Abstract
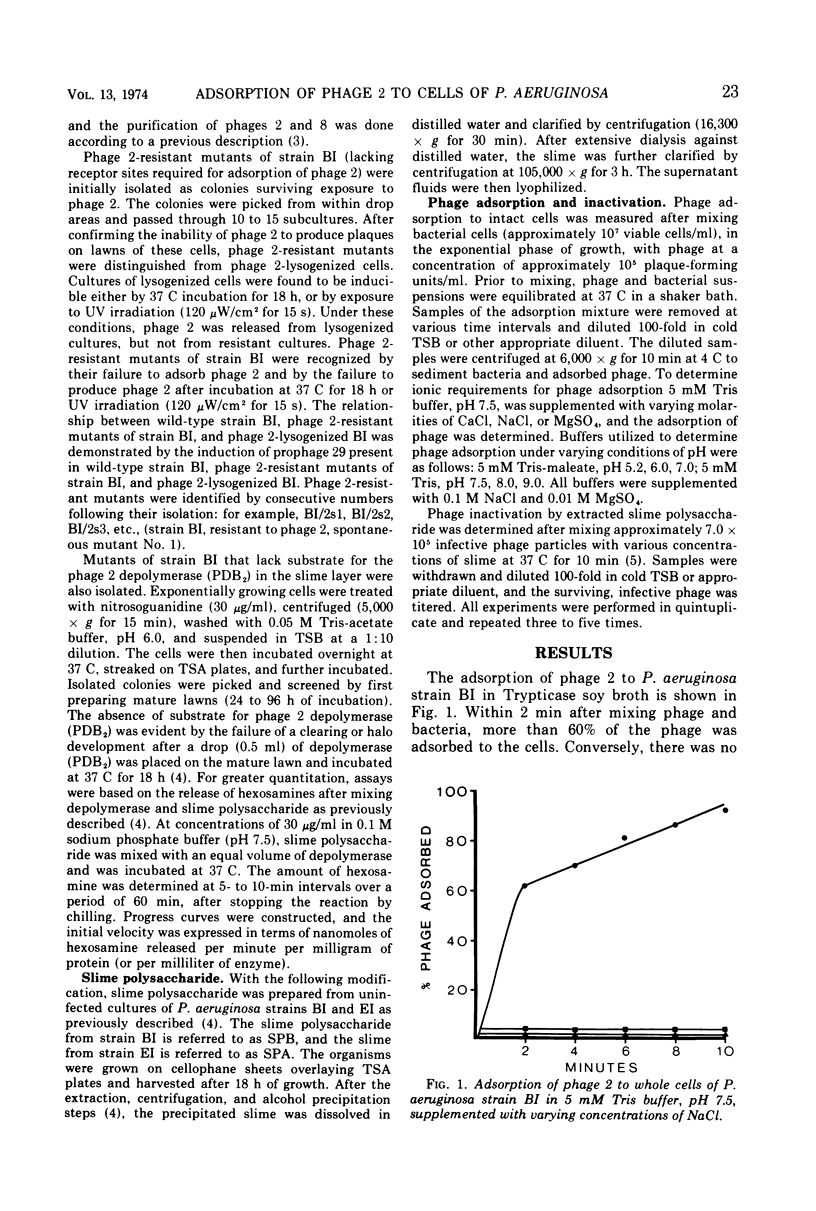
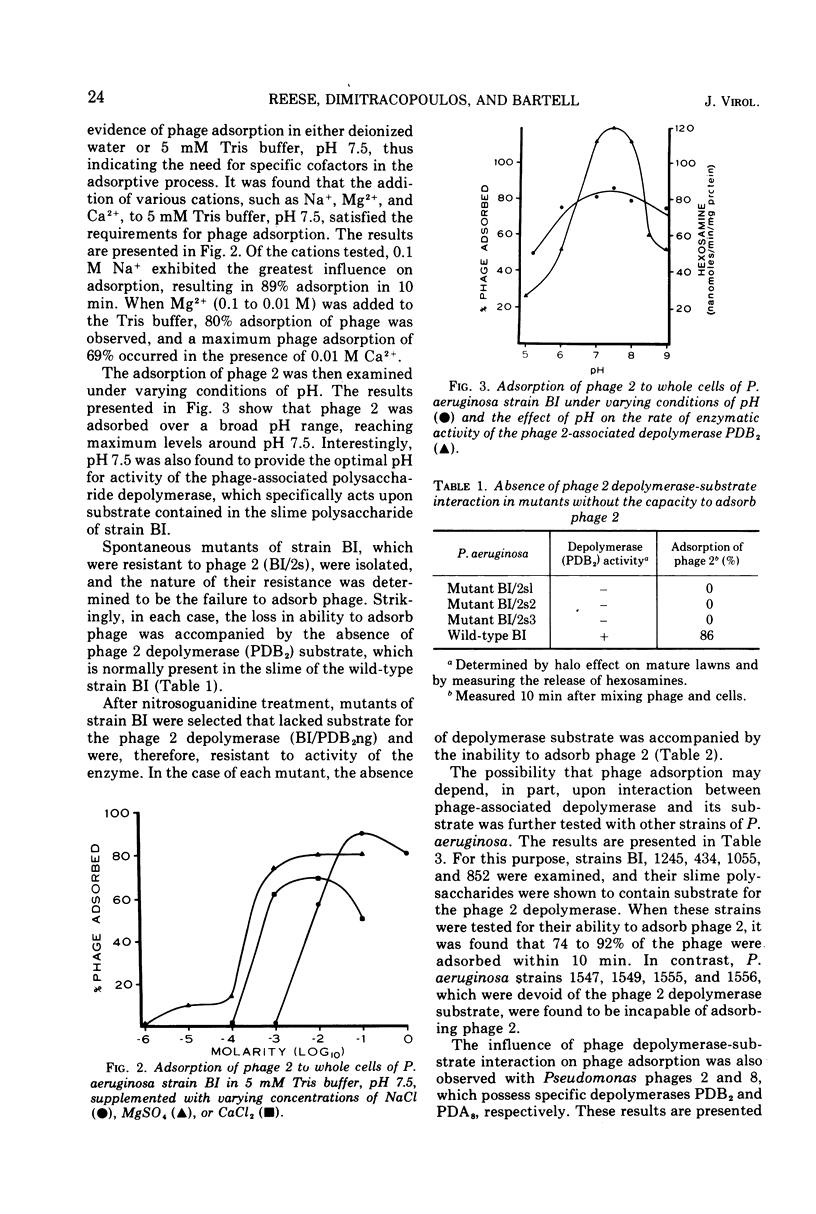
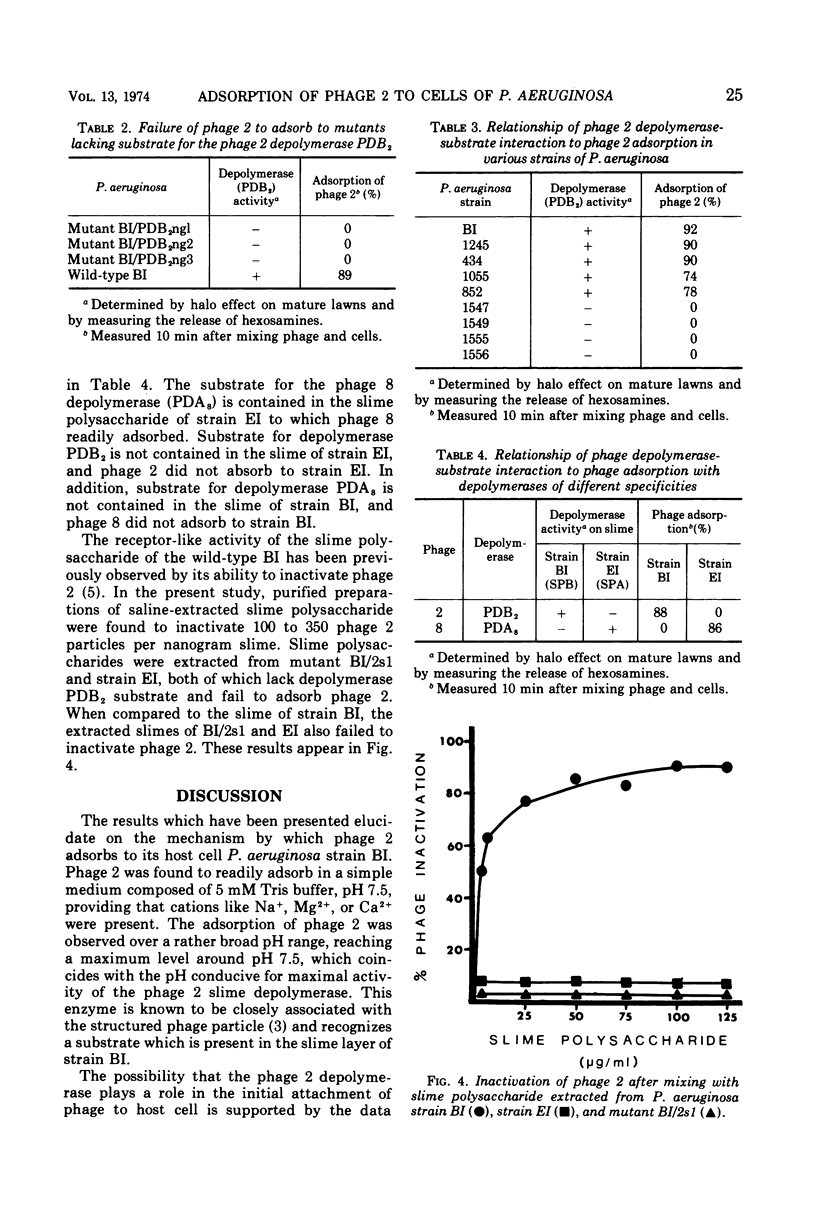
Phage 2 adsorbed to Pseudomonas aeruginosa strain BI in 5 mM Tris buffer, providing that cations like Na+, Mg2+, or Ca2+ were present. Adsorption was observed over a broad pH range, reaching a maximum level around pH 7.5, which coincided with the pH required for maximal activity of the phage 2-associated slime polysaccharide depolymerase. Mutants of strain BI and other strains of P. aeruginosa possessing slime layers that were devoid of phage 2 depolymerase substrate were incapable of adsorbing phage 2. On the other hand, those strains containing substrate for the phage 2 depolymerase in the slime layer were capable of adsorbing phage 2. The same relationship of phage depolymerase-substrate interaction to phage adsorption was observed with Pseudomonas phage 8, which possesses a depolymerase that differs in its specificity from the phage 2 depolymerase. The receptor-like activity of purified slime containing the specific substrate for the phage-associated depolymerase was demonstrable by its ability to inactivate phage. However, receptor-like activity or phage inactivation was not observed with those slimes that were devoid of the depolymerase substrate.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS M. H., PARK B. H. An enzyme produced by a phage-host cell system. II. The properties of the polysaccharide depolymerase. Virology. 1956 Dec;2(6):719–736. doi: 10.1016/0042-6822(56)90054-x. [DOI] [PubMed] [Google Scholar]
