SUMMARY
Chronic rhinosinusitis with nasal polyposis is considered to be a multifactorial disease where different stimuli (mechanical, viral, bacterial, fungal infection, immunological disorders or dysreactivity, environmental pollution), acting on the mucosa of nasal cavities and paranasal sinuses, lead to epithelial damage and mucosal inflammation. Inflammatory cell infiltration (predominantly eosinophils, but also neutrophils, mast cells, macrophages and lymphocytes), cytokine release and sub-epithelial oedema are the histological pictures that are associated, from the clinical point of view, with nasal congestion, secretion and/or post-nasal drip and facial pain/headache. Recently, the importance of the HMG B-1 protein in the pathogenesis of several inflammatory diseases has been demonstrated. This protein is released from necrotic/damaged cells or immune-activated cells, and by acting on specific membrane receptors causes the release of pro-inflammatory mediators, endothelial activation and the survival of inflammatory cells. The objective of the present study was: i) to determine whether HMG B1 is augmented in chronic rhinosinusitis with nasal polyps; ii) if its expression is associated with eosinophils, TNF-α, IL 5 and IL 8 cytokines typically present in chronic inflammation of the nose and paranasal sinuses; iii) to investigate a hypothetical role of this protein in the pathogenesis of nasal polyposis. Nasal polyps tissue from 21 patients affected by CRSwNP and nasal mucosa from 8 controls was collected at the ENT Department of the Chinese PLA General Hospital and underwent immunohistological staining for detection of HMG B1 protein and IL -5, IL -8 and TNF-α inflammatory cytokines. The degree of HMG B1 protein expression was evaluated by dividing the stained sections in 4 portions: 1) nucleus of epithelial cells, 2) cytoplasm of epithelial cells, 3) focal extracellular infiltration, 4) inflammatory cells. HMG B1 was more expressed in the nucleus of epithelial cells of patients compared with controls. In contrast, epithelial cytoplasm HMG B1 staining was significant lower in patients. Sub-epithelial focal infiltration of HMG B1 protein expression was lower in controls, whereas the expression of HMG B1 in the inflammatory cells in patients was significantly increased in comparison with controls. These data, together with the correlation we found between HMG B1 protein expression in different portions and the number of eosinophils infiltrating cells, or IL -5, IL -8 and TNF-α positive cells in patients, suggest that HMG B1 may play a crucial role in the pathogenesis of chronic rhinosinusitis with nasal polyps.
KEY WORDS: HMGB1 chromosomal protein, Chronic rhinosinusitis, Nasal polyposis
RIASSUNTO
La rinosinusite cronica associata o meno a poliposi nasale è considerata una patologia multifattoriale nella quale stimoli di diversa natura (traumi meccanici, infezioni virali, batteriche, fungine, deficit o disreattività immunologica, inquinamento ambientale) agendo sulla mucosa delle cavità nasali e dei seni paranasali ne danneggiano l'epitelio e portano ad infiammazione sub-epiteliale cronica. L'infiltrazione eosinofila, l'espressione di citochine infiammatorie e l'edema sono il corrispettivo istologico del quadro clinico caratterizzato da ostruzione/congestione nasale, secrezione nasale anteriore e posteriore, algie cranio-facciali. Recentemente è stata dimostrata l'importanza della proteina cromosomica HMGB1 nella patogenesi di diverse e numerose patologie infiammatorie; tale proteina viene rilasciata dalle cellule necrotiche/danneggiate o dalle cellule attivate da stimoli immunologici e agendo su specifici recettori di membrana, determina il rilascio di mediatori pro-infiammatori, con richiamo dall'endotelio, attivazione e sopravvivenza di cellule infiammatorie. Obiettivo del presente studio è stato quello di determinare se la proteina HMGB1 è aumentata nella sinusite cronica associata a poliposi nasale; se la sua espressione è associata ad un aumento degli eosinofili, e dell'espressione di IL-5, IL-8 e TNF-α (citochine tipicamente presenti nell'infiammazione cronica dei seni paranasali) al fine di verificare l'ipotesi di un ruolo di questa proteina nella patogenesi della poliposi nasale. Campioni di mucosa naso-sinusale