Abstract
The effects of norepinephrine (NOR) infusion on hemodynamic alterations induced with sedative doses of acepromazine (ACP) were evaluated. Infusion of NOR at 1 μg/kg body weight (BW)/minute for 15 min was administered to 5 standing horses 45 min (T45) after intravenous injection of ACP at 0.1 mg/kg BW. Systolic arterial blood pressure (SAP) and hemodynamic parameters were evaluated on the median artery. Parameters were evaluated every 5 min from 45 to 65 min (T65) at 75 (T75), 90 (T90), and 105 (T105) minutes after ACP administration, and the vessel’s surface (SURF), diameter (DIAM), circumference (CIRC), peak systolic velocity (PSV), end diastolic velocity (EDV), mean velocity (MV), volumetric flow (VF) and resistivity index (RI) of the flow were calculated. Acepromazine induced hypotension and vasodilation with a significant rise in SURF, DIAM, CIRC, PSV, EDV, MV, and VF and a reduction in RI and SAP, which were significantly counteracted from T50 to T60 for EDV, VF, MV and RI, and to T65 for SAP, from T50 to T90 for CIRC and SURF and to T60 for DIAM. These findings demonstrate that a 1 μg/kg BW/minute NOR infusion can reverse ACP’s vasodilatory effects, restoring hemodynamic parameters and blood pressure in horses.
Résumé
Évaluation d’altérations hémodynamiques induites par l’acépromazine et inversion par une infusion de norépinéphrine chez des chevaux debout. Les effets d’une infusion de norépinéphrine (NOR) sur les altérations hémodynamiques induites avec des doses sédatives d’acépromazine (ACP) ont été évalués. Une infusion de NOR à 1 μg/kg poids corporel (PC)/minute pendant 15 minutes a été administrée à 5 chevaux debout 45 minutes (T45) après une injection intraveineuse d’ACP à 0,1 mg/kg PC. La tension artérielle systolique (TAS) et les paramètres hémodynamiques ont été évalués sur l’artère médiane. Les paramètres ont été évalués toutes les 5 minutes, de 45 à 65 minutes (T65), puis 75 (T75), 90 (T90) et 105 (T105) minutes après l’administration d’ACP et la surface (SURF), le diamètre (DIAM), la circonférence (CIRC), le pic de vélocité systolique (PVS), la vélocité en fin de diastole (VFD), la vélocité moyenne (VM) et l’écoulement volumétrique (EV) du vaisseau ainsi que l’indice de résistivité (IR) du débit ont été calculés. L’hypotension et la vasodilatation induites par l’acépromazine causant une hausse significative de SURF, de DIAM, de CIRC, de PVS, d’EV, de VM et de EV ainsi qu’une réduction d’IR et de TAS ont été significativement compensées de T50 à T60 pour EDV, VF, MV et RI, à T65 pour SAP, de T50 à T90 pour CIRC et SURF et à T60 pour DIAM. Ces constatations démontrent qu’une infusion de 1 μg/kg PC/minute NOR peut inverser les effets vasodilatoires d’ACP, rétablissant les paramètres hémodynamiques et la tension artérielle chez les chevaux.
(Traduit par Isabelle Vallières)
Introduction
Acepromazine (ACP), a phenothiazine (PHE) commonly used in horses as a sedative agent in preanaesthetic protocols (1), or in the treatment of laminitis (2) has peripheral hemodynamic effects in horses: it induces an increase in the blood flow and has a concomitant vasodilatory effect on the digital vasculature (3,4), metatarsal artery (5), and microcirculation in the coronary band and laminae (4). The peripheral vasodilation, evident after intramuscular injection of 0.05 and 0.055 mg/kg body weight (BW) is the major side effect (5,6). Undesirable effects of ACP are particularly prominent and may become life-threatening when horses suffer from hypotension, anemia, or dehydration (1). In normovolemic and hemodynamically stable horses, the drop in blood pressure is without major consequences, but the risk of hypotensive crisis and subsequent collapse is high if the patient shows volume depletion (7). This low blood pressure results from blockade of the α1-adrenergic receptors (8) or from depression of the central vasomotor center (9).
Other pharmacological properties of ACP are anti-inflammatory and antioxidant effects (2). Acepromazine diminishes monocyte TNF-α production, inhibits the differentiation of monocytes into macrophages (10,11), and decreases the production of reactive oxygen species (2,12). These properties account for the therapeutic value of ACP in equine patients with inflammatory diseases. However, in order to safely administer ACP to high-risk patients, it is essential that a hypotensive crisis be avoided.
Vasoconstrictor drugs, including α-sympathomimetics, such as norepinephrine (NOR) may help to modulate the vasodilatory effects of ACP. Norepinephrine is an endogenous catecholamine, acting as a sympathetic neural and humoral transmitter in most mammalian species (13), having a potent agonist effect on α1- and α2-adrenergic receptors, a lesser effect on β1-adrenergic receptors, and a minor effect on β2-adrenergic receptors (14). Overall, NOR action results in an intense vasopressor effect that induces an increase in systolic and diastolic arterial blood pressures, which translates into positive inotropic and chronotropic effects (13). Therefore, NOR is primarily used for its intense vasoconstrictor effect in the hemodynamic management of horses under anesthesia, specifically when low arterial blood pressure is refractory to dobutamine (15) or when horses suffer from hyperdynamic shock, in which intravascular fluid resuscitation fails to reverse a low mean arterial blood pressure and systemic vascular resistance is decreased (14).
