Abstract
Exudative epidermitis (EE) is a common skin disease of young pigs, caused mainly by Staphylococcus hyicus. Increased prevalence of EE and poor response to treatment are reported. Common strategies used by Ontario pork producers to treat pigs with EE were determined using a survey. Injection of penicillin G was reported as the most common parenteral antibiotic choice. Antimicrobial resistance patterns of S. hyicus and Staphylococcus aureus isolated from clinical cases (30 herds with samples from approximately 6 pigs per farm) showed that 97% of S. hyicus isolates were resistant to penicillin G and ampicillin; 71% of these isolates were resistant to ceftiofur. Similar resistance was noted among S. aureus isolates. Antimicrobial resistance has become a problem in the treatment of EE in Ontario.
Résumé
Enquête sur l’épidermite exsudative (eczéma séborrhéique du porc) et les tendances d’antibiorésistance deStaphylococcus hyicuset deStaphylococcus aureusisolés des cas cliniques. L’épidermite exsudative (EE) est une maladie cutanée courante des porcelets qui est causée principalement par Staphylococcus hyicus. Une prévalence accrue d’EE et une réponse mitigée au traitement sont signalées. Les stratégies couramment utilisées par les producteurs de porcs pour traiter les porcs atteints d’EE ont été déterminées à l’aide d’un sondage. L’injection de pénicilline G a été signalée comme le choix d’antiobiotique parentéral le plus courant. Les tendances d’antibiorésistance de S. hyicus et de Staphylococcus aureus isolés de cas cliniques (30 troupeaux avec des échantillons provenant d’environ 6 porcs par ferme) ont montré que 97 % des isolats de S. hyicus étaient résistants à la pénicilline G et à l’ampicilline; 71 % de ces isolats étaient résistants au ceftiofur. Une résistance semblable a été signalée pour les isolats de S. aureus. L’antibiorésistance est devenue un problème dans le traitement d’EE en Ontario.
(Traduit par Isabelle Vallières)
Introduction
Exudative epidermitis (EE), commonly known as “greasy pig disease” is a generalized or localized skin disease of piglets characterized by exfoliation, sebaceous exudation, and formation of a crust that may cover the entire body (1). The disease is most commonly caused by strains of Staphylococcus hyicus that produce exfoliative toxins (2,3). Less frequently, it was reported that the disease can also be caused by toxin-producing strains of Staphylococcus aureus and Staphylococcus chromogenes(4,5) since some of their staphylococcal exfoliative toxins can cleave the target molecule, swine desmoglein1 (6,7). Exfoliative toxin-producing staphylococci may also penetrate the epidermis directly since the exfoliative toxins cleave cell-to-cell adhesion in mammalian skin and then destroy the barrier function of the skin, with subsequent blister formation (7). Further, damage from biting (particularly newborns with unclipped needle teeth), or from scratches from rough bedding or rubbing against projections on pen walls, can expose the dermis and facilitate the entry of staphylococci, which are commonly present on pigs and in the environment, to start infection (1,8).
The disease occurs worldwide and is a sporadic endemic problem on most farms, but occasionally, major outbreaks involve large numbers of piglets. The recent trend by the swine industry to discontinue the practice of cutting the tips of needle teeth at birth, coupled with the trend of increased litter size, may lead to a rise in the prevalence of EE. There have been anecdotal reports that the disease has become more common and more difficult to treat.
The main objectives of this study were to determine what treatments for EE were being used in Ontario swine, to isolate S. hyicus and S. aureus from cases of EE, and to determine their antimicrobial resistance profiles.
Materials and methods
Animal use was approved by the University of Guelph Animal Care Committee and was in keeping with Canadian Council of Animal Care Guidelines.
Survey
A survey of pork producers (n = 58) was conducted to obtain information regarding treatment of EE. The researcher completed a questionnaire by interviewing pig farmers who attended a regional trade show (28/58), or alternatively, by interviewing pork producers who participated in a cross-sectional study of farms with cases of EE (30/58). Questions were related to herd type, treatments, and perception of the efficacy of medication, as well as questions about other approaches to control the disease, such as improving hygiene, management changes, and autogenous vaccine use. A survey of swine veterinarians (n = 15) was also conducted in order to obtain their opinions regarding recommendations for treatment and prevention, and whether or not they thought the disease was becoming more difficult to control. The questionnaire was distributed at a regional meeting of Ontario swine veterinarians and was completed during the meeting by all swine veterinarians in attendance. The responding swine veterinarians constituted 53.6% of all Ontario swine veterinarians who practiced in the region (15/28).
