Abstract
Ligand-conjugated liposomes and other nano-sized constructs are attractive drug carriers due to their extended plasma circulation; however, limited data are available as to whether their cargo can traverse the endothelium of solid organs. To determine whether the cargo of endothelially-targeted liposomes is internalized by endothelial cells and transported into tissue, and to evaluate whether such liposomes can accumulate in models of cardiovascular disease, we tracked the fate of the cargo (a hydrophilic fluorescent dye) and shell (conjugated with a radioisotope) of a heart-homing liposome (CRPPR-conjugated). The ex vivo heart was imaged with confocal microscopy and the in vivo heart with positron emission tomography in sham treated mice and models of ischemia/reperfusion (I/R) and myocardial infarction (MI). Within 30 min of injection of 20mg/kg CRPPR-liposomes, fluorescence increased by 47 fold in the tissue surrounding the vascular lumen, as compared with non-targeted liposomes. Both the accumulation on the endothelium and the interstitial fluorescence saturated at an injected dose of 20 mg/kg. In both I/R and MI models, CRPPR-liposomes accumulated in diseased sites, although less than in surrounding healthy tissue. The accumulation in the diseased sites increased with time post injury: the ratio of accumulated radioactivity in the diseased and healthy cardiac tissue increased from 0.20±0.04, to 0.58±0.12 and 0.61±0.19 for 1, 7, and 99 days post MI, indicating the potential for adequate delivery and therapeutic efficacy if the targeted particles are injected at 7 or more days post MI. In summary, CRPPR- liposomes accumulated in normal and diseased hearts, and the cargo accumulated in the tissue within minutes and remained detectable after 24 hours.
Keywords: endothelium targeting, cell penetrating peptides, cardiovascular diseases, targeted delivery
1 Introduction
Liposomes and other nano-sized constructs are attractive drug carriers due to their extended plasma circulation; however, delivery to solid organs is challenging since these vehicles cannot ordinarily traverse the intact endothelium. By targeting organ- or disease-specific endothelial receptors, the accumulation of multivalent constructs has been reported to be enhanced in various organs, including brain [1], lungs [2], atherosclerotic lesions [3] and heart [4], although endothelial penetration is not necessarily increased. In vivo phage display technology has been widely used to identify tissue- and disease-specific markers and peptides targeted to the vasculature of the brain [5, 6], lung [7], heart [8], prostate [9], or breast [10] have been isolated.
One such peptide, CRPPR, was identified as a heart-homing peptide by in vivo phage display and cysteine-rich protein-2 (CRIP-2) was proposed as the receptor [8]. In our previous study using positron emission tomography (PET) to track liposomes, amide-terminated CRPPR-conjugated liposomes accumulated in the heart with a concentration of 44 ± 9% injected dose per gram of tissue (%ID/g) within 100 seconds, 9.4 fold greater than non-targeted liposomes, and we found that exposure of the CRPPR peptide above the polyethyleneglycol(PEG) layer was required for effective accumulation [4]. Also, CRPPR follows the RXXR motif, and the R/KXXR sequence terminated with a carboxyl group (CendR peptide) has been reported to bind to endothelial neuropilin-1 and to transport a payload efficiently into the interstitium [11]. Systemic co-injection of a peptide incorporating this motif was shown to improve the therapeutic index of small molecule therapeutics, nanoparticles, and monoclonal antibodies [12]. However, our preliminary studies indicate that CRPPR-conjugated particles with a terminal free amide accumulate on murine aortic endothelial cells to a greater extent than particles conjugated with the same sequence terminated with a free carboxyl group, therefore, here we evaluate both accumulation and trans-endothelial transport resulting from conjugation with the amide-terminated peptide.
Success has also been reported in delivering large fractions of the injected dose to other target organs. For example, 70% of the injected dose of immunoliposomes conjugated with an antibody specific to pulmonary endothelial cells accumulated in the mouse lung 5 minutes post intravenous (i.v.) injection [2]. VHSPNKK-conjugated magneto-fluorescent nanoparticles accumulated on vascular cell adhesion molecule 1-expressing endothelial cells in atherosclerotic lesions in cholesterol-fed apoE−/− mice [3]. Further, conjugation of RI7217 isotype IgG2a,k increased liposomal uptake in the brain capillaries and parenchyma up to 10 and 4.3 times, respectively, compared to non-targeted liposomes, 12 hours after i.v. injection [1]. Targeting to myocardial infarction has been reported with peptides and with an antimyosin antibody [13-15].
Vascular targeting has also been shown to increase efficacy in cancer [16-21] and cardiovascular disease [22-25]. For example, CRPPR-gp91ds increased the antioxidant effect and reduced systolic blood pressure to a greater extent than gp91ds alone in a rat model of genetic hypertension [26]. PEG-coated liposomes targeted with NGR or APRPG peptides increased the anti-tumor effect of doxorubicin [27, 28].
