Abstract
Thrombin stimulates proliferation, invasion and metastasis by cleaving PAR1 on a human prostate cancer cells. Current direct thrombin inhibitors pose risks for bleeding in the cancer patients. We have developed an oral reversible direct thrombin inhibitor called FM19. FM19 inhibits thrombin-induced calcium mobilization of PC3 cells with an IC50 of 15 μM with a 95% confidence interval of 7.3–31.6 μM. Thrombin stimulation increases PC3 cell invasion 3-fold from 27.1 ± 11.4 to 66 ± 11.6. FM19 or bivalirudin reduces cell invasion at ≥ 0.1 μM (p≤0.02). After inoculation with PC3 cells, nude mice were treated with oral FM19 at 3 mg/ml in the drinking water. The treated mice do not have long bleeding times and only a 1.4-fold increase in their thrombin clotting time. However, with treatment, the mice have a reduced rate of tumor growth 0.26 ± 0.17 fold change/day versus 0.55 ± 0.35 for untreated (p = 0.038), reduced fold change in tumor size 5.3 ± 0.47 to 8.9 ± 1.8 (untreated) (p=0.048), and reduced overall tumor weight 0.5 ± 0.31 g versus 0.82 ± 0.32 g (untreated) (p=0.04). On microscopic examination, FM19 treatment reduces the number of large vessels in the tumors from 4.6±2.1 per high-powered field in untreated samples to 1.4±1.4 in treated samples (p≤0.04). These studies show FM19 reduces prostate tumor growth in vivo at a concentration below that needed for anticoagulation. These data suggest novel opportunities for oral direct thrombin inhibitors in cancer therapy.
Keywords: prostate cancer, oral direct thrombin inhibitor, anticoagulation, cancer treatment
Introduction
The vascular system provides novel cancer targets. Inhibition of angiogenesis and cell-cell interactions mediated by integrins or cofactors or substrates of plasma coagulation enzymes are targets against cancers (1,2). Endogenous thrombin inhibition blocks tumor seeding and metastasis and spontaneous tumor growth in mouse models (3,4). Thrombin stimulates adhesion, proliferation or invasion in various tumor types by activating protease activated receptor 1 (PAR1). PAR1 expression on invasive prostate cancer cells is now recognized as an important target on prostate cancer cells (5–9). Tumor cell PAR1 expression correlates with invasion and metastasis (5,10–14). Lung metastasis of the human prostate cancer cell line M24met after tail vein injection is reduced when the cells were pretreated with a functional anti-PAR1 blocking antibody (15). Hu et al. demonstrated that TRAMP mice, a mouse model of human prostate cancer, have reduced tumor growth when treated with hirudin (3). hPar1 contains a functional androgen response element promoter that contributes to prostate tumor angiogenesis and progression (16). Modulation of hPar1 promoters through Egr-1 (early growth response -1 protein) contributes to tumor invasiveness (17).
The difficulty to date in considering thrombin inhibitors for cancer treatment is that all presently approved agents are parenteral and potent, putting the cancer patient at bleeding risk. We have developed an oral, reversible direct thrombin inhibitors based the angiotensin converting enzyme (ACE) breakdown product of bradykinin, BK1–5 or RPPGF (18–21). FM19 consists of D-isomers and unusual amino acid substitutions to increase stability and allow for oral activity (20). FM19 is a direct thrombin inhibitor with a Ki of 4.4±2.4 μM that inhibits the threshold of α-thrombin-induced human platelet aggregation at 8.4±4.7 μM (21). On crystallography, FM19 is a retrobinder in thrombin’s active site (21). When administered in drinking water at 5 mg/ml, but not at 3 mg/ml, it delays induced arterial thrombosis (21). We determined if oral FM19 at 3 mg/ml in the drinking water is sufficient to decrease prostate cancer growth in vivo.
Materials and Methods
Materials
FM19 [D-Arg-Oic-Pro, D-Ala-Phe(pMe), rOicPaF(p-Me)], where Oic is (2S,3aS, 7aS)-octahyroindol-2-carboxlic acid, was prepared as previously described (20). Human α-thrombin (3000–3250 U mg−1) was from Haematological Technologies (Essex Junction, VT). The human prostate cancer cell line PC-3 stably expressing luciferase (PC3-luc) were provided by Dr. Ken Pienta (University of Michigan) (22). PC3-luc cells were cultured in DMEM supplemented with 10% fetal bovine serum (Hyclone, Logan, UT).