Cross-sectional study: Bacterial culture and antimicrobial susceptibility test
Thirty pig farms from southwestern Ontario (Canada) were purposively selected for the study. The inclusion criteria for the cross-sectional study included farms that veterinary practitioners identified as having an outbreak of EE, as well as local farms that were conveniently chosen and identified by the researcher as having pigs with clinical signs of EE. One hundred and eighty-six pigs from the 30 farms were included in the study. An average of 6 pigs per farm (range: 4 to 10) was chosen for sampling. Pigs with localized or systemic clinical signs of EE were chosen. Generally, pigs with the most severe lesions were selected over pigs with mild clinical signs. When large numbers of pigs with clinical disease were present, attempts were made to select from different pens and rooms. However, if only a small number of affected pigs were available, then multiple piglets from the same litter or the same pen were sometimes included. Skin sampling from the facial lesions of pigs affected by EE was accomplished with 1 scraping and 1 swab per pig. Skin scabs from pig facial lesions were scraped into a sterile container by using a melon-baller. The melon-baller was cleaned and disinfected with 70% isopropyl alcohol between pigs. Skin swabs were collected using cotton-tipped swabs after application of 1 mL 0.9% sodium chloride to the lesions. Skin scrapings were placed in empty clean tubes and cotton-tipped swabs were placed in liquid Stuart’s medium and transported in a container in ambient temperature. All samples were submitted on the day of collection by taking them directly to the Animal Health Laboratory (AHL), University of Guelph, Ontario, Canada. Bacterial culture from skin samples and swabs was done, and isolates were identified as S. hyicus and S. aureus by standard laboratory techniques including colony morphology, hemolysis, Gram-stain, catalase reaction, and coagulase reaction. The recovery rates of the 2 pathogens were determined at the herd and pig levels. Antimicrobial susceptibility to penicillin G, ampicillin, ceftiofur, spectinomycin, sulphonamide, tetracycline, tiamulin, and trimethoprim/sulfamethoxazole was determined by the disk diffusion method (Kirby-Bauer Procedure) defined by the Clinical and Laboratory Standards Institute (9). For the purpose of analysing the data, intermediate level was included with the resistant level (4 of the S. aureus isolates). The antimicrobial resistance patterns of S. hyicus and S. aureus isolates were compared between 2 categories of farms: commercial farms that used antibiotics and “antibiotic-free” farms. The first group were the farms on which antibiotics were generally used routinely in-feed and by injection as needed to prevent or treat disease. The second group of farms (“antibiotic-free”) raised pigs for a special market so that pigs from birth-to-market were raised without receiving antibiotics. On these farms, if a pig became sick and needed treatment, it would be identified and removed from the production stream. Seven of the 30 farms in the cross-sectional study were categorized as “antibiotic-free” farms.
Data management and statistical analysis
The survey data from the questionnaires for farmers and swine veterinarians were entered into EpiData Entry v.3 (The EpiData Association, Odense, Denmark) and verified manually for accuracy of entry. Descriptive statistics were carried out using Stata10.1 (Statistics/Data Analysis, Texas, and USA). The results of the antimicrobial susceptibility tests of S. hyicus and S. aureus isolates were entered into Microsoft Office Excel 2007 and subsequently all data were transferred to Stata10.1 for statistical analysis.
A statistical analysis to compare the difference in antimicrobial resistance to tetracycline between S. hyicus and S. aureus isolates was performed using logistic regression with herd as a random effect on the intercept. The dependent variable was the tetracycline-resistance status of an isolate (yes/no) and the independent variable was species designation of Staphylococcus (i.e., S. hyicus or S. aureus).
The association of the prevalence of antimicrobial resistance to each of the antimicrobials (penicillin G, ampicillin, ceftiofur, tetracycline, streptomycin, tiamulin, sulphonamide, and trimethoprim/sulfamethoxazole) of S. hyicus and S. aureus separately between the commercial farms that used antibiotics and the “antibiotic-free” farms was evaluated using 2 sets of univariable Poisson regression models. Number of S. hyicus or number of S. aureus isolates that were resistant to each antimicrobial was used as a dependent variable in a separate univariable Poisson model. Number of antimicrobial susceptibility tests performed for the corresponding staphylococci was used as an offset, and herd-level categorical variable indicating antimicrobial usage on a farm was used as an independent variable (i.e., “antibiotic-free” farms versus commercial farms that used antibiotics).