While many studies have assessed systemic pharmacokinetics and resulting efficacy, few imaging studies have followed the trans-endothelial delivery of the liposomal cargo after accumulation [1, 29], and even fewer studies have quantified transport processes [1] or the distribution of the cargo among various cell types. One aim of our study is to address the factors affecting the trans-endothelial transport of such particles and their cargo, including the time, dose, and exposure of the ligand, by tracking the in vivo fate of the cargo at the tissue and cellular levels. The second aim is to study the effect of disease on the accumulation of CRPPR-targeted liposomes.
2 Materials and Methods
By loading a fluorescent probe as a model drug, targeted liposomes and their cargo were tracked after accumulation using confocal microcopy of the excised heart and by flow cytometry of the disassociated cells. By injecting radioactively-labeled, peptide-conjugated liposomes into diseased mice, the biodistribution and the pharmacokinetics were examined in the presence of disease to validate preferential accumulation in the diseased heart (as compared with surrounding tissue).
2.1 Dose preparation
A schematic of the liposome is found in Figure 1. All lipids were obtained from Avanti Polar Lipids Inc. (Alabaster, AL), and Lipo-Peg-Peptides (LPP) were synthesized manually with standard Fmoc chemistry, and the liposomes were made by an extrusion technique as previously described [4].
Figure 1.

Schematic of radiolabeled, peptide-conjugated liposomes encapsulating fluorescent dye (pink center).
For ex vivo confocal imaging, 2 mM Alexa555 (Invitrogen Inc., CA) was encapsulated in liposomes. For the cell isolation study, 2 mM Alexa488 (Invitrogen Inc., CA) was loaded within the liposomal interior and 60 mg/kg of CRPPR-conjugated liposomes injected. For PET imaging, a radioactively-labeled lipid, 18F-fluorodipalmitin (18F-FDP) was incorporated within the liposome shell during the assembly of the liposomes, as previously described [4]. In initial studies (data not shown), CRPPR-conjugated liposomes ending with a free amide or carboxyl group were compared; binding to murine arterial endothelial cells was significantly greater for the peptide ending in a free amide and therefore was the focus of this study.
The size of the liposomes was measured with the Zeta Potential/Particle Sizer Nicomp™ 380ZLS (Particle Sizing Systems, CA). The fluorescence of the liposomes was read with Infinite® M1000 plate reader from Tecan Systems, Inc. (San Jose, CA). The fluorescence intensity of the liposomes, together with a standard curve of fluorescence intensity vs concentration of free dye, was used to calculate the loading efficiency. By decomposing the liposomes with Triton X-100, and comparing the fluorescence intensity before and after treatment, we studied whether the fluorescent dye is quenched inside the liposomes. The stability of the liposomes was characterized by the change of size and fluorescence intensity in the particles.
2.2 In vivo studies
All animal studies were conducted under a protocol approved by the University of California, Davis Animal Use and Care Committee (Davis, CA). The ex vivo hearts were imaged with confocal microscopy and cells were isolated from the heart and characterized with flow cytometry (total of n=66). The pharmacokinetics and biodistribution were studied by incorporating 18F-FDP within the liposomes and evaluating liposomal pharmacokinetics in mouse models of myocardial infarction (MI) and ischemia/reperfusion (I/R), as compared to the sham-operated mice (control) (n=30).
2.2.1 Confocal study of the excised fluorescent heart after tail vein injection of targeted particles
For the trans-endothelial delivery study, two-month old, male FVB mice (20-25 g) were purchased from Charles River (Wilmington, MA). Ten different conditions are described in Table 1, including no-treatment controls (a), free dye (b), non-targeted liposomes (c), CRPPR-buried liposomes, where the ligand is conjugated to a 1200 MW PEG-lipid and is buried within the 2000 MW PEG brush layer of the liposomes (d), and CRPPR-exposed liposomes, where CRPPR is conjugated to a 3600 MW PEG-lipid and extends out of the 2000 MW PEG brush layer of the liposomes (e-j). For CRPPR-exposed liposomes, doses of 7 mg/kg (e and h), 20 mg/kg (f and i), and 60 mg/kg (g and j) at time points including 0.5 hours (e-g) and 24 hours (h-j) post injection were studied. For each of the ten conditions listed in Table 1, 3~8 mice (listed in Table 1) were studied and averaged to generate the mean and standard deviation.
Table 1.
