | 106.1 | 16 360 | … | ||
| Genotype | 139.3 | 110.5 | 16 730 | 1000 | ||
| Delay = 12 mo | ||||||
| Genotype | 125.5 | 101.0 | 16 120 | … | ||
| No genotype | 132.3 | 106.1 | 16 360 | 600 | ||
| Efficacy of continued NNRTI-based ART after first-line ART failuree (base case = 45%) | Efficacy >10% | Efficacy >17% | ||||
| Efficacy = 20% | ||||||
| No genotype | 132.3 | 106.1 | 16 360 | … | ||
| Genotype | 133.2 | 106.6 | 16 530 | 3900 | ||
| Efficacy = 70% | ||||||
| No genotype | 132.3 | 106.1 | 16 360 | … | ||
| Genotype | 140.0 | 110.9 | 16 550 | 500 | ||
| Efficacy of PI-based second-line after continued NNRTI-based ARTe (base case = 60%) | Efficacy >38% | Efficacy >38% | ||||
| Efficacy = 20% | ||||||
| Genotype | 129.5 | 104.1 | 16 210 | … | ||
| No genotype | 132.3 | 106.1 | 16 360 | 900 | ||
| Efficacy = 70% | ||||||
| No genotype | 132.3 | 106.1 | 16 360 | … | ||
| Genotype | 137.5 | 109.4 | 16 620 | 900 | ||
| Available third-line ART, $254/mo | … | Cost-saving | ||||
| Genotype | 203.6 | 149.0 | 37 100 | … | ||
| No genotype | 202.5 | 148.8 | 38 120 | Dominatedd | ||
| Probability of “late” failuref (base case = 1.3%) | ≥0.25% | 0.25%–0.9%g | ||||
| Probability = 0.1% | ||||||
| Genotype | 197.2 | 138.6 | 17 600 | … | ||
| No genotype | 198.1 | 139.0 | 17 840 | 5100 | ||
| Probability = 30% | ||||||
| No genotype | 82.1 | 73.2 | 13 980 | … | ||
| Genotype | 86.0 | 76.1 | 14 460 | 1900 | ||
| Genotype test cost (base case = $300) | … | <$100g | ||||
| Genotype test cost, $50 | ||||||
| Genotype | 135.9 | 108.3 | 16 310 | … | ||
| No genotype | 132.3 | 106.1 | 16 360 | Dominatedd | ||
| Genotype test cost, $600 | ||||||
| No genotype | 132.3 | 106.1 | 16 360 | … | ||
| Genotype | 135.9 | 108.3 | 16 850 | 2700 |
Abbreviations: ART, antiretroviral therapy; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; WT, wild-type; YLS, years of life saved.
a Clinical threshold represents the threshold value where genotype imparts increased clinical benefit, measured as increased life expectancy, compared with standard of care, no genotype.
b Cost-effectiveness threshold represents the threshold value where genotype is very cost-effective compared with no genotype. Guided by the World Health Organization, we consider an incremental cost-effectiveness ratio <1 times the South Africa per capita gross domestic product as “very cost-effective.”
c ART failure = 2 consecutive HIV RNA with >1 log increase.
d A strategy is “dominated” if it is less effective and more costly than the comparator strategy.
e ART efficacy expressed as week 24 HIV RNA <400 copies/mL
f “Late” failure = monthly probability of virologic failure after 24 weeks on suppressive ART.
g Cost-effectiveness threshold here represents the threshold value where genotype is cost-saving compared with no genotype. A strategy is cost-saving if it imparts more clinical benefit for less money than the comparator strategy.
Cost-effectiveness
In 1-way sensitivity analyses, 8 parameters exerted the greatest influence on the cost-effectiveness of genotype (Table 3, Supplementary Appendix Table 2): (1) prevalence of WT virus at first-line ART failure (base case = 20%): when WT virus was ≥12%, genotype was very cost-effective compared with no genotype; (2) CD4 count at first-line ART failure (base case = 173/μL): when CD4 count was >80/μL, genotype was very cost-effective; (3) genotype-associated delays in ART switching (base case = 3 months): genotype was very cost-effective when this delay was <5 months, but when the delay was ≥5 months, genotype reduced life expectancy compared with no genotype, making no genotype preferred; (4) efficacy of continued NNRTI-based ART (cohort Geno WT, base case = 45%): genotype was cost-effective when this efficacy was >15%, and very cost-effective at efficacies >17%; (5) efficacy of PI-based second-line ART (cohort Geno WT, base case = 60%): genotype was very cost-effective if the efficacy of second-line ART was >38%; (6) third-line ART (base case = $254 per month): if third-line ART was available, genotype became cost-saving; (7) monthly probability of “late” ART failure (base case = 1.3%): genotype was very cost-effective if the probability was ≥1% and cost-saving between 0.25% and 0.9%. When the probability was <0.25%, genotype reduced survival, making no genotype the preferred strategy; (8) genotype test cost (base case = $300): genotype was cost-saving when the test cost was <$100, and very cost-effective at costs greater than this.