There is controversy regarding the side effects of NOR. Some authors have noted a decrease in hepatosplanchnic (16) and renal (17) perfusion in patients under normal circulatory conditions; however, others have observed no alterations in renal function in normotensive foals, at a dose of 0.1 μg/kg BW/min (18). In addition, improved mean arterial blood pressure and renal function have been described in hyperdynamic shock in septic humans (19), sheep (20), dogs (17), and foals, in doses ranging from 0.1 to 1.5 μg/kg BW/min (21). The authors are not aware of any pharmacological antagonists to counteract acepromazine side effects in horses. Thus, the objective of this study was to determine if the hemodynamic effects induced by the intravenous administration of ACP at 0.1 mg/kg BW can be reversed by a continuous infusion of NOR at 1 μg/kg BW/min, which may allow a safer application of ACP to patients whose cardiovascular systems are compromised.
Materials and methods
The experimental protocol used in this study was approved by the animal ethical committee of the University of Liège. Five healthy resident research mares, 12 to 27 years old [20 ± 6 y, mean ± standard deviation (SD)] with weight between 420 and 620 kg (478 ± 81 kg) were used. All mares were housed in stocks, to which they were accustomed, and had the same management, health care, and follow-up. They were all acclimatized to the techniques employed in this study before it started. The mares had not been used in any other research for 2 wk prior to the study.
The mares were clipped before leaving the stocks. The trials took place in a quiet examination room for the setup and duration of the experiments. The setup of the experiments took about 15 min and immediately afterwards baseline measurements were done, followed directly by the administration of ACP.
Peripheral hemodynamic variables were measured ultrasonographically, with horses fully weight-bearing on all 4 limbs. Images were taken from the median artery of the left forelimb, immediately below the chestnut, after shaving the medial aspect of the region, using a Phased Array 7 MHz probe coupled to the ultrasound machine (model RT 6800; General Electric, Brussels, Belgium). No offset pad was used but a copious amount of coupling gel was applied. The artery was initially examined in B-mode, in transverse and then in longitudinal planes. The Doppler sample volume was placed centrally within the vessel in order to obtain the velocity waveforms. The angle between the probe and the vessel was always below 55° and the velocity waveforms chosen were those that presented the clearest visual and acoustic signal, and were the most homogenous. For each parameter evaluated, the mean of 3 successive measurements (throughout at least 10 cardiac cycles) was the final value retained.
From a B-mode ultrasonography, the hemodynamic parameters measured included the diameter (DIAM), circumference (CIRC), and surface (SURF) of the vessel. From the Doppler images, the peak systolic velocity (PSV) and the end diastolic velocity (EDV) were measured by placing the cursor at the apex of the maximal upward motion of blood flow, during systole, and at the minimal velocity of blood flow, during the end of diastole, respectively. The area under the velocity waveform (VTI) was measured by tracing the modal velocity envelope, represented by the brightest line in the spectral Doppler waveform. The heart rate (HR) was calculated by counting the number of Doppler curves per minute and the mean velocity (MV), volumetric flow (VF), and resistivity index (RI) were calculated using the following formulae:
The arterial velocity waveforms were also morphologically analyzed for shape of the systolic peak and amount and direction of blood flow during diastole.
Heart rate, cardiac rhythm, and a continuous base-apex ECG were continuously recorded by a Holter monitor (model Vista; Verimed Medical Supply, Wetteren, Belgium) during the study. Systolic arterial blood pressure was indirectly and manually measured at the tail, using an ultrasonic Doppler flow detector (model 811-B; Park’s Medical Electronics, 9.5 MHz probe) together with a 10 cm width cuff and a manometer. The SAP values were the mean of 3 consecutive measurements.
Before the beginning of the study, a 16 G catheter was aseptically inserted in the left jugular vein, in a clipped site, previously anesthetized with lidocaine 2%. Each mare received an intravenous bolus of ACP (Combistress; Kela Laboratoria, Hoogstraten, Belgium), 0.1 mg/kg BW and 45 min later an intravenous infusion of NOR (Levophed; Abbot N.V., Wavre, Belgium), 1 μg/kg BW/min for 15 min. Norepinephrine infusion would be interrupted if 1 of the following situations was presented: a HR higher than 60 beats/min, an increase in the SAP over 180 mmHg, abnormalities in cardiac rhythm, or excitement. The choice of the dose of NOR was based on a preliminary protocol, in which the mares received only NOR without ACP premedication.
The SAP was recorded immediately before administration of ACP (T0) and every 15 min for 45 min after administration. Then the infusion of NOR was immediately started and maintained for 15 min while the SAP was measured every 5 min and ultrasound images were taken. At the end of the NOR infusion SAP continued to be regularly measured until T105 and ultrasound images continued to be taken until T90. Overall SAP was measured at T0, T15, T30, T45, T50, T55, T60, T65, T75, T90, and T105 and ultrasonographic images of the median artery of the left forelimb were taken at T0, T45, T50, T60, T65, and T90 (Figure 1).
Figure 1.