Results
Survey for treatment of exudative epidemitis
The most common approach to treatment of EE (41/58 farmers) was topical therapy, including mixtures of topical antibiotics, antiseptics, and/or mineral oil, mostly in the form of a spray (Table 1). The most frequently used topical antibiotic treatment was a mixture of procaine penicillinG and novobiocin (Novodry; Pfizer Canada, Kirkland, Quebec) (69%), penicillin G (18.7%) and cephapirin benzathine (Cefadri; Wyeth Animal Health, Guelph, Ontario) plus cloxacillin benzathine (Dry-Clox; Wyeth Animal Health) (6.2%). In addition, 55.2% of respondents (32/58) stated that they used injectable antibiotics and most farmers using this method (93.8%; 30/32) indicated that they preferred to use injectable penicillin G. The other injectable antibiotics chosen by a small number of producers were trimethoprim/sulfadoxine (6.3%; 2/32), ceftiofur (3.1%; 1/32), and streptomycin (3.1%; 1/32). One farmer reported using ivermectin for treatment of clinical cases of EE.
Table 1.
Treatments for exudative epidermitis that farmers (n = 58) and veterinarians (n = 15) stated that they would use or recommend
| Treatment | Farmers (%) | Veterinarians (%) |
|---|---|---|
| Injectable antibiotic only | 17.2 | 6.7 |
| Topical oil only | 12.0 | 0 |
| Topical antibiotic + topical oil + injectable antibiotic | 10.3 | 13.3 |
| Antiseptic + injectable antibiotic | 8.6 | 0 |
| Topical oil + injectable antibiotic | 8.6 | 0 |
| Topical antibiotic + antiseptic + topical oil + injectable antibiotic | 5.2 | 66.7 |
| Topical antibiotic + topical oil | 5.2 | 0 |
| Antiseptic + topical oil + injectable antibiotic | 5.2 | 6.7 |
| Antiseptic + topical oil | 3.4 | 0 |
| Topical antibiotic + antiseptic + injectable antibiotic | 3.4 | 0 |
| Topical antibiotic + antiseptic + topical oil | 1.7 | 6.7 |
| Topical antibiotic + injectable antibiotic | 1.7 | 0 |
| Leave untreated | 17.2 | 0 |
Swine veterinarians commonly recommended novobiocin (66.7%; 10/15) as a topical treatment. In the case of antibiotics for injection, 40% of veterinarians (6/15) recommended penicillin G and 26.7% (4/15) of the veterinarians recommended ceftiofur, followed by 20% (3/15) for trimethoprim/sulfadoxine. Swine veterinarians reported that they also commonly recommended clipping needle teeth (12/15), reducing humidity (6/15), changing ventilation (4/15), and improving hygiene (14/15). Approximately a quarter of the veterinarians (4/15) recommended autogenous vaccines as an aid to controlling EE, but only 5% of the farmers (3/58) considered vaccination to be an option. Five swine practitioner respondents (33.3%) in surveys expressed some concern that response to treatment was poor.
Cross-sectional study: Bacteriology and antimicrobial susceptibility testing
The recovery rate of S. hyicus from skin samples was 76.9% (143/186) and the recovery rate of S. aureus was 48.9% (91/186) based on parallel interpretation of the 2 methods of sampling, skin scraping and skin swabs. Both S. hyicus and S. aureus were cultured from 39.8% of pigs (74/186), whereas S. hyicus was cultured alone from 33.9% of pigs (63/186) and S. aureus was cultured alone from 6.5% of the pigs (12/186). At the farm level, the recovery rate of S. hyicus was 100% (30/30) and the recovery rate of S. aureus was 80% (24/30), based on at least 1 positive isolate from a farm.
The overall antimicrobial resistance profiles are presented in Table 2. Antimicrobial susceptibility testing revealed that most S. hyicus and S. aureus isolates were resistant to the β-lactam antibiotics: penicillin G, ampicillin, and ceftiofur. Over 90% of isolates of S. hyicus and S. aureus were resistant to penicillin G and ampicillin. Over 70% of isolates of S. hyicus and S. aureus were resistant to ceftiofur. Antimicrobial resistance of S. hyicus (55.6%) and S. aureus (87.6%) to tetracycline was also common. Antimicrobial resistance patterns of the 2 pathogens were very similar, except that resistance to tetracycline was higher in S. aureus than in S. hyicus [odds ratio (OR): 14.29, 95% confidence interval (CI): 4.50–47.62, P < 0.01].
