SUMMARY
Noise-induced hearing loss (NHIL) is a significant source of hearing loss in industrialized countries. Recent research on the cellular bases of NIHL has led to new avenues for protection through prophylactic drugs. Although in experimental animal models several compounds have shown a protective effect in NIHL, limited data are available in humans. Many authors are focusing their attention on the role of antioxidant on hearing protection. Alpha-lipoic acid (ALA), an essential cofactor in mitochondrial enzymes, is a novel biological antioxidant and a potent free radical scavenger and, in animal models, it has been shown to protect from age-induced and cisplatin-induced hearing loss. The aim of our study was to evaluate the effect of alpha-lipoic acid on temporary threshold shift measured 2 minutes after the end of exposure (TTS2) induced by a 3 kHz tone in young normally hearing subjects. Thirty young normal hearing volunteers served as control subjects. Individuals were randomly assigned to three groups. Group A (10 subjects) subjects were exposed to a 90 dB HL 3 kHz pure tone for 10 min. Group B (10 subjects) subjects were exposed to a 90 dB HL 3 kHz pure tone one hour after oral ingestion of 600 mg of ALA. Group C (10 subjects) were exposed to a 90 dB HL 3 kHz pure tone after 10 days of oral ingestion of 600 mg of ALA. Statistical analysis showed that prior to the exposure the hearing thresholds did not differ significantly among the three groups. TTS2 of group C was significantly lower that TTS2 of Groups A and B at 6 kHz (p 0.03), and TEOAEs amplitude change after noise exposure was lower for group C compared to Groups A (p = 0.089) and B (p = 0.03). ALA is a powerful lipophilic antioxidant and free radical scavenger currently used in clinical practice. A single dose of 600 mg of dose ALA did not induce any protection on the TTS2 induced by a 90 dB HL 3 kHz tone, while 10 days of therapeutic dosage assumption of ALA was associated with significant protection at 6 kHz. The results of this study show that a short course of ALA protects from TTS2 in humans, and therefore further studies are needed to better define the role of ALA in the prevention of noise induced hearing loss.
KEY WORDS: Noise-induced hearing loss, Hearing protection, Antioxidants, Alpha-lipoic acid
RIASSUNTO
L'ipoacusia da rumore è un'importante causa di sordità nei paesi industrializzati. Recenti ricerche sulle basi biologiche della sordità da rumore hanno condotto a nuove prospettive per quanto riguarda la prevenzione farmacologica. Sebbene gli esperimenti condotti su animali abbiano dimostrato l'efficacia di numerosi composti nella prevenzione del danno da rumore, pochi dati sono ad oggi disponibili nell'uomo. Molti autori stanno concentrando la loro attenzione sul ruolo di protezione degli antiossidanti dal danno da rumore. L'acido alfa-lipoico (ALA), un importante cofattore degli enzimi mitocondriali, è un nuovo antiossidante biologico ed un potente scavenger dei radicali liberi. In modelli animali è stata dimostrata la sua efficacia nella protezione dall'ipoacusia età-correlata e cisplatino-indotta. L'obiettivo del nostro studio è stato quello di valutare l'effetto dell'acido alfa lipoico sull'innalzamento temporaneo di soglia indotto da un tono puro a 3 kHz in giovani soggetti normoudenti. Trenta giovani volontari sono stati utilizzati come soggetti di studio e suddivisi in tre gruppi. Il Gruppo A (10 soggetti) è stato esposto ad un tono puro di 90 dB HL a 3 kHz per dieci minuti. Il Gruppo B (10 soggetti) è stato esposto ad un tono puro di 90 dB HL a 3 kHz un'ora dopo l'assunzione per os di 600 mg di acido alfa-lipoico (ALA). Il Gruppo C (10 soggetti) é stato esposto ad un tono puro di 90 dB HL a 3 kHz dopo dieci giorni dall'assunzione per os di 600 mg di ALA. Prima dell'esposizione a rumore la soglia uditiva dei tre gruppi non mostrava differenze statisticamente significative. La TTS2 del Gruppo C é risultata invece significativamente piú bassa di quella dei Gruppi A e B a 6 kHz (p 0.03) e la variazione di ampiezza delle TEOAEs per il Gruppo C é risultata significativamente piú bassa di quella del Gruppo A (p = 0.089) e del gruppo B (p = 0.03). L'ALA é pertanto un potente antiossidante lipofilico e scavenger dei radicali liberi attualmente utilizzato nella pratica clinica. Una singola dose di ALA di 600 mg non induce alcuna protezione sulla TTS2 provocata dall'esposizione ad un tono puro di 90 dB HL a 3 kHz, ma é possibile ottenere una protezione statisticamente significativa attraverso l'assunzione per dieci giorni di ALA a dosaggio terapeutico. Il nostro studio dimostra quindi che un breve trattamento con ALA protegge dal danno da rumore nell'uomo e pertanto ulteriori ricerche sono necessarie per meglio definire il ruolo di ALA nella prevenzione del danno da rumore.
