Abstract
A 2-year-old gelding was referred for evaluation of severe right forelimb lameness. The horse was grade 4/5 lame on the right forelimb. Clinical, laboratory, and radiographic findings were consistent with septic arthritis and osteomyelitis. Due to poor prognosis the owner elected euthanasia. Histopathology confirmed chronic arthritis and osteomyelitis with intralesional yeast (Candida species).
Résumé
Ostéomyélite àCandidachez un hongre. Un hongre âgé de 2 ans a été référé pour l’évaluation d’une boiterie grave du membre antérieur droit. Le cheval avait une boiterie de stade 4/5 du membre antérieur droit. Les constatations cliniques ainsi que les résultats de laboratoire et des radiographies étaient conformes à l’arthrite septique et à l’ostéomyélite. En raison d’un pronostic sombre, le propriétaire a choisi l’euthanasie. L’histopathologie a confirmé l’arthrite chronique et l’ostéomyélite avec des levures intralésionnelles (espèce Candida).
(Traduit par Isabelle Vallières)
A 2-year-old Standardbred gelding was referred to the Atlantic Veterinary College, Veterinary Teaching Hospital (AVC-VTH) for evaluation of a severe right forelimb (RF) lameness of 2-weeks duration. It was suspected, although not confirmed, that the horse had been treated prior to his last race with an intra-articular injection of corticosteroid. The horse had raced 3 wk prior to evaluation and was then turned out to pasture. He had been found in the pasture with non-weight bearing lameness on the RF 2 wk prior to presentation at the AVC-VTH. The referring veterinarian examined the horse at that time and discovered a non-weight bearing RF lameness with severe soft tissue swelling and pain after flexion. Radiographs of the RF metacarpophalangeal (MCP) joint were obtained for suspected fracture but were within normal limits.
The referring veterinarian placed the horse on penicillin G procaine (PenPro; Vétoquinol, Lavaltrie, Quebec) 22 000 IU/kg body weight (BW), IM, q12h, phenylbutazone (Vétoquinol), 4.4 mg/kg BW, IV q24h, and strict stall rest then re-examined the horse 1 wk later. At this time there was no improvement in clinical signs and radiographs were repeated. Mild osteolysis of the proximal sesamoid bones was found and was assumed to be a result of limb disuse. No other abnormal findings were noted. Arthocentesis of the RF MCP joint was also performed at this time and joint fluid was submitted for cytology. Cytology revealed a mild increase in nucleated cell count (5.1 × 109/L; normal value < 5.0 × 109/L) but a marked increase in total protein (70 g/L; reference range: 8 to 25 g/L). Phenylbutazone was continued at the previous dose but the antibiotic was switched to oxytetracycline (Oxymycine LP; Wyeth Animal Health, Guelph, Ontario), 7.5 mg/kg BW, IV, q24h. No improvement in clinical signs was seen over the next week and the horse was referred to the AVC-VTH.