Genotype remained very cost-effective under plausible variations in the costs of second-line ART, routine care, and an adherence intervention for individuals with WT virus (Geno WT) as well as the discount rate (Supplementary Appendix Table 2).
Multiway Analyses
Efficacy of Continued NNRTI-Based ART and Prevalence of WT Virus
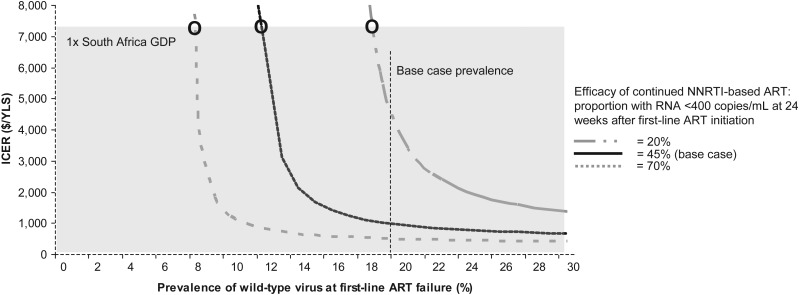
In 2-way sensitivity analysis, holding the efficacy of continued NNRTI-based ART at 20%, genotype was very cost-effective when prevalence of WT virus was ≥19%, cost-effective at 18% (Figure 2), and dominated by no genotype at <17% (region not displayed on Figure 2). When the efficacy of continued NNRTI-based ART was increased to 70%, genotype was very cost-effective when the prevalence of WT virus was ≥8%, and dominated by no genotype when prevalence was <8% (region not displayed on Figure).
Figure 2.
Two-way sensitivity analysis to examine the impact of prevalence of wild-type (WT) virus at first-line antiretroviral therapy (ART) failure and efficacy of continued nonnucleoside reverse transcriptase inhibitor (NNRTI)–based ART on suppressing human immunodeficiency virus RNA to <400 copies/mL at 24 weeks. The vertical axis represents the incremental cost-effectiveness ratio (ICER) of genotype compared with no genotype. The horizontal axis represents the prevalence of WT virus at first-line ART failure. Combinations of efficacy of continued NNRTI-based ART and prevalence of WT virus that yield ICERs above and to the left of the marked efficacy curves represent scenarios where genotype is “dominated” by no genotype, and the horizontal axis extends beyond the 30% mark to represent scenarios where genotype is very cost-effective compared with no genotype. The solid curve represents the base-case efficacy of continued NNRTI-based ART assuming a 3-month genotype-associated delay in care. The shaded gray region represents cases where the ICER of genotype is ≤1 times the South Africa gross domestic product ($7100) and very cost-effective. Circles represent the threshold prevalence of WT virus below which genotype becomes very cost-effective. Abbreviations: ART, antiretroviral therapy; GDP, gross domestic product; ICER, incremental cost-effectiveness ratio; NNRTI, nonnucleoside reverse transcriptase inhibitor; YLS, years of life saved.
CD4 Count and Genotype-Associated Delays in ART Switching
In a 2-way sensitivity analysis, we varied both CD4 count at first-line ART failure (base case = 173 cells/μL) and genotype-associated delay in ART switching (base case = 3 months; Supplementary Appendix Table 3). Higher CD4 counts at first-line ART failure permitted longer genotype-associated delays, while still achieving gains in life expectancy. For example, at a CD4 count of 500/μL, genotype was very cost-effective when the delay was ≤3 months though the survival benefit declined with increasing delay. Lower CD4 counts at first-line ART failure required shorter delays for genotype to remain clinically effective and cost-effective; when CD4 count was 50/μL at failure, genotype improved survival as long as the genotype-associated delay was <2 months, once delay was ≥3 months no genotype was preferred due to this decrease in survival.
Projected Costs of Genotype Testing at First-line ART Failure
Under base case assumptions at 5 years, the cumulative undiscounted cost per person for genotype was $8830 and for no genotype was $9020 (Supplementary Appendix Figure 1). Of total HIV costs, genotype testing represented 3%, ART 34%, laboratory monitoring 9%, and all other HIV care costs (eg, cost of clinic visits, opportunistic infection prophylaxis and events, routine care, and death) 54%.