Introduction
Noise-induced hearing loss (NHIL) is a significant source of hearing loss in industrialized countries 1. Currently, prevention of NIHL focuses on the use of hearing protecting devices, frequent hearing screening for at risk populations and education on the causes of hearing loss 2 3.
Recently, research on the cellular bases of NIHL has led to new avenues for protection through prophylactic drugs. Although in experimental animal models several compounds have shown a protective effect in NIHL 4-7, only limited data are available in humans 8 9.
Noise exposure causes a broad set of physical changes in the major cellular system of the cochlea that lead to temporary threshold shift (TTS) and permanent threshold shift (PTS) 4. The reversible nature of TTS indicates that the underlying mechanisms associated with hearing loss are most probably of a metabolic nature 4 8 10 11.
The generators of this oxidative stress likely include acoustically-induced ischaemia reperfusion, glutamate excitotoxicity and an increase in mitochondrial free radical production due to higher metabolic demand as well as through less efficient energy production due to mitochondrial damage 12-14.
Several studies have shown that during and after noise exposure reactive oxygen species (ROS), reactive nitrogen species (RNS) and lipid peroxides all increase 4 5. Yamane et al. 15 showed an increase of ROS in marginal cells of stria vascularis, while Nicotera et al. 16 were able to localize ROS within outer hair cells (OHC) after noise exposure. Furthermore, Ciorba et al. 17 provided evidence, by spectrophotometric analyses, of the presence and production of superoxide in inner ear perilymph of human subjects affected by profound hearing loss and treated with cochlear implantation.
The origin of increased ROS into the cochlea is somewhat speculative. However, the increased energy demand induced by the noise exposure may lead to the use of large amount of oxygen and the formation of peroxide as a byproduct of phosphorylation 18. In addition, noise exposure decreases cochlear blood flow, and its reduction is associated with increased ROS formation 4.
Several molecules with antioxidant and scavenging properties including α-tocopherol 19-21, idebenone 22 23, the water-soluble formulation of coenzyme Q10 24 25, glutathione 26, N acetylcysteine 27, D-methionine 28, ferulic acid (FA, 4-hydroxy 3-methoxycinnamic acid) 29 30 have been tested for their ability to reduce oxidative stress-induced hair cell death after intense sound exposure in animals.
Alpha-lipoic acid (ALA), an essential cofactor in mitochondrial enzymes, is a novel biological antioxidant and a potent free radical scavenger 31-33. In animal models, ALA has been shown to protect from both age-induced and cisplatin-induced hearing loss 34 35. The aim of this study was to evaluate the effects of ALA on reversible cochlear alterations using a TTS model in humans.
Materials and methods
A total of 30 volunteers served as subjects were randomly divided into three groups. Mean age was 23.9 years (range 20-30 years). Group A subjects were only exposed to noise, Group B subjects were exposed to noise 1 hour after assumption of 600 mg of ALA (Alfa Wassermann S.p.A., Alanno-PE, Italy) and group C subjects were exposed to noise after 10 days of oral daily assumption of 600 mg of ALA. All subjects signed an informed consent and the drug was kindly provided by Alfa Wassermann S.p.A. The study was approved by the Local Review Board.