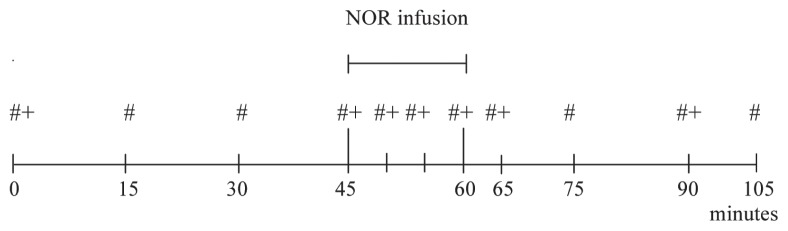
Chronology of the study. Baseline measures were done, immediately before the intravenous administration of acepromazine at 0.1 mg/kg BW, at 0 minutes; from 45 to 60 minutes the norepinephrine infusion was administered at 1 μg/kg BW/minute.
# SAP measure; + ultrasonographic images.
Data were analyzed using Statistical Analysis System software (SAS Institute, Cary, North Carolina, USA) with a mixed model (PROC MIXED). This model evaluates the variations over time induced by ACP, from T0 to T45, and by NOR, from T50 to T60, and takes into consideration repeated measures on different individuals. The Shapiro-Wilk test was used to evaluate normality. When normality failed, a Friedman’s test was conducted, as an alternative to analysis of variance (ANOVA), to evaluate the differences between repeated measures. Statistical differences were denoted at P < 0.05 with values expressed as least square means ± standard deviation (SD).
Results
Acepromazine induced a significant drop in the SAP from T15 to T45. However, during the NOR infusion, the SAP significantly increased at T50, T55 and T60, compared with T0 and T45, respectively. At T65 the SAP rapidly decreased, but remained statistically higher compared to T45. From T75 to T105, SAP was no longer statistically higher than at T45. From T90 onwards only the effect of ACP was present (Figure 2, Table 1).
Figure 2.
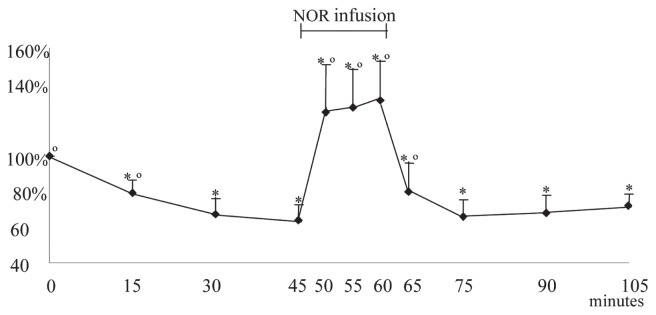
Mean (± SD) of the variation percentage of the systolic arterial pressure before, during, and after norepinephrine (NOR) infusion. At 0 minutes acepromazine at 0.1 mg/kg BW was administered intravenously; from 45 to 60 minutes the norepinephrine (NOR) infusion was administered at 1 μg/kg BW/minute.
* significantly different from T0. ∘ significantly different from T45.
Table 1.
Values for systolic arterial pressure (SAP) before (T0) and 45 minutes after (T45) administration of acepromazine (ACP), during continuous infusion of norepinephrine (NOR) between T45 and T60 and after NOR infusion, from T60. Results are given as mean ± SD
| Time ACP/NOR | SAP (mmHg) |
|---|---|
| T0 | 108.9∘ ± 11.24 |
| T15 | 85.3*∘ ± 4.31 |
| T30 | 72.4* ± 6.68 |
| T45 | 68.7* ± 7.39 |
| T50 | 133.7*∘ ± 24.98 |
| T55 | 137.3*∘ ± 20.77 |
| T60 | 141.0*∘ ± 19.53 |
| T65 | 86.3*∘ ± 11.27 |
| T75 | 71.1* ± 9.31 |
| T90 | 73.9* ± 9.52 |
| T105 | 78.3* ± 5.97 |
Each value of SAP is significantly different (P < 0.05) from T0 if marked with * and from T45 if marked with ∘.
Acepromazine induced a significant increase in the DIAM, CIRC and SURF, at T45, in comparison with T0. However, during the NOR infusion, those parameters significantly decreased from T45, and became similar to the values measured at T0. At T65 and T90; the CIRC and SURF, although numerically increased, stayed statistically smaller than at T45, while the DIAM, at T65 and T90, increased again, to values similar to those at T45 (Figure 3, Table 2).
Figure 3.
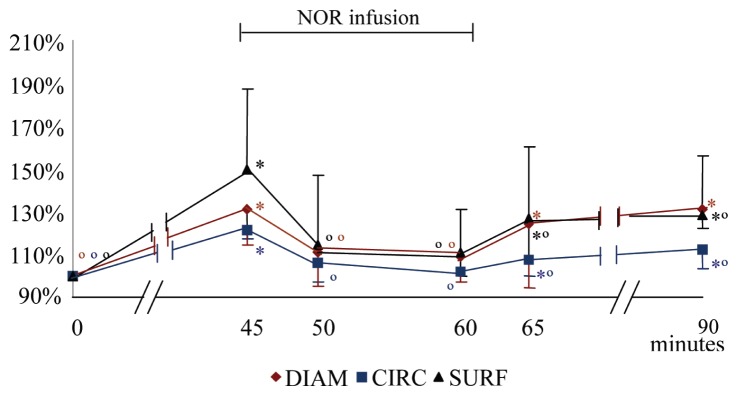
Mean (± SD) of the variation percentage of the diameter (DIAM), circumference (CIRC) and surface (SURF) of the median artery before, during, and after norepinephrine infusion (NOR). At 0 minutes acepromazine at 0.1 mg/kg BW was administered intravenously; from 45 to 60 minutes the norepinephrine (NOR) infusion was administered at 1 μg/kg BW/minute.