DISCUSSION
Effective and efficient management of first-line ART failure in resource-limited settings is critical due to limited availability of ART regimens and relatively higher costs of second-line ART. Several studies have examined the clinical and economic impact of genotype testing in the United States [30, 31] and Europe [32]. While ART rollout has accelerated in resource-limited settings, there has been limited analysis of the clinical and economic impact of individual genotype testing at first-line ART failure in these settings.
We used a validated simulation model of HIV disease and found that genotype testing at first-line ART failure increased the projected survival of HIV-infected patients by 2.2 months compared to no genotype testing, the current standard of care [6]. This gain in life expectancy is comparable to other HIV-related laboratory monitoring interventions in resource-limited settings [33]. Average time on PI-based second-line ART was 5 months less in a strategy of genotype testing compared with standard of care. The deferral of a more expensive second-line regimen in patients with persistent WT virus provides clinical as well as economic benefits by continuing less expensive ART in patients who may resuppress with improvement in ART adherence. As a result, genotype testing was very cost-effective compared to switching all patients failing first-line ART to PI-based second-line ART. The benefits of genotype testing persisted even if the reported prevalence of WT virus was reduced by 40% from the modeled base case (from 20% to 12%) or the test costs were >2-fold greater than current estimates (from $300 to $600).
Cost has been a major barrier to the rollout of PI-based second-line ART. Drug-related costs contribute almost three-quarters of the expense of second-line ART in South Africa [34]. Recently, the cost of ART has decreased through negotiations with drug manufacturers and approval of generic regimens [35]. The availability of less expensive second-line ART did not affect our main conclusions, consistent with a cost-consequence analysis of genotype testing in South Africa [36]. Furthermore, a genotype testing strategy at first-line ART failure may be cost-saving if the test cost were <$100 or third-line ART were to become available, since the one-time cost of genotype testing is outweighed by ART and other recurring HIV care costs.
The clinical and economic benefits of genotype testing are particularly critical in patients with low CD4 counts at ART failure where genotype results must lead promptly to ART switching. This is consistent with prior reports of the high-risk for serious clinical events and death for patients failing first-line NNRTI-based ART particularly with WT virus [20]. Therefore, program planners should consider operational strategies to reduce delays in processing of the genotype test and delivering results. Individuals with advanced immunosuppression may require immediate switching to potent ART when prompt implementation of genotype test results cannot be assured.
No genotype at first-line ART failure was the preferred strategy when the efficacy of a renewed trial of an NNRTI-based regimen was significantly reduced (≤10%). Such low efficacies with this second chance at NNRTI-based ART, although much lower than reported to date [20], might result from repeated nonadherence or the selection of drug resistance mutations over time.
This analysis has several limitations. First, model inputs for ART efficacy did not capture information on the relationship between ART adherence and ART efficacy [20, 22]. Second, in resource-limited settings, the effect of viral resistance on the efficacy of PI-based second-line ART has been described infrequently [37–39]. Third, we derived model inputs for sex distribution from a large observational cohort in South Africa and this assumption may not represent settings where sex-associated rates of adherence differ. To address these issues, we modeled the effect of ART adherence indirectly, through assumptions based on available data (Figure 1) and sensitivity analyses on ART efficacies. Lastly, while clinical and immunologic monitoring is more common than virologic monitoring in resource-limited settings, we modeled a scenario where genotype and viral load technologies were both available, since genotyping is contingent on detectable viremia. However, we addressed the impact of delayed detection of virologic failure by simulating delays in ART switching and lower CD4 counts at first-line ART failure.
In conclusion, we project that genotype testing, performed in settings where the prevalence of WT virus in those failing first-line ART is ≥12% and informing clinical decision-making <5 months from that failure, will improve survival in HIV-infected individuals. The upfront cost of the genotype test is largely offset by the deferral of a more expensive second-line regimen in patients who fail ART due to nonadherence, rendering genotype resistance testing very cost-effective.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We gratefully acknowledge the contributions of the entire Cost-Effectiveness of Preventing AIDS Complications International team.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (T32 AI007433, R01 AI058736, K01AI078754, K24 AI062476, AI48526); National Heart, Lung, and Blood Institute (HL090312); Fogarty International Center (RTW007370); and Harvard Center for AIDS Research (P30 AI060354).
