Abstract
Magnetic resonance imaging (MRI) is a clinical imaging modality effective for anatomical and functional imaging of diseased soft tissues, including solid tumors. MRI contrast agents have been routinely used for detecting tumor at an early stage. Gadolinium based contrast agents are the most commonly used contrast agents in clinical MRI. There have been significant efforts to design and develop novel Gd(III) contrast agents with high relaxivity, low toxicity and specific tumor binding. The relaxivity of the Gd(III) contrast agents can be increased by proper chemical modification. The toxicity of Gd(III) contrast agents can be reduced by increasing the agents’ thermodynamic and kinetic stability, as well as optimizing their pharmacokinetic properties. The increasing knowledge in the field of cancer genomics and biology provides an opportunity for designing tumor-specific contrast agents. Various new Gd(III) chelates have been designed and evaluated in animal models for more effective cancer MRI. This review outlines the design and development, physicochemical properties, and in vivo properties of several classes of Gd(III)-based MR contrast agents for tumor imaging.
1. Introduction
Magnetic resonance imaging (MRI) is a powerful medical imaging modality to display anatomical structures of body, especially useful for the detection and characterization of diseased soft tissues such as solid tumors. MRI has many advantages as it has no ionizing radiation and provides three-dimensional images with high spatial resolution and high contrast. In the past three decades, the quality of MR body images, including spatial resolution, signal-to-noise (SNR) and contrast-to-noise (CNR) ratios, has been substantially improved. In addition to stronger magnets, the development of safe and effective MRI contrast agents (CA) has played an important role for improving the image quality by enhancing the image contrast between normal and diseased tissues.1 MRI contrast agents are biocompatible magnetic materials that alter the longitudinal (T1) and transverse (T2) relaxation rates of the surrounding water protons, therefore enhancing image contrast in tissues of interest.2 MRI contrast agents are generally categorized as T1 and T2 contrast agents based on their magnetic properties and relaxation mechanisms. Gadolinium Gd(III) chelates are effective for increasing T1 relaxation rate (1/T1) and commonly used as T1 contrast agents, generating a positive image contrast. Superparamagnetic iron oxide nanoparticles are more effective for increasing T2 relaxation rate (1/T2) and commonly used as T2 contrast agents, producing negative image contrast. To date, the majority of MRI contrast agents used in clinical practice are Gd(III) chelates, with over 10 million contrast enhanced MRI scans on an annual basis, because of their high paramagnetism, favorable properties in term of relaxation enhancement, relatively high stability and inertness in the body.
Since the observation of difference in the nuclear magnetic relaxation times of normal tissues and tumors,3 MRI has played a significant role in cancer prediction and diagnosis. Paramagnetic contrast agents have been utilized to further enhance the image contrast for more accurate cancer detection and diagnosis, and timely evaluation of therapeutic efficacy. Many efforts have been made to develop better contrast agents with high relaxivity, low toxicity, and tumor specificity.4–10 This review summarizes the design and development, physicochemical properties, and in vivo properties of several classes of Gd(III) based MRI contrast agents for tumor imaging.
2. Relaxivity, Safety and Tumor-specificity
High relaxivity of a contrast agent is critical for effective contrast enhanced MRI. Relaxivity of the contrast agent describes its ability in changing water proton relaxation rates. Gd(III)-based contrast agents can increase both T1 and T2 relaxation rates (1/T1,2) of water protons. The observed water proton relaxation rates include the contributions from the relaxation rates (1/T1,2)d without a contrast agent and the increased relaxation rates (1/T1,2)p by the contrast agent. The increased relaxation rates of water protons are linearly related to the concentration of the contrast agent within the range of clinically relevant concentrations. The relaxivity is defined as the concentration-dependent increase in relaxation rate of water protons by the contrast agent in the units of mM−1S−1 (eq. 1).
| (1) |
The relaxivities of conventional small molecular contrast agents can vary if they bind to macromolecules such as plasma proteins. For the contrast agents binding to proteins, the relaxivities can altered by the concentrations of the agents and plasma proteins.11 (Figure 1) Moreover, the relaxivities depend on magnetic field strength.12, 13 For small molecular Gd(III) chelates with negligible protein binding (e.g. Magnevist®), the relaxivity has relatively low dependence on the field strength and the relaxivity usually decreases with increasing field strength. For protein binding small molecular Gd(III) chelates (e.g. MS-325) and macromolecular Gd(III) chelates (e.g. Gadomer), the relaxivities usually reach maxima at medium field strength and then decreases with increasing field strength. (Figure 1)
Figure 1.
The r1 relaxivities of MAGNEVIST (■), GADOMER (●) (polylysine dendrimer and Gd-DOTA complexes) and MS-325 (▲) in water or plasma at different magnetic field intensity. Data were obtained from literature.11
Safety is another important parameter of MRI contrast agents for clinical applications. Gd(III) ions cannot be administered directly because they are highly toxic in ionic form, acutely interfering with calcium channels and protein binding sites.14, 15 Free Gd ions accumulate in the liver, spleen, kidney and bones and have an LD50 = 0.2 mmol kg−1 in mice. Gd(III) ions are complexed with chelating ligands to prevent tissue interaction and minimize toxic side effects. Toxic Gd(III) ions may still be released from some of the chelates by transmetallation with other metal ions such as Zn2+, Ca2+ and Cu2+ in the body and protonation of the ligands at low pH that may cause chelate dissociation in vivo.16, 17 A serious adverse reaction called nephrogenic systemic fibrosis (NSF) may be associated to the use of Gd(III) based contrast agents in a very small percentage of patients with renal malfunctions due to incomplete excretion and, possibly, chelate disassociation of the agents.18 The FDA has issued a blackbox warning to prohibit the use of Gd(III) based contrast agents in the patients with renal malfunctions. Therefore, Gd(III) chelate based MRI contrast agents with good safety profiles should have high thermodynamic and kinetic stability. The contrast agents should be excreted after the contrast enhanced MRI within hours of administration.
Targeted delivery of contrast agents into tumor tissues is crucial in cancer diagnostic MRI. Tumor is physiologically different from normal tissue and has leaky blood vasculature. Thus, nanosized contrast agents can passively accumulate in the tumor via the tumor vascular permeability to macromolecules.19 Tumor tissues also express numerous cancer-related biomarkers on tumor cell surface, extracellular matrix or angiogenic microvessels, which can be targeted for molecular imaging. Abundantly expressed cancer biomarkers and ligands with high-binding affinity are essential for designing targeted contrast agents in order to generate sufficient contrast enhancement for visualizing these biomarkers with contrast enhanced MRI.
2. Gadolinium(III) chelates
Clinical MRI contrast agents are mostly small molecular Gd(III) chelates with high stability. The first MRI contrast agent Gd-DTPA (Magnevist®) was approved for clinical use in 1988.20 Its initial application was to detect the breakage of the blood brain barrier in brain tumors. Subsequently, many other Gd(III) chelates as shown in Figure 2 have been developed for clinical use. Although superparamagnetic iron oxide nanoparticles have been approved by the FDA for human uses, these agents have not been well received in the community of radiology, possibly due to negative contrast enhancement and their prolonged in vivo contrast enhancement, up to several months. Clinical Gd(III) based MRI contrast agents provide strong positive contrast enhancement and eliminate rapidly from the body after the MRI examinations. Currently, Gd(III) based MRI contrast agents are most commonly used in clinical practice.
Figure 2.
Structures of the Gd(III) based MRI contrast agents currently used in the clinical practice.
Gd(III) based MRI contrast agents are mainly categorized into two groups: linear Gd(III) chelates and macrocyclic chelates. In general, macrocyclic chelates have higher thermodynamic and kinetic stability than linear chelates.21 The clinical agents Magnevist® (gadopentetate dimeglumine, Gd-DTPA), Dotarem® (gadoterate, Gd-DOTA), Prohance® (gadoteridol, Gd(HP-DO3A)) and Omniscan® (gadodiamide, Gd(DTPA-BMA)) have similar r1 relaxivity in the range of 3.5–3.8 mM−1s−1 (20 MHz and 37 °C). These agents distribute rapidly between plasma and interstitial spaces following intravascular injection. They are ultimately eliminated through the renal route with half-lives of 1–2 h and excreted intact in urine (more than 95% of the injected dose in 24 h).22 Protein binding clinical agents Multihance® (Gd-BOPTA), Eovist® (Gd(EOB-DTPA)) and Ablacar® (MS-325) have increased relaxivity in plasma because of their non-covalent binding to albumin which slows down the molecular rotation. In particular, MS-325 has an r1 relaxivity as high as 28±1 mM−1s−1 when measured at 0.47 T and 37 °C in plasma.12 The dose of these small molecular contrast agents in clinical use is usually 100 times lower than their LD50. These low molecular weight Gd(III) complexes are most effective in detection and diagnosis of disrupted blood-brain barrier in the central nervous system (CNS) such as multiple sclerosis and brain tumor.23
3. Macromolecular Gd(III) complexes
Small molecular Gd(III) chelates have a relatively low relaxivity and extravasate non-selectively from blood into the interstitium of both normal tissue and tumor, which has been a major limitation for their clinical applications. Macromolecular Gd(III) complexes have been proposed and designed to improve relaxivity and pharmacokinetics of Gd(III) based agents. Attaching Gd(III) chelates to macromolecules slows down the rotational motion of the complexes, thus increases relaxivities.24 Macromolecular Gd(III) chelates have relatively higher r1 relaxivity per Gd(III) ion than that of small molecular Gd(III) chelates. For example, Gd-DTPA conjugated to polylysine with the molecular weights ranging from 3.3 up to 102 kDa has a r1 per Gd(III) ion 2.5 times higher than that of Gd-DTPA (measured at 2.4 T).25 P792 (Vistarem®), a macromolecular Gd-DOTA derivative (Figure 3), has very r1 relaxivity as high as 39 mM−1s−1 per Gd(III) ion (0.47 T, 37 °C, in water) compared with that of 3.4 mM−1s−1 for Gd-DOTA (0.47 T, 37 °C, in water).26, 27 Macromolecular Gd(III) complexes with higher relaxivity can generate more efficient contrast enhancement and can be used at significantly reduced doses. In addition, macromolecular contrast agents have a longer blood circulation and can preferentially accumulate in tumor tissue with leaky vasculature via passive targeting by the EPR effect. They also have longer tumor retention, which increases the tumor-to-background contrast over time and provides longer imaging time window. 28
Figure 3.
Examples of macromolecule-based Gd(III) contrast agents.
Macromolecular MRI contrast agents are mainly prepared by conjugating Gd-DOTA and Gd-DTPA to biocompatible macromolecules. Various bifunctional chelates of DTPA and DOTA have been developed with functional groups such as anhydride, N-hydroxysuccinimide activated carboxylic acid, maleimide, alkynyl and azide for conjugation. Bifunctional ligands and small molecular Gd chelates can be readily attached to many biocompatible macromolecules such as proteins, polymers and dendrimers to produce macromolecular contrast agents. The structures of some macromolecular Gd(III) based contrast agents are shown in Figure 3.