da 21 pazienti affetti da rinosinusite cronica e poliposi nasale e da 8 soggetti di controllo sono stati raccolti presso l'ENT Department del Chinese PLA General Hospital di Pechino e sottoposti a colorazione immunoistochimica per la ricerca della proteina HMGB1 e delle citochine proinfiammatorie IL-5, IL-8, e TNF-α. Il grado di espressione di HMGB1 è stato valutato dividendo le sezioni colorate in 4 porzioni: 1) nucleo delle cellule epiteliali; 2) citoplasma delle cellule epiteliali; 3) infiltrazione extracellulare focale; 4) cellule infiammatorie. HMGB1 è risultata più espressa nel nucleo delle cellule epiteliali dei pazienti rispetto ai controlli. Al contrario la colorazione positiva per HMGB1 nel citoplasma delle cellule epiteliali era meno evidente nei pazienti, così come l'espressione sub-epiteliale focale è risultata meno evidente nei soggetti di controllo. L'espressione di HMGB1 nelle cellule infiammatorie dei pazienti era aumentata in modo significativo rispetto ai controlli. Questi dati, unitamente alla correlazione da noi trovata tra l'espressione di HMGB1 nelle diverse localizzazioni e il numero di eosinofili o di cellule IL-5, IL-8 and TNF-α positive nei pazienti, suggeriscono che HMGB1 possa giocare un ruolo cruciale nella patogenesi della rinosinusite cronica e della poliposi nasale.
Introduction
Chronic rhinosinusitis with/without nasal polyps represents a common endpoint in several disease processes 1 2.
Factors contributing to rhinosinusitis include infections (viral, bacterial, fungal) muco-ciliary clearance impairment (such as in cystic fibrosis and primary ciliary dyskinesia), immune system defects or dysreactivity such as in allergic rhinitis, intolerance to non-steroid anti-inflammatory drugs (NSAIDs), gastro-oesophageal reflux disease (GERD) and pollution. In each of these cases we can observe, from a histological viewpoint, damage to the respiratory epithelium accompanied by the infiltration of inflammatory cells (predominantly eosinophils, but also neutrophils, mast cells, macrophages and lymphocytes) and subepithelial oedema.
As inflammation, in the broadest sense, should be considered as a protective physiological response to infection and injuries of diverse nature, these inflammatory cells, in turn, release a range of mediators and cytokines: Il-5, IL-8, TNF-α, IFN-γ, RANTES, MBP and ECP are responsible for new recruitment and survival of inflammatory cells, leading to the amplification of the inflammatory process 3. Recently, the importance of the HMG B1 protein in the pathogenesis of several inflammatory diseases has been demonstrated: this protein is released from necrotic/damaged cells 4 or immune-activated cells 5 and, acting on specific membrane receptors, causes the release of pro-inflammatory mediators, endothelial activation and increased survival of inflammatory cells, mainly eosinophils 6.
HMG B1 is an evolutionary ancient protein that is constitutively present in cells, such as leukocytes and epithelial cells, in granules, cytoplasm and nucleus as a deoxyribonucleic acid (DNA)-binding protein with "alarmin" activity 7. HMG B1 is actively released in the extra-cellular medium as a late mediator of inflammation by activated macrophages/ monocytes. After stimulation with exogenous bacterial products such as endotoxin, or with endogenous proinflammatory cytokines such as TNF-α and IFN-γ, cultures of macrophages and monocytes actively release HMG B1 in a time- and dose-dependent manner. In addition to its active release, HMG B1 can also be released passively from necrotic or damaged cells including epithelial cells.
Once released in the extracellular medium, HMGB1 has pleomorphic effects including activation of NF-kB, diffuse endothelial activation, epithelial leak and systemic activation of inflammatory cells 8.
Recently 9, it has been demonstrated that the transcription factor NF-kB is more expressed in NP compared to normal nasal mucosa. This discovery in NP is important both from pathogenic and therapeutic points of view as it is known that NF-kB induces the transcription of cytokines, chemokines and adhesion molecules, which play an important role in the inflammatory process. Moreover, transcription factors influence the response to corticosteroids, which are the basis of NP treatment.