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Heart, Lung, and Blood Institute, the Fogarty International Center, or the National Institutes of Health.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach: 2010 revision. Geneva, Switzerland: World Health Organization; 2010. Available at: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. Accessed 22 March 2012. [PubMed] [Google Scholar]
- 2.Clinton Foundation HIV/AIDS Initiative. Antiretroviral price list 2009. Available at: www.clintonfoundation.org/files/chaiarvpricelistaugust2009english.pdf. Accessed 22 March 2012. [Google Scholar]
- 3.Riddler SA, Haubrich R, DiRienzo AG, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirsch MS, Gunthard HF, Schapiro JM, et al. Antiretroviral drug resistance testing in adult HIV-1 infection: 2008 recommendations of an International AIDS Society–USA panel. Clin Infect Dis. 2008;47:266–85. doi: 10.1086/589297. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann CJ, Charalambous S, Sim J, et al. Viremia, resuppression, and time to resistance in human immunodeficiency virus (HIV) subtype C during first-line antiretroviral therapy in South Africa. Clin Infect Dis. 2009;49:1928–35. doi: 10.1086/648444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Department of Health South Africa. Clinical guidelines for the management of HIV and AIDS in adults and adolescents, 2010. Available at: http://www.fidssa.co.za/guidelines/2010_adult_ART_guidelines.pdf. Accessed 22 March 2012. [Google Scholar]
- 7.Soares EA, Santos AF, Sousa TM, et al. Differential drug resistance acquisition in HIV-1 of subtypes B and C. PLoS One. 2007;2:e730. doi: 10.1371/journal.pone.0000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- 9.International Monetary Fund (2009) World economic outlook database, October 2010. Available at: http://www.imf.org/external/pubs/ft/weo/2010/02/weodata/index.aspx. Accessed 13 March 2012. [Google Scholar]
- 10.World Health Organization. Report of the Commission on Macroeconomics and Health: Macroeconomics and Health: Investing in health for economic development. Geneva, Switzerland: WHO; 2001. Available at: http://www.who.int/choice/en/ Accessed 13 March 2012. [Google Scholar]
- 11.Cantor SB, Ganiats TG. Incremental cost-effectiveness analysis: the optimal strategy depends on the strategy set. J Clin Epidemiol. 1999;52:517–22. doi: 10.1016/s0895-4356(99)00021-9. [DOI] [PubMed] [Google Scholar]
- 12.Walensky RP, Wolf LL, Wood R, et al. When to start antiretroviral therapy in resource-limited settings. Ann Intern Med. 2009;151:157–66. doi: 10.7326/0003-4819-151-3-200908040-00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–9. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 14.Cole SR, Hernan MA, Robins JM, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158:687–94. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 15.Losina E, Yazdanpanah Y, Deuffic-Burban S, et al. The independent effect of highly active antiretroviral therapy on severe opportunistic disease incidence and mortality in HIV-infected adults in Côte d'Ivoire. Antivir Ther. 2007;12:543–51. [PMC free article] [PubMed] [Google Scholar]
- 16.Deeks SG, Barbour JD, Martin JN, Swanson MS, Grant RM. Sustained CD4+ T cell response after virologic failure of protease inhibitor-based regimens in patients with human immunodeficiency virus infection. J Infect Dis. 2000;181:946–53. doi: 10.1086/315334. [DOI] [PubMed] [Google Scholar]
- 17.Keiser O, Tweya H, Braitstein P, et al. Mortality after failure of antiretroviral therapy in sub-Saharan Africa. Trop Med Int Health. 2010;15:251–8. doi: 10.1111/j.1365-3156.2009.02445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–16. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 19.Hammond R, Harry TC. Efficacy of antiretroviral therapy in Africa: effect on immunological and virological outcome measures—a meta-analysis. Int J STD AIDS. 2008;19:291–6. doi: 10.1258/ijsa.2007.007248. [DOI] [PubMed] [Google Scholar]
- 20.Murphy RA, Sunpath H, Lu Z, et al. Outcomes after virologic failure of first-line ART in South Africa. AIDS. 2010;24:1007–12. doi: 10.1097/QAD.0b013e3283333639. Selected data presented at 16th Conference on Retroviruses and Opportunistic Infections, Montreal, Canada, 8–11 February 2009. Paper 658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marconi VC, Sunpath H, Lu Z, et al. Prevalence of HIV-1 drug resistance after failure of a first highly active antiretroviral therapy regimen in KwaZulu Natal, South Africa. Clin Infect Dis. 2008;46:1589–97. doi: 10.1086/587109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bubupuradah T, Ploenchan C, Ananworanich J, et al. HIV Star study team. Second line lopinavir/ritonavir monotherapy was inferior to lamivudine/lopinavir/ritonavir in patients who failed NNRTI-regimen: HIV Star study. 18th Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, 27 February–2 March 2011 Paper 584. [Google Scholar]
- 23.Tuboi SH, Brinkhof MW, Egger M, et al. Discordant responses to potent antiretroviral treatment in previously naive HIV-1-infected adults initiating treatment in resource-constrained countries: the Antiretroviral Therapy in Low-Income Countries (ART-LINC) collaboration. J Acquir Immune Defic Syndr. 2007;45:52–9. doi: 10.1097/QAI.0b013e318042e1c3. [DOI] [PubMed] [Google Scholar]
- 24.Gallant JE, DeJesus E, Arribas JR, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 25.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–94. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 26.Pozniak AL, Gallant JE, DeJesus E, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes—a 96-week analysis. J Acquir Immune Defic Syndr. 2006;43:535–40. doi: 10.1097/01.qai.0000245886.51262.67. [DOI] [PubMed] [Google Scholar]
- 27.Oanda Corporation. Historical currency exchange rates. Available at: http://www.oanda.com/currency/historical-rates. Accessed 22 March 2012.
