Abstract
A 6-month-old male castrated Labrador retriever was presented for coughing and forelimb lameness. Blastomyces dermatitidis was identified in cytology of sputum and synovial fluid. Repeat arthrocentesis 7 months later revealed resolution of septic arthritis. Fungal septic arthritis should be considered for cases of monoarthritis and may respond to oral itraconazole treatment.
Résumé
Blastomycose carpienne intra-articulaire chez un Labrador retriever. Un Labrador retriever mâle castré âgé de 6 mois a été présenté pour une toux et une boiterie du membre antérieur. Blastomyces dermatitidis a été identifié lors d’une cytologie de l’expectoration et du liquide synovial. Une nouvelle arthrocentèse 7 mois plus tard a révélé la résolution de l’arthrite septique. L’arthrite septique fongique devrait être considérée pour les cas de mono-arthrite et elle peut réagir au traitement à l’itraconazole oral.
(Traduit par Isabelle Vallières)
Blastomyces dermatitidis is a thermally dimorphic fungus endemic to parts of North America, Central America, and Africa that causes systemic infection in dogs and humans (1–4). Blastomyces dermatitidis forms conidia in the environment, which are inhaled by the host. If the host cannot clear the conidia via cell-mediated immunity, the conidia may transform into yeast at body temperature, reproduce asexually, and cause pulmonary disease (1,4). In yeast form, B. dermatitidis can disseminate throughout the body via hematogenous or lymphatic spread (4). Preferred sites of infection include lungs, skin, and eyes (1,4). Bony involvement has also been described in 14% to 60% of canine and human patients (1,2,4). However, while confirmed synovial involvement in blastomycosis has been reported in humans and, rarely, in dogs (5–9), treatment options for canine blastomycotic septic arthritis have not been reported in the veterinary literature.
Case description
A 6-month-old male castrated Labrador retriever was presented to the Ontario Veterinary College Health Sciences Centre (OVCHSC) for further evaluation of acute onset of coughing of 2-weeks duration. The dog was current on vaccinations and had received monthly heartworm prophylaxis. He also had a history of travel to Georgian Bay, Ontario. The dog had been clinically healthy at the time of castration 2 wk prior to presentation. Within 12 h of anesthesia, a non-productive cough was noted and oral antibiotics (amoxicillin-clavulanate, Clavamox; Pfizer Animal Health, Kirkland, Quebec), 12.5 mg/kg body weight (BW), PO, q12h, were prescribed. The cough persisted despite antibiotic therapy and the dog became progressively lethargic and anorexic. The dog was re-assessed by an emergency hospital 24 h prior to presentation to the OVCHSC for persistent cough, one episode of self-limiting epistaxis, one episode of vomiting, and acute right forelimb lameness. The lameness was localized to the right carpus. Thoracic radiographs performed at the emergency hospital identified a moderate, generalized, multi-focal interstitial to nodular pattern. Right carpal and orthogonal radiographs of the left and right humeri were unremarkable, as assessed by a board-certified radiologist. The dog was referred for further diagnostic testing and supportive care.
Upon presentation to the OVCHSC, the dog was quiet, alert, and responsive. The dog was thin, with a body condition score of 2/5 and was assessed as 7% dehydrated. The dog was febrile [rectal temperature = 40.0°C, reference interval (RI): 37.5 to 39.0°C], tachycardic (heart rate = 180 beats/min, RI: 80 to 160 beats/min) and tachypneic (respiratory rate = 66 breaths/ min, RI: 12 to 30 breaths/min), with pulmonary crackles auscultated bilaterally. The left prescapular lymph node was mildly enlarged and firm. Orthopedic examination identified pain upon palpation of the diaphysis of all appendicular long bones and upon manipulation of the right carpus. Oxygen saturation was decreased on room air (oxygen saturation = 88%, normal value: > 95%) and the dog coughed frequently and produced a firm, mucoid sputum. The remainder of the physical examination was unremarkable.
A complete blood (cell) count (CBC) identified a mild leukocytosis characterized by neutrophilia with a marked left shift (leukocyte count = 16.6 × 109/L, RI: 4.9 to 15.4 × 109/L; segmented neutrophil count = 12.12 × 109/L, RI: 2.9 to 10.6 × 109/L; band neutrophil count = 1.66 × 109/L, RI: 0.0 to 0.3 × 109/L). Moderate toxic changes were present on the blood smear. The biochemical profile was unremarkable. Cytology of the sputum identified mixed inflammation (75% markedly degenerative neutrophils and 25% macrophages) and frequent extracellular round to oval yeasts. The yeast measured 8 to 25 μm in diameter, with clear, non-staining walls and stippled to basophilic cytoplasm. Occasional broad-based budding of the yeast was noted. Sputum cytology was consistent with active blastomycosis infection.