Calcium mobilization
Calcium mobilization studies were carried out as previously described (20). Briefly, the cells were removed from plates with versene and labeled with Fura2-AM (Molecular Probes) in Hepes-Tyrodes buffer supplemented with magnesium and calcium. Cells (2×105) were placed in 96-well plates, stimulated with α-thrombin and read in a NOVOstar plate reader (BMG Labtech, Durham, NC). Initial changes in calcium flux were determined at early time points where the signal was linear.
Proliferation assay
103 PC3 cells were plated in 96 well plates and allowed to adhere overnight. After washing and replacement with serum free media, they were treated with 0.1 to 30 nM α-thrombin or 10% fetal bovine serum and incubated overnight. Proliferation was measured using CellTiter96 Aqueous One Solution Cell Proliferation Assay (Promega, Madison, WI) according to manufacturer’s instructions. Briefly, 20 μl of MTS solution was added to 100 μl of cells and the OD at 490 nm was read at 60, 120 and 180 min.
Matrigel invasion assay
PC3-luc cells (3.5×104) cell were plated in the top well of Matrigel coated filters (BD Biocoat, BD Biosciences) in serum free DMEM and stimulated with α-thrombin (1 nM) in the presence of increasing concentration of FM19 (0.01–100 μM) or bivalirudin (0.001–10 μM). The bottom well contained DMEM containing 10% fetal bovine serum. The cells were removed from the top chamber with a cotton swab and those cells that migrated to the bottom of the filter were stained with Diff-Quick. Data are expressed as the mean number of cells/field ± SD at 100x magnification for 3 independent experiments using each thrombin inhibitor. In each experiment, 10 fields were counted.
Mice
Six week old male nude mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in the pathogen free animal facility at Case Western Reserve University. All animals studies were performed according to institution approved guidelines and protocols. For in vivo imaging, animals were injected with 5×106 cells/site in each hind quarter. Five animals (10 tumors) were treated for 35 days with FM19 in the drinking water (3 mg/ml) containing 5% sucrose; 4 untreated mice (8 tumors) received 5% sucrose water alone. The water was changed twice a week. The tumor growth was monitored by non-invasive bioluminescence imaging using a Xenogen IVISs 200 bioluminescence scanner (Xenogen, Hopkinton, MA, USA). Mice were imaged on day 7, 14, 21 and 28. To quantitate images, the intensity was determined for a fixed region of interest (ROI). The rate of growth was determined by the change in bioluminescence intensity and each injection site was normalized for its signal on day 7. In other experiments, mice were treated 5 days with FM19 in the drinking water (3 mg/ml) containing 5% sucrose; untreated mice received 5% sucrose water alone. Mouse bleeding times, plasma collection, and thrombin clotting times were performed as previously reported (18,19).
Staining of tumors
Excised tumors were imbedded in OCT Tissue Tek (Sakura Finetek USA Inc., Torrance CA) and sectioned. Samples were rehydrated with PBS and blocked with PBS with 10% goat serum. Samples were then incubated with anti-PECAM (anti-CD31) (clone MEC13.3) (Santa Cruz Biotech, Santa Cruz CA) at 1:100 dilution (2 μg/ml) at 4°C overnight. Samples were washed in PBS 3 times and incubated with a second antibody conjugated to Alexa 594 (Molecular Probes, Carlsbad, CA) for 1 hour at room temperature. Samples were washed 3 times in PBS then mounted with Vectashield containing DAPI (Vector Laboratories Inc., Burlingame, CA). Slides were visualized and photographed using a Nikon TE2000S fluorescent microscope at 200x magnification. The total number of vessels and the number of large vessels were counted in each of five high-powered-field (hpf)/tumor for 4 different animals in both the untreated and treated groups. Data were expressed as the mean±SD of 4 different experiments.
Data analysis
The IC50 for FM19 inhibiting calcium mobilization was determined by fitting the data to a sigmoidal dose response curve with Prism (Graphpad, San Diego, CA) (23). The data from all experiments were analyzed simultaneously (global analysis) (23,24). In the other experiments, the data were analyzed by unpaired t test. Significance was defined as a p value < 0.05.