- 28.Yazdanpanah Y, Fagard C, Descamps D, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009;49:1441–9. doi: 10.1086/630210. [DOI] [PubMed] [Google Scholar]
- 29.Supply Chain Management System (SCMS) Available at: http://scms.pfscm.org/scms/ecatalog/arvs. Accessed 21 July 2011.
- 30.Sax PE, Islam R, Walensky RP, et al. Should resistance testing be performed for treatment-naive HIV-infected patients? A cost-effectiveness analysis. Clin Infect Dis. 2005;41:1316–23. doi: 10.1086/496984. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein MC, Goldie SJ, Losina E, et al. Use of genotypic resistance testing to guide HIV therapy: clinical impact and cost-effectiveness. Ann Intern Med. 2001;134:440–50. doi: 10.7326/0003-4819-134-6-200103200-00008. [DOI] [PubMed] [Google Scholar]
- 32.Yazdanpanah Y, Vray M, Meynard J, et al. The long-term benefits of genotypic resistance testing in patients with extensive prior antiretroviral therapy: a model-based approach. HIV Med. 2007;8:439–50. doi: 10.1111/j.1468-1293.2007.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimmel AD, Weinstein MC, Anglaret X, et al. Laboratory monitoring to guide switching antiretroviral therapy in resource-limited settings: clinical benefits and cost-effectiveness. J Acquir Immune Defic Syndr. 2010;54:258–68. doi: 10.1097/QAI.0b013e3181d0db97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS. 2010;24:915–9. doi: 10.1097/QAD.0b013e3283360976. [DOI] [PubMed] [Google Scholar]
- 35.Holmes CB, Coggin W, Jamieson D, et al. Use of generic antiretroviral agents and cost savings in PEPFAR treatment programs. JAMA. 2010;304:313–20. doi: 10.1001/jama.2010.993. [DOI] [PubMed] [Google Scholar]
- 36.Rosen S, Long L, Sanne I, Stevens WS, Fox MP. The net cost of incorporating resistance testing into HIV/AIDS treatment in South Africa: a Markov model with primary data. J Int AIDS Soc. 2011;14:24. doi: 10.1186/1758-2652-14-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Khatib Z, Ekstrom AM, Ledwaba J, et al. Viremia and drug resistance among HIV-1 patients on antiretroviral treatment: a cross-sectional study in Soweto, South Africa. AIDS. 2010;24:1679–87. doi: 10.1097/QAD.0b013e32833a097b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levison JH, Orrell C, Gallien S, et al. Virologic failure of protease inhibitor-based second-line antiretroviral therapy without resistance in a large HIV treatment program in South Africa. PLoS One. 2012;7:e32144. doi: 10.1371/journal.pone.0032144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallis CL, Mellors JW, Venter WD, Sanne I, Stevens W. Protease inhibitor resistance is uncommon in HIV-1 subtype C infected patients on failing second-line lopinavir/r-containing antiretroviral therapy in South Africa. AIDS Res Treat. 2011;2011:769627. doi: 10.1155/2011/769627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mellors JW, Munoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 41.Gauteng Department of Health. Gauteng hospitals numeric. Gauteng Province, South Africa: Available at: http://www.healthlink.org.za/uploads/files/sahr05_chapter5.pdf . [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.