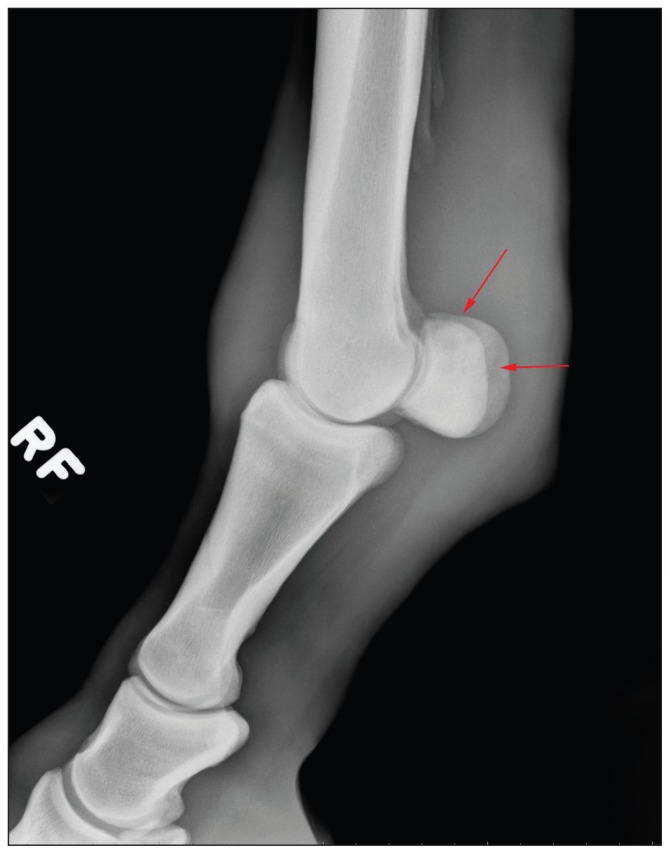
Upon presentation, the horse was grade 4/5 lame on the RF with severe soft tissue swelling of the distal limb and palpable heat in the palmar aspect of the fetlock area. Forced flexion of the MCP joint induced a severe pain response. Radiographs of the MCP joint were obtained and revealed severe soft tissue enlargement with small radiolucent areas in a mottled pattern within the proximal sesamoid bones. These changes were consistent with osteomyelitis (Figure 1). Ultrasound examination of the MCP joint revealed thickening of the synovium within the joint, and marked periarticular edema but no significant joint effusion. Following imaging, a high suspicion of joint sepsis and concurrent osteomyelitis of the proximal sesamoid bones prompted arthrocentesis and cytologic examination of the joint fluid of the RF MCP joint. Cytology revealed moderate to marked neutrophilic inflammation with a total nucleated cell count of 17.2 × 109/L and a total protein of 69 g/L, consistent with severe joint inflammation. No etiologic agent of joint sepsis could be identified on cytology. A poor prognosis for return to racing was given to the owner; the horse was euthanized and a postmortem examination was done.
Figure 1.
Lateral radiograph of the right metacarpophalangeal joint demonstrating the mottled radiolucent areas in the proximal sesamoid bones (arrows). Significant soft tissue enlargement is noted.
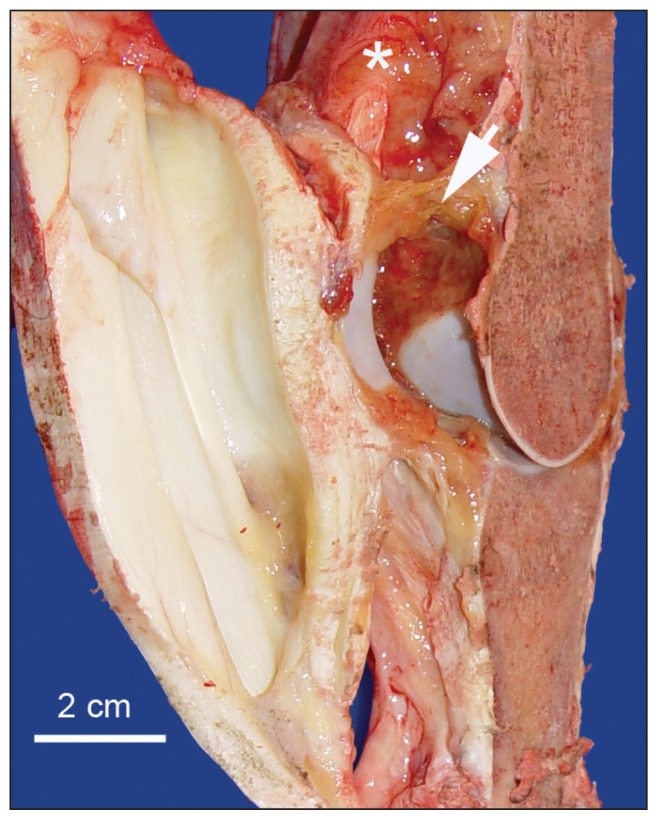
Significant postmortem lesions were restricted to the right MCP joint that appeared notably swollen due to subcutaneous edema and periarticular fibrosis. There was no evidence of synovial effusion but the synovial membrane was notably swollen and edematous with fibrin on the surface (Figure 2). This synovial swelling extended 7 cm proximally and 3 cm distally from the MCP joint articulation. The articular cartilage was grossly normal except for a small area (1 to 2 mm) in which the cartilage was moderately pitted. Longitudinal sections of affected bones did not reveal any gross evidence of inflammation or osteoporosis. Three swabs were taken from 2 areas of the right MCP joint for microbiologic culture. Pieces of the MCP bones and both of the proximal sesamoid bones were placed in decalcifying solution, and pieces of synovial membrane, articular capsule, and periarticular tissues were fixed in formalin for processing and microscopic examination.
Figure 2.
Right metacarpophalangeal joint. Note extensive edematous swelling of the synovial membrane (asterisk) and a plaque of fibrin on the synovial surface (arrow).