Overview of the study applying confocal microscopy to image the excised heart 0.5 or 24 hours after tail vein injection of targeted particles
| Name | DPPC:DSPE-PEG2000:LPP, mol/mol |
Dose (mg/kg) |
Time1 (hr) |
n | |
|---|---|---|---|---|---|
| a | No-treatment control | N/A | N/A | -- | 8 |
| b | Free Alexa 555 | N/A | N/A | 0.5 | 4 |
| c | Non-targeted liposomes | 88:12:0; | 20 | 0.5 | 4 |
| d | CRPPR-buried liposomes2 | 88:6:6; LPP: Lip-PEG1200 –CRPPR |
20 | 0.5 | 3 |
| e | CRPPR liposomes3, 7mg/kg | 88:6:6; LPP: Lip-PEG3600 –CRPPR |
7 | 0.5 | 4 |
| f | CRPPR liposomes, 20mg/kg | 20 | 7 | ||
| g | CRPPR liposomes, 60mg/kg | 60 | 4 | ||
| h | CRPPR liposomes, 7mg/kg | 7 | 24 | 4 | |
| i | CRPPR liposomes, 20mg/kg | 20 | 5 | ||
| j | CRPPR liposomes, 60mg/kg | 60 | 4 |
between injection and dissection for imaging;
the ligand CRPPR was buried underneath of the PEG (MW 2000) brush layer of the liposome, because of the shorter PEG length (MW 1200) on the LPP;
the ligand CRPPR was exposed beyond the PEG (MW 2000) brush layer of the liposomes, because of the longer PEG length (MW 3600) on the LPP.
The fluorescent liposomes, freshly prepared on the same day, were injected via tail vein catheter. For confocal ex vivo imaging, either 15 minutes or 24 hours after injection, fluorescein-labeled Lycopersicon Esculentum (Tomato) Lectin (VectorLab, CA) (50 μg each mouse) was injected to label the endothelium [30]. Fifteen minutes later, the animals were sacrificed, and the hearts were harvested and soaked in 1% paraformaldehyde (PFA) in PBS on ice for one hour before imaging. Several tissue preparation protocols were evaluated, including no fixation, fixation with 1% PFA, and fixation with Tissue-Tek Optimum Cutting Temperature (OCT) Compound. Treatment of the liposomes with 1% PFA overnight at room temperature did not change the size and the fluorescence loading of liposomes and resulted in clear, highly fluorescent images. The alternative techniques resulted in liposome destruction or blurring of the resulting images due to motion of the (non-fixed) tissue.
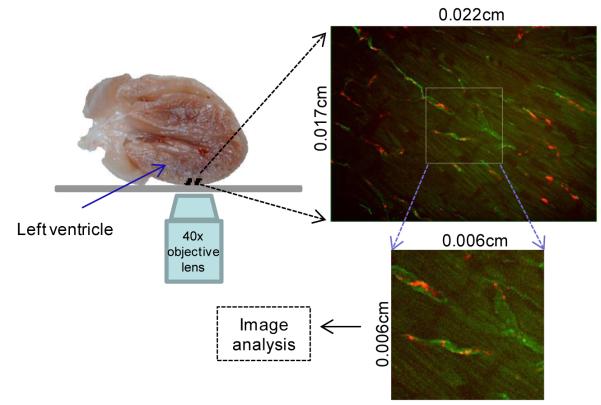
A custom designed confocal microscope was used in the ex vivo study, which was based on a Zeiss Axio Observer equipped with a Yokogawa spinning disk confocal system and a CoolSnap HQ II CCD camera with two solid-state lasers (488 and 561 nm). The microscope system was controlled by Slidebook software (Intelligent Imaging Innovations). The images were acquired with a 40x intravital lens (Objective LD C-Apochromat 40x/1.1 W Corr M27, from Carl Zeiss Microscopy, LLC, United States), with an exposure time of 1000 ms for both 488 and 561 nm wavelengths. A glass bottom culture dish (diameter: 35mm; thickness: No.1.5) (Mat Tek Corporation, MA) was used to position the mouse heart on the microscope stage with the epicardial surface of the left ventricle wall (Figure 2) down for imaging. For each mouse heart, 5 z-stacks (z step size: 1 μm; each stack has 20 steps starting from the surface of the heart) were acquired at different xy positions.
Figure 2.
Schematic of confocal image collection of fluorescent cargo in the excised heart, 0.5 or 24 hours after in vivo injection of targeted liposomes in the tail vein. Red indicates liposomal cargo and green indicates vascular lectin.
Image analysis was performed with ImageJ (http://rsbweb.nih.gov/ij/). Smaller z-stacks (squares of 4.14×10−5 cm2 at the image center, 5 μm thick centering at 10 μm depth of the heart tissue) were cropped from the original z-stacks and analyzed (Figure 2). For the conditions shown in Table 1 (a, b, c, h, i and j), no significant differences were observed over the stacks, and the first 3 z-stacks were analyzed and averaged for each mouse; for the conditions in Table 1 (d, e, f, and g), 5 z-stacks were analyzed and averaged.