3.1. Protein-based macromolecular Gd(III) complexes
Albumin-(Gd-DTPA)x, first reported in 1987 by Ogan et al.,29 is a prototype macromolecular agent for intravascular MR imaging.30 This contrast agent was obtained by covalently attaching multiple Gd-DTPA chelates (typically 25–35) to albumin. The complexes had an average molecular weight about 92 kDa and a diameter around 6 nm. The relaxivity of the agent was 14.9 mM−1s−1 per Gd(III) at 0.25 T, which was three times higher than that of unconjugated Gd-DTPA. It has a blood half-life of approximately 3 h. Albumin-(Gd-DTPA)x was effective in characterizing the vascularity of various tumors, including breast, sarcoma and prostate tumors, and for early assessment of tumor response to antiangiogenic therapy.31–33 For example, Bhujwalla et al.33 investigated the effectiveness of albumin-(Gd-DTPA)x as a macromolecular MRI contrast agent for determining the efficacy of an antiangiogenic agent TNP-470 based on the measurement of tumor vascular volume and permeability in a MatLyLu prostate cancer model. Dynamic contrast enhanced MRI (DCE-MRI) with the agent was able to determine significant changes in tumor vascular characteristics after treatment with TNP-470. However, elimination of the agent from the body is extremely slow.30 Consequently, in vivo Gd(III) chelate dissociation34 and potential immunogenicity35 observed for the agent have limited its use only in preclinical studies.
3.2. Linear polymer-based macromolecular Gd(III) complexes
Polymer-based macromolecular Gd(III) complexes have also been studied and evaluated as potential MRI contrast agents. Many biocompatible synthetic polymers, such as polylysine (PLL)36–39 and polyethylene glycol (PEG)40, have been used as carriers to prepare polymeric MRI contrast agents.41 Schuhmann-Giampieri et al36 first reported PLL-(Gd-DOTA)n (Mn=48.7 kDa), which was synthesized by conjugating Gd-DOTA to the free amines of polylysine. Other versions of polylysine-based Gd(III) complexes containing either Gd-DTPA37 or Gd-DOTA39 have been subsequently synthesized and evaluated. The r1 relaxivity of polylysine chelates was three times greater than that of their corresponding small molecular chelates. The pharmacokinetics of polylysine based macromolecular contrast agents have been investigated in a wide range of molecular weights. As the molecular weight of PLL-(Gd-DTPA) increased from 36 kDa to 480 kDa, their blood half-life increased from 64.9 to 428.6 minutes in rats.38 The pharmacokinetic studies showed that PLL-(Gd-DTPA) had a high LD50 and it was cleared primarily through the kidney.42 However, high molecular weight polylysine conjugates took a long time to be excreted from the body. More than 3% of the injected PLL-(Gd-DTPA) (48 kDa) resided in the liver, kidneys, and bone of rats at 7 days postinjection.36 In general, PLL based macromolecular contrast agents have relatively low immunogenicity, but show relatively poor hemodynamic tolerance at higher doses.
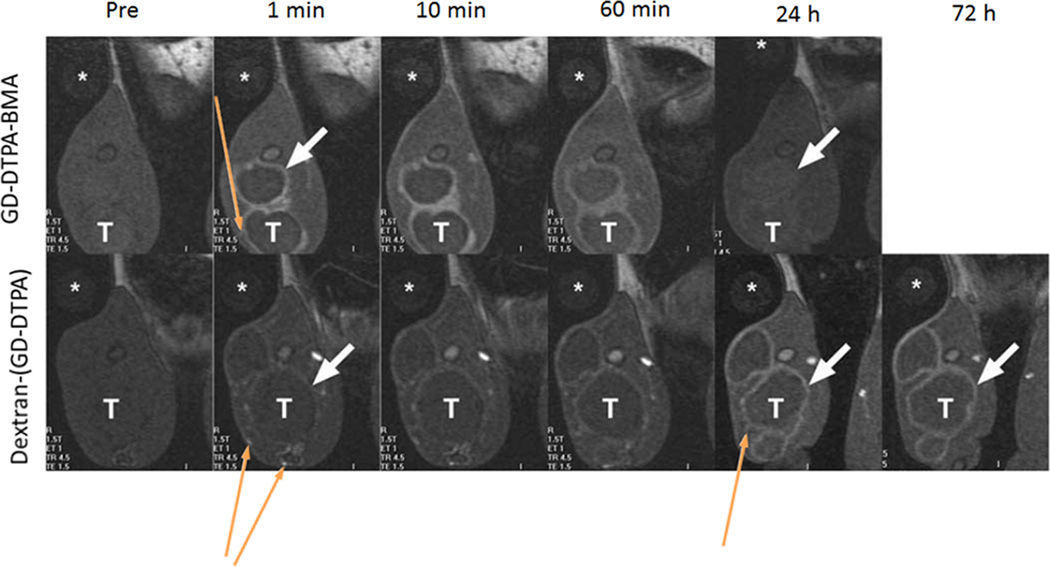
Dextran, a linear polysaccharide, was also well studied as a macromolecular carrier for MRI contrast agents.43–45 Dextran has been used clinically for over 50 years as a synthetic plasma expander. Gd-DOTA or Gd-DTPA can be readily conjugated to dextran, while DTPA dianhydride may cause cross-linking between dextran chains.46 The advantages for dextran-based contrast agents were inexpensive, good biocompatibility and easy synthesis. The r1 relaxivity of a dextran conjugated with 15 Gd-DTPA chelates (Mw=~75 kDa) was 10.5 mM−1s−1 per Gd(III) at 0.25 T and 37 °C, more than twice as that of Gd-DTPA.43 It was found that the relaxivity of dextran-(Gd-DTPA) did not change with molecular weight (Mw between 332 and 865 kDa) or the distance between dextran and DTPA.44 The blood retention time of dextran-Gd(III) contrast agents could be adjustable by dextran molecular weights. Sirlin et al.45 synthesized a high molecular weight (165 kDa) of dextran-(Gd-DTPA) with 187 Gd(III) chelates per dextran chain and a size of 17.6 nm. This agent had an intravascular half-life of approximately 58 h. Dextran-(Gd-DTPA) produced prolonged enhancement in tumor rim in rats with thigh VX2 tumors for up to 72 hours due to its slow leakage from tumor capillaries, as shown in Figure 4. In contrast, the small molecular weight agent, Gd(DTPA-BMA), resulted in rapid and more intense enhancement but progressively diminished over one hour. The increased enhancement by dextran-(Gd-DTPA) also permitted clear delineation of small peritumoral vessels.
Figure 4.
Coronal T1-weighted gradient echo images of VX2 tumor bearing rabbits at 1.5T showing tumor rim (white, short, thick arrow) and peritumoral vessels (yellow, thin, long arrows) before and at 1, 10 and 60 minutes and 24 and 72 hours after iv injection of Gd(DTPA-BMA) (Omniscan®) and Dextran-(Gd-DTPA) at 0.1 mmol-Gd kg−1. (*, water phantom; T, tumor). Adapted from ref. 39.
3.3. Dendrimer-based contrast agents
Dendrimers are highly branched macromolecules with precise three-dimensional nanosized molecular structures, which provide a platform for numerous biomedical applications. Dendrimers has many advantages, including precise molecular structure and a large number of surface functionalities for bioconjugation, as compared to linear polymers. The first dendrimeric MRI contrast agents were reported by Adam et al.47 and Wiener et al.21 Since then, many dendrimeric MRI contrast agents have been reported using various dendrimers, including poly(amidoamine) (PAMAM),21, 47, 48 poly(propylene imine) (PPI),49, 50 and polylysine (PLL)51. Dendrimeric Gd(III) complexes showed higher relaxivity due to the more rigid structure than their linear polymeric counterparts. Langereis et al.49 reported that a Gd-DTPA conjugated poly(propylene imine) dendrimer (the fifth generation G5, Mw = 51kDa) had r1 relaxivity as high as 19.7 mM−1s−1 per Gd(III) (1.5 T, 20 °C), which was 4.7 times of that of Gd-DTPA. Dendrimer-based Gd(III) chelates were effective for contrast enhanced MRI of the kidneys, vasculature, liver, or tumor.52, 53 They showed well defined size-dependent pharmacokinetic properties.53, 54 As shown in Figure 5, PAMAM-(Gd-DTPA) conjugates of low generations (G2, G3, G4) with relatively small sizes (3, 5 and 6 nm) excreted primarily via the kidneys, while the agents of high generations (G6-G10) and larger sizes (10–15 nm) showed minimal renal excretion.7, 54, 55 The dendrimers with a size around 5 nm preferentially leaked into tumor tissue rather than normal tissue, but further size increase (> 8 nm) would decrease their tumor accumulation. For example, a G4 agent (6 nm) leaked into tumor much more rapidly than both G6 (10 nm) and G8 (13 nm) dendrimeric agents.53 Thus, the design of dendrimer-based contrast agents with proper generations and sizes are essential for their application in MR imaging.
Figure 5.
Whole body MR images of mice injected with 0.03 mmol-Gd/kg of PAMAM G9 (a), G7 (b), and G3 (c) Gd-DTPA conjugates, and 0.1 mmol-Gd/kg of Gd-DTPA (d) at 3 min post-injection. Reprinted from ref.53.
3.4. Biodegradable macromolecular Gd(III) based MRI contrast agents
The clinical application of macromolecule-based Gd(III) contrast agents have been limited by their potential toxicity due to slow body excretion. In order to minimize the toxicity of macromolecular Gd(III) chelates while maintaining their favorable properties such as high relaxivity, preferential tumor accumulation, we have designed and prepared novel biodegradable macromolecular contrast agents.56, 57 These macromolecular Gd(III) contrast agents contain disulfide bonds in the polymer backbones.57 These disulfide bonds can be gradually reduced by endogenous or exogenous thiols via disulfide-thiol exchange reactions. Consequently, the macromolecular agents were reduced into small molecular weight chelates to facilitate their clearance from the body.58, 59
Polydisulfide Gd(III) complexes were synthesized by condensation polymerization of DTPA dianhydride and cystamine or other disulfide containing monomers followed by Gd(III) complexation,57 as shown in Figure 6. The molecular weight of these complexes ranged from 10 to 60 kDa with relaxivity ranging from 4.4 to 12 mM−1s−1 per Gd(III) at 3 T. The structure effect of these biodegradable macromolecular contrast agents on physicochemical and tumor imaging was evaluated in mice.60 The degradation rate of the polydisulfide Gd(III) chelates decreased with the increase of steric hindrance around the disulfide bonds. The polydisulfide chelates with negative charges around the disulfides had slow degradation rate due to the impeded access of negatively charged free thiols, including cysteine, to disulfide bonds at physiological pH. The pharmacokinetics of these agents revealed that they were excreted relatively quickly via renal filtration, resulted in much lower long-term tissue retention than the non-degradable macromolecular agents. The polydisulfide contrast agents showed better tumor enhancement than a clinical contrast agent Gd(DTPA-BMA) in mice bearing MDA-MB-231 human breast carcinoma xenografts.59
Figure 6.