- BRINTON C. C., Jr, GEMSKI P., Jr, CARNAHAN J. A NEW TYPE OF BACTERIAL PILUS GENETICALLY CONTROLLED BY THE FERTILITY FACTOR OF E. COLI K 12 AND ITS ROLE IN CHROMOSOME TRANSFER. Proc Natl Acad Sci U S A. 1964 Sep;52:776–783. doi: 10.1073/pnas.52.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E. Distinct slime polysaccharide depolymerases of bacteriophage-infected Pseudomonas aeruginosa: evidence of close association with the structured bacteriophage particle. J Virol. 1969 Nov;4(5):580–584. doi: 10.1128/jvi.4.5.580-584.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Lam G. K. Polysaccharide depolymerase associated with bacteriophage infection. J Bacteriol. 1966 Jul;92(1):56–62. doi: 10.1128/jb.92.1.56-62.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Reese J. F., Imaeda T. Interaction of Pseudomonas bacteriophage 2 with the slime polysaccharide and lipopolysaccharide of Pseudomonas aeruginosa strain B1. J Virol. 1971 Sep;8(3):311–317. doi: 10.1128/jvi.8.3.311-317.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro L. G., Schnös M. The attachment of the male-specific bacteriophage F1 to sensitive strains of Escherichia coli. Proc Natl Acad Sci U S A. 1966 Jul;56(1):126–132. doi: 10.1073/pnas.56.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A. M., Niblack J. F., Gunsalus I. C. A phage-initiated polysaccharide depolymerase in Pseudomonas putida. Virology. 1967 Jul;32(3):532–534. doi: 10.1016/0042-6822(67)90305-4. [DOI] [PubMed] [Google Scholar]