The tissue fluorescence intensity (Itissue), endothelial fluorescence intensity (Iendothelium), and the punctate liposome fluorescence intensity (Ipunctate), were quantified separately. The Itissue was calculated by drawing and analyzing regions of interest (ROI) excluding the blood vessels, 5 squares of 6.9×10−7 cm2 on each z-stack. Iendothelium was recorded by drawing and quantifying ROIs based on the lectin staining of the endothelium. The fluorescence from punctate regions of fluorescence (assumed to result from liposome clusters) was quantified by the “Analyze Particles” function in ImageJ (minimum intensity: (mean + 6 × standard deviation) of Itissue; minimum area: 5.1×10−9 cm2). The punctates fluorescence was recorded as the sum of the intensity from all the pixels within the punctate regions, ΣIpunctate
All the fluorescence intensity readings were normalized with the following equation:
Where In is the normalized intensity; I is the intensity for each row in Table 1 and for each value of Itissue, Iendothelium, and Ipunctate;
Iimage is the fluorescence intensity of the image (squares of 4.14×10−5 cm2), which including both tissue and blood vessels;
Iimage,a and Iimage,f are the image fluorescence intensities at conditions a and f (Table 1).
2.2.2 Flow cytometric analyses of cargo distribution in cardiac cells
For the cellular delivery study, two-month old, male FVB mice (20-25 g) were purchased from Charles River (Wilmington, MA). Our preliminary results showed low numbers of cardiomyocytes were fluorescent at 1 hour after liposome injection, and our previous experiments with radioactivity on the liposome shell showed a wash-out of CRPPR-liposomes from the heart over time (only 44±5%, and 23±2% of initial radioactivity remained at 6 and 18 hours, n=4), therefore, we chose 4 and 12 hours post injection to ensure sufficient time for cells to pick up fluorescence and to avoid wash-out.
At 4 or 12 hours, the mice were euthanized with pentobarbital (80 mg/kg, Premier Pharmacy Labs Inc, FL), the hearts were removed and placed in Tyrode’s solution and mounted on a Langendorff apparatus. The coronary arteries were retrogradely perfused first with Tyrode’s solution and then with enzyme solution (collagenase type 2, 1 mg/ml, Worthington Biochemical Corporation, NJ). The digested hearts were gently divided into small pieces in high-K+ solution, and filtered through a 200-μm cell strainer to obtain a single cell suspension. After fixation with 0.4% PFA at room temperature for 10 min, the cells were stained with various antibodies, in Ca2+ and Mg2+ free PBS, with 0.1% Triton-X 100, 5% donkey serum, and 20 μg/ml DNAse-free RNAse at room temperature for one hour. The following antibodies were used 1) Zenon Alexa Fluor 647 (Invitrogen, Carlsbad, CA)-conjugated sarcomeric myosin heavy chain-specific antibody (MF20, Developmental Studies Hybridoma Bank, IA) (1 μg/ml) was used to label cardiomyocytes, 2) Phytoerythrin (PE) conjugated anti-mouse Thy1.1 antibody (Biolegend, San Diego, CA) (0.7μg/ml) was used to mark fibroblasts, 3) PE-conjugated anti-CD31 antibody (BD Bioscience, MD) was used to identify endothelial cells, and 4) 7 -amino-actinomycin D (7AAD, BD Bioscience, MD) (40 μg/ml), was used to stain nucleated cells prior to flow cytometry.
Flow cytometry data were collected with a Becton Dickinson FACScan flow cytometer using CellQuest software (BD Bioscience, MD) and analyzed with FlowJo (ver9.0.1 Treestar Inc., San Carlos). Each sample was evaluated in triplicate. For each condition (cell types and time points), 3-7 mice were examined to generate the mean and standard deviation (Table 2).
Table 2.
Number of animals studied with flow cytometry to assess accumulation within cardiac endothelial cells, cardiomyocytes or fibroblasts after the injection of targeted particles within the tail vein.
| Endothelial cells (CD31+) |
Cardiomyocytes (MF20+) |
Fibroblasts (Thy1.1+) |
|
|---|---|---|---|
| No-treatment | 7 | 7 | 4 |
| CRPPR, 4 Hrs | 7 | 7 | 5 |
| CRPPR, 12 Hrs | 5 | 5 | 3 |
2.2.3 PET studies of accumulation in diseased hearts using 18F-FDP-labeled liposomes
For studies of the accumulation of targeted liposomes in models of myocardial disease, 2-month old, male C57BL/6 mice (20-30g) were purchased from Charles River (Wilmington, MA).
Two heart disease models were used in our PET study—models of ischemia/reperfusion (I/R) and myocardial infarction (MI), both resulting in damage to the left anterior descending artery (LAD). I/R was induced by the ligation of the LAD for 1 hour after which the ligation was released. Twenty-four hours later, the mice were injected with liposomes and imaged, and the biodistribution was then assessed. Two MI studies were performed, both with permanent occlusion at the LAD. In the first cohort, at 7 days post MI, radioactive liposomes were injected and the mice were imaged and dissected for biodistribution. In the second cohort, LAD occlusion surgeries were performed and the mice were injected with liposomes and imaged to generate the Day 1 and Day 2 data. On the following day, mice were injected and imaged again, resulting in Day 3 and Day 4 data. Ninety-five days later, the surviving animals were injected and imaged again, and then were dissected for a biodistribution study at Day 99. For each condition (PET images or Biodistribution at different days), 3–13 mice were examined to generate the mean and standard deviation (Table 3).