* significantly different from T0. ∘ significantly different from T45.
Table 2.
Values for surface (SURF), circumference (CIRC), diameter (DIAM), peak systolic velocity (PSV), end diastolic velocity (EDV), mean velocity (MV), volumetric flow (VF) and resistivity index (RI) of the left median artery with time, before (T0) and 45 minutes after (T45) administration of acepromazine (ACP), during continuous infusion of norepinephrine (NOR) between T45 and T60 and after NOR infusion, from T60. Results are given as mean ± SD
| Time | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| ACP/NOR | T0 | T45 | T50 | T60 | T65 | T90 |
| DIAM (cm) | 0.30∘ ± 0 | 0.393* ± 0.04 | 0.333∘ ± 0.04 | 0.327∘ ± 0.03 | 0.373* ± 0.07 | 0.393* ± 0.03 |
| CIRC (cm) | 1.113∘ ± 0.04 | 1.347* ± 0.05 | 1.18∘ ± 0.08 | 1.133∘ ± 0.06 | 1.193*∘ ± 0.14 | 1.247*∘ ± 0.09 |
| SURF (cm) | 0.085∘ ± 0.01 | 0.127* ± 0.03 | 0.095∘ ± 0.02 | 0.093∘ ± 0.02 | 0.107*∘ ± 0.03 | 0.107*∘ ± 0.02 |
| PSV (m/s) | 0.294∘ ± 0.06 | 0.427* ± 0.15 | 0.456* ± 0.24 | 0.440* ± 0.17 | 0.473* ± 0.14 | 0.482* ± 0.14 |
| EDV (m/s) | 0.041∘ ± 0.02 | 0.165* ± 0.07 | 0.095∘ ± 0.11 | 0.078∘ ± 0.08 | 0.148* ± 0.08 | 0.172* ± 0.09 |
| MV (m/s) | 0.095∘ ± 0.03 | 0.265* ± 0.15 | 0.159 ± 0.13 | 0.141 ± 0.08 | 0.330* ± 0.24 | 0.261* ± 0.09 |
| VF (mL/min) | 47.51∘ ± 14.93 | 202.47* ± 139.35 | 87.76∘ ± 61.43 | 76.86∘ ± 37.31 | 207.13* ± 152.92 | 166.02* ± 60.82 |
| RI | 0.864∘ ± 0.04 | 0.618* ± 0.04 | 0.825∘ ± 0.09 | 0.845∘ ± 0.08 | 0.699* ± 0.09 | 0.655* ± 0.12 |
Each value is significantly different (P < 0.05) from T0 if marked with * and from T45 if marked with ∘.
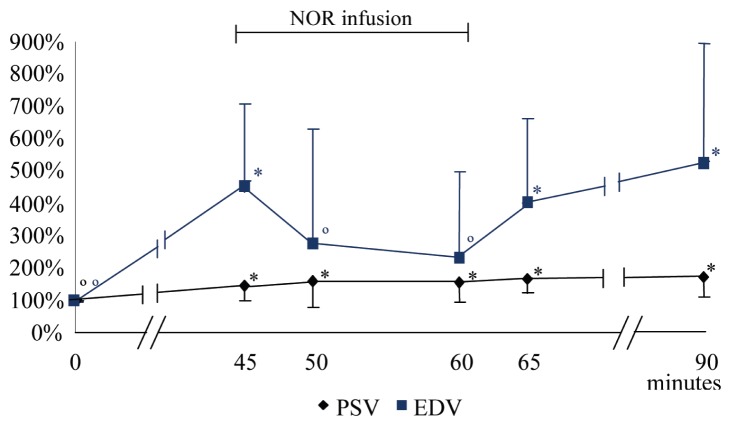
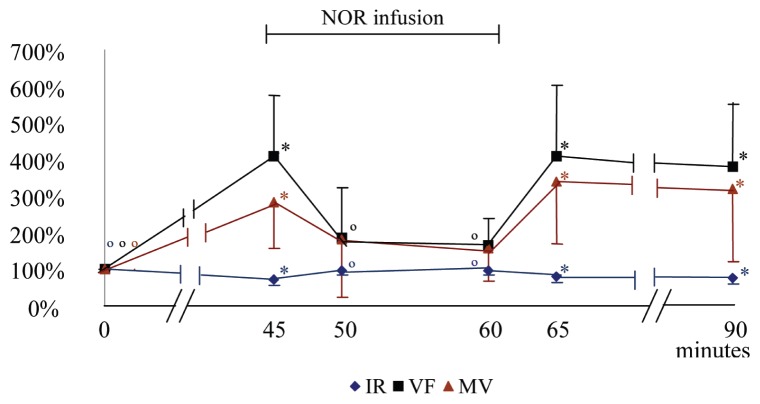
At T45, the PSV, EDV, MV, and VF significantly increased, while RI significantly decreased, compared with T0 values. During the NOR infusion, at T50 and T60, the EDV and VF significantly diminished with regard to T45, and the RI increased, with these parameters becoming similar to the values recorded at T0. From T45, PSV continuously increased compared with T0; however, the difference wasn’t significant and during this period it did not statistically change with respect to T45. The MV had a tendency to decrease at T50 (P < 0.093) and T60 (P < 0.052), compared to T45. When the NOR infusion ended, all hemodynamic parameters approached values previously recorded at T45 (Figures 4 and 5; Table 2).