Polydisulfide macromolecular Gd(III)-chelates with different chemical structures around the biodegradable disulfide bonds.
4. Liposomal Gd(III) based MRI contrast agents
Liposomes are clinically used nanosized drug carriers and have been applied as carriers for MRI contrast agents. Gd(III) chelates have been loaded to liposomal particles via covalently attachment to their surface or encapsulation into their interior core. The large surface-to-volume ratio of the nanoparticles enables high loading content of Gd(III) chelates. High loading of Gd(III) chelates in nanoparticles can greatly increase the sensitivity of the contrast agents for MR imaging. The size, surface charge, as well as the mechanical properties of nanoparticles can be modulated to optimize their biodistribution and pharmacokinetics and to promote their tumor accumulation for effective cancer MRI. For example, polyethylene glycol (PEG) is usually coated to liposomal surface to increase its blood retention and passive tumor targeting.61 Biologically active ligands, e.g. peptides or antibodies, can be anchored to the surface of the nanoparticles to achieve tumor targeting.
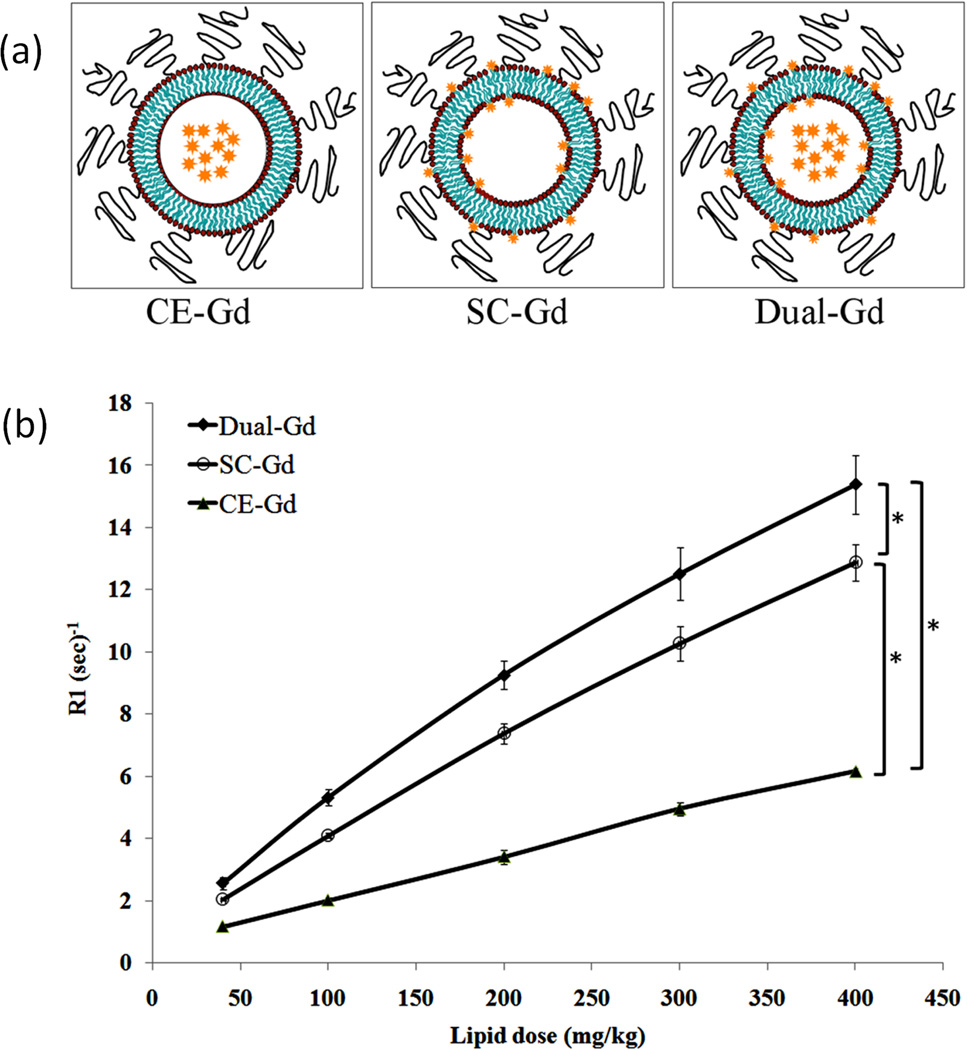
Gd(III) complexes including Gd-DTPA,62, 63 Gd(DTPA-BMA)64 and Gd-DOTA65 have been encapsulated in the core of liposomes to prepare nanosized MRI contrast agents.66 The relaxivity of liposomes encapsulated contrast agents is generally lower than that of the corresponding small molecular contrast agents. For example, unilamellar vesicles encapsulated Gd-DTPA had a r1 relaxivity of 0.42 to 3.43 mM−1s−1 per Gd(III) at 0.5 T,67, 68 and the relaxivity decreased with increasing particle size.67 This is presumably a simple consequence of a decrease in the exchange between bulk water and the water complexed to encaged Gd(III) chelates due to the permeability barrier imposed by the vesicle membrane.67 Covalently attaching Gd(III) chelates to the exterior of liposomes would greatly increase their relaxivity because bulk water could have an easy access to the surface Gd(III) chelates.64 A liposomal agent containing both core-encapsulated and surface-conjugated Gd(III) chelates had better r1 relaxivity (based on liposome) than liposomal agents with either encapsulated or conjugated gadolinium chelates (Figure 7).69 The higher r1 observed for this dual-mode liposomal contrast agents was due to the higher Gd concentration per unit of liposomal particle. The dual-mode gadolinium chelate containing liposomes could increase image contrast at relatively low particle doses.
Figure 7.
(a) Schemes of core-encapsulated gadolinium chelate (CE-Gd) liposomes, surface-conjugated gadolinium chelate (SC-Gd) liposomes and liposomes containing both encapsulated and conjugated gadolinium chelates (Dual-Gd). (b) T1 relaxation rates (R1) of liposomal-Gd formulations for different lipid concentrations. (*corresponds to p<0.05). Adapted from ref.69.
The liposome encapsulated Gd(III) complexes were initially developed as liver imaging contrast agents due to its preferential uptake by macrophages within the reticulo-endothelial system (RES) cells in the spleen and liver. The liposome-based Gd(III) contrast agents were effective for detecting tumors in the liver of rats with hepatic metastases.62, 63 Unger et al.62 found that liposomal Gd-DTPA resulted in significant contrast enhancement between the liver and tumor in T1-weighted MR images, thus these images permitted significant improvement in metastasis detection. Size effect was also studied with liposomes in a size range of 70 to 400 nm. Liposomal Gd-DTPA with a size of 70 nm created greater contrast enhancement possibly due to its larger surface-area-to-volume ratio.
The liposome encapsulated Gd(III) complexes have also shown some utility for MR imaging of brain tumor.70–73 Karathanasis et al.72 evaluated 100 nm liposomes loaded with Gd(DTPA-BMA) for brain tumor MRI in a rat glioma model. The r1 and r2 relaxivities of the liposomal contrast agents were 0.65 and 20.7 mM−1s−1 per Gd(III) chelate (25 °C, 9.4T), respectively. Different from usual T1-shortening Gd(III) contrast agents, this liposomal encapsulated agent was used as a T2 agent due to its high r2 relaxivity and produced negative signal enhancement (Figure 8). The tumor enhancement of the liposomal contrast agent was visible for a period of three days.
Figure 8.

Coronal T2 -weighted images of the rat brain with glioma obtained before and 5 min, 1 day and 3 days after administration of the Gd(III)-liposome at a dose of 0. 25 mmol-Gd/kg on a 9.4 T MRI. Reprinted from ref.72.
5. Targeted Gd(III) based MRI contrast agents
Targeted MRI contrast agents were explored as early as mid-1980s. Since then, there have been continuous efforts in developing targeted contrast agents for more accurate diagnostic imaging. Various targeted contrast agents have been prepared by conjugating Gd(III) chelates to targeting moieties, including monoclonal antibodies and peptides, specific to the biomarkers expressed on cancer cell surface. However, most of these agents could not generate sufficient contrast enhancement because of low sensitivity of contrast enhanced MRI and low concentration of biomarkers in the target tissues. In order to achieve distinct increase of signal intensity, a relatively large amount of the Gd(III) chelates is required at the target site for effective target-specific contrast enhanced MRI. Several approaches have been designed to address the limitations of targeted MRI contrast agents.
5.1. Tumor-targeting small molecular Gd(III) contrast agents
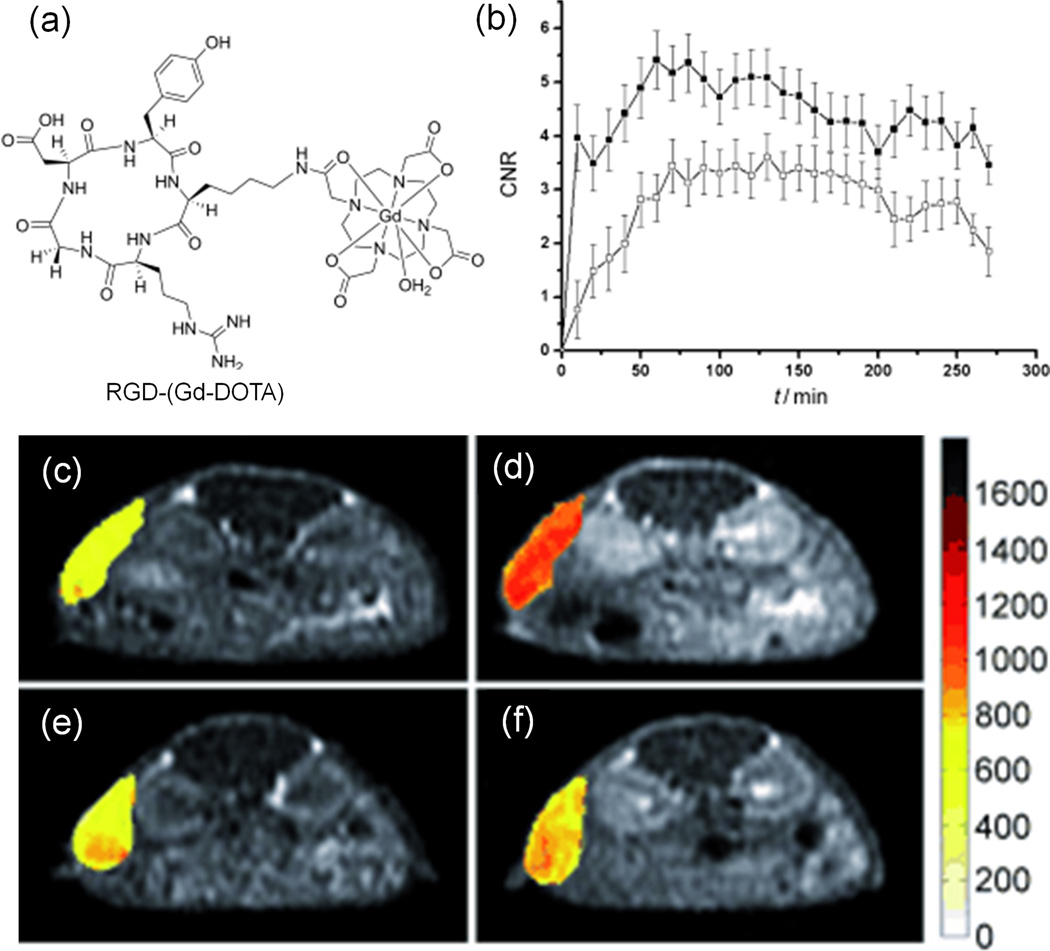
Tumor targeting MRI contrast agents were initially prepared by conjugating cancer specific moieties to the existing small molecular Gd(III) chelates of DTPA and DOTA. For example, cyclic RGD peptide has a relatively high affinity for aνβ3-integrin over-expressed in nascent endothelial cells during angiogenesis in various tumor types. Cyclic RGD was conjugated to Gd-DOTA for MR imaging of aνβ3-integrin in tumor tissues.74 RGD-(Gd-DOTA) has a higher r1 relaxivity than Gd-DOTA because of slower molecular tumbling after introduction of the RGD peptide (Figure 9). The targeting ability of RGD-(Gd-DOTA) for aνβ3-integrin was examined with the hepatocellular carcinoma in H-ras 12V transgenic mice. Specific enhancement was observed in the tumor with high expression of aνβ3-integrin. The tumor CNR generated by the targeted agent was approximately 45% higher than that obtained by the agent and pre-injection of c-(RGDYK), which blocked aνβ3-receptor binding (Figure 9). However, a very high dose (1.43 mmol/kg) of RGD-(Gd-DOTA) had to be used to produce an observable contrast.