Results
PAR1 has been recognized to be over-expressed in prostate cancer, but not in benign tumors (5). The cell line PC3 stably expressing luciferase (PC3-luc) mobilized intracellular calcium when stimulated with 1 nM α-thrombin (Figure 1A). FM19 at > 1–100 μM inhibited α-thrombin-induced calcium mobilization (Figure 1B). The IC50 of FM19 inhibiting calcium flux was 15 μM with a 95% confidence interval of 7.3–31.6 μM.
Figure 1.
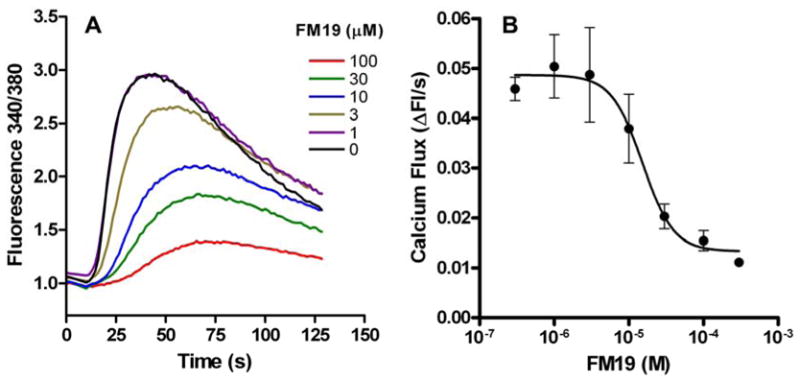
FM19 inhibits α-thrombin-induced intracellular calcium mobilization in PC3 prostate cancer cells. PC3-luc cells were loaded with fura2-AM and incubated with 0–100 μM FM19. After incubations, cells were treated with 1.0 nM α-thrombin. (A) Representative tracings for each concentration of FM19 are shown. (B) Initial rates of calcium mobilization were determined at early time points where the signal was linear. Data were fit to a sigmoid dose represent standard deviation of 3 independent experiments.
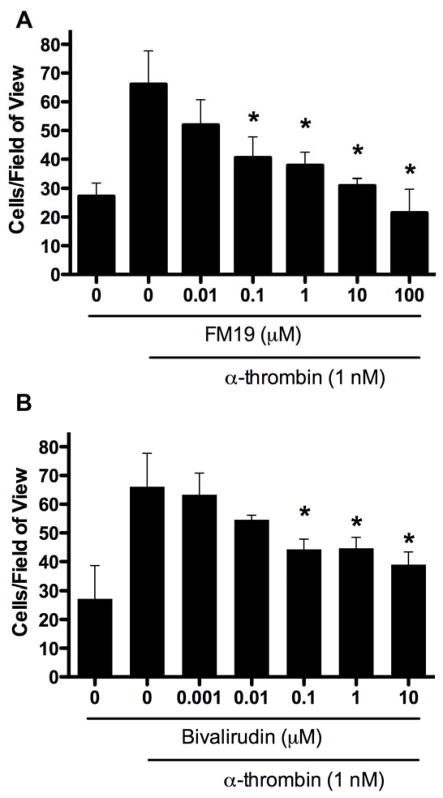
We determined if thrombin-stimulated PC3 cells invaded Matrigel. Stimulation of PC3 cells with α-thrombin increased PC3 cell invasion through Matrigel ~3-fold from 27.1 ± 11.4 to 66.1 ± 11.6 (Figure 2). The number of invading cells was increasingly blocked by 0.1 (p = 0.02) to 100 μM (p = 0.002) FM19 (Figure 2A). In independent experiments, bivalirudin also blocked the number of invading cells in a concentration dependent manner (Figure 2B). The lowest concentration that significantly decreased the number of invading cells also was 0.1 μM (p = 0.02). In contrast, α-thrombin did not stimulate proliferation of PC3 cells.
Figure 2.
FM19 inhibits α-thrombin-induced invasion through Matrigel in PC3 prostate cancer cells. PC3-luc cells were plated into the top well of an invasion chamber coated with Matrigel in the presence of 0–100 μM FM19 (A) or 0–10 μM bivalirudin (B). Cells were stimulated with 1 nM α-thrombin overnight. The data are represented as the number of cells per 100x field of view. In each experiment 10 fields of view were counted. Error bars represent the standard deviation from 3 independent experiments performed in the absence or presence of each concentration of each thrombin inhibitor.
