Abstract
Rationale
Netrin-4 regulates vascular development. Identity of netrin-4 endothelial receptor and its subsequent cell functions is controversial. We previously demonstrated that the inhibition of netrin-1 canonical receptors, Unc5B and neogenin, expressed by lymphatic endothelial cells, do not suppress netrin-4-induced cell signaling and functions. Netrin family members were shown to signal through a range of receptors, including integrins (such as α3β1, α6β1 and α6β4) in non-endothelial cells.
Objective
We tested whether integrins are netrin-4 receptors in the endothelium.
Methods and Results
α6β1 integrin is expressed by endothelial cells, and binds Netrin-4 in a dose dependent manner. Inhibition of α6 or β1 integrin subunits suppresses Netrin-4-induced endothelial cell migration, adhesion and focal adhesion contact. Netrin-4-stimulated phosphorylation of Src Kinase Family, effectors of endothelial cell migration, is also abolished by α6 or β1 inhibition. Finally, Netrin-4 and α6β1 integrin expression colocalize in mouse embryonic, intestine and tumor vasculature.
Conclusions
α6β1 integrin is a netrin-4 receptor in lymphatic endothelium and consequently represents a potential target to inhibit netrin-4-induced metastatic dissemination.
Keywords: Netrin, Integrin, endothelium
Introduction
Netrins are laminin-like secreted proteins, initially identified as axonal guidance molecules, which have been since shown to play roles in angiogenesis, lymphangiogenesis and tumor metastasis. Controversy exists regarding the identity of endothelial receptors mediating these effects in the vasculature. We and others have reported that the inhibition of netrin-1 canonical receptors, DCC, neogenin, and the Unc5s 1 did not abrogate netrin signaling 2-4 and additional findings have reinforced the idea that a one-to-one relationship between ligand and receptor is too simplistic: the angiogenic activity of the receptor Unc5B, for example, has been shown to be modulated by a non-netrin ligand, the extracellular domain of the Robo4 receptor 5; in non-vascular settings, non-canonical receptors such as A2b, DSCAM and integrins have been reported to modulate netrin activity in vivo and in vitro 4, 6-9.
We have previously shown that Netrin 4 stimulates both lymphangiogenesis and tumor metastasis in vivo 2. Because integrins have also been implicated in these same processes 10-13, we asked if integrins regulate netrin-4 functions in the endothelium. We found that netrin-4 binds endothelial α6β1 integrin and that netrin-4-induced lymphatic endothelial cell (EC) migration, adhesion, focal adhesion contact and phosphorylation of Src Family kinase (a key element of both integrin signaling and cell migration), are suppressed by inhibition of α6 or β1. Finally, we show that netrin-4 and α6β1 integrin gene expression co-localize in vivo.
Materials and Methods
Refer to Online Data Supplement at http://circres.ahajournals.org.
Cell Culture
Human EC are from Lonza.
Human integrin siRNAs were from Qiagen.
Integrin function-blocking antibodies were selected according to Yebra et al4 and were from Millipore. Netrin-4; VEGF-C and α6; αvβ5; β1 recombinant proteins were from R&D Systems and Abnova respectively.
In vitro migration assays were performed as previously described2.
Immunoblot and Immunostaining
Experiments were performed using phospho-specific tyrosine 416, total Src Family kinase (#2101 and 2110 respectively, Cell Signaling Technology) or activated β1 integrin (clone 9EG7, BD Pharmingen), anti LYVE1 (Abcam, ab14917), anti α6 integrin subunit (Millipore, GoH3), anti netrin-4 (R&D System, AF1132).
Statistical analysis
Data reported as mean ± SEM. Statistical analyses were performed using Statview (SAS) and standard Student two-tailed t tests. P values of <0.05 (*,#) were defined as statistically significant.