Figure 9.
The chemical structure of RGD-(Gd-DOTA) (a), normalized signal intensities of the tumor as a function of time measured by MRI with RGD-(Gd-DOTA) (●) and Omniscan (○) (b), and MR T1-weighted images of mice with hepatocellular carcinoma before (c, e) and after (d, f) injection of RGD-(Gd-DOTA) (f, injected with c-(RGDyK) 30 minutes before). Adapted from ref.74.
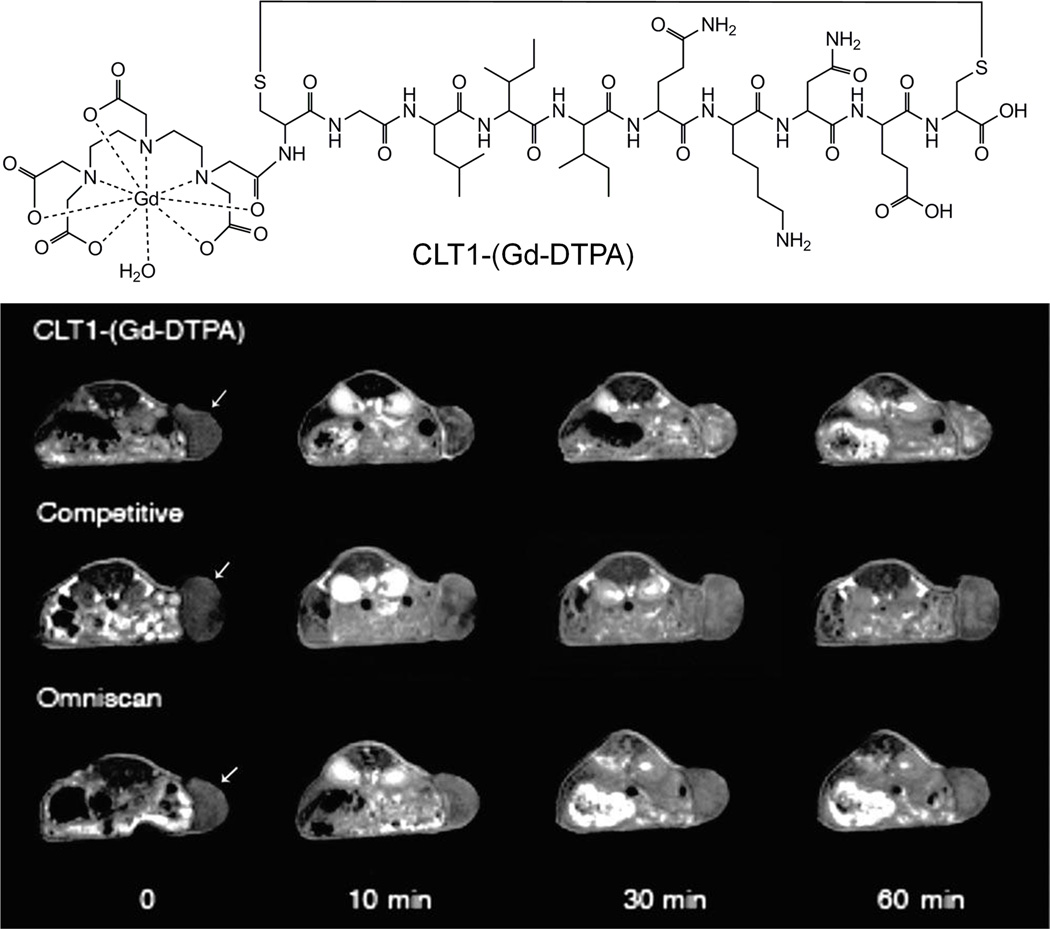
A cyclic decapeptide CGLIIQKNEC (CLT1) specifically binds to the fibronectin-fibrin complexes in the extracellular matrix of different tumors with little binding to normal tissues.75 We have developed an MRI contrast agent CLT1-(Gd-DTPA) for tumor imaging by conjugating CLT1 peptide to Gd-DTPA, as shown in Figure 10.76, 77 The r1 relaxivity of CLT1-(Gd-DTPA) was 4.22 mM−1s−1 at 3 T. This agent was evaluated in mice bearing HT-29 human colon carcinoma xenografts at dose of 0.1 mM/kg and Gd(DTPA-BMA) was used as a control. Significant enhancement was observed in tumor tissues for the mice injected with CLT1-(Gd-DTPA) and the enhancement was lasted more than 60 minutes as shown in Figure 10. In a competitive study, a 3-fold excess of free CLT1 peptide was co-injected with CLT1-(Gd-DTPA), resulted in much lower tumor enhancement. For the control experiment, there was little tumor enhancement in the mice injected with Gd(DTPA-BMA) at 60 minutes postinjection. CLT1 peptide was able to deliver a sufficient amount of Gd-DTPA chelates to its molecular target for effective tumor molecular imaging with MRI. It has demonstrated that MRI can be effective for molecular imaging if suitable molecular targets are identified.
Figure 10.
The structure of CLT1-(Gd-DTPA) and T1-weighted 2D spin-echo MR images of mice bearing HT-29 xenografts before contrast and at 10, 30, and 60 min postinjection of CLT1-(Gd-DTPA), Omniscan and competitive mixture of CLT1-(Gd-DTPA) and free CLT1. Arrow points to the tumor tissue. Adapted from ref.76.
5.2. Targeted protein-based Gd(III) contrast agents
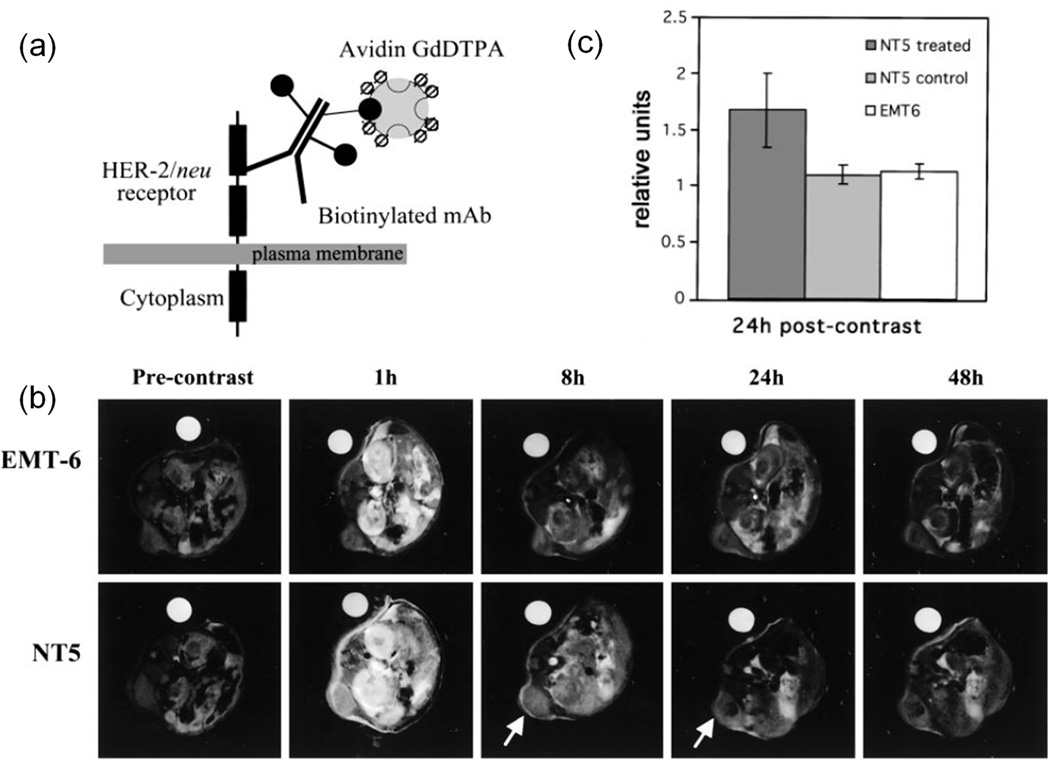
Targeted protein-based Gd(III) contrast agents have been developed by introducing tumor-specific ligands such as antibody.78–81 HER-2/neu receptor is a member of the epidermal growth factor family and is overexpressed on many cancers. Artemov et al.78 developed a two-component Gd-based avidin-biotin system for MR imaging of HER-2/neu receptors. The extracellular domain of HER-2/neu receptors was prelabeled by a biotinylated anti-HER/neu mAb, avidin-(Gd-DTPA)12.5 was then administered and bound to biotinylated mAb. The MR detection limit was estimated as around 106 receptors/cell by in vitro MRI study. In vivo MRI study was performed on female severe combined immunedeficient mice bearing NT-5 (overexpressing HER-2/neu receptors) and EMT-6 (control) murine mammary carcinomas. Mice were pre-injected with biotinylated antibody and imaged 12 h later using a 4.7 T MR scanner right after injection of avidin-(Gd-DTPA)12.5 at dose around 0.1 mmol-Gd/kg. The contrast of NT-5 tumors was retained for more than 24 h, and the contrast of EMT-6 tumors decreased to baseline levels at early time points.(Figure 11) NT-5 tumors without pretreatment of biotinylated antibody also didn’t retain contrast. This two-component Gd-based avidin-biotin system has shown great potential in screen receptor status and to combine with receptor-blocking therapy. However, it should be noticed that avidin-biotin systems could induce a profound immune response.82
Figure 11.