Subjects
There were 15 males and 15 females. Subject selection was based on age (20-30 years old) and hearing level (mean threshold for frequencies from 0.5 to 8 kHz better than 20 dB HL). All subjects were carefully screened for negative history of otological disease, noise exposure, ototoxic drugs, metabolic disease associated with hearing loss and family history of hearing loss.
Auditory threshold and otoacoustic emissions measurement
Pure tone audiometry was performed in a soundproof cabin using pure tones (250 msec duration, 25 msec rise/ fall time, 50% duty cycle) at octave frequencies from 125 Hz to 8000 Hz with a maximum intensity of 120 dB SPL with an Amplaid 319 audiometer.
TEOAEs were recorded for click stimulation. Clicks were generated by very short electrical pulses (< 50 μsec) with a wideband spectrum using an ILOV 6 system in the standard non-linear mode. The click level was 0.3 Pa in the ear canal. The intrameatal stimulus intensity was measured directly in the outer auditory canal and was adjusted by the software. The noise rejection level was set at 4.6 mPa corresponding to 47.3 dB SPL. The spectrum analyzer was triggered at 4 msec after the stimulus presentation to avoid acoustic ringing of the input stimuli, and the temporal window was set at 20 msec. 260 averages were recorded. TEOAEs were considered present when the wave reproducibility was greater than 70%. All recordings were performed in a soundproof booth.
Noise exposure and drug assumption
Subjects were randomly assigned to three groups. Group A (10 subjects) was exposed to a 90 dB HL 3 kHz pure tone for 10 min. Group B (10 subjects) was exposed to a 90 dB HL 3 kHz pure tone 1 hour after the oral assumption of 600 mg of ALA. Group C (10 subjects) was exposed to a 90 dB HL 3 kHz pure tone for 10 min following 10 days of 600 mg ALA. In all subjects, the right ear was exposed to noise.
The high frequency pure tone was generated by an Amplaid 319 audiometer and was delivered by Peltor H7A earphones.
The TTS value was measured at 3, 4 and 6 kHz 2 minutes (TTS2) after the end of the exposure. The thresholds for the 3, 4 and 6 kHz tones were measured in the test ear using a 2 dB down-1 dB up tracking method. The TTS2 value was the difference between pre and post exposure thresholds. TEOAEs were also recorded at the end of the noise exposure after the threshold measurements.
Statistical analysis
ANOV As were used to compare hearing levels, TTS and TEOAEs between the three groups, and values of p < 0.05 were considered significant. A post-hoc analysis was performed to compare the three groups.
Results
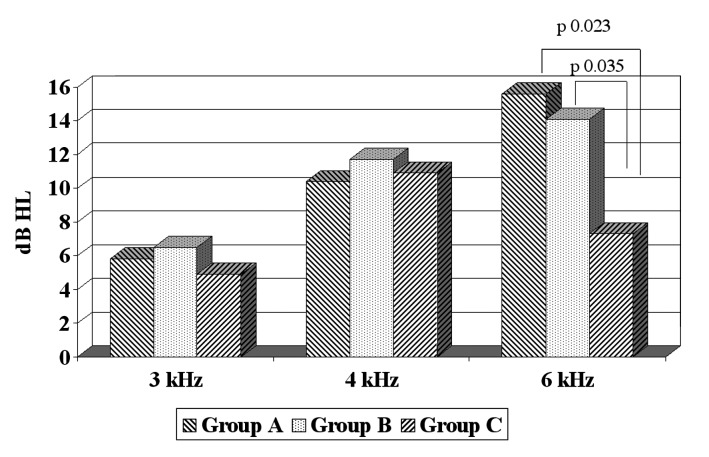