Ophthalmic examination performed by a board-certified veterinary ophthalmologist was unremarkable, except for an incidental incipient cataract in the left eye.
Treatment for pulmonary blastomycosis and presumptive panosteitis, based on patient age and clinical signs, was initiated with intravenous fluid therapy, nasal oxygen supplementation via unilateral nasal cannula, itraconazole (Sporanox 100 mg capsules; Janssen, Toronto, Ontario), 5 mg/kg BW, PO, q24h; dexamethasone (2 mg/mL injection; Schering-Plough Canada, Kirkland, Quebec), 0.05 mg/kg BW, IV, q24h; sedation upon presentation [butorphanol tartrate (Torbugesic 10 mg/mL injection; Fort Dodge/Pfizer Animal Health, Kirkland, Quebec)], 0.2 mg/kg BW, IV, PRN; analgesia (fentanyl citrate, 50 μg/mL injection USP, Sandoz Canada, Boucherville, Quebec), 2 to 6 μg/kg per hour, gabapentin (Neurontin 100 mg capsules; Pfizer Animal Health, Kirkland, Quebec), 10 mg/kg BW, PO, q12h, and gastroprotectants [omeprazole (Losec, 20 mg tablets; Proctor and Gamble, Toronto, Ontario), 1 mg/kg BW, PO, q24h; famotidine (Johnson & Johnson/Merck Consumer Pharmaceuticals, Guelph, Ontario)], 0.5 mg/kg BW, IV, q12h. Dexamethasone was administered at an anti-inflammatory dose in an attempt to avoid an exuberant pulmonary inflammatory response following institution of anti-fungal therapy. The dog was treated with antacids due to dexamethasone administration and the history of anorexia and single episode of vomiting. Famotidine was given concurrently with omeprazole for 3 d due to the lag time from administration of omeprazole to full clinical effects (10), following which famotidine therapy was discontinued. The dog remained febrile while in hospital, but his condition gradually improved, based on normalization of resting respiratory rate, and improved appetite and energy level. Oxygen supplementation was discontinued after 5 d in hospital, when the dog could maintain normal oxygen saturation on room air, and the dog was discharged 6 d after admission. Discharge instructions included exercise restriction and the following medications: itraconazole (5 mg/kg BW, PO, q24h), prednisone (0.5 mg/kg BW, PO, q24h), omeprazole (1 mg/kg BW, PO, q24h), gabapentin (10 mg/kg BW, PO, q8h), tramadol (2 mg/kg BW, PO, q8h, 50 mg capsules, compounded by the Ontario Veterinary College Pharmacy, Guelph, Ontario). Omeprazole and dexamethasone were discontinued soon after discharge, once the dog began eating normally at home.
The OVCHSC Emergency Service reassessed the dog 1 wk following discharge for recurrence of self-limiting epistaxis. Upon presentation, the dog was bright, alert, and responsive. A small 0.3 cm firm, freely mobile, alopecic mass was identified on the caudomedial aspect of the left elbow. The right carpus and long bones remained painful. In addition, the right carpus was moderately swollen secondary to joint effusion, which was a new physical examination finding since the initial presentation. Oxygen saturation was within normal limits. Complete blood cell count identified a mild neutrophilia (segmented neutrophil count = 14.36 × 109/L, RI: 2.9 to 10.6 × 109/L) with a left shift (band neutrophil count = 0.46 × 109/L, RI: 0.0 to 0.3 × 109/L), moderate monocytosis (2.00 × 109/L, RI: 0.0 to 1.1 × 109/L) and mild thrombocytosis (platelet count = 468 × 109/L, RI: 117 to 418 × 109/L). Coagulation profile (prothrombin time, PT; partial thromboplastin time, PTT) was assessed due to the recurrence of self-limiting epistaxis and was within normal limits. The dog’s owners declined further diagnostic testing or hospitalization due to financial limitations and treatment was continued with itraconazole, tramadol, and gabapentin.
The dog was presented to the OVCHSC 3 wk following initial presentation for a scheduled recheck examination. The respiratory signs continued to improve; however, the dog remained moderately lame on the right forelimb. Physical examination identified harsh respiratory signs bilaterally and a moderately swollen right carpus. The diaphyseal bone pain had resolved.