(a) The illustration of two-component Gd-based avidin-biotin system binding to HER-2/neu receptor. (b) T1-weighted MR images of mice bearing EMT-6 and NT5 tumors before contrast and at 1, 8, 24 and 48 h postinjection of avidin-(Gd-DTPA)12.5. Arrow points to the tumor tissue and water phantom is used as reference. (c) Signal enhancement in the tumor relative to the muscle tissue at 24 h after contrast. ‘NT5 treated’ and ‘EMT6’ groups were pretreated with anti-HER-2/neu biotinylated antibody. ‘NT5 control’ group was treated with BSA instead of antibody. Error bars represent the SE. The signal enhancement was significant (P <0.05) for prelabeled NT-5 tumors. Adapted from ref.78.
5.2. Targeted dendrimer-based Gd(III) contrast agents
Dendrimers have a high surface functionality. Both targeting agents and Gd(III) chelates can be readily conjugated on the surface of dendrimers to prepare targeted MRI contrast agents. Monoclonal antibodies83, peptides84–86 or folate87, 88 have been tested as the targeting agents. A relatively large number of Gd(III) chelates can be conjugated to high generation dendrimers to improve signal enhancement. For example, folic acid is necessary for cell proliferation and maintenance of new cells especially during periods of rapid cell division.89 Folate-targeted drug delivery systems90 or imaging agents91 have been developed to target folate receptor positive tumors. Folic acid and Gd-DOTA were conjugated to the fifth generation of PAMAM dendrimer to produce a folate-targeted dendrimeric MRI contrast agent.87 The r1 relaxivity of G5-FA-(Gd-DOTA) was 26.0 mM−1s−1 per Gd(III) (2 T, 20 °C), more than 6 times greater than that of Gd-DOTA (3.5 mM−1s−1). The targeted agent produced more significant tumor enhancement than a non-targeted G5-(Gd-DOTA) control agent in NOD C.B-17 SCID mice bearing KB tumor overexpressing folate receptor at a dose of 0.029 mmol-Gd/kg. Strong tumor enhancement of the targeted agent remained for 24 hours and then slowly decreased from 24 to 48 hours, while tumor enhancement of the non-targeted agent was present only 1 hour. The significant signal enhancement of targeted contrast agent was also observed in the kidneys and liver tissues due to the expression of folic acid receptors in these tissues.92
CLT1 peptide-targeted nanoglobular contrast agents were synthesized by conjugating Gd-DOTA monoamide and the peptide on the G2 and G3 nanoglobule (lysine dendrimers with a cubic silsesquioxane core),86 as shown in Figure 12. The targeted nanoglobular contrast agents resulted in greater enhancement within tumor tissues than the corresponding non-targeted nanoglobular agents in female nude mice bearing MB-231 human breast cancer xenograft. The targeted contrast agents generated strong prolonged enhancement in tumor tissue for at least 60 min postinjection, while the enhancement by the nontargeted agent was observed only in the first 10 min and gradually reduced overtime (Figure 13). The G3 nanoglobular contrast agent with a larger size than the G2 agent resulted in more prolonged enhancement in the heart and vasculature. At 48 h postinjection, the targeted G2 agent had much lower Gd(III) retention in the body than the G3 agent. In consideration of safety, rapid clearance is one of the key requirements for MRI contrast agents, thus the targeted G2 agent would be a more promising contrast agent than the G3 agent for further development.
Figure 12.
Synthesis of peptide CLT1-targeted nanoglobular contrast agents. Reprinted from ref.86.
Figure 13.
MR 3D maximum intensity projection images (a, b) and 2D axial T1-weighted spin-echo images of tumor tissue (c,d) of nu/nu female nude mice bearing MDA MB-231 tumor xenografts before and at various time points after intravenous injection of G2 (a, c) and CLT1 peptide-targeted G2 (b, d) nanoglobular MRI contrast agents at 0.03 mmol-Gd/kg. Arrows indicate tumor. Reprinted from ref.86.
5.3. Targeted liposomal MRI contrast agents
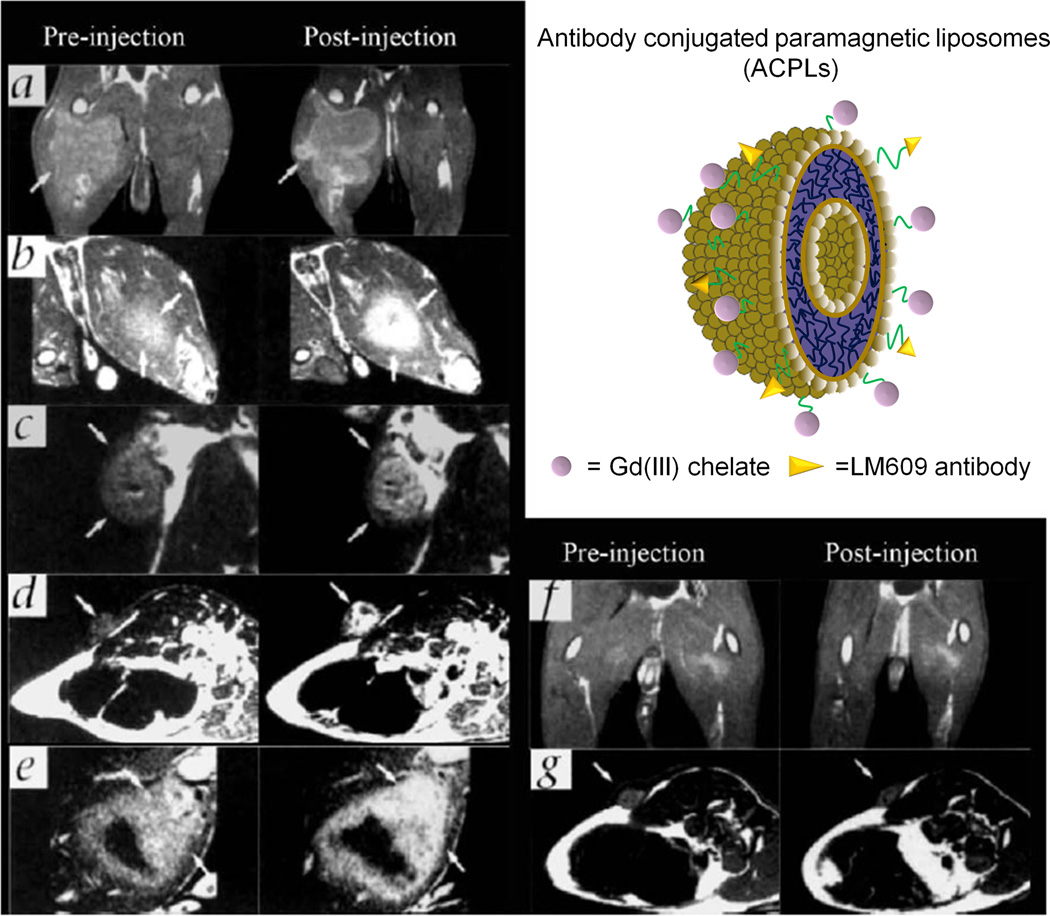
Targeting ligands, including folate acid93, peptide94, 95 and antibodies96, have also been incorporated into liposomes containing Gd(III) chelates to prepare targeted liposomal MRI contrast agents. An ανβ3 integrin specific monoclonal antibody was conjugated to paramagnetic liposomes as a targeted contrast agent for MR imaging of tumor angiogenesis.96 The exterior of polymerized liposome particles97 were conjugated with Gd(III)-chelates as imaging probes and biotinylated LM609 antibodies as targeting ligands specific binding to ανβ3 integrin (Figure 14). It had a mean size of 300–350 nm containing 30% of Gd(III) chelate-labeled lipid. This liposomal contrast agent with approximately fifty antibodies per particle exhibited significantly higher affinity for ανβ3 than equivalent concentrations of free antibody. MR imaging was performed on rabbit model of squamous cell carcinoma (V2). The signal in tumor was enhanced in animals receiving targeting liposomal agents while no significant enhancement was observed in the tumor with the corresponding non-targeting agents.(Figure 14) The targeting liposomal agents were also tested on mice bearing antibody (LM609) non-reactive tumors as a receptor-negative control experiment and no signal enhancement was detected. The immunohistochemical staining for ανβ3 integrin in the tumor margin was co-localized with the LM609 antibodies bound to the liposome.(Figure 15 a and b) It also revealed the ανβ3-positive vessel density was correlated with the degree of the MR signal enhancement. (Figure 15 c and d)
Figure 14.
T1 MR images of rabbit tumors before and 24 after injection of anti-ανβ3 (LM609) ACPLs. Arrows indicate tumors. a) Coronal images of tumor in the right thigh muscle; b) axial images of a intramuscle tumor; c) coronal images of a subcutaneously implanted V2 carcinoma; d) LM609 ACPLs improved visualization of a subcutaneous tumor; e) axial images of hyperintense intramuscle tumor with central necrosis and the post-contrast image with LM609 ACPLs; f) coronal image of tumor growing in left thigh muscle and minimal tumor enhancement at 24 h after injection of isotype-matched control ACPLs; g) axial images of a rabbit subcutaneous tumor and almost no enhancement 24 h after administration of avidin-conjugated control paramagnetic liposomes. Insert illustrates the structure of ACPLs. . Adapted from ref.96.
Figure 15.
The expression ανβ3 integrin and ACPL localization in tumor tissue sections. a) An immunohistochemical staining section for ανβ3 integrin from the tumor margin shown in Fig. 13 a; b) An adjacent tissue section stained for ACPLs; c) A staining section showed a high density of stained ανβ3 positive vessels from the tumor with strong MR enhancement with the ACPLs; d) A staining section showing a relative paucity of ανβ3 positive vessels from the tumor with relatively weak MR enhancement with ACPLs. Reprinted from ref.96.