The mean pre-exposure thresholds and TTS2 (with standard deviation-SD) for each group are reported in Table I. Mean pre and post-exposure TEOAEs amplitudes and reproducibility are reported in Table II. Mean threshold pre noise exposure for Group A was -0.9 ± 3.5 dB HL at 3 kHz, -2.8 ± 4.9 dB HL at 4 kHz and 9.5 ± 5.2 dB HL at 6 kHz. Mean threshold for Group B was 1.5 ± 5.4 dB HL at 3 kHz, -2.4 ± 4.9 dB HL at 4 kHz and 4.8 ± 5.9 dB HL at 6 kHz. Mean threshold pre noise exposure for Group C was 5.2 ± 8.4 dB HL at 3 kHz, -0.1 ± 6.5 dB HL at 4 kHz and 8.6 ± 6.5 dB HL at 6 kHz. TTS2 post noise exposure was 5.8 ± 4.5 dB HL at 3 kHz, 10.4 ± 7.9 dB HL at 4 kHz and 15.6 ± 7.3 dB HL at 6 kHz for Group A. TTS2 post noise exposure for Group B was 6.5 ± 3.7 dB HL at 3 kHz, 11.7 ± 4.2 dB HL at 4 kHz and 14.1 ± 5.6dB HL at 6 kHz. In Group C, it was respectively 4.9 ± 6.1 dB HL at 3 kHz, 10.9 ± 6.5 dB HL at 4 kHz and 7.3 ± 7.6dB HL at 6 kHz. TEOAEs mean amplitude measured pre-noise exposure was 16.8 ± 4.3 dB SPL for Group A, 16.6 ± 2.9 dB SPL for Group B and 15.8 ± 4.9 dB SPL for Group C. After noise exposure their change was, respectively, 0.7 ± 1.17, 1 ± 1 and -0.2 ± 0.96 dB SPL.
Table I.
Pre-exposure thresholds and TTS2 in the three groups. Mean values and standard deviation (SD) are shown. ANOVA test was used to compare groups.
| Threshold 3 kHz pre | Threshold 4 kHz pre | Threshold 6 kHz pre | TTS2 3 kHz | TTS2 4 kHz | TTS2 6 kHz | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (dB HL) |
SD | Mean (dB HL) |
SD | Mean (dB HL) |
SD | Mean (dB HL) |
SD | Mean (dB HL) |
SD | Mean (dB HL) |
SD | |
| Group A | -0.9 | 3.5 | -2.8 | 4.9 | 9.5 | 5.2 | 5.8 | 4.5 | 10.4 | 7.9 | 15.6 | 7.3 |
| Group B | 1.5 | 5.4 | -2.4 | 4.9 | 4.8 | 5.9 | 6.5 | 3.7 | 11.7 | 4.2 | 14.1 | 5.6 |
| Group C | 5.2 | 8.4 | -0.1 | 6.5 | 8.6 | 6.5 | 4.9 | 6.1 | 10.9 | 6.5 | 7.3 | 7.6 |
| p | 0.1007 | 0.424 | 0.1886 | 0.129 | 0.521 | 0.004 | ||||||
Table II.
Pre-exposure TEOAEs amplitudes and standard deviations (SD) pre-exposure and TEOAEs change after noise exposure are reported. ANOVA test was used to compare groups.
| TEOAEs pre exposure | TEOAEs change after noise exposure | |||
|---|---|---|---|---|
| Ampl (dB SPL) |
SD | Ampl (dB SPL) |
SD | |
| Group A | 16.8 | 4.3 | 0.7 | 1.17 |
| Group B | 16.6 | 2.9 | 1 | 1 |
| Group C | 15.8 | 4.9 | -0.2 | 0.96 |
| p | 0.835 | 0.0278 | ||
Statistical analysis showed that prior to exposure hearing thresholds and TEOAEs amplitude did not differ significantly between the three groups. After exposure, 4 and 6 kHz were the most affected frequencies in all groups (Table I and Fig. 1). Statistical analysis showed that TTS2 at 3 and 4 kHz was not significantly different in the three groups, but only for 6 kHz (p = 0.004). At 6 kHz, Group C showed a TTS2 that was significantly lower than both group A (p = 0.023 at t test) and Group B (p = 0.035 at t test).
Fig. 1.
Mean TTS2 for the three groups.