The discovery of HMGB1 protein in the nasal secretions of patients with allergic and non-allergic inflammation of the nose and paranasal sinuses mucosa may contribute to the inflammatory process becoming chronic: in fact, as demonstrated by Ek et al. 10 in Sjogren's syndrome, HMGB1, together with TNF-α and IL-1β, may form a proinflammatory loop that promotes the chronic inflammation of nasal-sinusal mucosa.
The objectives of the present study were:
To investigate the role of the HMGB1 protein in the pathogenesis of chronic rhinosinusitis with nasal polyps (CRSwNP).
To determine whether HMGB1 is increased in chronic rhinosinusitis with nasal polyps (CRSwNP) and is associated with eosinophils, TNF-α and IL5, IL8 cytokines typically expressed in nasal polyps.
To compare the HMGB1 protein in the eosinophil CRSwNP and non-eosinophil CRSwNP.
Materials and methods
The present work originates from an active collaboration among researchers from different Countries and Universities. The protocol was studied to analyze a nuclear protein implicated in the pathogenesis of inflammation. In a first phase, a frequent ORL pathology was examined, but other research is in progress on different inflammatory processes of the ENT district 11. The results have been examined and discussed by all the authors. At the Department of Otolaryngology at Beijing PLA (People Liberation Army) General Hospital, nasal polyps tissue from 21 patients with CRSwNP including 1 patient with asthma and 2 patients with allergic rhinitis and 8 healthy control subjects were collected.
Chronic rhinosinusitis with nasal polyps was confirmed by medical history, nasal endoscopy and computed tomography (CT) scan of the paranasal cavities according to the European Position Paper on rhinosinusitis and nasal polyps 3.
The atopic status was evaluated by skin prick test to a standard panel of aeroallergens. The diagnosis of asthma and aspirin sensitivity was based on patient history and physician diagnosis.
Patients with non-invasive fungal sinusitis and invasive fungal disease, chronic obstructive pulmonary disease or systemic disease, such as congenital mucociliary diseases, cystic fibrosis or severe asthma were excluded.
Individual symptoms of chronic rhinosinusitis (including nasal congestion, nasal discharge or postnasal drip, headache or facial pain and hyposmia) were evaluated on a scale of 0-10 before surgery. Nasal endoscopic examination was graded with the Lund-Kennedy classification 12; CT scans of paranasal cavities were graded with the Lund-Mackay classification 13. Control subjects were patients with rhinorrhoea of cerebrospinal fluid, fracture of the optic canal or a benign skull base tumour and did not have a history of sinus disease or asthma. Clinical characteristics of patients and control subjects are reported in Table I.
Table I.
Clinical characteristics of study participants.
| Control subjects | CRSwNP | p value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eos | non-Eos | |||||||||
| No. of subjects | 8 | 10 | 11 | – | ||||||
| Sex (male/female) | 5/3 | 8/2 | 8/3 | NS | ||||||
| Mean age(y), median range | 37(22-70) | 42 (30-54) | 38 (17-56) | NS | ||||||
| Nasal congestion | 1 | 9 | 11 | < 0.001 | ||||||
| Rhinorrhoea | 0 | 9 | 9 | 0.001 | ||||||
| Headache | 1 | 6 | 3 | NS | ||||||
| Facial pain/pressure | 0 | 1 | 1 | NS | ||||||
| Hyposmia | 0 | 8 | 6 | 0.014 | ||||||
| No. of bilateral polyps | 0 | 10 | 4 | NS | ||||||
| Recurrent history | 0 | 2 | 3 | NS | ||||||
| Atopy | Y | N | U | Y | N | U | Y | N | U | NS |
| 0 | 4 | 4 | 2 | 5 | 3 | 0 | 5 | 6 | ||
| Asthma | 0 | 8 | 0 | 1 | 9 | 0 | 0 | 11 | 0 | |
| Increased eosinophils* | 0 | 8 | 3 | 0.005 | ||||||
| Increased lymphocytes* | 0 | 2 | 1 | NS | ||||||
CRSwNP: chronic rhinosinusitis with nasal polyps;
Eos: eosinophilic;
Non-Eos: non eosinophilic;
N: No;
Y: yes;
U: unknown
In peripheral blood
Biopsy specimens of nasal mucosa and nasal polyps (II degree according to the Lund Kennedy grading system) were collected before surgery. Samples were subjected to haematoxylin-eosin (HE) staining and immunohistochemistry to investigate HMGB1 protein expression and its correlation with eosinophils and IL-5, IL-8 and TNF-α cytokines. Part of fresh tissue was snap-frozen at -180° for immunohistochemical staining.