Table 3.
Number of animals studied with radiolabeled particles using PET to assess accumulation after MI or I/R
| MI | I/R | WT | ||||||
|---|---|---|---|---|---|---|---|---|
| Day1 | Day2 | Day3 | Day4 | Day7 | Day99 | |||
| PET Images | 3 | 4 | 3 | 4 | 4 | 4 | 4 | 11 |
| Biodistribution | -- | -- | -- | -- | 4 | 4 | 6 | 13 |
PET imaging was performed on a microPET Focus scanner (Concorde Microsystems, Inc., TN). Mice were injected with 18F-FDP-labeled liposomes (0.1-0.2mg (3-7mg/kg) lipids, 50-200 uci, per mouse) and were imaged immediately for up to 60 minutes. Maximum a posteriori (MAP) files were then created and quantitative analysis was obtained with region-of-interest (ROI) analysis with ASIPro software (CTI Molecular Imaging). A 1470 Automatic Gamma Counter (Perkin Elmer Life Sciences, MA) was used for the biodistribution study.
2.4 Statistical Analysis
Data were recorded as mean ± standard deviation, which were calculated with Excel 2010 (Microsoft, Seattle, WA). The significance of differences between groups was assessed using a T test when two groups were compared. ANOVA followed by Tukey’s tests were applied for comparisons involving 3 or more groups. All significance tests were performed in Minitab 15 (Minitab, State College, PA), and a p value less than 0.05 indicated a statistically significant difference. Methodologies were reviewed by the UC Davis Biostatistics core personnel.
3 Results
3.1 The characterization and stability of the liposomes
The size, stability and loading efficiency are listed in Table 4. The diameter and fluorescence of liposomes loaded with Alexa 555 were unchanged over 50 days, whereas liposomes loaded with Alexa 488 were stable for 7 days; however the diameter increased between 7 and 50 days. Decomposing liposomes with Triton X-100 did not increase the fluorescence intensity, indicating that the dye within the liposomes was not quenched.
Table 4.
Characterization of the injected liposomes
| Liposomes | Label | Size (nm) |
Stability (days @ 4°C ) |
Loading efficiency (%) |
|---|---|---|---|---|
| Non-Targeted | Alexa 555 | 116±8 | >50 | 0.13±0.01 |
| CRPPR-buried | Alexa 555 | 121±1 | >50 | 1.08±0.34 |
| CRPPR | Alexa 555 | 143±12 | >50 | 0.27±0.04 |
| Alexa 488 | 172±20 | >7 | 0.16±0.12 |
3.2 The in vivo fate of heart-homing liposomes via confocal imaging
To determine the liposome localization in live tissues, we harvested the mouse heart and imaged using confocal microscopy. Following the injection of free dye (injected with a concentration that was comparable to the dye contained within 20mg/kg CRPPR liposomes), fluorescence decreased rapidly in the cardiac vasculature and surrounding heart tissue, resulting in a fluorescence intensity that was not significantly greater than the no-treatment control (Figures 3a, and b). The injection of non-targeted liposomes did not increase the cardiac fluorescence intensity significantly from the no treatment control (Figures 3a and c), although our previous PET results showed the long circulation of such liposomes in blood pool [4]. Thirty minutes after injection of CRPPR-conjugated liposomes at a dose of 20 or 60 mg/kg (Figures 3f and g), brightly fluorescent particles localized in the vasculature, with a subset of contiguous vessels heavily coated with particles, while surrounding vessels contained a sparse concentration. In [8], coronary and smaller cardiac arteries were strongly positive for the uptake of peptide CRPPR, whereas veins were weakly positive and this difference may result in the differing density.
Figure 3.
Representative ex vivo confocal images of heart (lectin –green, Alexa 555-red), after injection of (a) no treatment, (b) free dye, (c) no peptide (control) liposomes, (d) CRPPR-buried liposomes, (e-j) CRPPR-exposed liposomes, (b-g) at 0.5 hour, (h-j) at 24 hours, (e, h) 7mg/kg, (c, d, f, i) 20mg/kg, (g, j) 60mg/kg. Scale bar = 10μm. All images were normalized by subtracting tissue autofluorescence (a) and setting the mean fluorescence of (f) as 33%. Arrows indicate punctate fluorescence.