Results and Discussion
Netrin-4 activates endothelial α6β1 integrin
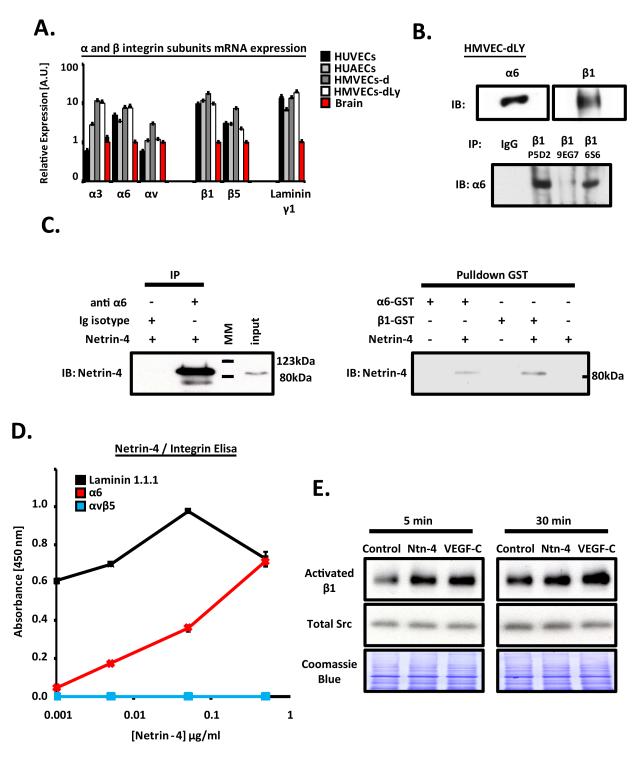
The laminin receptors, α3β1; α6β1 and α6β4 integrins, have been shown to induce netrin-1 and netrin-4 stimulated neuronal and epithelial cell migration and signaling 4, 8, 9. qRT-PCR, direct western-blotting, β1 immunoprecipitation or flow cytometry confirmed that these same integrins are expressed in ECs (Fig.1A-B and Online I). As shown in figure 1C, netrin-4 binding to integrin is detected only when the α6 or β1 subunit is specifically precipitated. Netrin-4 interacts in a dose dependent manner with the α6 or β1 (data not shown) subunits and with laminin-111 (as previously described) 14, but not with αvβ5 (Fig.1D), using two distinct binding sites (Online Fig.II). Moreover, netrin-4 enhanced, remarkably, α6 integrin/laminin-1 interaction (Online Fig.II), confirming both a cooperative, rather than competitive, interaction with different partners1 and the functional relevance of the heterotrimer netrin-4/α6β1/laminin-1 as described previously9. Finally, a robust activation of endothelial β1 integrin by netrin-4 or VEGF-C, detected specifically by the 9EG7 antibody, (Fig.1E) demonstrates the functional relevance of its interaction.
Figure 1. Netrin-4 binds to endothelial α6β1 integrin.
(A) Expression of different integrin subunits as well as laminin γ1 was determined in Human umbilical vein (HUVECs), umbilical artery (HUAECs), microvascular dermal blood (HMVEC-ds) and microvascular dermal lymphatic (HMVEC-dLys) endothelial cells by quantitative RT-PCR normalized to brain expression. (B, upper panel) Expression of α6 and β1 integrin subunits in HMVEC-dLys was confirmed by western blotting (WB). (B, lower panel). Determination of the α6β1 integrin heterodimer expression in HMVEC-dLys by sequential immunoprecipitation (IP) and western blotting (IB) with antibodies anti β1 (P5D2, 9EG7, 6S6) and α6 integrin subunits, respectively. (C, left panel) Immunoprecipitation with anti α6 integrin subunit antibody or its isotype control, followed by western blotting for netrin-4. Recombinant netrin-4 was used as a positive control (input). (MM molecular marker). (C, right panel) Pulldown between α6 or β1 -GST recombinant fusion proteins in the absence or presence of netrin-4, followed by netrin-4 western blotting. (D) Binding of netrin-4 (0.001 to 0.5 μg/ml) to microplate-coated proteins (10 μg/ml each); laminin-111 (black), α6 (red) or αvβ5 (blue) integrin was determined by ELISA. Three separate experiments were done in duplicate. (E) Activation of β1 integrin subunit was determined by seeding HMVEC-dLys on control (buffer only), netrin-4 or VEGF-C (1 μg/ml each) –coated wells for 5 or 30 minutes followed by western blotting using the anti β1 integrin antibody (9EG7) which recognizes specifically its active conformation. Total src and coomassie blue staining of the membranes served as loading controls.