Pre-exposure TEOAEs amplitude and repeatability was not significantly different between the three groups. Noise exposure determined a reduction in the amplitude of TEOAE for both group A and B, and no changes in group C. Statistical analysis showed that TEOAE amplitude change after noise exposure was lower for group C compared to Group A (p = 0.089 at t test) and group B (p = 0.03 at t test).
Discussion
The results of the present study show that a 10 day course, and not a single administration, of 600 mg of ALA can protect the cochlea from subsequent acoustic trauma. The protection was evident on both subjective (TTS2 at 6 kHz) and objective (TEAOEs) measures of hearing. In reality, an increased sensitivity of OAEs compared to pure tone audiometry in the monitoring of preclinical cochlear damage, has been demonstrated in literature 29 36 37.
Acoustic overstimulation is associated with increased oxidative stress due to ischaemia reperfusion, glutamate excitotoxicity and an increase in mitochondrial free radical production 12-15. ALA, an essential cofactor in mitochondrial enzymes, may protect against cochlear damage through a variety of mechanisms, such as providing a substrate for cochlear GSH synthesis, free radical scavenging and inhibition of cell death pathway activation 38.
ALA is readily absorbed from the diet, transported, taken up by cells and reduced to dihydrolipoate in various tissues, including the brain 38. Both lipoate and dihydrolipoate are antioxidants 19 and participate in reactions neutralizing RO S as well as in reduction of the oxidized forms of other antioxidants such as endogenous vitamin E, vitamin C and glutathione18. ALA administration has been shown to increase intracellular glutathione levels by 30-70% both in cell culture and in vivo 39, scavenge NO and inhibit the formation of NF-κB (20), a transcription factor whose nuclear transport has recently been shown to increase after acoustic trauma 40.
In animal models, ALA has been shown to protect from age-induced and cisplatin-induced hearing loss 34 35. Seidman et al. 34 supplemented Fischer rats aged 24 months for 6 weeks with either placebo, acetyl-L-carnitine or ALA. Animals treated with ALA showed a delay in progression of hearing loss, which was, however, significant only at 3 kHz; in addition, qualitative and quantitative analysis of mitochondrial DNA (mt-DNA) showed that in treated animals the common aging deletion was present to a lesser degree 34. Even better results were obtained in rats supplemented with acetyl-L-carnitine. The authors concluded that mitochondrial metabolites such as ALA or acetyl-L-carnitine may reduce age-associated deterioration in auditory sensitivity and improve cochlear function by their ability to protect and repair age-induced mt-DNA damage.
Rybak et al. 35 investigated the protective effect of ALA against cisplatin-induced ototoxicity in Wistar rats, which has been shown to be mediated by RO S. Rats treated with cisplatin plus ALA did not show significant elevations of hearing thresholds, and ALA further prevented the depletion of glutathione as well as the decrease of superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase activities 35. The protective effect of ALA was related to its action as a RO S scavenger and a chelator of platinum from the cochlea. Interestingly, the authors noted that the dose of ALA required to protect from cisplatin toxicity was 3 to 12 times lower than that of other chelating agents.
Animal and human studies have demonstrated that exogenous administration of ALA has therapeutic potential in diabetes and diabetic neuropathy, neurodegenerative disorders and heavy metal toxicity 38 41. In clinical practice, ALA is administered orally at a daily dosage of 600 mg. Although blood levels of lipoate and dihydrolipoate were not dosed in this study, the drug is rapidly absorbed in the stomach, crosses the blood-brain barrier41 and reaches a peak plasma concentration 60 min after oral assumption 42.
In the present study, the administration of a single dose of ALA 60 min before acoustic overstimulation did not have any protective effect, while a 10 day course led to significant protection at 6 kHz and on TEOAEs. We believe that the main effect of ALA in these patients is related to an increase in GSH synthesis and free radical scavenging that prevented the TTS.
To date, very few clinical studies have examined the protective effects of compounds in NIHL in humans. Attias et al. 8 have shown that the oral assumption of 122 mg of magnesium for 10 days can significantly reduce the TTS induced by a 90 dB SL white noise in normal subjects.