The injection of liposomes with the exposed CRPPR peptide (Figures 3e-g) resulted in an increase in the fluorescence intensity of the image by 64 fold as compared with free dye injection (Figure 3b), 47 fold greater than with liposomes without a conjugated peptide (Figure 3c), and 2.1 fold greater than liposomes with the CRPPR peptide buried within a PEG brush (Figure 3d). Together, these data suggest that the exposed peptide conjugated to the liposome was sufficient to target the endothelial lining of the heart and deliver the fluorescent dye into and across the endothelium.
Fluorescence intensity resulting from the no treatment group, from free dye injection and from the injection of a non-targeted (control) liposome was similar and therefore the control groups were combined and compared with other treatment groups (Figure 4). The fluorescence intensity of the tissue surrounding the blood vessels, resolvable fluorescent punctates and the endothelium increased as the injected dose increased from 7 to 20 mg/kg but did not increase further as the dose increased to 60 mg/kg (Figures 4 a, d, g). Thus, our results show that, the liposome accumulation on the surface of the blood vessel and the fluorescence penetration to the surrounding tissue reached a maximum at the 20 mg/kg dosage.
Figure 4.
Normalized fluorescence intensity (%) in: (a-c) tissue surrounding blood vessels; (d-f) endothelium; and (g-i) resolvable brightly fluorescent punctate regions, assumed to represent fluorescent liposome clusters, characterized by the summation of the normalized intensity of all pixels within the punctate regions. The left, middle and right columns represent the effect of dose, exposed ligand and time after injection, respectively. The group “Control”, combines the no-treatment, free-dye injection and no-ligand injection groups, was compared with: (a, d, g) CRPPR liposomes with 7, 20, 60 mg/kg dosages imaged at 0.5 hour after injection; (b, e, h) CRPPR liposomes with buried or exposed ligand, at 0.5 hour after injection of 20mg/kg; (c, f, i) CRPPR liposomes at 0.5 hour or 24 hours after injection, where the groups “0.5 hour” and “24 hours” were the summation of all doses (7, 20, and 60mg/kg) at the respective time points. The statistical significance was analyzed with ANOVA followed by Tukey’s tests, with the study sizes described in Table 1. Single symbol, p < 0.05; double symbols, p < 0.01; triple symbols, p < 0.001.
Injection of the CRPPR-buried liposomes resulted in a small but significant increase in the fluorescence intensity of the tissue surrounding the blood vessel, as well as in the blood vessels, but not in the fluorescence punctates, however the change was significantly smaller than the increased accumulation resulting from CRPPR-exposed liposomes (Figures 4 b, e, h). This agrees with our previous study which indicated that the exposure of the CRPPR peptide is required for vascular targeting [4, 31].
Twenty-four hours after injection, substantial fluorescence remained in the cardiac tissue, with a fluorescence intensity of ~40% of that observed at 0.5 hours post injection (Figures 3h-j, and 4c). The fluorescence intensity decreased in all regions between 0.5 and 24 hours after injection (Figures 4c, f, i). Yet, the fluorescence in the surrounding tissue and on the endothelium resulting from CRPPR-exposed injection remained significantly greater than the control group at 24 hours after injection (Figures 4 c and f). The fluorescence intensity on the endothelium was ~2 fold greater than the surrounding tissue at 0.5 hour after injection (comparing Figure 4c and 4f), although this difference was no longer significant at the 24 hour time point. These results indicate that the fluorescence was either washed away by the blood circulation or penetrated into the tissue or degraded between 0.5 and 24 hours.
3.3 Flow cytometry analyses
Hearts were excised from mice 4 or 12 hours post injection. Single cells were enzymatically isolated to quantify fluorescence within endothelial, cardiomyocyte, and fibroblast populations (Figure 5). Four hours after injection, the fluorescence intensity of 37.0% of endothelial (CD31+) cells, 0.63% of cardiomyocytes (MF20+), and 5.3% of fibroblasts (Thy 1.1+) was greater than in the control (non-treated) tissue. Twelve hours after injection, the fluorescence intensity remained above baseline in 29.1% of endothelial cells, 0.80% of cardiomyocytes, and 4.0% fibroblasts (Thy1.1+).
Figure 5.
Typical flow cytometry for cellular fluorescence and percentage of positive (fluorescent) cells from the mouse heart after tail vein injection of CRPPR-conjugated particles. Fluorescence was separately quantified for endothelial cells (a-c), cardiomyocytes (d-f), and Thy1.1 positive fibroblasts (g-i). Treatment groups include no injection (a,d,g), 4 hours post CRPPR-liposome injection (b,e,h) and 12 hours post CRPPR-liposome injection (c,f,i), Summary statistics for endothelial cells (j), cardiomyocytes (k), and Thy1.1 positive fibroblasts (l). The statistical significance was analyzed by ANOVA followed with Tukey’s tests, with study sizes described in Table 2. Single symbol, p < 0.05; double symbols, p < 0.01; triple symbols, p < 0.001.