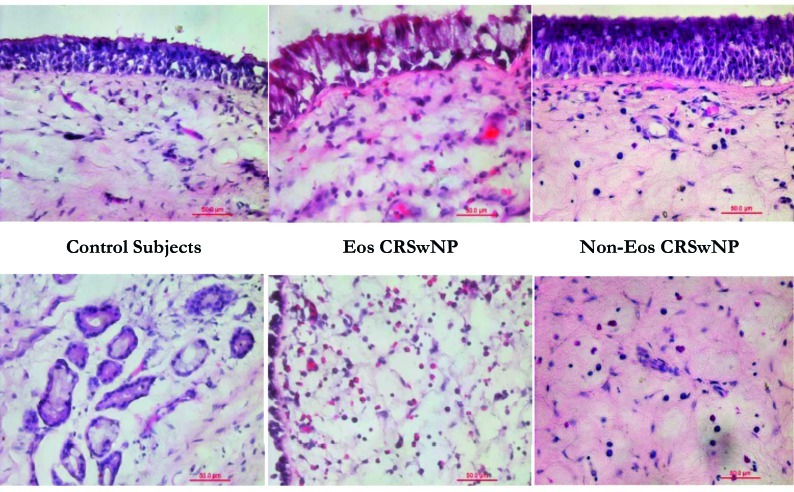
According to HE staining, subjects were divided into three groups: 8 control subjects, 10 eosinophilic CRSwNP patients and 11 non-eosinophilic CRSwNP patients. The degree of the infiltrating eosinophils was counted in 10 high power (HP) fields (x400 magnification). A patient was classified as Eos CRSwNP when the eosinophils in the specimen showed more than 10 eosinophils per HP field. Symptom scores, nasal endoscopic examination and CT results in eosinophil CRSwNP and non-eosinophil CRSwNP analysis are reported in Table II.
Table II.
CT scans, nasal endoscopic examination and symptoms score analysis in Eos CRSwNP and non-Eos CRSwNP (mean ± SD).
| Eos CRSwNP non- | non-Eos CRSwNP | p value | |
|---|---|---|---|
| MS | 2.40 ± 0.70 | 2.00 ± 0.78 | NS |
| AES | 3.20 ± 1.03 | 2.00 ± 1.61 | NS |
| PES | 2.60 ± 0.97 | 1.36 ± 1.29 | 0.023 |
| SS | 2.20 ± 1.03 | 0.91 ± 1.04 | 0.010 |
| FS | 2.40 ± 1.17 | 1.64 ± 1.43 | NS0 |
| OMCS | 3.60 ± 0.70 | 2.55 ± 1.64 | NS |
| ES | 5.80 ± 1.75 | 3.27 ± 2.76 | 0.023 |
| TS | 16.40 ± 4.17 | 10.45 ± 6.92 | 0.030 |
| Endoscopy | 3.40 ± 1.35 | 2.36 ± 1.43 | NS |
| Nasal congestion | 7.6 ± 1.58 | 6.64 ± 2.46 | NS |
| Rhinorrhoea | 5.30 ± 2.63 | 4.82 ± 3.13 | NS |
| Headache | 3.80 ± 2.70 | 1.27 ± 2.24 | 0.032 |
| Hyposmia | 7.00 ± 2.16 | 3.27 ± 3.58 | 0.010 |
| VAST | 23.70 ± 5.21 | 15.91 ± 7.57 | 0.014 |
MS: Maxillary sinus score; AES: Anterior ethmoid sinus score; PES: Posterior ethmoid sinus score; SS: Sphenoid sinus score; FS: Frontal sinus score; OMCS: Ostiomeatal complex score; ES: Total ethmoid sinus score; TS: Total CT score; VAST: Visual analogue scale total
Immunohistochemical staining
Biopsy samples were sectioned (7 μm) in a cryostat after fixation and dehydration. Sections were placed on chromegelatin-coated slides and air dried for 10 min permeabilized in PBS. Endogenous peroxidase activity was blocked with 3% H2O2 for 10 min at room temperature in the dark. The slides were rinsed three times in PBS for 2 min before blocking for 20 min with 10% normal horse serum in PBS. Slides were incubated with a HMG B1 rabbit polyclonal antibody (ab-18256, dilution 1:300), IL -5 mouse monoclonal