A recent study looked at the protective effects of N-acetylcysteine (NAC), a drug commonly used in clinical practice on TTS in voluntary discotheque attendees 43; an oral dose of 900 mg of NAC or placebo were administered 1 hour prior to attending a local discotheque. Upon exiting, audiometric measures were performed in a soundproof van, and no difference was found between the drug and placebo. Confounding factors in this study were the small number of subjects and the variability in noise exposure levels, and that only one dose of NAC was administered.
More favourable preliminary results have been reported by Kopke et al. 9 in military subjects who were supplemented with 2.7 gm/day of NAC, a dose three times higher than that used in discotheque attendees.
In this preliminary study, ALA appears to be protective for the ear from noise in humans. The dosage needed to obtain a protective effect is not higher than that proposed in clinical practice, and therefore ALA seems to have the characteristics of the "ideal" pharmacologic prophylaxis. In fact, it specifically addresses known mechanisms of acoustic trauma, is orally administered and is safe, effective and inexpensive 9. Further research is however warranted to evaluate the effects of ALA in a larger study population and its effects on TTS and permanent cochlear damage.
References
- 1.Nelson DI, Nelson RY, Concha-Barrientos M, et al. The global burden of occupational noise-induced hearing loss. Am J Ind Med. 2005;48:446–458. doi: 10.1002/ajim.20223. [DOI] [PubMed] [Google Scholar]
- 2.Dobie RA. Prevention of noise-induced hearing loss. Arch Otolaryngol Head Neck Surg. 1995;121:385–391. doi: 10.1001/archotol.1995.01890040011002. [DOI] [PubMed] [Google Scholar]
- 3.Lusk SL. Noise exposure: effects on hearing and prevention of noise induced hearing loss. AAOHN Journal. 1997;45:397–405. [PubMed] [Google Scholar]
- 4.Henderson D, Bielefield EC, Harris KC, et al. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 5.Poirrier AL, Pincemail J, Ackerveken P, et al. Oxidative stress in the cochlea: an update. Curr Med Chem. 2010;17:3591–3604. doi: 10.2174/092986710792927895. [DOI] [PubMed] [Google Scholar]
- 6.Choi CH, Chen K, Du X, et al. Effects of delayed and extended antioxidant treatment on acute acoustic trauma. Free Radic Res. 2011;45:1162–1172. doi: 10.3109/10715762.2011.605360. [DOI] [PubMed] [Google Scholar]
- 7.Cassandro E, Sequino L, Mondola P, et al. Effect of superoxide dismutase and allopurinol on impulse noise-exposed guinea pigs - electrophysiological and biochemical study. Acta Otolaryngol. 2003;123:802–807. [PubMed] [Google Scholar]
- 8.Attias J, Sapir S, Bresloff I, et al. Reduction in noise-indiced temporary threshold shift in humans followign oral magnesium intake. Clin Otolaryngol. 2004;29:635–641. doi: 10.1111/j.1365-2273.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- 9.Kopke RD, Jackson RL, Coleman JKM, et al. NAC for noise: from the bench top to the clinic. Hear Res. 2007;226:114–125. doi: 10.1016/j.heares.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Scheibe F, Haupt H, Ludwig C, et al. Intensity-dependent changes in oxygenation of cochlear perilymph during acoustic exposure. Hear Res. 1992;63:19–25. doi: 10.1016/0378-5955(92)90069-y. [DOI] [PubMed] [Google Scholar]
- 11.Scheibe F, Haupt H, Ludwig C, et al. Intensity-related changes in cochlear blood-flow in the guinea pig during and following acoustic exposure. Eur Arch otorhinolaryngol. 1993;250:281–285. doi: 10.1007/BF00186226. [DOI] [PubMed] [Google Scholar]