Microscopically, the synovial membrane was diffusely thickened and markedly hypercellular with large numbers of infiltrating plasma cells, lymphocytes, and fibroblasts and to a lesser extent macrophages and neutrophils (Figure 3). The surface of the synovial membrane was covered by organized fibrin admixed with some neutrophils, macrophages, and cellular debris. The cytoplasm of some macrophages contained karyor-rhectic debris. In addition to inflammatory cells, the fibrous portion of the synovial membrane was infiltrated by fibroblasts and showed abundant neovascularization. These proliferative changes extended deep into the surrounding articular capsule and tendon sheaths. A few small fragments of trabecular bone appeared impacted into the adjacent capsular fibrous tissue. The articular capsule had angiocentric infiltration of mononuclear cells and small foci of interstitial hemorrhage. Surprisingly, there was no evidence of synovial villous hypertrophy or hyperplasia.
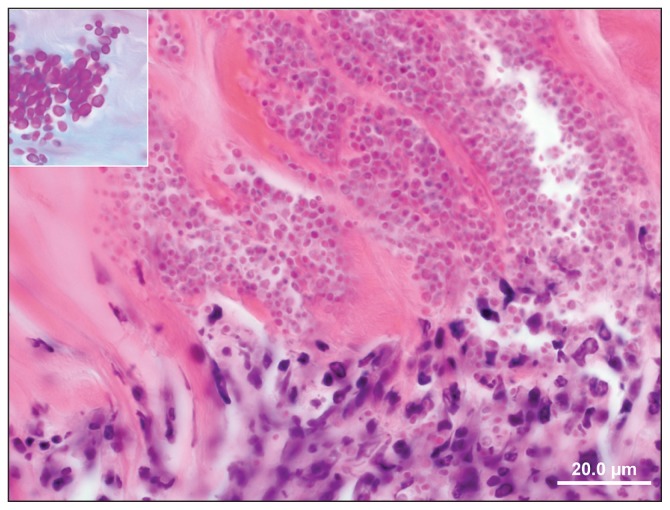
Figure 3.
Section of decalcified right metacarpophalangeal bone. Note neutrophils infiltrating the bone with a myriad of intralesional Candida yeasts (Hematoxylin and eosin). Inset: close-up view of yeasts (PAS stain).
Sections of right proximal sesamoid bones revealed remarkable microscopic changes characterized by cartilage fibrillation and subchondral microabscesses. These microabscesses consisted of large aggregates of neutrophils surrounded by a thick rim of macrophages, fibroblasts, and lymphocytes. At the junction of the bone and cartilage there were large colonies of yeasts which were microscopically visible by routine hematoxylin and eosin (H&E) staining (Figure 3). These organisms were round to oval, Periodic acid-Schiff (PAS) and Gomori’s methenamine silver (GMS) positive, measuring 3 to 7 μm in diameter. The swabs taken from the affected joint yielded scant growth of Escherichia coli and yeast. The yeast was identified by Matrix Assisted Laser Desorption Ionization Time of Flight mass spectrometry (MALDI-TOF) (microflex™ LT; Bruker Daltonics, Bruker Corporation, Milton, Ontario) as Candida glabrata with a 2.3 organism best match score value.
Candida species are ubiquitous commensal yeasts normally present in skin and mucous flora that, under suitable conditions, can become invasive and cause disease called candidiasis in humans and animals (1). Candidiasis affects particularly the skin and mucosal membranes, while invasive infections affecting internal organs are rare. Osseous and articular candidiasis is reported sporadically in humans with risk factors such as immunosuppression or concurrent debilitating disease; it can also be secondary to administration of broad-spectrum antibiotics (2). The prognosis for osseous candidiasis is good once the condition is diagnosed and appropriately treated with antifungals, with or without surgery. A large study in human patients with osseous candidiasis revealed predilection for vertebrae and sternum, but other bones such as limbs, fingers, and mandible can also be affected (3).
As in humans, infections with Candida species in animals are most frequently superficial and invasive, such as osseous candidiasis, and are only sporadically reported (4). Candida osteomyelitis is a rare but well-documented disease in horses (5). The port of entry to the bone by the yeast is often difficult to establish but, based on the clinical history, many authors propose that it is by wound contamination, traumatic implantation, or arthroscopy (4–5). The port of entry for the Candida for the horse described in this report was not identified as there was no confirmed history of trauma or intra-articular injection, although the latter was suspected. It is also known that Candida species can invade bones or joints by the blood circulation (candidemia), but this hematogenous form of dissemination typically occurs in immunocompromised patients (6). This route of infection, however, was unlikely herein since there were no risk factors such as immunosuppression in this horse. It could be speculated that genetics could have played a role in the susceptibility to invasive yeast osteomyelitis since experimental murine models have shown phenotypic predisposition to candidiasis (7). Broad-spectrum antibiotics administration has been associated with the development of osseous and articular candidiasis in humans and may have contributed to the development of disease in our case (2).