Netrin-4-induced in vitro effects are α6β1 integrin dependent
We next investigated whether netrin-4-stimulated in vitro EC functions depend on α6β1 integrin. Netrin-4 induces migration, adhesion and focal adhesion, in the presence of immunoglobulin isotype (IgG); while function-blocking antibodies against α6 or β1, but not αvβ5, significantly abrogate this induction (Fig.2A, Online IV-VI). VEGF-C-stimulated lymphatic EC functions are not affected by α6 function-blocking antibodies (Online Fig.III-VI). Efficient suppression by siRNA of either α6 or β1 subunit similarly inhibits netrin-4-promoted migration (Fig.2B).
Figure 2. Netrin-4-induced HMVEC-dLy migration and Src Family Kinase phosphorylation depends on α6β1 integrin.
(A) Chemotactic effects of netrin-4 (0.5 μg/ml) on HMVEC-dLys in Boyden chamber assays in the absence (-) or presence of an isotype control (IgG), α6 (left panel, 10 to 40 μg/ml) or β1 (right panel, 20 μg/ml) function-blocking antibodies. (B) HMVEC-dLys were transfected with anti α6 (left panel) or anti β1 integrin subunits (middle panel) siRNA. Silencing score was quantified by qRT-PCR and compared to control (Ctrl) siRNA transfected condition. Control or integrin subunits-transfected HMVEC-dLys underwent in vitro migration (right panel) stimulated by netrin-4 (0.5 μg/ml). (C, upper panels) α6 or β1 siRNA expression inhibits netrin-4 but not VEGF-C (1 and 5μg/ml, respectively, for 15 minutes) –induced SFK phosphorylation (Y416) as observed in control or α3 siRNA-transfected HMVEC-dLys; Inhibition of netrin-4 but not VEGF-C (1 and 5 μg/ml, for 15 minutes)-induced SFK phosphorylation (Y416) is observed using α6 or β1, but not isotype control (IgG) or α3 function blocking antibodies (40 μg/ml) (lower panels). Experiments were at least performed in triplicate and change in phosphorylation (Y416) over total SFK determined using Image J and graphed as fold change over respective controls (right panels). (*; no growth factor control condition, #; growth factor and antibody condition. P<0.05).
As previously reported 2, netrin-4, as well as VEGF-C, induces in a dose and time dependent manner in vitro, Src Family kinase (SFK) phosphorylation (Online Fig.VII), an essential mediator of cell migration and one of the most proximal activated integrin effectors. Inactivation of α6 or β1 subunits, but not α3, by siRNA or function-blocking antibodies, suppress netrin-4, but not VEGF-C, -stimulated SFK phosphorylation (Fig.2C), demonstrating that netrin-4-induced migration and intracellular signaling is α6β1 dependent. The integrin dependency of other the downstream effectors, ERK1/2, PI-3K, Akt, FAK, Rac1 and RhoA activated by netrin-4 2, 9 remains to be fully evaluated in the context of VEGFs / α9β1 signaling 15, 16.
Netrin-4 and α6β1 integrin expression colocalize in the vasculature
To determine in vivo expression, we probed mouse E14.5dpc (days post coitum) embryo, adult intestine and human breast MCF7 tumor with antibodies against netrin-4, α6β1 integrin and the lymphatic marker, LYVE-1. Netrin-4 and α6β1 co-staining was detected in ECs in all these different tissues (Online Fig.VIII A-I and J-O). Specific integrins regulate angiogenesis, tumor growth and dissemination. Though nothing is known regarding α6β1 integrin role in lymphangiogenesis, promising results are expected as blockade of α4β1 inhibits tumor lymphangiogenesis and metastasis 12, while α9β1 knockout mice develop lethal lymphatic defects in correlation with VEGFs-associated processes 17.
Despite the failure of us or others to report any suppression of netrin-induced effects by inhibition of netrin-1 endothelial canonical receptors, Unc5s, DCC and neogenin, and that non canonical netrin receptors are employed in non vascular systems 2-4, 7-9, 14, there has been a singular focus on canonical netrin receptors in the vascular system. Our report is the first to show binding, functional and expression data indicating that a non canonical netrin receptor, α6β1 integrin, plays a role in netrin-4 function in the endothelium. This work, implicating new non canonical receptors for netrin signaling in the endothelium and a recent report demonstrating new endothelial ligands for canonical netrin receptors 5 indicates that a simple view of netrins only interacting with canonical receptors and vice versa in the vascular system must be reevaluated. Moreover, this data suggests a complexity in netrin signaling that may partially explain the lack of concordance of phenotypes in mice lacking canonical netrins or netrin receptors, and encourages investigations of other non-canonical classes of netrin receptors in the endothelium.