Table 2.
Antimicrobial resistance profiles for S. hyicus (n = 142) and S. aureus (n = 89) isolates from pigs with clinical signs of exudative epidermitis (all 30 farms)
| S. hyicus | S. aureus | |||
|---|---|---|---|---|
|
|
|
|||
| Antimicrobial | % Resistant | 95% CI | % Resistant | 95% CI |
| Penicillin G | 97.2 | 94–100 | 92.1 | 86–98 |
| Ampicillin | 97.2 | 94–100 | 92.1 | 86–98 |
| Ceftiofur | 71.1 | 64–77 | 76.4 | 67–85 |
| Spectinomycin | 45.1 | 37–53 | 48.3 | 38–59 |
| Sulfonamide | 8.5 | 4–13 | 13.5 | 6–21 |
| Tetracycline | 55.6 | 47–64 | 87.6 | 81–91 |
| Tiamulin | 31.0 | 23–39 | 15.7 | 8–23 |
| Trimethoprim/sulfa | 2.1 | 0–5 | 0 | N/A |
CI — Confidence interval.
N/A — Not available.
Resistance to 1 or more antimicrobials was detected in 99.3% (142/143) of S. hyicus isolates. Resistance to 5 or more antimicrobials was detected in 40.6% (58/143) of S. hyicus isolates. The most common resistance patterns for S. hyicus isolates were penicillin G-ampicillin-ceftiofur (24.5%, 35/143), penicillin G-ampicillin-ceftiofur-spectinomycin-tetracycline-tiamulin (12.6%, 18/143), penicillin G-ampicillin-spectinomycin-tetracycline-tiamulin (11.2%, 16/143), and penicillin G-ampicillin-ceftiofur-tetracycline (9.1%, 13/143). Resistance to 1 or more antimicrobials was detected in 98.9% (90/91) of S. aureus isolates. Resistance to 5 or more antimicrobials was detected in 39.6% (36/91) of S. aureus isolates. The most common resistance patterns of S. aureus isolates were penicillin G-ampicillin-ceftiofur-tetracycline (28.6%, 26/91), penicillin G-ampicillin-ceftiofur-spectinomycin-tetracycline (22.0%, 20/91), penicillin G-ampicillin-tetracycline (8.8%, 8/91), and penicillin G-ampicillin-ceftiofur (7.7%, 7/91).
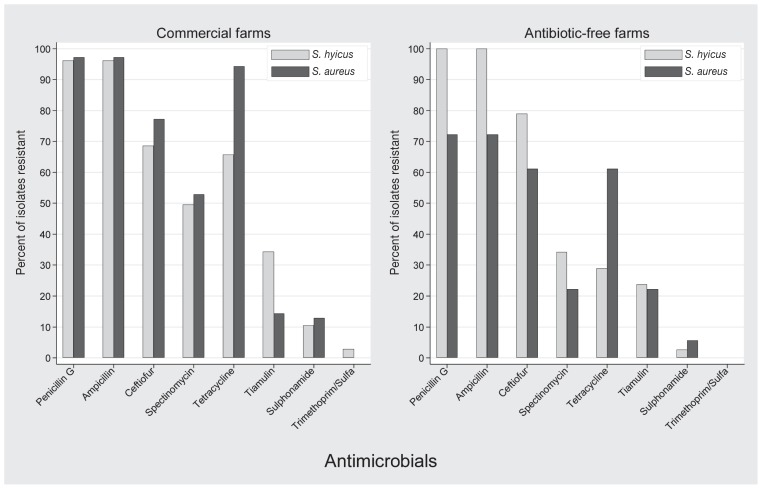
When examined descriptively, the difference in prevalence of resistance of S. aureus isolates to penicillin G and ampicillin between commercial farms that used antibiotics and “antibiotic-free” farms was 24.9% (Figure 1). Similarly, there was a lower prevalence of resistance of S. aureus isolates from “antibiotic-free” farms to all other antimicrobials (with the exception of tiamulin) (Figure 1). In contrast, the prevalence of resistance of S. hyicus isolates from “antibiotic-free” farms to penicillin G, ampicillin, and ceftiofur was numerically higher than for isolates from farms that used antibiotics (Figure 1). However, there was no significant difference in prevalence of antimicrobial resistance of S. hyicus isolates (P > 0.328) or S. aureus isolates (P > 0.486) to any antimicrobial when “antibiotic-free” farms and commercial farms that used antibiotics were compared.