antibody (ab25034, dilution 1:200), TNF-α mouse monoclonal antibody (ab1793, dilution 1:200) or IL -8 goat polyclonal antibody (ab10769, dilution 1:200) (Abcam, Cambridge, USA) at 4° overnight in a humidified chamber. Slides were then rinsed three times in PBS for 5 min. The secondary antibody was PV-9000 Kit or PV-9003. Finally, slides were developed with a diaminobenzidine kit (ZSJQ) for 10 min. Slides were counterstained with Mayer's haematoxylin and mounted using neutral resin. Negative control slides were probed with PBS and normal rabbit serum (normal mouse serum) under the same experimental conditions.
All statistical analyses were performed using SPSS13.0 software. Data are presented as the mean ± SEM unless otherwise noted. One-way repeated measures ANOV A with post hoc multiple comparisons using LDS method were used to compare means among groups. For nonparametric data, differences between two groups were analyzed by the Mann-Whitney U test. Correlations were assessed by using the Pearson correlation or Spearman rank correlation. Multivariate regression analysis was undertaken to evaluate the relationship between HMGB1 protein expression and eosinophils, IL-5, IL-8, TNF-α positive cells.
A p value less than 0.05 was considered statistically significant.
Results
In Figure 1, haematoxylin-eosin stained sections from control subjects, patients with eosinophil chronic rhinosinusitis with nasal polyps and patients with non-eosinophil chronic rhinosinusitis with nasal polyps are shown.
Fig. 1.
Haematoxilin-eosin stained sections (x40).
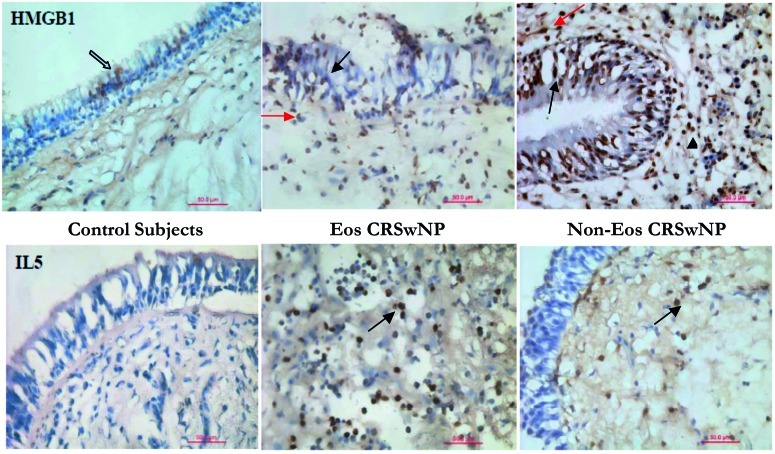
Figures 2 and 3 show immunohistochemically stained sections for HMGB1, Il-5, IL-8 and TNF-α in control subjects, patients with eosinophil chronic rhinosinusitis with nasal polyps and patients with non-eosinophil chronic rhinosinusitis with nasal polyps. Immunohistochemical staining revealed that HMGB1 protein was widely detectable in both controls and patients with CRSwNP. The degree of HMGB1 protein expression was evaluated by dividing the stained sections in 4 portions: 1) nucleus of epithelial cells, 2) cytoplasm of epithelial cells, 3) focal extracellular infiltration, 4) inflammatory cells.