- 12.Lamm K, Arnold W. The effect of blood flow promoting drugs on cochlear blood flow, perilymphatic pO(2) on auditory function in the normal and noise-damaged hypoxic and ischemic guinea pig inner ear. Hear Res. 2000;141:199–219. doi: 10.1016/s0378-5955(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 13.Poderoso JJ, Boveris A, Cadenas E. Mitochondrial oxidative stress: a self-propagating process with implications for signaling cascades. Biofactors. 2000;11:43–45. doi: 10.1002/biof.5520110112. [DOI] [PubMed] [Google Scholar]
- 14.Puel JL, Ruel J, Gervais d'Aldin C, et al. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998;9:2109–2114. doi: 10.1097/00001756-199806220-00037. [DOI] [PubMed] [Google Scholar]
- 15.Yamane H, Nakai Y, Takayama M, et al. The emergence of free radicals after acoustic trauma and strial blood flow. Acta Otolaryngol Suppl. 1995;519:87–92. doi: 10.3109/00016489509121877. [DOI] [PubMed] [Google Scholar]
- 16.Nicotera T, Henderson D, Zheng XY, et al. Paper presented at the 22nd Annual Midwinter Meeting of the Association for Research in Otolaryngology. St. Petersbourg, Florida: Reactive oxygen species, apoptosis and necrosis in noise-exposed cochleas of chinchillas. [Google Scholar]
- 17.Ciorba A, Gasparini P, Chicca M, et al. Reactive oxygen species in human inner ear perilymph. Acta Otolaryngol. 2010;130:240–246. doi: 10.3109/00016480903143978. [DOI] [PubMed] [Google Scholar]
- 18.Halliwell B, Gutteridge J. Free radicals in biology and disease. Oxford: Oxford University Press; 1999. [Google Scholar]
- 19.Fetoni AR, Sergi B, Ferraresi A, et al. Alphatocopherol protective effects on gentamicin ototoxicity: an experimental study. Int J Audiol. 2004;43:166–171. doi: 10.1080/14992020400050023. [DOI] [PubMed] [Google Scholar]
- 20.Fetoni AR, Sergi B, Ferraresi A, et al. Protective effects of alpha-tocopherol and tiopronin against cisplatininduced ototoxicity. Acta Otolaryngol. 2004;124:421–426. doi: 10.1080/00016480410016559. [DOI] [PubMed] [Google Scholar]
- 21.Prell CG, Hughes LF, Miller JM. Free radical scavengers vitamins A, C, and E plus magnesium reduce noise trauma. Free Radic Biol Med. 2007;42:1454–1463. doi: 10.1016/j.freeradbiomed.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sergi B, Fetoni AR, Paludetti G, et al. Protective properties of idebenone in noise-induced hearing loss in the guinea pig. Neuroreport. 2006;17:857–861. doi: 10.1097/01.wnr.0000221834.18470.8c. [DOI] [PubMed] [Google Scholar]
- 23.Fetoni AR, Ferraresi A, Greca CL, et al. Antioxidant protection against acoustic trauma by coadministration of idebenone and vitamin E. Neuroreport. 2008;19:277–281. doi: 10.1097/WNR.0b013e3282f50c66. [DOI] [PubMed] [Google Scholar]
- 24.Fetoni AR, Piacentini R, Fiorita A, et al. Water-soluble coenzyme Q10 formulation (Q-ter) promotes outer hair cell survival in a guinea pig model of noise induced hearing loss (NIHL) Brain Res. 2009;1257:108–116. doi: 10.1016/j.brainres.2008.12.027. [DOI] [PubMed] [Google Scholar]
- 25.Fetoni AR, Eramo SL, Rolesi R, et al. Antioxidant treatment with coenzyme Q-ter in prevention of gentamycin ototoxicity in an animal model. Acta Otorhinolaryngol Ital. 2012;32:103–110. [PMC free article] [PubMed] [Google Scholar]
- 26.Hight NG, McFadden SL, Henderson D, et al. Noise-induced hearing loss in chinchillas pre-treated with glutathione monoethylester and R-PIA. Hear Res. 2003;179:21–32. doi: 10.1016/s0378-5955(03)00067-4. [DOI] [PubMed] [Google Scholar]