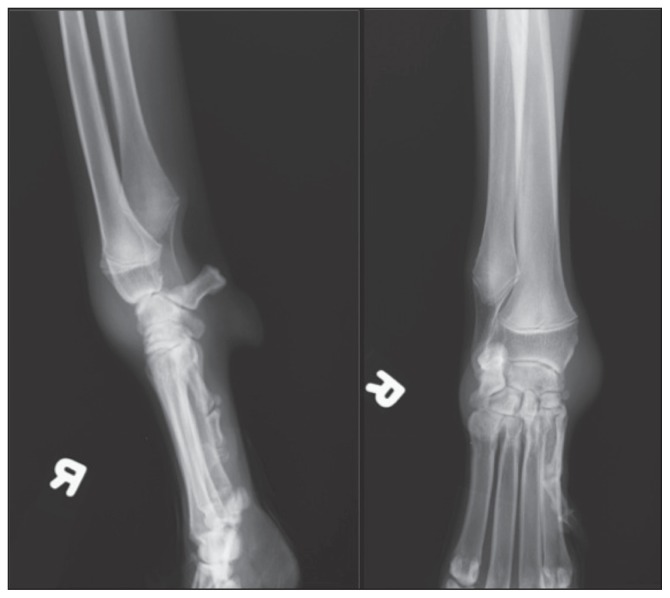
Biochemical profile identified a mild elevation in blood urea nitrogen (9.6 mmol/L, RI: 3.5 to 9.0 mmol/L), but was otherwise unremarkable. Two view orthogonal thoracic radiographs identified a mild diffuse nodular interstitial pulmonary pattern and a moderately diffuse bronchial pulmonary pattern. Radiographs of the right carpus identified increased soft tissue opacity, confined to the joint capsule. The carpal bones did not appear to be affected by the swelling (Figure 1). The dog was sedated with butorphanol tartrate (Torbugesic; Fort Dodge/ Pfizer Animal Health) 0.2 mg/kg BW, IV for arthrocentesis of the right carpus. Cytology of the joint fluid identified increased cellularity (nucleated cell count > 70 × 109 cells/μL) characterized by 77% non-degenerate neutrophils and 23% mononuclear cells. Occasional fibroblasts and multinucleated giant cells were identified. Yeasts, measuring 10 to 15 μm in diameter with a refractile, deeply basophilic cell wall were scattered amongst the inflammatory cells. Cytology was consistent with blastomycotic septic arthritis and suppurative inflammation.
Figure 1.
Lateral (left) and dorsopalmar (right) radiographs of the right carpus, which identify increased soft tissue opacity confined to the carpal joint capsule. Bony structures are unremarkable.
Treatment options including chronic oral anti-fungal therapy alone and oral anti-fungal therapy with surgical lavage of the carpal joint were discussed with the owners. They elected to continue with oral anti-fungal therapy alone, with instructions to continue itraconazole until resolution of pulmonary radiographic changes.
Seven months following initial presentation, the dog was clinically well. The right carpal swelling resolved 2 mo into therapy. Serial biochemical profiles have been within normal limits. Repeat thoracic radiographs identified improvement in pulmonary radiographic changes to a mild bronchointerstitial pattern. Repeat arthrocentesis of the right carpus identified mild mononuclear inflammation. No yeast organisms or suppurative inflammation were identified. The cytology of the right carpal synovial fluid was consistent with degenerative joint disease, which may be a sequel to previous septic fungal arthritis. The dog is still being treated with oral itraconazole, which will be continued until the dog’s pulmonary radiographic changes are resolved or until they are stable, along with a negative urine blastomycosis antigen test.
Discussion
This case report describes systemic blastomycosis infection in a dog that was presented with septic monoarthritis. The blastomycotic septic monoarthritis was treated successfully with oral itraconazole. In addition to arthritis, the dog had evidence of pulmonary involvement, which is consistent with the pathogenesis of systemic blastomycosis (1,3–4). An enlarged prescapular lymph node was identified on initial physical examination, which may have also been related to systemic blastomycosis; however, as the lymph node was not aspirated this cannot be confirmed. Blastomyces dermatitidis can affect almost every organ and body system; however, fungal arthritis is considered a rare cause of canine lameness (1,11–12). While blastomycosis has been previously identified in canine synovial fluid (9), specific treatment recommendations for blastomycotic septic arthritis do not exist.