Figure 4.
Mean (± SD) of the variation percentage of the peak systolic velocity (PSV) and the end diastolic velocity (EDV) of the median artery before, during, and after norepinephrine infusion (NOR). At 0 minutes acepromazine at 0.1 mg/kg BW was administered intravenously; from 45 to 60 minutes the norepinephrine (NOR) infusion was administered at 1 μg/kg BW/minute.
* different from T0. ∘ significantly different from T45.
Figure 5.
Mean (± SD) of the variation percentage of the resistivity index (RI), volumetric flow (VF), and mean velocity (MV) of the median artery before, during, and after norepinephrine infusion (NOR). At 0 minutes acepromazine at 0.1 mg/kg BW was administered intravenously; from 45 to 60 minutes the norepinephrine (NOR) infusion was administered at 1 μg/kg BW/minute.
* significantly different from T0. ∘ significantly different from T45.
The Doppler waveforms observed at T0 were biphasic, with a small spectral window and no reverse flow, which is characteristic of an intermediate resistance flow pattern. During systole, there was a sharp rise in flow velocity and a rapid decline toward baseline, and during diastole an oscillatory portion with alternating acceleration and deceleration of the antegrade blood flow. At T45, the waveforms matched a low resistance flow pattern, with a diastolic velocity higher than at T0, and a smaller Doppler shift on the initial alternating acceleration-deceleration phase. During the NOR infusion the Doppler curves indicated a smaller diastolic velocity, comparable to those from T0.
During the study, horses appeared sedated after ACP administration; those signs were absent during NOR infusion and reappeared after it, with less magnitude. None of the mares had excessively high arterial blood pressures or arrhythmias and all recovered uneventfully after completing the experimental protocol.
Discussion
The present study confirms the hemodynamic effects of intravenous ACP administration and reveals that NOR infusion at 1 μg/kg BW/min can reverse ACP’s vasodilatory effects, restoring hemodynamic parameters and blood pressure in a group of healthy adult horses. Throughout the study, the horses were tranquilized; however, as evaluation of sedation was not an objective, the study only evaluated the hemodynamic effect of ACP and NOR at the given doses.
Acepromazine’s vasodilatory properties can be expressed through a significant decrease in SAP and RI, and a significant increase in DIAM, CIRC, SURF, VF, PSV, EDV, and MV. In this study, such responses were observed during the period in which horses were exclusively under the effect of ACP, before, from T0 to T45, and after, from T65 onwards, NOR infusion. Results from our study are in agreement with those previously published and confirm a decrease in SAP (23,24), and an increase in DIAM (5,25) after ACP administration. The augmentation of CIRC and SURF, from T0 to T45, observed in the current study confirms results from previous reports, demonstrating that ACP administration induces a common vasodilatory effect (3). As well there was augmentation of VF, at T45 in the current study, previously reported in healthy horses after administration of ACP (3,5,24). The augmentation of VF has been associated with increases in the vessel diameter and the maximal flow velocity and a decrease in the peripheral resistance (22), which are compatible with the augmentation in PSV and MV and the diminution of RI. Additionally, the present study demonstrates that the morphology of Doppler waveforms varies in parallel, as it is consistent with vasodilation observed after ACP administration, when waveforms turn from an initial intermediate resistance flow pattern to a low resistance one. When compared with an intermediate resistance flow, the low resistance pattern is characterized by a higher diastolic velocity, and a greater amount of diastolic flow, which is consistent with peripheral vasodilation (26) and diminution of the Doppler shift following the systolic peak and lower pulsatility waveform (27).
Forty-five minutes after ACP administration, when all the signs of vasodilation were present in the horses, a 15-minute NOR infusion progressively reversed ACP’s effect on the majority of parameters studied. Indeed, between T50 and T65, the SAP not only significantly increased after NOR infusion, but became higher than the values recorded at T0, which agrees with results previously reported in foals (15,18,28), dogs (17,29), sheep (20), and humans (30,31). The NOR infusion induced a significant increase in SAP for 15 min and then was progressively attenuated from T65 onwards. Based on clinical experience, similar reports have been cited with adult horses (15), and are in agreement with our results. As the values of SAP became higher during NOR infusion than at T0, we could question if the doses of NOR should be lower. To determine this, further studies could investigate the effect of different doses of NOR administered to horses under ACP premedication. During the period of NOR infusion, after T45, the CIRC and the SURF significantly decreased, from T65 to T90 and the DIAM from T45 to T65, proving there was an induced vasoconstriction and being in agreement with the significant diminution of EDV and the VF (32), while the increase in peripheral resistance was supported by an increase in RI (33). Besides, during NOR infusion, the PSV and the MV did not statistically change, with respect to T45; only MV had a tendency to decrease, from T50 to T60, which is also consistent with vasoconstriction (32). When analyzing changes in the morphology of Doppler waveforms during NOR infusion, with respect to T45, these are compatible with an augmentation in the vascular resistance, resulting in vasoconstriction. This effect can occur through augmentation of the oscillations, and consequent higher pulsatility (27), or through a smaller diastolic velocity (32), as observed.