Supplementary Material
Novelty and Significance.
What is known
Netrins are laminin-like secreted proteins. They regulate angiogenesis, lymphangiogenesis, and tumor metastasis.
The identity of the endothelial receptors mediating the vascular effects of netrins remains unclear.
Non-canonical receptors, such as integrin α6β1 have been shown to modulate netrin functions in non-vascular context.
What new information does this article contribute
The α6β1, a laminin-1 receptor, expressed by human lymphatic endothelial cells, binds netrin-4 on a site different from its laminin-1 interaction domain.
This interaction regulates netrin-4-induced cell adhesion, migration and focal adhesion contact, as α6 orβ1 function-blocking antibodies or siRNA abrogate netrin-4-stimulated effects.
Both α6 and netrin-4 expression co-localize in vivo.
We previously showed that netrin-4 stimulates endothelial cell function, independently of netrin-1 canonical receptors Unc5B and neogenin, expressed on the endothelium. Although netrins interact and signal with non-canonical receptors such as integrins in non-vascular environment, and mice lacking netrins ligands or canonical receptors display different phenotypes, no alternative receptors have been identified in the endothelium. We found that α6β1, previously shown to mediate the effects of netrin-4 in neuronal cells, is expressed in ECs. It is activated by binding to netrin-4 leading to the stimulation of paxillin and Src Moreover, netrin-4-induced cell adhesion, migration and focal adhesion contact formation is inhibited by α6 orβ1 function blocking antibodies or siRNA. These results demonstrate for the first time not only that netrins can signal, in the endothelium, using non-canonical receptors such as integrins, but also that netrin / receptor interaction is far more complex than expected. Because both netrin-4 and α6β1 induce angiogenesis, lymphangiogenesis and metabolism , they represent a potential targets for preventing tumor dissemination.
Acknowledgments
We thank Drs. Murtaugh and Wang for help with microscopy and Elisa respectively.
Sources of Funding
Support came from the Fondation pour la Recherche Médicale (FLL), the American Heart Association (FLL and D.Y.L.), the Huntsman Cancer Institute and Foundation (A.L.W.), the US Department of Defense Breast Cancer Research Program (A.L.W. and D.Y.L.), the US National Institutes of Health, the H.A. and Edna Benning Foundation, the JDRF (D.Y.L.).
Non-standard Abbreviations and Acronyms
- EC
Endothelial cell
- DCC
Deleted in colorectal cancer
- HUVEC
Human umbilical vein endothelial cell
- HMVEC-d
Human dermal blood microvascular endothelial cell
- HMVEC-dLy
Human dermal lymphatic microvascular endothelial cell
- HUAEC
Human umbilical artery endothelial cell
- SFK
Src Family Kinase
- VEGF-C
Vascular endothelial growth factor-C
- ERK
Extracellular signal-regulated kinase
- PI-3K
Phosphatidylinositol 3-kinase
- FAK
Focal adhesion kinase
- dpc
Days post-coitum
- CB
Coomassie Blue
Footnotes
Authorship Contribution: F.L.L. and A.L.W. performed experiments, F.L.L; K.R.T. and D.Y.L. designed the research, analyzed results and wrote the paper.