Joint and bone pain is a common presenting complaint in human patients with systemic blastomycosis (5). However, blastomycotic arthritis is infrequent, with fungal organisms identified within synovial fluid of only 3% to 8% of human patients (5–6). Arthritis may be the primary reason for presentation and the most common clinical signs include joint effusion, pain on manipulation, and decreased range of motion (7). Septic fungal arthritis in humans is typically monoarticular, with acute onset of severe pain (5,7–8). Knees, elbows, and ankles are most commonly affected, although small joints of the hands and feet may also be involved (7–8); most patients exhibit concurrent extra-articular signs of blastomycosis (5). Pathogenesis of fungal septic arthritis is suspected to be secondary to juxta-articular osteomyelitis or hematogenous spread, but predisposing osseous injury secondary to trauma has also been hypothesized (5–6). The diagnosis is best made via cytology of synovial fluid, which is a sensitive and specific diagnostic test (5).
The clinical features of the dog in this case report are similar to those described in humans. The dog experienced acute onset of carpal lameness, with pain identified on physical examination. Carpal effusion was identified at the first recheck examination and B. dermatitidis organisms were identified in synovial fluid. It should be noted that the pyogranulomatous inflammation expected secondary to blastomycosis tends to be mild in synovial fluid, compared with solid tissues, and yeast cells may become crenated in fluid cytology which can mimic the appearance of starch granules (e.g., talcum powder) (9). On orthopedic examination, generalized diaphyseal long bone pain was also identified. Due to the age of the patient, presence of long bone pain, and lack of radiographic changes, we initially began treatment for presumed panosteitis. As dexamethasone was being administered to prevent pulmonary inflammation, non-steroid anti-inflammatory drugs, which are the treatment of choice for panosteitis (13), could not be concurrently administered. Panosteitis is a common cause of lameness in medium to large breed dogs and can affect multiple limbs (13). Panosteitis is less likely in this case as serial forelimb radiographs did not identify changes consistent with the disease, such as medullary radiolucency, granular radi-opacity in the region of the nutrient foramen, and formation of new endosteal bone (13–14). It is possible that the dog in our case had fungal osteomyelitis, but that his osseous lesions were too minimal to produce radiographic changes. A bone scan or computed tomography may be more sensitive than radiographs for identification of inflammatory bony lesions, such as fungal osteomyelitis (4,13); however, this was not pursued in this case due to financial limitations. In addition, fungal osteomyelitis is often noted at the epiphysis distal to the elbow and/or stifle and diaphyseal pain would be an atypical presentation (1,4). A second hypothesis is that the dog was experiencing generalized myalgia and arthralgia secondary to systemic blastomycosis infection, as is reported in the human literature (5,15).
There are no specific treatment guidelines for fungal septic arthritis in humans as these cases are often considered in conjunction with fungal osteomyelitis (15). A review of orthopedic blastomycosis (6) found that the majority of patients were treated with a combination of medical antifungal therapy (amphotericin B and/or other oral antifungal drugs) and surgical debridement. Few patients in that study experienced treatment failures; however, some patients were successfully treated with non-surgical therapy alone (6). Similarly, a case series of blastomycotic osteomyelitis found that medical antifungal therapy was often sufficient treatment (7). Another case series of orthopedic blastomycosis identified that treatment failures were associated with inadequate antifungal therapy, as opposed to type of antifungal therapy or surgical treatment (5). Therefore, surgical debridement of fungal osteomyelitis in humans is only recommended when medical management has failed (15).
While joint involvement of blastomycosis is frequently mentioned in the veterinary literature, case reports and specific treatment guidelines for canine blastomycotic septic arthritis are rare. A review of cytologic findings in 43 dogs with naturally occurring blastomycosis identified B. dermatitidis organisms in synovial fluid of 3 dogs; however, therapies and treatment outcomes for these dogs were not discussed (9). There is a previous case report of isolated patellar blastomycosis in a dog, which resulted in arthritis and stifle lameness (16). Lytic lesions were identified in the left patella and histopathology of surgical synovium and bone biopsies identified Blastomyces organisms (16). However, no microorganisms were identified within the joint fluid itself (16). The dog responded to treatment with surgical debridement of the patella, a 90-day course of oral itraconazole, and physical therapy; however, successful treatment was based solely on clinical signs, physical examination findings, and patellar radiographs (16). Repeat cytology, histology, or fungal culture, which are the only ways to definitively diagnose blastomycosis (1), were not performed to confirm resolution of infection in this dog (16). Itraconazole is considered the treatment of choice for systemic canine blastomycosis, as long as there is no central nervous system involvement, with a 70% to 90% response rate reported (1,4,17). Both surgical and medical treatment options were considered for the dog in this case report. Due to financial limitations, the owners declined arthrotomy and lavage. The dog responded well to oral itraconazole and clinical signs associated with carpal septic arthritis resolved within 2 mo of therapy. Repeat arthrocentesis of the right carpus and cytology performed following 7 mo of itraconazole therapy was negative for B. dermatitidis organisms and suppurative inflammation. This case report represents the first documented resolution of blastomycotic arthritis with confirmation based upon repeated arthrocentesis. This finding is significant as it indicates that canine septic fungal arthritis secondary to generalized blastomycosis may resolve without surgical intervention, thus sparing a patient from unnecessary anesthesia and increased morbidity.