Since 3 mg/ml oral FM19 in drinking water does not alter the time to arterial thrombosis in mice, we examined if that concentration lengthened tail bleeding and thrombin clotting times (21). After FM19 treatment, the mean tail bleeding time was 92±8 sec (n = 6) in the treated mice versus 79±11 sec (n = 5) in the untreated animals (p = 0.38). Although FM19 at 3 mg/ml in drinking water did not influence the bleeding time or time to carotid artery thrombosis, the treated mice had a 1.4-fold increase in the thrombin clotting time from 16±1 sec (n = 6) for untreated mice to 22±1 sec (n = 6) for treated mice (p < 0.003) (21).
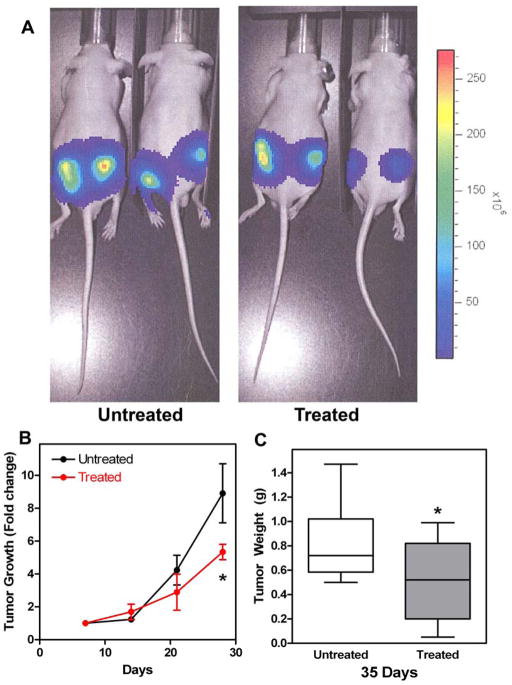
FM19’s influence on PC3 tumor cell-inoculated mice next was determined (21). Tumor growth was followed for 4 weeks by bioluminescence imaging weekly for 4 weeks (Figure 3A). The mean tumor growth rate was significantly reduced in the treated animals from day 14 to day 28, 0.26 ± 0.17 fold change/day versus 0.55 ± 0.35 for untreated (p = 0.038). The overall fold change in tumor size was reduced from 8.9 ± 1.8 to 5.3 ± 0.47 in treated compared to untreated animals (p = 0.048) (Figure 3B). At the end of 5 weeks, the animals were sacrificed and the tumors were excised and weighed. The mean tumor weight in the treated mice was 0.5 ± 0.31 g versus 0.82 ± 0.32 g for the untreated mice, p = 0.04 (Figure 3C). At excision, the PC3 tumors from the FM19-treated animals were more circumscribed and less exophytic than those in the untreated animals. The number of PECAM positive cells in the tumors from the untreated and treated animals was characterized to determine if that could account for tumor size differences (Figure 4A and B). The mean total PECAM positive vessels in the untreated tumors was 10.6 ± 4.5/hpf versus 4.9 ± 2.4/hpf for treated tumors (p = 0.06) (Figure 4C). However, when the counting was restricted to readily visualized large vessels, i.e. vessels > 10 microns, more large vessels were present in the untreated samples (4.6 ± 2.1/hpf) when compared to the number of large vessels seen (1.4 ± 1.4/hpf) in the treated samples (p = 0.04).
Figure 3.
Panel A. Representative bioluminescence imaging of tumors in untreated and treated nude mice. Animals were imaged on days 7, 14, 21 and 28. The figure is from day 28. Panel B. FM19 delayed tumor growth in treated nude mice. Mice were prepared for non-invasive bioluminescence imaging during treatment with FM19 in the drinking water (3 mg/ml). The data were normalized to day 7 for each injection site and the fold change for each subsequent scan is plotted. Error bars indicate standard deviation from 4 (untreated) or 5 (treated) animals each with 2 injections. Panel C. FM19 reduced the overall tumor weight in nude mice. Mice were treated for with FM19 in the drinking water (3 mg/ml) for 5 weeks, tumors were excised and weighed. Error bars represent the standard deviation of 8 (untreated) or 10 (treated) tumors.
Figure 4.