Figure 1.
Antimicrobial resistance profiles of S. hyicus and S. aureus isolates from commercial farms that used antibiotics (n = 23) and isolates from “antibiotic-free” farms (n = 7).
Discussion
Exudative epidermitis (EE) is a sporadic disease that causes significant problems and economic losses on certain farms, particularly those that are newly populated (1). Mortality and morbidity may be high during an outbreak of EE. However, even mild expressions of the disease can negatively influence the price of feeder pigs, because the readily visible skin lesions make weanling pigs with clinical signs of EE difficult to sell. It is possible that recent trends in the industry such as an increase in litter size and a move to not clip needle teeth at birth may be leading to an increase in EE.
The traditional treatment for EE has been the prompt use of antiseptics for wounds or injection of clinically affected pigs with procaine penicillin G (10). The surveys of pork producers and veterinarians demonstrate that penicillin G is still considered an appropriate drug for treatment of EE, but antimicrobial susceptibility results strongly contradict this idea. Studies from other countries have also demonstrated a high level of resistance to penam penicillins among S. hyicus isolates (11–18).
The Danish national monitoring program showed the antimicrobial resistance profiles of bacteria from diagnostic submissions (17). In the report from this program, S. hyicus isolates from cases of skin disease (2001 to 2008) showed a moderately high resistance to penicillin G (60% to approximately 80%) (19). The present study of Ontario pigs shows a much higher proportion of resistance with over 90% of isolates from cases of EE resistant not only to penicillin G, but in most cases, to other members of the β-lactam family of antibiotics, including ampicillin and ceftiofur. These results help to explain the poor response to treatment of EE reported by farmers, because penicillin G as seen in the study was the farmers treatment of choice to resolve EE. There should be more timely and regional antimicrobial resistance profiles to provide guidelines for using effective antimicrobials (20,21).
Overall the antimicrobial resistance patterns for S. hyicus and S. aureus were similar in all isolates from 30 farms; however, tetracycline resistance was more common in S. aureus isolates compared with S. hyicus isolates with the penicillin G-ampicillin-ceftiofur resistance pattern being the most prevalent in S. hyicus isolates and penicillin G-ampicillin-ceftiofur-tetracycline resistance pattern being the most prevalent pattern in S. aureus isolates. When examined descriptively, frequency of resistance of S. aureus to β-lactam antibiotics appeared lower for isolates from “antibiotic-free” farms compared with isolates from farms that used antibiotics. The reverse was true for descriptive examination of S. hyicus isolates: the frequency of resistance to β-lactam antibiotics appeared lower for isolates from commercial farms compared with isolates from “antibiotic-free farms.” It appeared that reduced antibiotic pressure was associated with a reduction of resistance in the S. aureus population but not in the S. hyicus population. Selection of S. aureus strains with resistance plasmids or chromosomally encoded resistance genes might be an explanation of this phenomenon. Information on how long farms had maintained their antibiotic-free status was not available for the present study and this knowledge might have been useful in interpreting the resistance data. In general, one can conclude that whether or not antimicrobials are being used on the farm, S. hyicus and S. aureus will likely be resistant to penam penicillins, at least according to in-vitro testing.