A case of monoarticular fungal septic arthritis with concomitant pulmonary infection in a dog is presented. While rare, blastomycotic septic arthritis should be considered as a differential diagnosis for bone and joint infection in patients which have travelled to endemic areas. This condition may respond to oral antifungal therapy alone. Clinical trials are needed to prospectively compare treatment outcomes of antifungal therapy alone to antifungal therapy in combination with surgical intervention in dogs with septic blastomycotic arthritis. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Taboada J, Grooters AM. Histoplasmosis, blastomycosis, sporothrichosis, candidiasis, pythiosis, and langenidiosis. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 7th ed. St Louis, Missouri: Saunders Elsevier; 2010. pp. 975–980. [Google Scholar]
- 2.Moore RM, Green NE. Blastomycosis of bone. J Bone Joint Surg. 1982;64:1097–1101. [PubMed] [Google Scholar]
- 3.Maggs DJ, Miller PE, Ofri R. Slatter’s Fundamentals of Veterinary Ophthalmology. 4th ed. St Louis, Missouri: Saunders Elsevier; 2008. [Google Scholar]
- 4.Legendre AM. Blastomycosis. In: Greene CE, editor. Infectious Diseases of Dogs and Cats. 4th ed. St Louis, Missouri: Saunders Elsevier; 2012. pp. 806–814. [Google Scholar]
- 5.George AL, Hays JT, Graham BS. Blastomycosis presenting as monarticular Arthritis: The role of synovial fluid cytology. Arthritis Rheum. 1985;28:516–521. doi: 10.1002/art.1780280508. [DOI] [PubMed] [Google Scholar]
- 6.MacDonald PB, Black GB, MacKenzie R. Orthopaedic manifestations of blastomycosis. J Bone Joint Surg. 1990;72A:860–864. [PubMed] [Google Scholar]
- 7.Oppenheimer M, Embil JM, Black B, et al. Blastomycosis of bones and joints. South Med J. 2007;100:570–578. doi: 10.1097/SMJ.0b013e3180487a92. [DOI] [PubMed] [Google Scholar]
- 8.Liggett AS, Silberman Z. Blastomycosis of the knee joint. J Bone Joint Surg. 1970;52:1445–1449. [PubMed] [Google Scholar]
- 9.Garma-Avina A. Cytologic findings in 43 cases of blastomycosis diagnosed ante-mortem in naturally-infected dogs. Mycopathologia. 1995;131:87–91. doi: 10.1007/BF01102884. [DOI] [PubMed] [Google Scholar]
- 10.Plumb DC. Plumb’s Veterinary Drug Handbook. 7th ed. Ames, Iowa: Blackwell Publishing; 2011. [Google Scholar]
- 11.Legendre AM, Walker M, Buyukmihci N, et al. Canine blastomycosis: A review of 47 clinical cases. J Am Vet Med Assoc. 1981;178:1163–1168. [PubMed] [Google Scholar]
- 12.Buyukmihci N. Ocular lesions of blastomycosis in the dog. J Am Vet Med Assoc. 1982;180:426–431. [PubMed] [Google Scholar]
- 13.Johnson KA. Skeletal diseases. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 7th ed. St Louis, Missoiuri: Saunders Elsevier; 2010. pp. 819–845. [Google Scholar]
- 14.Thrall DE. Textbook of Veterinary Diagnostic Radiology. 5th ed. St Louis, Missouri: Saunders Elsevier; 2007. p. 272. [Google Scholar]
- 15.Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Mycobiol Rev. 2010;23:367–381. doi: 10.1128/CMR.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshin A, Griffon D, Lemberger K, et al. Patellar blastomycosis in a dog. J Am Anim Hosp Assoc. 2009;45:239–244. doi: 10.5326/0450239. [DOI] [PubMed] [Google Scholar]
- 17.Mazepa AS, Trepanier LA, Foy DS. Retrospective comparison of the efficacy of fluconazole or itraconazole for the treatment of systemic blastomycosis in dogs. J Vet Intern Med. 2011;25:440–445. doi: 10.1111/j.1939-1676.2011.0710.x. [DOI] [PubMed] [Google Scholar]