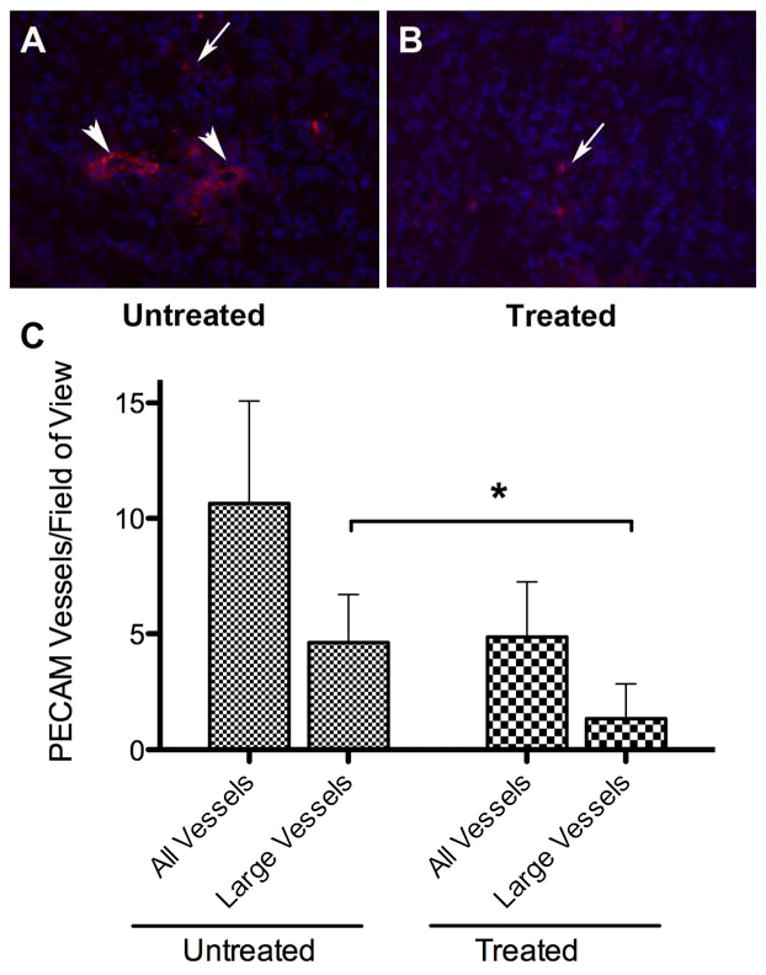
FM19 treated tumors have less PECAM positive large vessels. Frozen tumor sections from untreated (Panel A) or FM19 treated (Panel B) were stained for PECAM and counterstained with DAPI to determine cell nuclei. Representative sections are shown in A and B. Large vessels are indicated with a large arrowhead, other PECAM positive cells are indicated with a small arrow. Panel C: Quantification of all PECAM positive vessels. Five fields of view were counted for each of 4 tumors analyzed for the total number of vessels and the number of large vessels (≥ 10 μm) from both untreated and treated samples. Error bars indicate the standard deviation of the number of vessels in each group. The * indicates that the p value was <0.05.
Discussion
These data demonstrate that FM19, a direct thrombin inhibitor, reduces tumor growth in vivo at concentrations below that which influence bleeding time or thrombosis prevention. The ability of FM19 to be adsorbed from the GI tract must rely on two features. 1) FM19 does not have peptides bonds between two natural amino acids, reducing its rate of degradation by physiologic enzymes. 2) Since FM19 is an analogue of bradykinin and its breakdown product RPPGF, it must be binding to the di- and tri-peptide transporter in the GI tract (25). The oral pharmacokinetics of FM19 are not known since it has not been formulated for oral adsorption. When administered intravenously, it has a t1/2αof 32.5 ± 2.9 min with an AUC of 803 ± 542 mg/1 x min and a clearance of 8.2 ± 5.1 ml/min/kg (21). When administered orally at 3 mg/ml in the drinking water, it has a steady-state plasma concentration after 5 days of 80 ng/ml with a 1.4 times prolongation of the plasma thrombin clotting time (21). At this concentration, it does not prolong the mouse bleeding time or delay time to carotid artery occlusion on the rose Bengal assay (21). Alternatively, 5 mg/ml steady-state oral FM19 has a plasma concentration of 173 ± 16 ng/ml with 2.7-fold prolongation of the arterial thrombosis time and a 7.3-fold prolongation of the thrombin clotting time (21). FM19 influences tumor cell growth at 0.112 μM plasma concentration, a value half of that needed to prevent thrombosis.