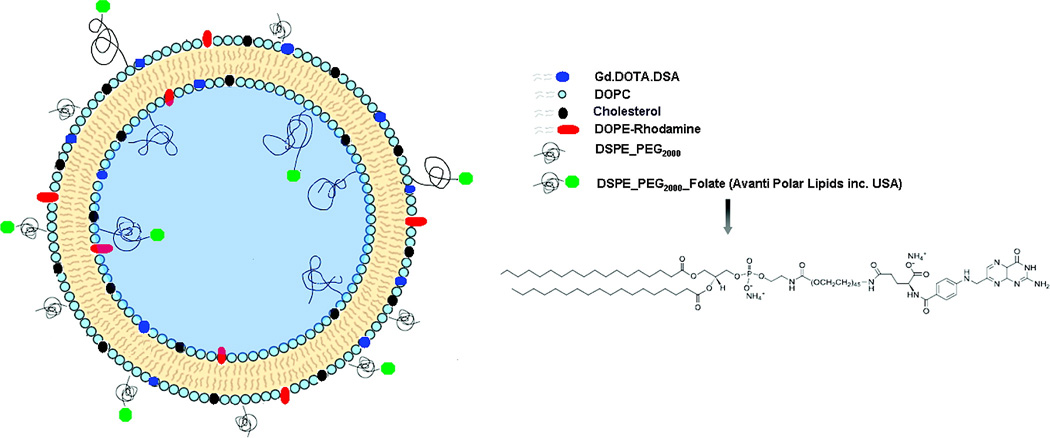
Kamaly et al.93 prepared folate targeted bimodal liposomes for tumor MRI by incorporating folate acid with a PEG spacer, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[folate-(PEG-2000)] (DSPE-PEG (2000)folate). A Gd(III) chelate with long alkyl chain (Gd(DOTA-DSA)) and phosphatidylethaolamine Lissamine rhodamine B (DOPE-Rhodamine) were incorporated into the liposome for both targeted MRI and fluorescent imaging. The targeted liposomes were consisted of Gd-DOTA-DSA/DOPC/Cholesterol/DSPE-PEG2000/DSPE-PEG(2000) Folate/DOPE-Rhodamine 30/32/30/4/3/1 mol% (Figure 16). The folate receptor targeted bimodal liposomes were tested in tumor-bearing mice with human ovarian carcinoma (IGROV-1) xenograft overexpressing folate receptor. Tumor targeting was confirmed by histological study of tumor using fluorescence microscopy. The targeted liposomes produced 4-fold tumor contrast enhancement in T1-weighted MRI at 2 h postinjection due to the folate-mediated active tumor targeting as well as the EPR effect. The non-targeted liposomes resulted in similar tumor contrast enhancement at 24 h post-injection by the EPR effect only.
Figure 16.
The illustration of folate-targeted targeted bimodal liposomes containing Gd(III) chelates for MR imaging of ovarian cancer. Reprinted from ref.93.
6. CONCLUSIONS
Contrast agents have been increasingly used in clinical MRI. Currently, approximately 40% of MRI patients receive Gd(III) based contrast agents. The Gd(III) contrast agent assisted MRI has greatly improved cancer detection and diagnosis. In the last three decades, there has been a significant progress in the design and development of new Gd(III) based MRI contrast agents to further improve the safety and effectiveness for cancer imaging. The relaxivities of Gd(III) chelates can be significantly increased by conjugating biocompatible macromolecules and nanoparticles. The excretion of Gd(III) chelates on the nanoparticles and macromolecules after the MRI examinations can be facilitated by incorporating biodegradable structures. Some of targeted Gd(III) contrast agents specifically binding to tumor markers can produce significant tumor contrast enhancement at reduced doses for effective cancer MR molecular imaging. Preclinical studies have shown that these new agents exhibit improved contrast enhanced MRI as compared to currently available clinical contrast agents in animal models. Further detailed toxicological and pharmaceutical evaluations are required to demonstrate the safety of the agents before they can be preceded into clinical development.
Acknowledgements
This research was supported in part by the NIH grant R01 CA097465.
REFERENCES
- 1.Lauffer RB. Paramagnetic metal-complexes as water proton relaxation agents for NMR Imaging-theory and design. Chem Rev. 1987;87:901–927. [Google Scholar]
- 2.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: structure, dynamics, and applications. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 3.Damadian R. Tumor detection by nuclear magnetic resonance. Science. 1971;171:1151–1153. doi: 10.1126/science.171.3976.1151. [DOI] [PubMed] [Google Scholar]
- 4.Werner EJ, Datta A, Jocher CJ, Raymond KN. High-relaxivity MRI contrast agents: where coordination chemistry meets medical imaging. Angew Chem Int Ed. 2008;47:8568–8580. doi: 10.1002/anie.200800212. [DOI] [PubMed] [Google Scholar]
- 5.Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 6.Villaraza AJL, Bumb A, Brechbiel MW. Macromolecules, dendrimers, and nanomaterials in magnetic resonance imaging: the interplay between size, function, and pharmacokinetics. Chem Rev. 2010;110:2921–2959. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aime S, Crich SG, Gianolio E, Giovenzana GB, Tei L, Terreno E. High sensitivity lanthanide(III) based probes for MR-medical imaging. Coord Chem Rev. 2006;250:1562–1579. [Google Scholar]
- 8.Kamaly N, Miller AD, Bell JD. Chemistry of tumour targeted T1 based MRI contrast agents. Curr Top Med Chem. 2010;10:1158–1183. doi: 10.2174/156802610791384199. [DOI] [PubMed] [Google Scholar]
- 9.Gore JC, Manning HC, Quarles CC, Waddell KW, Yankeelov TE. Magnetic resonance in the era of molecular imaging of cancer. Magn Reson Imaging. 2011;29:587–600. doi: 10.1016/j.mri.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glunde K, Artemov D, Penet MF, Jacobs MA, Bhujwalla ZM. Magnetic resonance spectroscopy in metabolic and molecular imaging and diagnosis of cancer. Chem Rev. 2010;110:3043–3059. doi: 10.1021/cr9004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caravan P, Cloutier NJ, Greenfield MT, McDermid SA, Dunham SU, Bulte JW, Amedio JC, Jr, Looby RJ, Supkowski RM, Horrocks WD, Jr, et al. The interaction of MS-325 with human serum albumin and its effect on proton relaxation rates. J Am Chem Soc. 2002;124:3152–3162. doi: 10.1021/ja017168k. [DOI] [PubMed] [Google Scholar]
- 12.Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40:715–724. doi: 10.1097/01.rli.0000184756.66360.d3. [DOI] [PubMed] [Google Scholar]
- 13.Caravan P. Strategies for increasing the sensitivity of gadolinium based MRI contrast agents. Chem Soc Rev. 2006;35:512–523. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 14.Lansman JB. Blockade of current through single calcium channels by trivalent lanthanide cations-effect of ionic radius on the rates of ion entry and exit. J Gen Physiol. 1990;95:679–696. doi: 10.1085/jgp.95.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biagi BA, Enyeart JJ. Gadolinium blocks low-threshold and high-threshold calcium currents in pituitary-cells. Am J Physiol. 1990;259:C515–C520. doi: 10.1152/ajpcell.1990.259.3.C515. [DOI] [PubMed] [Google Scholar]
- 16.Laurent S, Elst LV, Copoix F, Muller RN. Stability of MRI paramagnetic contrast media-A proton relaxometric protocol for transmetallation assessment. Invest Radiol. 2001;36:115–122. doi: 10.1097/00004424-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg SA. Zinc transmetallation and gadolinium retention after MR imaging: case report. Radiology. 2010;257:670–673. doi: 10.1148/radiol.10100560. [DOI] [PubMed] [Google Scholar]
- 18.Thomsen HS. Nephrogenic systemic fibrosis: a serious late adverse reaction to gadodiamide. Eur Radiol. 2006;16:2619–2621. doi: 10.1007/s00330-006-0495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torchilin V. Tumor delivery of macromolecular drugs based on the EPR effect. Adv Drug Del Rev. 2011;63:131–135. doi: 10.1016/j.addr.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 20.Weinmann HJ, Brasch RC, Press WR, Wesbey GE. Characteristics of gadolinium-DTPA complex-a potential NMR contrast agent. Am J Roentgenol. 1984;142:619–624. doi: 10.2214/ajr.142.3.619. [DOI] [PubMed] [Google Scholar]
- 21.Wiener EC, Brechbiel MW, Brothers H, Magin RL, Gansow OA, Tomalia DA, Lauterbur PC. Dendrimer-based metal-chelates-a new class of magnetic-resonance-imaging contrast agents. Magn Reson Med. 1994;31:1–8. doi: 10.1002/mrm.1910310102. [DOI] [PubMed] [Google Scholar]
- 22.Perazella MA. Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephro. 2009;4:461–469. doi: 10.2215/CJN.06011108. [DOI] [PubMed] [Google Scholar]
- 23.Leach MO, Brindle KM, Evelhoch JL, Griffiths JR, Horsman MR, Jackson A, Jayson G, Judson IR, Knopp MV, Maxwell RJ, et al. Assessment of antiangiogenic and antivascular therapeutics using MRI: recommendations for appropriate methodology for clinical trials. Br J Radiol. 2003;76:S87–S91. doi: 10.1259/bjr/15917261. [DOI] [PubMed] [Google Scholar]
- 24.Botta M, Tei L. Relaxivity enhancement in macromolecular and nanosized GdIII-based MRI contrast agents. Eur J Inorg Chem. 2012:1945–1960. [Google Scholar]
- 25.Spanoghe M, Lanens D, Dommisse R, Vanderlinden A, Alderweireldt F. Proton relaxation enhancement by means of serum-albumin and poly-L-lysine labeled with DTPA-Gd3+-relaxivities as a function of molecular-weight and conjugation efficiency. Magn Reson Imaging. 1992;10:913–917. doi: 10.1016/0730-725x(92)90445-6. [DOI] [PubMed] [Google Scholar]
- 26.Port M, Corot C, Rousseaux O, Raynal I, Devoldere L, Idee JM, Dencausse A, Le Greneur S, Simonot C, Meyer D. P792: a rapid clearance blood pool agent for magnetic resonance imaging: preliminary results. Magn Reson Mater Phy Biol Med. 2001;12:121–127. doi: 10.1007/BF02668093. [DOI] [PubMed] [Google Scholar]
- 27.Fries P, Runge VM, Bucker A, Schurholz H, Reith W, Robert P, Jackson C, Lanz T, Schneider G. Brain tumor enhancement in magnetic resonance imaging at 3 tesla intraindividual comparison of two high relaxivity macromolecular contrast media with a standard extracellular Gd-chelate in a rat brain tumor model. Invest Radiol. 2009;44:200–206. doi: 10.1097/RLI.0b013e31819817ff. [DOI] [PubMed] [Google Scholar]
- 28.Daldrup H, Shames DM, Wendland M, Okuhata Y, Link TM, Rosenau W, Lu Y, Brasch RC. Correlation of dynamic contrast-enhanced magnetic resonance imaging with histologic tumor grade: comparison of macromolecular and small-molecular contrast media. Pediatr Radiol. 1998;28:67–78. doi: 10.1007/s002470050296. [DOI] [PubMed] [Google Scholar]
- 29.Ogan MD, Schmiedl U, Moseley ME, Grodd W, Paajanen H, Brasch RC. Albumin labeled with Gd-DTPA-an intravascular contrast-enhancing agent for magnetic-resonance blood pool imaging - preparation and characterization. Invest Radiol. 1987;22:665–671. [PubMed] [Google Scholar]