In the present study, the disk diffusion method (Kirby-Bauer Procedure) was used to test for antimicrobial susceptibility of S. hyicus and S. aureus. The antimicrobial susceptibility test results apply to the population of animals on the farm, but not necessarily to individual animals. The disk diffusion method has some limitations in extrapolation of the data to minimum inhibitory concentration (MIC) (22). Furthermore, when we apply the results of antimicrobial susceptibility testing to clinical cases, to achieve better treatment success, pharmacokinetic- pharmacodynamic parameters should be considered: the bound versus unbound state of the agent, tissue versus plasma concentrations, drug degradation over time, variations among micro-organisms, and factors associated with the specific environment at the infection site (23). Failing to consider these parameters contributes to discrepancies between in-vitro results of antimicrobial susceptibility tests and clinical outcomes following using of the selected antimicrobials (24). Ceftiofur was the injectable antimicrobial second most frequently recommended by veterinarians in the present study. This antimicrobial is resistant to penicillinases so that it would seem more likely to be effective in the treatment of a staphylococcal infection that is resistant to penam penicillins. However, ceftiofur is not a good choice for staphylococcal infection because of its relatively high MIC90 (Minimal Inhibitory Concentration needed to inhibit the growth of 90% of the bacterial population, 1.0 μg/mL). In addition, the MIC90 of desfuroylceftiofur (a metabolite of ceftiofur in the body) is 4.0 to 8.0 μg/mL, in contrast to that for other organisms such as Pasteurella multocida and Actinobacillus pleuropneumoniae (MIC90, 0.03 μg/mL). Thus, a higher dosage of ceftiofur is required to treat a S. hyicus infection than to treat other bacterial infections (22).
The antimicrobial agents that were tested in the study were limited; for example, the use of novobiocin was reported frequently, but it was not included in our antimicrobial susceptibility test. Information on antimicrobial resistance of S. hyicus and S. aureus to novobiocin would be useful for farmers and veterinarians.
In conclusion, the likely reason for the poor response to treatment of EE in the southwestern Ontario region in this study was the presence of widespread antimicrobial resistance of S. hyicus and S. aureus isolates, especially to β-lactam antibiotics. Therefore, pork producers and swine veterinarians would benefit from having bacterial culture and antimicrobial susceptibility tests done prior to treating EE diseased pigs; if that is not feasible trimethoprim-sulfa appears to be a reasonable choice in that almost all staphylococcal isolates examined in this study appeared to be susceptible in vitro.
Acknowledgments
The research was funded by Ontario Pork, the Animal Health Strategic Initiative Fund, Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) and the University of Guelph. We are grateful to the farmers who allowed us to sample pigs and who answered our survey, and Brian Bloofield for technical assistance. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Wegener HC, Skov-Jensen EW. Exudative epidermitis. In: Straw BE, Zimmerman JJ, D’Allaire S, Taylor DJ, editors. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Pub; 2006. pp. 675–679. [Google Scholar]
- 2.Tanabe T, Sato H, Sato H, et al. Correlation between occurrence of exudative epidermitis and exfoliative toxin-producing ability of Staphylococcus hyicus. Vet Microbiol. 1996;48:9–17. doi: 10.1016/0378-1135(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 3.Andresen LO. Production of exfoliative toxin by isolates of Staphylococcus hyicus from different countries. Vet Rec. 2005;157:376–378. doi: 10.1136/vr.157.13.376. [DOI] [PubMed] [Google Scholar]
- 4.van Duijkeren E, Jansen MD, Flemming SC, et al. Methicillin-resistant Staphylococcus aureus in pigs with exudative epidermitis. Emerg Infect Dis. 2007;13:1408–1410. doi: 10.3201/eid1309.061268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andresen LO, Ahrens P, Daugaard L, Bille-Hansen V. Exudative epidermitis in pigs caused by toxigenic Staphylococcus chromogenes. Vet Microbiol. 2005;105:291–300. doi: 10.1016/j.vetmic.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Nishifuji K, Fudaba Y, Yamaguchi T, Iwasaki T, Sugai M, Amagai M. Cloning of swine desmoglein 1 and its direct proteolysis by Staphylococcus hyicus exfoliative toxins isolated from pigs with exudative epidermitis. Vet Dermatol. 2005;16:315–323. doi: 10.1111/j.1365-3164.2005.00474.x. [DOI] [PubMed] [Google Scholar]