Overall, once NOR infusion was completed, at T60, apart from CIRC and SURF, the rest of the parameters that varied during NOR infusion, returned to values similar to those at T45, when the only effect was vasodilation from ACP’s action. Consequently throughout a NOR infusion of 1 μg/kg BW/min the hypotension induced by ACP can be reversed.
Practically, the use of NOR to counteract hemodynamic effects of ACP may seem extreme, but results from our study provide equine clinicians and anesthesiologists a new clinical approach to counteract those effects, in particular in patients intolerant to ACP. To the authors’ knowledge the hemodynamic response on standing adult hypotensive horses to a NOR infusion has not been reported previously. As a consequence of α-adrenergic activity of NOR, which primarily causes vasoconstriction (13), the effects observed in the present study are those expected. Furthermore, the fact that no sign of epinephrine reversal was observed, following the concomitant administration of ACP and NOR, confirms the minor β2-adrenergic receptor activation by NOR. Additionally the infusion of NOR not only reverted ACP’s α-adrenergic blocking effect, but also induced a significantly higher SAP compared with the baseline values. The present study appears to be the first to report such results, where there are no significant changes from the baseline of systemic arterial blood pressure, following the administration of a different α-adrenergic agonist drug, such as romifidine, subsequent to ACP’s administration, although romifidine is known to induce a transitory initial period of arterial hypertension (34).
The capacity of NOR to reverse ACP’s induced hypotension could eventually allow a better and more frequent use of ACP in the pre-anaesthetic medication protocol in some patients, permitting equine practitioners to take advantage of the protective effect of ACP during general anesthesia (35). Indeed, when horses from the same group at risk received an α2-adrenergic receptor agonist for preanesthetic medication, there was a reduction in the cardiac output (36). It would be of interest to evaluate the effect of using a reduced dose of preanesthetic α2-adrenergic receptor agonist, concurrent with ACP administration, as an alternative protocol option in horses. This could be possible if NOR administration could be used to modulate the hypotensive and vasodilatory effects of ACP, although this administration would need to be closely monitored.
During the preliminary phase of this experiment, the same 5 mares that received an intravenous infusion of NOR at 1 μg/kg BW/min, without being premedicated with ACP, showed second degree atrioventricular blocks (2AVB). Although other types of arrhythmia can be linked to sympathetic activation (37,38), to the authors’ knowledge, the induction of 2AVB by NOR administration has not been reported. Additionally, when these mares were premedicated with ACP before the infusion of NOR, they developed a statistically lower frequency of 2AVB. It has been reported that ACP has the capacity to increase the arrhythmogenic dose of epinephrine (39), thus the ability of ACP to enhance the baroreceptor reflex (40), which mediates rapid changes in sympathetic and parasympathetic activity in response to changes in blood pressure (41), is most probably related to the ACP’s protective effect observed in this study. It is possible that the benefits of ACP are related to its α-adrenergic blocking effect and vasodilation; therefore, the NOR infusion, even when allowing a safer ACP administration to horses at risk, could reduce the beneficial properties induced by ACP. Studies are needed to investigate maintenance of the beneficial effects of ACP, when administered with NOR, to counteract its hemodynamic effects.
In the present study, the use of Doppler ultrasonography proved to be sensitive enough to detect vascular and hemodynamic alterations in the standing horse, while evaluating the effect of drugs with opposite properties. These results help to confirm that Doppler ultrasonography can be successfully applied as a noninvasive technique to measure hemodynamic changes in horses with blood flow alteration disorders, and promote its use in studies to control the hemodynamic effects of treatments on the standing horse.
We conclude that a 15-minute continuous infusion of NOR at 1 μg/kg BW/min has the capacity to reverse the hypotension and vasodilation induced by ACP at 0.1 mg/kg BW, restoring hemodynamic parameters in healthy standing horses. In particular, the significant rise in SURF, DIAM, CIRC, PSV, EDV, MV, and VF and the reduction in RI and SAP which were significantly counteracted from T50 to T60 for EDV, VF, MV, and RI, and to T65 for SAP, from T50 to T90 for CIRC and SURF, and from T50 to T60 for DIAM.