Fig. 2.
Immunohistochemical staining for HMGB1 and IL-5 (x40).
Immunolocalization of HMGB1 in nasal polyposis. Immunohistochemical staining revealed that HMGB1 protein is widely detectable in both controls and patients with CRSwNP, and is expressed in the nucleus and cytoplasm.Il-5 is detectable in the subepithelial layer of patients with CRSwNP.Black arrows show HMGB1 expression in epithelial cells, while red arrows show focal infiltration in subepithelial layers.
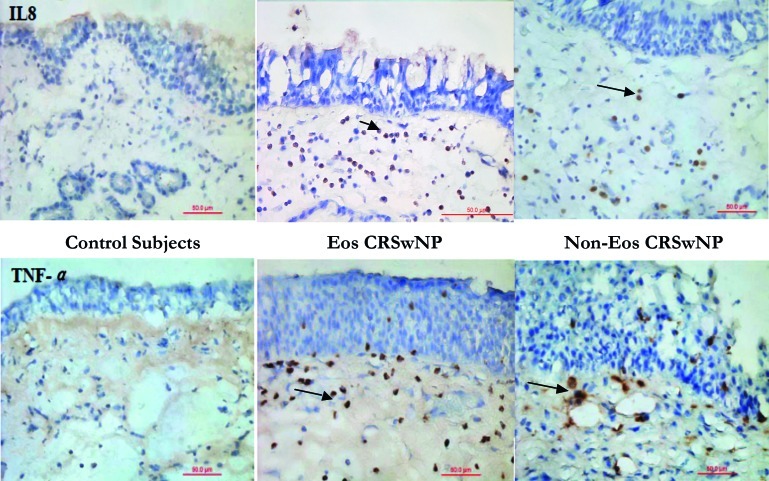
Fig. 3.
Immunohistochemicalstaining staining for IL-8 and TNF-α (x40).
Il-8 and TNF-α are detectable in the subepithelial layer of patients with CRSwNP, but not in controls.
HMGB1 was more expressed in the nucleus of epithelial cells of patients compared with controls, and the difference was statistically significant between controls and patients with non-eosinophil CRSwNP. In contrast, epithelial cytoplasm HMGB1 staining was significantly lower in patients with eosinophil CRSwNP and non-eosinophil CRSwNP.
Subepithelial focal infiltration of HMGB1 protein expression was lower in controls and in patients with eosinophil chronic rhinosinusitis with nasal polyps.
Expression of HMG B1 in inflammatory cells in patients with both eosinophil and non-eosinophil chronic rhinosinusitis with nasal polyps was significantly increased compared to the controls. Semi-quantitative analysis of this data is represented as box plots in Figures 4 and 5.
Fig. 4.
Semiquantitative analysis of cells staining positive for HMGB1 protein presented as box plots.
Data are presented as box plots, where the boxes represent the 25th to 75th percentiles, the line within the boxes represent the median and the lines outside the boxes represent the minimum and maximum values. P values are compared among groups.
* p < 0.05; ** p < 0.01; *** p < 0.001
Fig. 5.
Semiquantitative analysis of cells staining for eosinophils, IL-5, IL-8 and TNF-α.
Data are presented as box plots, where the boxes represent the 25th to 75th percentiles, the line within the boxes represent the median, and the lines outside the boxes represent the minimum and maximum values. P values are compared among groups.