Disclosures
The authors are or were previously employed by the University of Utah, which has filed intellectual property surrounding the therapeutic uses of vascular guidance cues and with the intent to license this body of intellectual property for commercialization.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nikolopoulos SN, Giancotti FG. Netrin-integrin signaling in epithelial morphogenesis, axon guidance and vascular patterning. Cell Cycle. 2005;4:e131–135. [PubMed] [Google Scholar]
- 2.Larrieu-Lahargue F, Welm AL, Thomas KR, Li DY. Netrin-4 induces lymphangiogenesis in vivo. Blood. 2010;115:5418–5426. doi: 10.1182/blood-2009-11-252338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nacht M, St Martin TB, Byrne A, Klinger KW, Teicher BA, Madden SL, Jiang Y. Netrin-4 regulates angiogenic responses and tumor cell growth. Exp Cell Res. 2009;315:784–794. doi: 10.1016/j.yexcr.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Yebra M, Montgomery AM, Diaferia GR, Kaido T, Silletti S, Perez B, Just ML, Hildbrand S, Hurford R, Florkiewicz E, Tessier-Lavigne M, Cirulli V. Recognition of the neural chemoattractant netrin-1 by integrins alpha6beta4 and alpha3beta1 regulates epithelial cell adhesion and migration. Dev Cell. 2003;5:695–707. doi: 10.1016/s1534-5807(03)00330-7. [DOI] [PubMed] [Google Scholar]
- 5.Koch AW, Mathivet T, Larrivee B, Tong RK, Kowalski J, Pibouin-Fragner L, Bouvree K, Stawicki S, Nicholes K, Rathore N, Scales SJ, Luis E, del Toro R, Freitas C, Breant C, Michaud A, Corvol P, Thomas JL, Wu Y, Peale F, Watts RJ, Tessier-Lavigne M, Bagri A, Eichmann A. Robo4 maintains vessel integrity and inhibits angiogenesis by interacting with unc5b. Dev Cell. 2011;20:33–46. doi: 10.1016/j.devcel.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Corset V, Nguyen-Ba-Charvet KT, Forcet C, Moyse E, Chedotal A, Mehlen P. Netrin-1-mediated axon outgrowth and camp production requires interaction with adenosine a2b receptor. Nature. 2000;407:747–750. doi: 10.1038/35037600. [DOI] [PubMed] [Google Scholar]
- 7.Ly A, Nikolaev A, Suresh G, Zheng Y, Tessier-Lavigne M, Stein E. Dscam is a netrin receptor that collaborates with dcc in mediating turning responses to netrin-1. Cell. 2008;133:1241–1254. doi: 10.1016/j.cell.2008.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanco A, Szekeres C, Patel N, Rao S, Campbell K, Kreidberg JA, Polleux F, Anton ES. Netrin-1-alpha3beta1 integrin interactions regulate the migration of interneurons through the cortical marginal zone. Proc Natl Acad Sci U S A. 2009;106:7595–7600. doi: 10.1073/pnas.0811343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staquicini FI, Dias-Neto E, Li J, Snyder EY, Sidman RL, Pasqualini R, Arap W. Discovery of a functional protein complex of netrin-4, laminin gamma1 chain, and integrin alpha6beta1 in mouse neural stem cells. Proc Natl Acad Sci U S A. 2009;106:2903–2908. doi: 10.1073/pnas.0813286106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee TH, Seng S, Li H, Kennel SJ, Avraham HK, Avraham S. Integrin regulation by vascular endothelial growth factor in human brain microvascular endothelial cells: Role of alpha6beta1 integrin in angiogenesis. J Biol Chem. 2006;281:40450–40460. doi: 10.1074/jbc.M607525200. [DOI] [PubMed] [Google Scholar]
- 11.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garmy-Susini B, Avraamides CJ, Schmid MC, Foubert P, Ellies LG, Barnes L, Feral C, Papayannopoulou T, Lowy A, Blair SL, Cheresh D, Ginsberg M, Varner JA. Integrin alpha4beta1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 2010;70:3042–3051. doi: 10.1158/0008-5472.CAN-09-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Primo L, Seano G, Roca C, Maione F, Gagliardi PA, Sessa R, Martinelli M, Giraudo E, di Blasio L, Bussolino F. Increased expression of alpha6 integrin in endothelial cells unveils a proangiogenic role for basement membrane. Cancer Res. 2010;70:5759–5769. doi: 10.1158/0008-5472.CAN-10-0507. [DOI] [PubMed] [Google Scholar]
- 14.Schneiders FI, Maertens B, Bose K, Li Y, Brunken WJ, Paulsson M, Smyth N, Koch M. Binding of netrin-4 to laminin short arms regulates basement membrane assembly. J Biol Chem. 2007;282:23750–23758. doi: 10.1074/jbc.M703137200. [DOI] [PubMed] [Google Scholar]
- 15.Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, Sheppard D. Integrin alpha9beta1 directly binds to vascular endothelial growth factor (vegf)-a and contributes to vegf-a-induced angiogenesis. J Biol Chem. 2007;282:15187–15196. doi: 10.1074/jbc.M609323200. [DOI] [PubMed] [Google Scholar]
- 16.Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors vegf-c and -d are ligands for the integrin alpha9beta1. J Biol Chem. 2005;280:4544–4552. doi: 10.1074/jbc.M412816200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr., Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.