There is potential interest of using a targeted thrombin inhibitor below its anticoagulation dose to reduce cancer cell growth in vivo. In the present studies, bivalirudin, a 55-fold more potent direct thrombin inhibitor was not a better inhibitor of the Matrigel invasion assay by PC3 cells than FM19. Previous unpublished studies with a predecessor of FM19, rOicPGF, show that it is equipotent to hirudin in limiting murine 4T1 tumor growth (unpublished). These studies indicate that thrombin inhibition potency alone does not confer improved cancer growth limiting features. How a direct thrombin inhibitor may be working to limit tumor growth is not completely clear. It is not known what level of thrombin is being generated at the site of tumor implantation. Further, it is currently not known the mechanism(s) by which this thrombin may be acting. Is it a requirement that the tumor cells have a thrombin receptor (e.g. PAR1) or does thrombin alone act on host factors independent of its PAR substrate? Previous studies by Hu et al. suggest that host thrombin formation itself is important for tumor implantation, seeding, and metastasis (4).
In conclusion, these investigations show that an oral thrombin inhibitor is able to limit growth of a tumor in vivo at concentrations below that needed for anticoagulation. This observation suggests new opportunities for oral direct thrombin inhibitors not previously appreciated. Additional investigations are needed to better understand how thrombin contributes to cancer growth and metastasis.
Supplementary Material
Table 1.
|
References
- 1.Sanders RJ, Mainiero F, Giancotti FG. The role of integrins in tumorigenesis and metastasis. Cancer Invest. 1998;16:329–344. doi: 10.3109/07357909809084653. [DOI] [PubMed] [Google Scholar]
- 2.Schnitzer JE. Vascular targeting as a strategy for cancer therapy. N Engl J Med. 1998;339:472–474. doi: 10.1056/NEJM199808133390711. [DOI] [PubMed] [Google Scholar]
- 3.Hu L, Ibrahim S, Liu C, Skaar J, Pagano M, Karpatkin S. Thrombin induces tumor cell cycle activation and spontaneous growth by down-regulation of p27Kip1, in association with the up-regulation of Skp2 and MiR-222. Cancer Res. 2009;69:3374–3381. doi: 10.1158/0008-5472.CAN-08-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu L, Lee M, Campbell W, Perez-Soler R, Karpatkin S. Role of endogenous thrombin in tumor implantation, seeding, and spontaneous metastasis. Blood. 2004;104:2746–2751. doi: 10.1182/blood-2004-03-1047. [DOI] [PubMed] [Google Scholar]
- 5.Chay CH, Cooper CR, Gendernalik JD, Dhanasekaran SM, Chinnaiyan AM, Rubin MA, et al. A functional thrombin receptor (PAR1) is expressed on bone-derived prostate cancer cell lines. Urology. 2002;60:760–765. doi: 10.1016/s0090-4295(02)01969-6. [DOI] [PubMed] [Google Scholar]
- 6.Tantivejkul K, Loberg RD, Mawocha SC, Day LL, John LS, Pienta BA, et al. PAR1-mediated NFkappaB activation promotes survival of prostate cancer cells through a Bcl-xL-dependent mechanism. J Cell Biochem. 2005;96:641–652. doi: 10.1002/jcb.20533. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Bastian M, Kohlschein P, Schuff-Werner P, Steiner M. Expression of functional protease-activated receptor 1 in human prostate cancer cell lines. Urol Res. 2003;31:163–168. doi: 10.1007/s00240-003-0309-2. [DOI] [PubMed] [Google Scholar]
- 8.Yuan TC, Lin MF. Protease-activated receptor 1: a role in prostate cancer metastasis. Clin Prostate Cancer. 2004;3:189–191. doi: 10.3816/cgc.2004.n.030. [DOI] [PubMed] [Google Scholar]
- 9.Kaushal V, Kohli M, Dennis RA, Siegel ER, Chiles WW, Mukunyadzi P. Thrombin receptor expression is upregulated in prostate cancer. Prostate. 2006;66:273–282. doi: 10.1002/pros.20326. [DOI] [PubMed] [Google Scholar]