3.4 The accumulation of CRPPR-conjugated liposomes in the diseased heart via PET
Compared with sham-operated control mice, mice post-MI demonstrated substantial left ventricular thinning, and impaired left ventricular wall motion (Figure 6). At one day after the MI, the lesion encompassed 41% of the heart volume (n=3), similar to literature [32], and one day after I/R, the lesion comprised ~7% of the heart volume (n=4) (both from PET image analysis).
Figure 6.
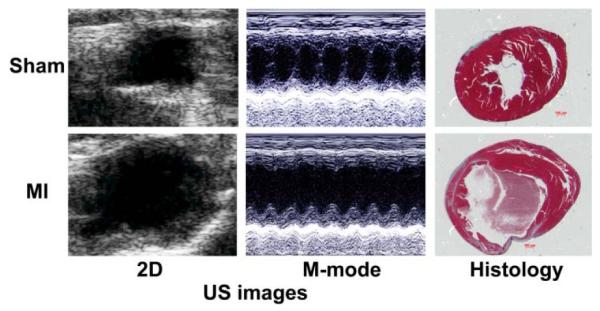
In vivo echocardiogram and histology images of the sham and MI mice, left and right ventricles are displayed on the left and right, respectively.
Compared with control mice, CRPPR-conjugated liposomal accumulation in the whole heart decreased by 19.9%, 38.0%, and 17.4%, in MI-7-day, MI-99-day, and I/R models (Figure 7), respectively. Still, the accumulation within the heart was substantially greater than other muscle. For example, heart to muscle ratio in mice 7 days post myocardial infarction was 85% of that in age-matched control mice, and the heart to muscle ratio exceeded 9 in the ischemia reperfusion group and 14 in mice 7 days post myocardial infarction.
Figure 7.

Results from the quantitation of radioactivity based on the biodistribution in the diseased heart. The accumulation of CRPPR liposomes in the diseased heart, normalized by the no-treatment counterparts, demonstrates the reduced accumulation in models of disease. n = 4-6; p values result from the comparison to their WT counterparts, with T tests in Minitab 15. *, p< 0.05.
In addition, PET images of liposomal accumulation clearly delineated the damaged regions (Figure 8a). In the control mice, a ring-shaped left ventricle demonstrates that the radioactivity was evenly distributed throughout the left ventricular wall. One day after surgery, the accumulation in the diseased site decreased to 20±4% of that of the surrounding tissue. The ratio of accumulation in the diseased versus healthy tissue increased to 58±12% and 61±19% when tested 7 and 99 days post-surgery, respectively, and was 71±4% for the I/R model (Figure 8b).
Figure 8.
Results of PET imaging and biodistribution in models of cardiovascular disease. a) PET images of mouse hearts from sham, MI-1Day, MI-7Day, and I/R mice, with the white circles highlighting the diseased sites; b) PET image-derived region of interest results for the accumulation of radioactivity in the diseased regions normalized by the radioactivity in surrounding healthy tissue in diseased and sham-treated mouse models. Comparisons were performed by ANOVA followed with Tukey’s test, with study sizes in Table 3. Single symbol, p < 0.05; double symbols, p < 0.01; triple symbols, p < 0.001.
Overall, we find that while the accumulation of vascularly-targeted liposomes decreases with MI or I/R, accumulation is substantially greater than that in skeletal muscle and increases quickly over the first days post MI.
4 Discussion
Cardiovascular disease is the leading cause of death worldwide and the prevalence is expected to increase in the coming decades. Specific delivery of drugs and genes to the heart is desirable to avoid the systemic toxicity, for example, amiodarone (or Desethylamiodarone) [33, 34]. Using vascular zip code-based targeting strategies and cell penetrating peptides to deliver drugs, genes, or nanoparticles to the heart represents a promising new strategy [12, 35]. The delivery of cardiovascular therapeutics using vascular zip codes could be applied to treat the entire heart muscle in early disease, limiting systemic exposure and reducing the injected dose. Vascularly-targeted nanotherapeutics can carry RNAi or DNA to the heart muscle, where delivery of such hydrophilic cargo is otherwise problematic. Yet, the kinetics of transport from liposomes through the endothelium is not well characterized [36]. Therefore, here, we have evaluated the accumulation of targeted liposomes in the heart in models of cardiovascular disease and have tracked the cargo as it enters the endothelium.
Taken together, our data indicate that specific delivery of liposomal cargo to cardiac endothelial cells is feasible and while delivery was reduced after MI or as a result of I/R, accumulation remains substantially higher than in surrounding tissues and increases in the days after the MI event. In the normal heart, delivery of the targeted cargo to the cardiac endothelial cells was efficient, with 37% of cardiac endothelial cells demonstrating fluorescence above control cells, as compared with ~5% of fibroblasts and <1% of myocytes. The low level of fluorescence within the myocytes may result from the far greater size of myocytes as compared with endothelial cells or may simply represent a low efficiency of transfer of the hydrophilic cargo from the endothelium to the surrounding muscle. Thus, in the myocytes and fibroblasts, low levels of fluorescence, below our level of detection, may go unrecognized.