Acknowledgment
We thank Dr. Fabrice Péters for his contribution to this study. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.McKelvey D, Hollingshead W. The preanesthetic period. In: McKelvey D, Hollingshead W, editors. Veterinary Anaesthesia and Analgesia. 3rd ed. St. Louis, Missouri: Mosby; 2003. pp. 1–50. [Google Scholar]
- 2.Péters F, Franck T, Pequito M, et al. In vivo administration of acepromazine or promethazine to horse decreases the reactive oxygen species production response of subsequently isolated neutrophils to stimulation with phorbol myristate acetate. J Vet Pharmacol Ther. 2009;32:541–547. doi: 10.1111/j.1365-2885.2009.01077.x. [DOI] [PubMed] [Google Scholar]
- 3.Hunt RJ, Brandon CI, McCann ME. Effects of acetylpromazine, xylazine, and vertical load on digital arterial blood flow in horses. Am J Vet Res. 1994;55:375–378. [PubMed] [Google Scholar]
- 4.Adair HS, III, Goble DO, Shires GMH, et al. Evaluation of laser Doppler flowmetry for measuring coronary band and laminar microcirculatory blood flow in clinically normal horses. Am J Vet Res. 1994;55:445–449. [PubMed] [Google Scholar]
- 5.Walker M, Geiser D. Effects of acetylpromazine on the haemodynamics of the equine metatarsal artery, as determined by two-dimensional real-time and pulsed Doppler ultrasonography. Am J Vet Res. 1986;47:1075–1078. [PubMed] [Google Scholar]
- 6.Parry BW, Anderson GA, Gay CC. Hypotension in the horse induced by acepromazine maleate. Aust Vet J. 1982;59:148–152. doi: 10.1111/j.1751-0813.1982.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 7.Muir WW., III . Equine Anesthesia Monitoring and Emergency Therapy. St. Louis, Missouri: Mosby; 1991. Anesthetic complications and cardiopulmonary resuscitation in the horse; pp. 461–483. [Google Scholar]
- 8.Mason D. Anesthetics, tranquilizers and opioid analgesics. In: Bertone JJ, Horspool LJI, editors. Equine Clinical Pharmacology. Edinburgh, UK: Saunders; 2004. pp. 267–309. [Google Scholar]
- 9.Brock N. Acepromazine revisited. Can Vet J. 1994;35:458–459. [PMC free article] [PubMed] [Google Scholar]
- 10.Serteyn D, Deby-Dupont G, Péters F, Deby C. Acepromazine modulates the cell differentiation: In vitro experiments on monocytes stimulated by Chlamydia pneumoniae toxins. Proc Spring Meet Assoc Vet Anaesth. 2001;46 doi: 10.1046/j.1467-2987.2001.00064.x-i10. [DOI] [PubMed] [Google Scholar]
- 11.Serteyn D, Deby-Dupont G, Péters F, Deby C. Comparative study of the effects of acepromazine and structurally related compounds on the TNF-α release by monocytes stimulated by Chlamydia pneumoniae. Proc Autumn Meet Assoc Vet Anaesth. 2002:35. [Google Scholar]
- 12.Sandersen C, Mouithys-Mickalad A, de la Rebière G, Deby G, Serteyn D, Franck T. Modulating effects of acepromazine on the reactive oxygen species production by stimulated equine neutrophils. Vet Anaesth Analg. 2011;38:83–93. doi: 10.1111/j.1467-2995.2010.00583.x. [DOI] [PubMed] [Google Scholar]
- 13.Adams HR. Adrenergic agonists and antagonists. In: Riviere JE, Papich MG, editors. Veterinary Pharmacology and Therapeutics. 9th ed. Ames, Iowa: Wiley-Blackwell; 2009. pp. 125–156. [Google Scholar]
- 14.Corley KT. Inotropes and vasopressors in adults and foals. Vet Clin North Am Equine Pract. 2004;20:77–106. doi: 10.1016/j.cveq.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Craig CA, Haskins SC, Hildebrand SV. The cardiopulmonary effects of dobutamine and norepinephrine in isoflurane-anesthetized foals. Vet Anaesth Analg. 2007;34:377–387. doi: 10.1111/j.1467-2995.2006.00304.x. [DOI] [PubMed] [Google Scholar]
- 16.Reinelt H, Radermacher P, Kiefer P, et al. Impact of exogenous beta-adrenergic receptor stimulation on hepatosplanchnic oxygen kinetics and metabolic activity in septic shock. Crit Care Med. 1999;27:325–331. doi: 10.1097/00003246-199902000-00039. [DOI] [PubMed] [Google Scholar]
- 17.Bellomo R, Kellum JA, Wisniewski SR, Pinsky MR. Effects of norepinephrine on the renal vasculature in normal and endotoxemic dogs. Am J Respir Crit Care Med. 1999;159:1186–1192. doi: 10.1164/ajrccm.159.4.9802055. [DOI] [PubMed] [Google Scholar]
- 18.Hollis AR, Ousey JC, Palmer L, Stoneham SJ, Corley KTT. Effects of norepinephrine and a combined norepinephrine and dobutamine infusion on systemic haemodynamics and indices of renal function in normotensive neonatal thoroughbred foals. J Vet Intern Med. 2006;20:1437–1442. doi: 10.1892/0891-6640(2006)20[1437:eonaac]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Bellomo R, Di Giantomasso D. Noradrenaline and the kidney: Friends or foes? Crit Care. 2001;5:294–298. doi: 10.1186/cc1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Giantomasso D, May CN, Bellomo R. Norepinephrine and vital organ blood flow during experimental hyperdynamic sepsis. Intensive Care Med. 2003;29:1774–1781. doi: 10.1007/s00134-003-1736-9. [DOI] [PubMed] [Google Scholar]