- 7.Nishifuji K, Sugai M, Amagai M. Staphylococcal exfoliative toxins: “Molecular scissors” of bacteria that attack the cutaneous defense barrier in mammals. J Dermatol Sci. 2008;49:21–31. doi: 10.1016/j.jdermsci.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Nagase NF, Sasaki AF, Yamashita KF, et al. Isolation and species distribution of staphylococci from animal and human skin. J Vet Med Sci. 2002;64:245–250. doi: 10.1292/jvms.64.245. [DOI] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard-third edition. CLSI document. 2008;28:M31–A3. [Google Scholar]
- 10.Drug therapy and prophylaxis. In: Friendship RM, Prescott JF, editors; Straw BR, Zimmerman JJ, D’Allaire S, Taylor DJ, editors. Diseases of Swine. 9th ed. Ames, Iowa: Blackwell Pub; 2006. p. 1131. [Google Scholar]
- 11.Devriese L. Antibiotic susceptibility of Staphylococcus hyicus strains isolated from exudative epidermitis cases in Belgium. Vlaams Diergeneeskd Tijdschr. 1977;46:143–144. [Google Scholar]
- 12.Teranishi H, Shimizu A, Kawano J, Kimura S. Antibiotic resistance of Staphylococcus hyicus subsp. hyicus strains isolated from pigs, cattle and chickens. Nippon Juigaku Zasshi. 1987;49:427–432. doi: 10.1292/jvms1939.49.427. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz S, Blobel H. Isolation and restriction endonuclease analysis of a tetracycline resistance plasmid from Staphylococcus hyicus. Vet Microbiol. 1990;24:113–122. doi: 10.1016/0378-1135(90)90058-4. [DOI] [PubMed] [Google Scholar]
- 14.Schwarz S, Wegener H, Blobel H. Plasmid-encoded resistance to macrolides and lincosamides in Staphylococcus hyicus. J Appl Bacteriol. 1990;69:845–849. doi: 10.1111/j.1365-2672.1990.tb01582.x. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz S, Blobel H. A new streptomycin-resistance plasmid from Staphylococcus hyicus and its structural relationship to other staphylococcal resistance plasmids. J Med Microbiol. 1990;32:201–205. doi: 10.1099/00222615-32-3-201. [DOI] [PubMed] [Google Scholar]
- 16.Aarestrup FM, Bager F, Jensen NE, Madsen M, Meyling A, Wegener HC. Surveillance of antimicrobial resistance in bacteria isolated from food animals to antimicrobial growth promoters and related therapeutic agents in Denmark. APMIS. 1998;106:606–622. doi: 10.1111/j.1699-0463.1998.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 17.Aarestrup FM, Bager F, Jensen NE, Madsen M, Meyling A, Wegener HC. Resistance to antimicrobial agents used for animal therapy in pathogenic-, zoonotic- and indicator bacteria isolated from different food animals in Denmark: A baseline study for the Danish integrated antimicrobial resistance monitoring programme (DANMAP) APMIS. 1998;106:745–770. doi: 10.1111/j.1699-0463.1998.tb00222.x. [DOI] [PubMed] [Google Scholar]
- 18.Wegener HC, Watts JL, Salmon SA, Yancey RJ., Jr Antimicrobial susceptibility of Staphylococcus hyicus isolated from exudative epidermitis in pigs. J Clin Microbiol. 1994;32:793–795. doi: 10.1128/jcm.32.3.793-795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen VF, The DANMAP. 2008: Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, foods and humans in Denmark. Statens Serum Institut. 2008:94–95. [Google Scholar]
- 20.McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clin Infect Dis. 2002;34:S93–S106. doi: 10.1086/340246. [DOI] [PubMed] [Google Scholar]
- 21.Wells SJ, Fedorka-Cray PJ, Dargatz DA, Ferris KF, Green A. Fecal shedding of Salmonella spp. by dairy cows on farm and at cull cow markets. J Food Prot. 2001;64:3–11. doi: 10.4315/0362-028x-64.1.3. [DOI] [PubMed] [Google Scholar]
- 22.Apley MD. Predicting antimicrobial efficacy: Pharmacokinetic data and MICs or “Avoiding really big mistakes and at least getting in the ballpark”. Proc George A Young Swine Conf. 2010:3–42. [Google Scholar]
- 23.Martinez M, Toutain P, Walker RD. The pharmacokinetic-pharmacodynamic (PK/PD) relationship of antimicrobial agents. In: Giguere S, Prescott J, Baggot J, Walker R, Dowling PM, editors. Antimicrobial Therapy in Veterinary Medicine. 4th ed. Ames, Iowa: Blackwell Publishing; 2006. pp. 81–106. [Google Scholar]
- 24.Li RC, Zhu M, Schentag JJ. Achieving an optimal outcome in the treatment of infections: The role of clinical pharmacokinetics and pharmacodynamics of antimicrobials. Clin Pharmacokinet. 1999;37:1–16. doi: 10.2165/00003088-199937010-00001. [DOI] [PubMed] [Google Scholar]