Clinical diagnosis of systemic candidiasis such as yeast osteomyelitis is challenging as it requires invasive techniques such as bone biopsy or yeast culture which are time consuming. Recently, DNA-based tests such as PCR have been proposed for rapid detection of invasive candidiasis (8). Candida species arthritis has been successfully treated with systemic amphotericin B administration and joint lavage in the horse (9). Aspergillus species osteomyelitis of the axial border of the proximal sesamoid bones was also successfully treated with regional limb perfusion, joint lavage, and long-term, systemic itraconazole administration in a horse (10). That horse remained lame following resolution of infection due to the sequelae of osteoarthritis of the MCP joint. In our case, the invasiveness of the osteomyelitis of the proximal sesamoid bones and severe inflammation of the MCP joint provided a poor prognosis for return to racing with or without treatment, and the owner elected euthanasia. However, these previous reports, and reports in humans, suggest fungal osteomyelitis and arthritis can be resolved with appropriate treatment, but prognosis for athletic soundness should remain poor. Although clinical diagnosis of fungal osteomyelitis is difficult, histopathological diagnosis is simple because yeast can be easily identified microscopically.
This report presents a case of Candida species osteomyelitis of the proximal sesamoid bones following suspected intraarticular injection. Systemic fungal infections in the horse are rare but should be considered as a differential diagnosis for horses with joint sepsis and/or osteomyelitis. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Fleming L, Ng A, Paden M, Stone P, Kruse D. Fungal osteomyelitis of calcaneous due to Candida albicans: A case report. J Foot Ankle Surg. 2011;51:212–214. doi: 10.1053/j.jfas.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Kaldau NC, Brorson S, Jensen PE, Schultz C, Arpi M. Bilateral poly-microbial osteomyelitis with Candida tropicalis and Candida krusei: A case report and an updated literature review. Inter J Infect Dis. 2012;16:e16–e22. doi: 10.1016/j.ijid.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Arias F, Mata-Essayag S, Landaeta ME, et al. Candida albicans osteomyelitis: Case report and literature review. J Infect Dis. 2004;8:307–314. doi: 10.1016/j.ijid.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 4.García ME, Blanco JL. Principales enfermedades fúngicas que afectan a los animales domésticos. Rev Iberam Micol. 2000;17:S2–S7. [PubMed] [Google Scholar]
- 5.Riley CB, Yovich JV, Robertson JP, O’Hara FL. Fungal arthritis due to infection by Candida famata in a horse. Aust Vet J. 1992;69:65–66. doi: 10.1111/j.1751-0813.1992.tb07453.x. [DOI] [PubMed] [Google Scholar]
- 6.Grubb SEW, Murdoch C, Sudbery PE, Saville SP, Lopez-Ribot JL, Thornhill MH. Candida albicans-endothelial cell interactions: A key step in the pathogenesis of systemic candidiasis. Infect Immun. 2008;76:4370–4377. doi: 10.1128/IAI.00332-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radovanovic I, Mullick A, Gros P. Genetic control of susceptibility to infection with Candida albicans in mice. PLoS One. 2011;6:e18957. doi: 10.1371/journal.pone.0018957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wahyuningsih R, Freisleben HJ, Sonntag HG, Schnitzler P. Simple and rapid detection of Candida albicans DNA in serum by PCR for diagnosis of invasive candidiasis. J Clin Microbiol. 2000;38:3016–3021. doi: 10.1128/jcm.38.8.3016-3021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madison JB, Reid BV, Raskin RE. Amphotericin B treatment of candida arthritis in two horses. J Am Vet Med Assoc. 1995;206:338–341. [PubMed] [Google Scholar]
- 10.Sherman KM, Myhre GD, Heymann EI. Fungal osteomyelitis of the axial border of the proximal sesamoid bones in a horse. J Am Vet Med Assoc. 2006;229:1607–1611. doi: 10.2460/javma.229.10.1607. [DOI] [PubMed] [Google Scholar]