- 10.Yin YJ, Salah Z, Maoz M, Ram SC, Ochayon S, Neufeld G, et al. Oncogenic transformation induces tumor angiogenesis: a role for PAR1 activation. FASEB J. 2003;17:163–174. doi: 10.1096/fj.02-0316com. [DOI] [PubMed] [Google Scholar]
- 11.Yin YJ, Salah Z, Grisaru-Granovsky S, Cohen I, Even-Ram SC, Maoz M, et al. Human protease-activated receptor 1 expression in malignant epithelia: a role in invasiveness. Arterioscler Thromb Vasc Biol. 2003;23:940–944. doi: 10.1161/01.ATV.0000066878.27340.22. [DOI] [PubMed] [Google Scholar]
- 12.Wojtukiewicz MZ, Tang DG, Ciarelli JJ, Nelson KK, Walz DA, Diglio CA, et al. Thrombin increases the metastatic potential of tumor cells. Int J Cancer. 1993;54:793–806. doi: 10.1002/ijc.2910540514. [DOI] [PubMed] [Google Scholar]
- 13.Nierodzik ML, Chen K, Takeshita K, Li JJ, Huang YQ, Feng XS, et al. Protease-activated receptor 1 (PAR-1) is required and rate-limiting for thrombin-enhanced experimental pulmonary metastasis. Blood. 1998;92:3694–3700. [PubMed] [Google Scholar]
- 14.Kamath L, Meydani A, Foss F, Kuliopulos A. Signaling from protease-activated receptor-1 inhibits migration and invasion of breast cancer cells. Cancer Res. 2001;61:5933–5940. [PubMed] [Google Scholar]
- 15.Shi X, Gangadharan B, Brass LF, Ruf W, Mueller BM. Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol Cancer Res. 2004;2:395–402. [PubMed] [Google Scholar]
- 16.Salah Z, Maoz M, Cohen I, Pizov G, Pode D, Runge MS, et al. Identification of a novel functional androgen response element within hPar1 promoter: implications to prostate cancer progression. FASEB J. 2005;19:62–72. doi: 10.1096/fj.04-2386com. [DOI] [PubMed] [Google Scholar]
- 17.Salah Z, Maoz M, Pizov G, Bar-Shavit R. Transcriptional regulation of human protease-activated receptor 1: a role for the early growth response-1 protein in prostate cancer. Cancer Res. 2007;67:9835–9843. doi: 10.1158/0008-5472.CAN-07-1886. [DOI] [PubMed] [Google Scholar]
- 18.Hasan AA, Warnock M, Nieman M, Srikanth S, Mahdi F, Krishnan R, et al. Mechanisms of Arg-Pro-Pro-Gly-Phe inhibition of thrombin. Am J Physiol Heart Circ Physiol. 2003;285:H183–H193. doi: 10.1152/ajpheart.00490.2002. [DOI] [PubMed] [Google Scholar]
- 19.Nieman MT, Warnock M, Hasan AA, Mahdi F, Lucchesi BR, Brown NJ, et al. The preparation and characterization of novel peptide antagonists to thrombin and factor VIIa and activation of protease-activated receptor 1. J Pharmacol Exp Ther. 2004;311:492–501. doi: 10.1124/jpet.104.069229. [DOI] [PubMed] [Google Scholar]
- 20.Burke FM, Warnock M, Schmaier AH, Mosberg HI. Synthesis of novel peptide inhibitors of thrombin-induced platelet activation. Chem Biol Drug Des. 2006;68:235–238. doi: 10.1111/j.1747-0285.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 21.Nieman MT, Burke F, Warnock M, Zhou Y, Sweigart J, Chen A, et al. Thrombostatin FM compounds: direct thrombin inhibitors - mechanism of action in vitro and in vivo. J Thromb Haemost. 2008;6:837–845. doi: 10.1111/j.1538-7836.2008.02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, et al. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology. 2005;146:1727–1736. doi: 10.1210/en.2004-1211. [DOI] [PubMed] [Google Scholar]
- 23.Johnson ML, Faunt LM. Parameter estimation by least-squares methods. Methods Enzymol. 1992;210:1–37. doi: 10.1016/0076-6879(92)10003-v. [DOI] [PubMed] [Google Scholar]
- 24.Beechem JM. Global analysis of biochemical and biophysical data. Methods Enzymol. 1992;210:37–54. doi: 10.1016/0076-6879(92)10004-w. [DOI] [PubMed] [Google Scholar]
- 25.Yuasa H, Amidon GL, Fleisher D. peptide carrier-mediate transport in interstinal brush border membrane vesicles of rats and rabbits: cephradine uptake and inhibition. Pharm Res. 1993;10:400–404. doi: 10.1023/a:1018940306394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.