- 30.Schmiedl U, Ogan M, Paajanen H, Marotti M, Crooks LE, Brito AC, Brasch RC. Albumin labeled with Gd-DTPA as an intravascular, blood pool enhancing agent for MR imaging-biodistribution and imaging studies. Radiology. 1987;162:205–210. doi: 10.1148/radiology.162.1.3786763. [DOI] [PubMed] [Google Scholar]
- 31.Raatschen HJ, Simon GH, Fu YJ, Sennino B, Shames DM, Wendland MF, McDonald DM, Brasch RC. Vascular permeability during antiangiogenesis treatment: MR imaging assay results as biomarker for subsequent tumor growth in rats. Radiology. 2008;247:391–399. doi: 10.1148/radiol.2472070363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dafni H, Kim SJ, Bankson JA, Sankaranaravanapillai M, Ronen SM. Macromolecular dynamic contrast-enhanced (DCE)-MRI detects reduced vascular permeability in a prostate cancer bone metastasis model following anti-platelet-derived growth factor receptor (PDGFR) therapy, indicating a drop in vascular endothelial growth factor receptor (VEGFR) activation. Magn Reson Med. 2008;60:822–833. doi: 10.1002/mrm.21727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhujwalla ZM, Artemov D, Natarajan K, Solaiyappan M, Kollars P, Kristjansen PEG. Reduction of vascular and permeable regions in solid tumors detected by macromolecular contrast magnetic resonance imaging after treatment with antiangiogenic agent TNP-470. Clin Cancer Res. 2003;9:355–362. [PubMed] [Google Scholar]
- 34.Sherry AD, Cacheris WP, Kuan KT. Stability-constants for Gd3+ binding to model DTPA-conjugates and DTPA-proteins-implications for their use as magnetic-resonance contrast agents. Magn Reson Med. 1988;8:180–190. doi: 10.1002/mrm.1910080208. [DOI] [PubMed] [Google Scholar]
- 35.Baxter AB, Melnikoff S, Stites DP, Brasch RC. Immunogenicity of gadolinium-based contrast agents for magnetic-resonance-imaging-induction and characterization of antibodies in animals. Invest Radiol. 1991;26:1035–1040. doi: 10.1097/00004424-199112000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Schuhmanngiampieri G, Schmittwillich H, Frenzel T, Press WR, Weinmann HJ. In vivo and in vitro evaluation of Gd-DTPA-polylysine as a macromolecular contrast agent for magnetic-resonance-imaging. Invest Radiol. 1991;26:969–974. doi: 10.1097/00004424-199111000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Curtet C, Maton F, Havet T, Slinkin M, Mishra A, Chatal JF, Muller RN. Polylysine-Gd-DTPAn and polylysine-Gd-DOTAn coupled to anti-CEA F(ab')2 fragments as potential immunocontrast agents. Relaxometry, biodistribution, and magnetic resonance imaging in nude mice grafted with human colorectal carcinoma. Invest Radiol. 1998;33:752–761. doi: 10.1097/00004424-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Vexler VS, Clement O, Schmittwillich H, Brasch RC. Effect of varying the molecular-weight of the MR contrast agent Gd-DTPA-polylysine on blood pharmacokinetics and enhancement patterns. J Magn Reson Imaging. 1994;4:381–388. doi: 10.1002/jmri.1880040325. [DOI] [PubMed] [Google Scholar]
- 39.Wendland MF, Saeed M, Yu KK, Roberts TPL, Lauerma K, Derugin N, Varadarajan J, Watson AD, Higgins CB. Inversion-recovery EPI of bolus transit in rat myocardium using intravascular and extravascular gadolinium-based MR contrast-media-dose effects on peak signal enhancement. Magn Reson Med. 1994;32:319–329. doi: 10.1002/mrm.1910320307. [DOI] [PubMed] [Google Scholar]
- 40.Desser TS, Rubin DL, Muller HH, Qing F, Khodor S, Zanazzi G, Young SW, Ladd DL, Wellons JA, Kellar KE, et al. Dynamics of tumor imaging with Gd-DTPA polyethylene-glycol polymers-dependence on molecular-weight. J Magn Reson Imaging. 1994;4:467–472. doi: 10.1002/jmri.1880040337. [DOI] [PubMed] [Google Scholar]
- 41.Frank H, Weissleder R, Bogdanov AA, Brady TJ. Detection of pulmonary emboli by using MR-angiography with mPEG-Pl-GdDTPA-an experimental-study in rabbits. Am J Roentgenol. 1994;162:1041–1046. doi: 10.2214/ajr.162.5.8165978. [DOI] [PubMed] [Google Scholar]
- 42.Vanhecke P, Marchal G, Bosmans H, Johannik K, Jiang Y, Vogler H, Vanongeval C, Baert AL, Speck U. NMR imaging study of the pharmacodynamics of polylysine-gadolinium-DTPA in the rabbit and the rat. Magn Reson Imaging. 1991;9:313–321. doi: 10.1016/0730-725x(91)90417-k. [DOI] [PubMed] [Google Scholar]
- 43.Wang SC, Wikstrom MG, White DL, Klaveness J, Holtz E, Rongved P, Moseley ME, Brasch RC. Evaluation of Gd-DTPA labeled dextran as an intravascular MR contrast agent-imaging characteristics in normal rat-tissues. Radiology. 1990;175:483–488. doi: 10.1148/radiology.175.2.1691513. [DOI] [PubMed] [Google Scholar]
- 44.Rebizak R, Schaefer M, Dellacherie E. Polymeric conjugates of Gd3+-diethylenetriaminepentaacetic acid and dextran. 2. Influence of spacer arm length and conjugate molecular mass on the paramagnetic properties and some biological parameters. Bioconjugate Chem. 1998;9:94–99. doi: 10.1021/bc9701499. [DOI] [PubMed] [Google Scholar]
- 45.Sirlin CB, Vera DR, Corbeil JA, Caballero MB, Buxton RB, Mattrey RF. Gadolinium-DTPA-dextran: a macromolecular MR blood pool contrast agent. Acad Radiol. 2004;11:1361–1369. doi: 10.1016/j.acra.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Armitage FE, Richardson DE, Li KCP. Polymeric contrast agents for magnetic resonance imaging: synthesis and characterization of gadolinium diethylonetriaminepentaacetic acid conjugated to polysaccharides. Bioconjugate Chem. 1990;1:365–374. doi: 10.1021/bc00006a001. [DOI] [PubMed] [Google Scholar]
- 47.Adam G, Neuerburg J, Spuntrup E, Muhler A, Scherer K, Gunther RW. Gd-DTPA-cascade-polymer-potential blood-pool contrast agent for MR-imaging. J Magn Reson Imaging. 1994;4:462–466. doi: 10.1002/jmri.1880040336. [DOI] [PubMed] [Google Scholar]
- 48.Venditto VJ, Regino CAS, Brechbiel MW. PAMAM dendrimer based macromolecules as improved contrast agents. Mol Pharm. 2005;2:302–311. doi: 10.1021/mp050019e. [DOI] [PubMed] [Google Scholar]
- 49.Langereis S, de Lussanet QG, van Genderen MHP, Backes WH, Meijer EW. Multivalent contrast agents based on gadolinium-diethylenetriaminepentaacetic acid-terminated poly(propylene imine) dendrimers for magnetic resonance imaging. Macromolecules. 2004;37:3084–3091. [Google Scholar]
- 50.Langereis S, de Lussanet QG, van Genderen MHP, Meijer EW, Beets-Tan RGH, Griffioen AW, van Engelshoven JMA, Backes WH. Evaluation of Gd(III)DTPA-terminated poly(propylene imine) dendrimers as contrast agents for MR imaging. NMR Biomed. 2006;19:133–141. doi: 10.1002/nbm.1015. [DOI] [PubMed] [Google Scholar]
- 51.Luo K, Liu G, She WC, Wang QY, Wang G, He B, Ai H, Gong QY, Song B, Gu ZW. Gadolinium-labeled peptide dendrimers with controlled structures as potential magnetic resonance imaging contrast agents. Biomaterials. 2011;32:7951–7960. doi: 10.1016/j.biomaterials.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi H, Brechbiel MW. Dendrimer-based macromolecular MRI contrast agents: characteristics and application. Mol Imaging. 2003;2:1–10. doi: 10.1162/15353500200303100. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi H, Brechbiel MW. Nano-sized MRI contrast agents with dendrimer cores. Adv Drug Del Rev. 2005;57:2271–2286. doi: 10.1016/j.addr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 54.Sato N, Kobayashi H, Hiraga A, Saga T, Togashi K, Konishi J, Brechbiel MW. Pharmacokinetics and enhancement patterns of macromolecular MR contrast agents with various sizes of polyamidoamine dendrimer cores. Magn Reson Med. 2001;46:1169–1173. doi: 10.1002/mrm.1314. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi H, Sato N, Hiraga A, Saga T, Nakamoto Y, Ueda H, Konishi J, Togashi K, Brechbiel MW. 3D-micro-MR angiography of mice using macromolecular MR contrast agents with polyamidoamine dendrimer core with reference to their pharmacokinetic properties. Magn Reson Med. 2001;45:454–460. doi: 10.1002/1522-2594(200103)45:3<454::aid-mrm1060>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 56.Lu ZR, Mohs AM, Zong Y, Feng Y. Polydisulfide Gd(III) chelates as biodegradable macromolecular magnetic resonance imaging contrast agents. Int J Nanomed. 2006;1:31–40. doi: 10.2147/nano.2006.1.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu ZR, Wu XM. Polydisulfide-based biodegradable macromolecular magnetic resonance imaging contrast agents. Isr J Chem. 2010;50:220–232. doi: 10.1002/ijch.201000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu ZR, Parker DL, Goodrich KC, Wang X, Dalle JG, Buswell HR. Extracellular biodegradable macromolecular gadolinium(III) complexes for MRI. Magn Reson Med. 2004;51:27–34. doi: 10.1002/mrm.10656. [DOI] [PubMed] [Google Scholar]
- 59.Zong YD, Wang XH, Goodrich KC, Mohs AM, Parker DL, Lu ZR. Contrast-enhanced MRI with new biodegradable macromolecular Gd(III) complexes in tumor-bearing mice. Magn Reson Med. 2005;53:835–842. doi: 10.1002/mrm.20402. [DOI] [PubMed] [Google Scholar]