- 27.Kopke RD, Jackson RL, Coleman JK, et al. NAC for noise: from the bench top to the clinic. Hear Res. 2007;226:114–125. doi: 10.1016/j.heares.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Campbell KC, Meech RP, Klemens JJ, et al. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007;226:92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Fetoni AR, Mancuso C, Eramo SL, et al. In vivo protective effect of ferulic acid against noise-induced hearing loss in the guinea-pig. Neuroscience. 2010;169:1575–1588. doi: 10.1016/j.neuroscience.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Fetoni AR, Troiani D, Eramo SL, et al. Efficacy of different routes of administration for Coenzyme Q10 formulation in noise-induced hearing loss: systemic versus transtympanic modality. Acta Otolaryngol. 2012;132:391–399. doi: 10.3109/00016489.2011.652307. [DOI] [PubMed] [Google Scholar]
- 31.Biewenga GP, Haenen GRRR, Bast A. The pharmacology of the antioxidant lipoic acid. Gen Pharmacol. 1997;29:315–331. doi: 10.1016/s0306-3623(96)00474-0. [DOI] [PubMed] [Google Scholar]
- 32.Haramaki N, Han D, Handelman GJ, et al. Cytosolic and mitochondrial systems for NADH- and NADHPH-dependent reduction of alpha-lipoic acid. Free Rad Biol Med. 1997;23:535–542. doi: 10.1016/s0891-5849(96)00400-5. [DOI] [PubMed] [Google Scholar]
- 33.Packer L, Witt EH, Trischler HJ. Alpha-lipoic acid as a biological antioxidant. Free Rad Biol Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 34.Seidman MD, Khan MJ, Shirwany N, et al. Biologic activity of mitochondrial metabolites on aging and age-related hearing loss. Am J Otol. 2000;21:161–167. doi: 10.1016/s0196-0709(00)80003-4. [DOI] [PubMed] [Google Scholar]
- 35.Rybak LP, Husain K, Whitworth C, et al. Dose dependent protection by lipoic acid against cisplatin-induced ototoxicity in rats: antioxidant defense system. Pharmacol Toxicol. 2000;86:234–241. doi: 10.1034/j.1600-0773.2000.d01-41.x. [DOI] [PubMed] [Google Scholar]
- 36.Sisto R, Chelotti S, Moriconi L, et al. Otoacoustic emission sensitivity to low levels of noise-induced hearing loss. J Acoust Soc Am. 2007;122:387–401. doi: 10.1121/1.2737668. [DOI] [PubMed] [Google Scholar]
- 37.Helleman HW, Jansen EJ, Dreschler WA. Otoacoustic emissions in a hearing conservation program: general applicability in longitudinal monitoring and the relation to changes in pure-tone thresholds. Int J Audiol. 2010;49:410–419. doi: 10.3109/14992020903527616. [DOI] [PubMed] [Google Scholar]
- 38.Packer L, Trischler HJ, Wessel K. Neuroprotection by the metabolic antioxidant α-lipoic acid. Free Rad Biol Med. 1997;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 39.Busse E, Zimmer G, Schopohl B, et al. Influence of alphalipoic acid on intracellular glutathione in vitro and in vivo. Arznei Forschung. 1992;42:829–831. [PubMed] [Google Scholar]
- 40.Tahera Y, Meltser I, Johansson P, et al. NF-kappaB mediated glucocorticoid response in the inner ear after acoustic trauma. J Neurosci Res. 2006;83:1066–1076. doi: 10.1002/jnr.20795. [DOI] [PubMed] [Google Scholar]
- 41.Bilska A, Wlodek L. Lipoic acid-the drug of the future? Pharmacol Rep. 2005;57:570–577. [PubMed] [Google Scholar]
- 42.Teichert J, Kern J, Trischler HJ, et al. Investigations on the pharmacokinetics of α-lipic acid in healthy volunteers. Int J Clin Pharmacol Ther. 1998;36:625–628. [PubMed] [Google Scholar]
- 43.Kramer S, Dreisbach L, Lockwood J, et al. Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music. J Am Acad Audiol. 2006;17:265–278. doi: 10.3766/jaaa.17.4.5. [DOI] [PubMed] [Google Scholar]