- Eklund C., Wyss O. ENZYME ASSOCIATED WITH BACTERIOPHAGE INFECTION. J Bacteriol. 1962 Dec;84(6):1209–1215. doi: 10.1128/jb.84.6.1209-1215.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH G., DRYER W. J. Characterization of an enzyme of phage T2 as a lysozyme. Virology. 1958 Aug;6(1):291–293. doi: 10.1016/0042-6822(58)90079-5. [DOI] [PubMed] [Google Scholar]
- Kanegasaki S., Wright A. Studies on the mechanism of phage adsorption: interaction between phage epsilon15 and its cellular receptor. Virology. 1973 Mar;52(1):160–173. doi: 10.1016/0042-6822(73)90406-6. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski B., Taylor A. Two-step attachment of Vi-phage I to the bacterial surface. Acta Microbiol Pol A. 1970;2(1):13–20. [PubMed] [Google Scholar]
- MEYNELL E. W. A phage, phi chi, which attacks motile bacteria. J Gen Microbiol. 1961 Jun;25:253–290. doi: 10.1099/00221287-25-2-253. [DOI] [PubMed] [Google Scholar]
- MURPHY J. S. A phage-associated enzyme of Bacillus megaterium which destroys the bacterial cell wall. Virology. 1957 Dec;4(3):563–581. doi: 10.1016/0042-6822(57)90086-7. [DOI] [PubMed] [Google Scholar]