- 60.Zong YD, Wang XL, Jeong EK, Parker DL, Lu ZR. Structural effect on degradability and in vivo contrast enhancement of polydisulfide Gd(III) complexes as biodegradable macromolecular MRI contrast agents. Magn Reson Imaging. 2009;27:503–511. doi: 10.1016/j.mri.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A. 1991;88:11460–11464. doi: 10.1073/pnas.88.24.11460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unger EC, Winokur T, MacDougall P, Rosenblum J, Clair M, Gatenby R, Tilcock C. Hepatic metastases: liposomal Gd-DTPA-enhanced MR imaging. Radiology. 1989;171:81–85. doi: 10.1148/radiology.171.1.2928550. [DOI] [PubMed] [Google Scholar]
- 63.Unger EC, Macdougall P, Cullis P, Tilcock C. Liposomal Gd-DTPA-effect of encapsulation on enhancement of hepatoma model by MRI. Magn Reson Imaging. 1989;7:417–423. doi: 10.1016/0730-725x(89)90491-8. [DOI] [PubMed] [Google Scholar]
- 64.Bui T, Stevenson J, Hoekman J, Zhang SR, Maravilla K, Ho RJY. Novel Gd nanoparticles enhance vascular contrast for high-resolution magnetic resonance imaging. PLoS One. 2010;5:pii: e13082. doi: 10.1371/journal.pone.0013082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hak S, Sanders HMHF, Agrawal P, Langereis S, Grull H, Keizer HM, Arena F, Terreno E, Strijkers GJ, Nicolay K. A high relaxivity Gd(III)DOTA-DSPE-based liposomal contrast agent for magnetic resonance imaging. Eur J Pharm Biopharm. 2009;72:397–404. doi: 10.1016/j.ejpb.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 66.Mulder WJM, Strijkers GJ, van Tilborg GAF, Griffioen AW, Nicolay K. Lipid-based nanoparticles for contrast-enhanced MRI and molecular imaging. NMR Biomed. 2006;19:142–164. doi: 10.1002/nbm.1011. [DOI] [PubMed] [Google Scholar]
- 67.Tilcock C, Unger E, Cullis P, Macdougall P. Liposomal Gd-DTPA-preparation and characterization of relaxivity. Radiology. 1989;171:77–80. doi: 10.1148/radiology.171.1.2928549. [DOI] [PubMed] [Google Scholar]
- 68.Unger E, Shen D, Wu GL, Fritz T. Liposomes as MR contrast agents-pros and cons. Magn Reson Med. 1991;22:304–308. doi: 10.1002/mrm.1910220229. [DOI] [PubMed] [Google Scholar]
- 69.Ghaghada KB, Ravoori M, Sabapathy D, Bankson J, Kundra V, Annapragada A. New dual mode gadolinium nanoparticle contrast agent for magnetic resonance imaging. PLoS One. 2009;4:pii:e7628. doi: 10.1371/journal.pone.0007628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krauze MT, Forsayeth J, Park JW, Bankiewicz KS. Real-time imaging and quantification of brain delivery of liposomes. Pharm Res. 2006;23:2493–2504. doi: 10.1007/s11095-006-9103-5. [DOI] [PubMed] [Google Scholar]
- 71.Mamot C, Nguyen JB, Pourdehnad M, Hadaczek P, Saito R, Bringas JR, Drummond DC, Hong KL, Kirpotin DB, McKnight T, et al. Extensive distribution of liposomes in rodent brains and brain tumors following convection-enhanced delivery. J Neurooncol. 2004;68:1–9. doi: 10.1023/b:neon.0000024743.56415.4b. [DOI] [PubMed] [Google Scholar]
- 72.Karathanasis E, Park J, Agarwal A, Patel V, Zhao F, Annapragada AV, Hu X, Bellamkonda RV. MRI mediated, non-invasive tracking of intratumoral distribution of nanocarriers in rat glioma. Nanotechnology. 2008;19:315109. doi: 10.1088/0957-4484/19/31/315101. [DOI] [PubMed] [Google Scholar]
- 73.Saito R, Bringas JR, McKnight TR, Wendland MF, Mamot C, Drummond DC, Kirpotin DB, Park JW, Berger MS, Bankiewiez KS. Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res. 2004;64:2572–2579. doi: 10.1158/0008-5472.can-03-3631. [DOI] [PubMed] [Google Scholar]
- 74.Park JA, Lee JJ, Jung JC, Yu DY, Oh C, Ha S, Kim TJ, Chang YM. Gd-DOTA conjugate of RGD as a potential tumor-targeting MRI contrast agent. ChemBioChem. 2008;9:2811–2813. doi: 10.1002/cbic.200800529. [DOI] [PubMed] [Google Scholar]
- 75.Pilch J, Brown DM, Komatsu M, Jarvinen TA, Yang M, Peters D, Hoffman RM, Ruoslahti E. Peptides selected for binding to clotted plasma accumulate in tumor stroma and wounds. Proc Natl Acad Sci U S A. 2006;103:2800–2804. doi: 10.1073/pnas.0511219103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ye FR, Jeong EK, Jia ZJ, Yang TX, Parker D, Lu ZR. A peptide targeted contrast agent specific to fibrin-fibronectin complexes for cancer molecular imaging with MRI. Bioconjugate Chem. 2008;19:2300–2303. doi: 10.1021/bc800211r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye FR, Jeong EK, Parker D, Lu ZR. Evaluation of CLT1-(Gd-DTPA) for MR molecular imaging in a mouse breast cancer model. Chin J Magn Reson Imaging. 2011;2:325–330. [PMC free article] [PubMed] [Google Scholar]
- 78.Artemov D, Mori N, Ravi R, Bhujwalla ZM. Magnetic resonance molecular imaging of the HER-2/neu receptor. Cancer Res. 2003;63:2723–2727. [PubMed] [Google Scholar]
- 79.Artemov D. Molecular magnetic resonance imaging with targeted contrast agents. J Cell Biochem. 2003;90:518–524. doi: 10.1002/jcb.10660. [DOI] [PubMed] [Google Scholar]
- 80.Chen K, Xie J, Chen XY. RGD-human serum albumin conjugates as ffficient tumor targeting probes. Mol Imaging. 2009;8:65–73. [PMC free article] [PubMed] [Google Scholar]
- 81.Qiao JJ, Li SY, Wei LX, Jiang J, Long R, Mao H, Wei L, Wang LY, Yang H, Grossniklaus HE, et al. HER2 targeted molecular MR imaging using a de novo designed protein contrast agent. PLoS One. 2011;6:pii:e18103. doi: 10.1371/journal.pone.0018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paganelli G, Bartolomei M, Ferrari M, Cremonesi M, Broggi G, Maira G, Sturiale C, Grana C, Prisco G, Gatti M, et al. Pre-targeted locoregional radioimmunotherapy with 90Y-biotin in glioma patients: phase I study and preliminary therapeutic results. Cancer Biother Radiopharm. 2001;16:227–235. doi: 10.1089/10849780152389410. [DOI] [PubMed] [Google Scholar]
- 83.Xu H, Regino CAS, Koyama Y, Hama Y, Gunn AJ, Bernardo M, Kobayashi H, Choyke PL, Brechbiel MW. Preparation and preliminary evaluation of a biotin-targeted, lectin-targeted dendrimer-based probe for dual-modality magnetic resonance and fluorescence Imaging. Bioconjugate Chem. 2007;18:1474–1482. doi: 10.1021/bc0701085. [DOI] [PubMed] [Google Scholar]
- 84.Huang RQ, Han L, Li JF, Liu SH, Shao K, Kuang YY, Hu X, Wang XX, Lei H, Jiang C. Chlorotoxin-modified macromolecular contrast agent for MRI tumor diagnosis. Biomaterials. 2011;32:5177–5186. doi: 10.1016/j.biomaterials.2011.03.075. [DOI] [PubMed] [Google Scholar]
- 85.Han LA, Li JF, Huang SX, Huang RQ, Liu SH, Hu X, Yi PW, Shan D, Wang XX, Lei H, et al. Peptide-conjugated polyamidoamine dendrimer as a nanoscale tumor-targeted T1 magnetic resonance imaging contrast agent. Biomaterials. 2011;32:2989–2998. doi: 10.1016/j.biomaterials.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 86.Tan MQ, Wu XM, Jeong EK, Chen QJ, Lu ZR. Peptide-targeted nanoglobular Gd-DOTA monoamide conjugates for magnetic resonance cancer molecular imaging. Biomacromolecules. 2010;11:754–761. doi: 10.1021/bm901352v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Swanson SD, Kukowska-Latallo JF, Patri AK, Chen CY, Ge S, Cao ZY, Kotlyar A, East AT, Baker JR. Targeted gadolinium-loaded dendrimer nanoparticles for tumor-specific magnetic resonance contrast enhancement. Int J Nanomed. 2008;3:201–210. [PMC free article] [PubMed] [Google Scholar]
- 88.Konda SD, Aref M, Wang S, Brechbiel M, Wiener EC. Specific targeting of folate-dendrimer MRI contrast agents to the high affinity folate receptor expressed in ovarian tumor xenografts. Magn Reson Mater Phy Biol Med. 2001;12:104–113. doi: 10.1007/BF02668091. [DOI] [PubMed] [Google Scholar]
- 89.Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71:121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- 90.Lu YJ, Low PS. Folate targeting of haptens to cancer cell surfaces mediates immunotherapy of syngeneic murine tumors. Cancer Immunol Immun. 2002;51:153–162. doi: 10.1007/s00262-002-0266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reddy JA, Xu L-C, Parker N, Vetzel M, Leamon CP. Preclinical evaluation of 99mTc-EC20 for imaging folate receptor–positive tumors. J Nucl Med. 2004;45:857–866. [PubMed] [Google Scholar]
- 92.Parker N, Turk MJ, Westrick E, Lewis JD, Low PS, Leamon CP. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338:284–293. doi: 10.1016/j.ab.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 93.Kamaly N, Kalber T, Thanou M, Bell JD, Miller AD. Folate receptor targeted bimodal liposomes for tumor magnetic resonance imaging. Bioconjugate Chem. 2009;20:648–655. doi: 10.1021/bc8002259. [DOI] [PubMed] [Google Scholar]
- 94.Vaccaro M, Accardo A, D'Errico G, Schillen K, Radulescu A, Tesauro D, Morelli G, Paduano L. Peptides and Gd complexes containing colloidal assemblies as tumor-specific contrast agents in MRI: physicochemical characterization. Biophys J. 2007;93:1736–1746. doi: 10.1529/biophysj.107.107417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Accardo A, Tesauro D, Roscigno P, Gianolio E, Paduano L, D'Errico G, Pedone C, Morelli G. Physicochemical properties of mixed micellar aggregates containing CCK peptides and Gd complexes designed as tumor specific contrast agents in MRI. J Am Chem Soc. 2004;126:3097–3107. doi: 10.1021/ja039195b. [DOI] [PubMed] [Google Scholar]
- 96.Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KCP. Detection of tumor angiogenesis in vivo by anb3-targeted magnetic resonance imaging. Nat Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 97.Storrs RW, Tropper FD, Li HY, Song CK, Kuniyoshi JK, Sipkins DA, Li KCP, Bednarski MD. Paramagnetic polymerized liposomes - synthesis, characterization, and applications for magnetic-resonance-imaging. J Am Chem Soc. 1995;117:7301–7306. [Google Scholar]