* p < 0.05; ** p < 0.01; *** p < 0.001
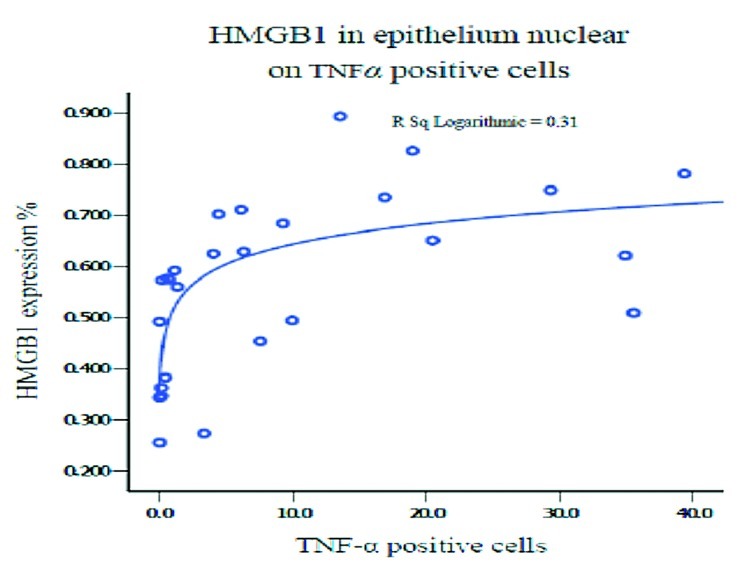
When exploring the correlation between HMGB1 protein expression in different portions and the number of eosinophil infiltrating cells, or IL-5, IL-8 and TNF-α positive cells in patients with CRSwNP and controls, a significant positive correlation was found between expression in the nucleus of epithelial cells and eosinophils, IL-5, IL-8 and TNF-α. A positive correlation was also found between HMGB1 expression in the cytoplasm of epithelial cells and eosinophils, and the expression in inflammatory cells and IL-8 positive cells (Figs. 6 and 7).
Fig. 6.
Multivariate regression analysis between HMGB1 protein expression and eosinophils, and IL-5 and IL-8 positive cells.
Fig. 7.
Multivariate regression analysis between HMGB1 protein expression and TNF-α positive cells.
Conclusions
Chronic rhinosinusitis with or without nasal polyposis is a clinical syndrome characterized by persistent inflammation of the mucosa of the nose involving secondly the sinus mucosa. Whereas there is no evidence for a causal correlation between nasal anatomic variations and the incidence of CRS, and as the inflammation is observed mainly at the interface with the external environment, the hypothesis that CRS results from a defective or excessive immune response to foreign agents with persistent inflammatory cells influx has been recently postulated 14. In the last decades, due to the difficulty in understanding the aetiologic factors and pathogenic mechanisms, CRSwNP was considered as a multifactorial disease. The real innovation of the "immune and mechanical barrier" hypothesis is that it focuses on host susceptibility as the main factor for CRS with/without nasal polyposis and does not on a single microbial or environmental factor.
The mechanical barrier of the sinus mucosa consists of ciliated cells, goblet cells and respiratory epithelial cells linked by tight junctions. Ciliated and goblet cells are entrusted with the mucociliary transport first line of defense against foreign materials and microbial agents. Breakdown of the mechanical components of the epithelial barrier plays an important role in permitting microbial colonization and foreign proteins to stimulate an immune response.
It has been recently demonstrated that in addition to the physical barrier, sinonasal epithelial cells play an active role in both the innate and acquired immune response 15. On airway epithelial cells 16-18, pattern recognition receptors (PRRs) that recognize pathogen associated molecular patterns (PAMPs) have been identified; recognition of PAMPs by host epithelial cells through PRRs results in the release of innate protective agents as well as chemokines and cytokines that attract innate cellular defenses. In addition to PAMPs, cells also sense cellular damage through damage-associated molecular patterns (DAMPs) 19.
Among DAMPs, HMGB1 was recognized three decades ago, and has been identified in patients affected by inflammatory processes of the lower airways 20.
Our study showed that the HMGB1 protein is increased in the nucleus of epithelial cells, or as focal subepithelial infiltration and in inflammatory cells in patients with CRSwNP; HMGB1 protein is decreased in the cytoplasm of epithelial cells. We did not find a significant difference in HMGB1 expression between Eos CRSwNP and non-Eos CRSwNP, suggesting that HMGB1 may play a crucial role in the pathogenesis of chronic rhinosinusitis with nasal polyps independently of the aetiologic stimuli. Eosinophils and IL-5, IL-8 and TNF-α positive cells may be involved in the regulation of HMGB1.