- 21.Corley KT, McKenzie HC, Amoroso LM, Furr MO. Initial experience with norepinephrine infusion in hypotensive critically ill foals. J Vet Emerg Crit Care. 2000;10:267–276. [Google Scholar]
- 22.Schumcker N, Schatzmann U, Budde K, Gundel M, Jäggin C, Meier H. Duplex-ultrasonographic evaluation of the common carotid artery in the resting, sedated and anesthetized horse. Vet Radiol Ultrasound. 2000;41:168–171. doi: 10.1111/j.1740-8261.2000.tb01472.x. [DOI] [PubMed] [Google Scholar]
- 23.Miller PJ, Martin ICA, Kohnke JR, Rose RJ. Responses of horses to acepromazine maleate administered orally in a paste. Res Vet Sci. 1987;42:318–325. [PubMed] [Google Scholar]
- 24.Leise BS, Fugler LA, Stokes AM, Eades SC, Moore RM. Effects of intramuscular administration of acepromazine on palmar digital blood flow, palmar digital arterial pressure, transverse facial arterial pressure, and packed cell volume in clinically healthy, conscious horses. Vet Surg. 2007;36:717–723. doi: 10.1111/j.1532-950X.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- 25.Menzies-Gow NJ. Effects of sedation with acepromazine on echocardiographic measurements in eight healthy thoroughbred horses. Vet Rec. 2008;163:21–25. doi: 10.1136/vr.163.1.21. [DOI] [PubMed] [Google Scholar]
- 26.Raisis AL, Young LE, Blissit KJ, et al. A comparison of the haemodynamic effects of isoflurane and halotane anaesthesia in horses. Equine Vet J. 2000;32:318–326. doi: 10.2746/042516400777032282. [DOI] [PubMed] [Google Scholar]
- 27.Szatmári V, Sótonyi P, Voros K. Normal duplex doppler waveforms of major abdominal blood vessels in dogs: A review. Vet Radiol Ultrasound. 2001;42:93–107. doi: 10.1111/j.1740-8261.2001.tb00911.x. [DOI] [PubMed] [Google Scholar]
- 28.Valverde A, Giguère S, Sanchez LC. Effects of dobutamine, norepinephrine, and vasopressin on cardiovascular function in anesthetized neonatal foals with induced hypotension. Am J Vet Res. 2006;67:1730–1737. doi: 10.2460/ajvr.67.10.1730. [DOI] [PubMed] [Google Scholar]
- 29.Peng ZY, Critchley LA, Fok BS. The effects of increasing doses of noradrenaline on systemic and renal circulations in acute bacteraemic dogs. Intensive Care Med. 2005;31:1558–1563. doi: 10.1007/s00134-005-2741-y. [DOI] [PubMed] [Google Scholar]
- 30.Richer M, Robert S, Lebel M. Renal haemodynamics during norepinephrine and low-dose dopamine infusions in man. Crit Care Med. 1996;24:1150–1156. doi: 10.1097/00003246-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Vincent JL, Biston P, Devriendt J, Bresseaur A, De Backer D. Dopamine versus norepinephrine: Is one better? Minerva Anesthesiol. 2009;75:333–337. [PubMed] [Google Scholar]
- 32.Raisis AL, Young EL, Meire HB, et al. Measurements of hindlimb blood flow recorded using Doppler ultrasound during administration of vasoactive agents in halothane-anesthetized horses. Vet Radiol Ultrasound. 2000;41:64–72. doi: 10.1111/j.1740-8261.2000.tb00429.x. [DOI] [PubMed] [Google Scholar]
- 33.Oates C. What the Doppler spectral waveforms show. In: Oates C, editor. Cardiovascular Haemodynamics and Doppler Waveforms. 1st ed. Cambridge, UK: Cambridge University Press; 2008. pp. 9–24. [Google Scholar]
- 34.Marntell S, Nyman G, Funkquist P, Hedenstierna G. Effects of acepromazine on pulmonary gas exchange and circulation during sedation and dissociative anaesthesia in horses. Vet Anaesth Analg. 2005;32:83–93. doi: 10.1111/j.1467-2995.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 35.Johnston GM, Eastment JK, Wood JLN, Taylor PM. The confidential enquiry into perioperative equine fatalities (CEPEF): Mortality results of Phases 1 and 2. Vet Anaesth Analg. 2002;29:159–170. doi: 10.1046/j.1467-2995.2002.00106.x. [DOI] [PubMed] [Google Scholar]
- 36.Nyman G, Marntell S, Edner A, Funkquist P, Morgan K, Hedenstierna G. Effect of sedation with detomidine and butorphanol on pulmonary gas exchange in the horse. Acta Vet Scand. 2009;7:51–22. doi: 10.1186/1751-0147-51-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esler M. The autonomic nervous system and cardiac arrhythmias. Clin Auton Res. 1992;2:133–135. doi: 10.1007/BF01819669. [DOI] [PubMed] [Google Scholar]
- 38.Schlaich MP, Socratous F, Hennebry S, et al. Sympathetic activation in chronic renal failure. J Am Soc Nephrol. 2009;20:933–939. doi: 10.1681/ASN.2008040402. [DOI] [PubMed] [Google Scholar]
- 39.Muir WW, Werner LL, Hamlin RL. Effects of xylazine and acetylpromazine upon ventricular fibrillation in dogs anesthetized with thiamylal and halothane. Am J Vet Res. 1975;36:1299–1303. [PubMed] [Google Scholar]
- 40.Lemke KA. Anticholinergics and sedatives. In: Tranquilli WJ, Thurmon JC, Grium KA, editors. Lumb & Jones’ Veterinary Anesthesia and Analgesia. 4th ed. Ames, Iowa: Blackwell Publishing; 2007. pp. 414–415. [Google Scholar]
- 41.O’Donaughy TL, Resta TC, Walker BR. Laboratory demonstration of baroreflex control of heart rate in conscious rats. Advan Physiol Educ. 2002;26:309–316. doi: 10.1152/advan.00040.2001. [DOI] [PubMed] [Google Scholar]