- PARK B. H. An enzyme produced by a phage-host cell system. I. The properties of a Klebsiella phage. Virology. 1956 Dec;2(6):711–718. doi: 10.1016/0042-6822(56)90053-8. [DOI] [PubMed] [Google Scholar]
- REITER B., ORAM J. D. GROUP N STREPTOCOCCAL PHAGE LYSIN. J Gen Microbiol. 1963 Jul;32:29–32. doi: 10.1099/00221287-32-1-29. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W., Jann K., Jann B. The isolation of O-acetylated fragments from the K antigen of Escherichia coli 08:K27 (A):H by the action of phage-induced enzymes from Klebsiella aerogenes. Eur J Biochem. 1970 Feb;12(2):285–288. doi: 10.1111/j.1432-1033.1970.tb00848.x. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W., Wilkinson J. F. Depolymerases for bacterial exopolysaccharides obtained from phage-infected bacteria. J Gen Microbiol. 1965 Jun;39(3):373–383. doi: 10.1099/00221287-39-3-373. [DOI] [PubMed] [Google Scholar]
- TAYLOR K., KWIATKOWSKI B. Adsorption of Vi-phage II on the Vi-receptor coated erythrocyte membranes, examined in the electron microscope. Acta Microbiol Pol. 1963;12:107–112. [PubMed] [Google Scholar]
- Takeda K., Uetake H. In vitro interaction between phage and receptor lipopolysaccharide: a novel glycosidase associated with Salmonella phage 15 . Virology. 1973 Mar;52(1):148–159. [PubMed] [Google Scholar]
- Taylor K. Enzymatic deacetylation of Vi-polysaccharide by Vi-phage. II. Biochem Biophys Res Commun. 1965 Sep 22;20(6):752–756. doi: 10.1016/0006-291x(65)90081-1. [DOI] [PubMed] [Google Scholar]