Obviously, these results warrant further studies to determine the mechanisms and the relative importance of the HMGB1 protein in the pathogenesis of CRSwNP.
References
- 1.Passali D, Berstein JM, Passali FM, et al. Treatment of recurrent chronic hyperplastic sinusitis with nasal polyposis. Arch Otolaryngol Head Neck Surg. 2003;129:656–659. doi: 10.1001/archotol.129.6.656. [DOI] [PubMed] [Google Scholar]
- 2.Passali D, Bellussi L. Revision of the European Position Paper on Rhinosinusitis and nasal Polyposis (EP3OS) with particular attention to acute and recurrent rhinosinusitis. Acta Otorhinolaryngol Ital. 2007;27:1–21. [PubMed] [Google Scholar]
- 3.Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyposis group, authors. European Position Paper on rhinosinusitis and nasal polyposis. Rhinology. 2012;(Suppl.23):1–299. [PubMed] [Google Scholar]
- 4.Scaffidi P, Misteli B, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 5.Gardella S, Andrei C, Ferrera D, et al. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesciclemediated secretory pathway. EMBOR ep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi ME, Manfredi AA. High-mobility group box 1 (HMGB1) protein at the crossroads between innate and adaptive immunity. Immunol Rev. 2007;220:35–46. doi: 10.1111/j.1600-065X.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 7.Yang D, Rosa G, Tewary P, et al. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009;30:531–537. doi: 10.1016/j.it.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 9.Valera FCP, Queirozw R, Scrideliw C, et al. Expression of transcription factors NF-kB and AP-1 in nasal polyposis. Clin Exp Allergy. 2008;38:579–585. doi: 10.1111/j.1365-2222.2007.02929.x. [DOI] [PubMed] [Google Scholar]
- 10.Ek M, Popovic K, Harris HE, et al. Increased extracellular levels of the novel proinflammatory cytokine High Mobility Group Box Chromosomal protein 1 in minor salivary glands of patients with Sjogren's syndrome. Arthr Rheum. 2006;54:2289–2294. doi: 10.1002/art.21969. [DOI] [PubMed] [Google Scholar]
- 11.Passali D, Kern E, Lei Chen R, et al. High mobility group box 1 (HMGB 1): a new protein in the pathogenesis of ENT inflammatory and infectious diseases. Acta Otorhinolaryngol Ital. 2012;32:46–47. [PMC free article] [PubMed] [Google Scholar]
- 12.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117:35–40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 13.Lund VJ, Mackay IS. Staging in rhinosinusitis. Rhinology. 1993;107:183–184. [PubMed] [Google Scholar]
- 14.Kern RC, Conley DB, Walsh W, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinology. 2008;22:549–559. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schleimer RP, Lane AP, Kim J. Innate and acquired immunity and epithelial cell function in chronic rhinosinusitis. Clin Allergy Immunol. 2007;20:51–78. [PubMed] [Google Scholar]
- 16.Sha Q, Truong-Tran AQ, Plitt JR, et al. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- 17.Lane AP, Truong-Tran QA, Myers A, et al. Serum amyloid A, properdin, complement 3, and toll-like receptors are expressed locally in human sinonasal tissue. Am J Rhinology. 2006;20:117–123. [PMC free article] [PubMed] [Google Scholar]
- 18.Lane AP, Truong-Tran QA, Schleimer RP. Altered expression of genes associated with innate immunity and inflammation in recalcitrant rhinosinusitis with polyps. Am J Rhinology. 2006;20:138–144. [PMC free article] [PubMed] [Google Scholar]
- 19.Bianchi ME, Manfredi AA. Immunology. Dangers in and out. Science (New York) 2009;323:1683–1684. doi: 10.1126/science.1172794. [DOI] [PubMed] [Google Scholar]
- 20.Hou C, Zhao H, Liu L, et al. High mobility group protein B1 (HMGB1) in asthma: comparison of patients with chronic obstructive pulmonary disease and healthy controls. Mol Med. 2011;17:807–815. doi: 10.2119/molmed.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]