The time course of changes in fluorescence intensity was quantified in our study. Based on our previous PET studies, the liposomes accumulate on the cardiac endothelium within 1 minute after injection[4] and the current study demonstrated that within 30 min of injection of 20mg/kg CRPPR-liposomes, fluorescence increased by 47 fold in the tissue surrounding the vascular lumen, as compared with non-targeted liposomes. Twenty-four hours after the delivery, the fluorescence intensity was ~40% of the 0.5-hour values suggesting that the cargo had cleared from the heart tissue, was further disseminated, or had broken down. Evaluating the specific cell types, fluorescence within endothelial cells and fibroblasts decreased prior to 12 hours after injection but an increase was observed in myocyte intensity from 4 to 12 hours, although the changes were not significant between these time points.
With an increase in the injected dose of targeted liposomes from 7 to 20 mg/kg, the endothelial fluorescence increased, but then did not increase further with the injection of 60 mg/kg. We hypothesize that this fluorescence saturation is due to saturation of the vascular surface receptors. Other potential mechanisms that could account for this observation include the loss of particles that accumulated after injection of the higher dose within the first thirty minutes.
We have previously demonstrated that CRPPR-conjugated liposomes with a terminal free amide (postulated to be targeted to cysteine-rich protein-2) accumulated in the heart within tens to hundreds of seconds, with the accumulated radioactivity remaining localized in the heart for hours. In preliminary studies (data not shown), the carboxyl terminated peptide did not further increase delivery. Here, we demonstrated that both the radioactivity attached to the liposomal shell and the fluorescence loaded within the core accumulated in the heart. Using confocal microscopy and flow cytometry, we showed that the cargo accumulated in the endothelium, but also passed into and through the endothelium within 30 minutes of injection, resulting in a 47 fold increase in fluorescence, as compared with the injection of non-targeted liposomes. This rapid delivery of cargo into tissues by surface-modified nanoparticles was also observed in a previous study in brain tissue [37].
The early cellular events post MI include cardiomyocyte necrosis and apoptosis and the influx of inflammatory cells leading to the phagocytosis of the dead cells (day1-2). This is followed by the influx of fibroblasts that lay down new collagen and an increase in vascular endothelial growth factor (VEGF) with possible growth of collateral vessels [38]. The low uptake of CRPPR-liposomes one day post MI reflects reduced perfusion of the tissue distal to the vessel occlusion, with subsequent angiogenesis in collateral vessels likely resulting in the increased tissue uptake at later time points. Therefore, the injection of targeted liposomes 7 or more days post MI could result in effective delivery.
In summary, we conclude that heart-homing liposomes, targeting the endothelium, could be applied in cardiovascular interventions if administered at the proper time after infarction. Further, delivery to the endothelium is substantial and the payload was observed to penetrate into the tissue, accumulating within a large fraction of endothelial cells, and entering a limited number of fibroblasts and cardiomyocytes.
Acknowledgements
The authors would thank Dr. Hong Qiu for helpful discussion, Dr. Sandra Taylor for suggestions in data analysis, and Sarah Johnson for her help in animal work. This publication has been funded in part with the Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201000043C (K.W.F.), by NIHR01CA134659, NIHR01CA103828 (K.W.F.), NIHT32EB003827 (K.W.F.), R01 HL85844 (N.C.), R01 HL85727 (N.C.), T32 DC008072 (N.C.), T32 HL86350 (P.S.), and by VA Merit Review Grant 5 I01BX000576 (N.C.).
Abbreviations
- %ID/g
% injected dose per gram tissue
- 7AAD
7-amino- actinomycin D
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- DSPE-PEG2000
1,2-distearoylsn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt)
- I/R
ischemia/reperfusion
- i.v.
intravenous
- LAD
left anterior descending artery
- LPP
Lipo-Peg-Peptides
- MAP
Maximum a posteriori
- MI
myocardial infarct
- MW
Molecular weight
- PBS
phosphate buffered saline
- PE
Phytoerythrin
- PEG
Polyethylene glycol
- PET
Positron emission tomography
- PFA
paraformaldehyde
- ROI
regions of interest
- US
Ultrasound
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].van Rooy I, Mastrobattista E, Storm G, Hennink WE, Schiffelers RM. Comparison of five different targeting ligands to enhance accumulation of liposomes into the brain. Journal of Controlled Release. 2011;150(1):30–36. doi: 10.1016/j.jconrel.2010.11.014. [DOI] [PubMed] [Google Scholar]
- [2].Maruyama K, Kennel SJ, Huang L. Lipid-composition is important for highly efficient target binding and retention of immunoliposomes. Proc. Natl. Acad. Sci. U. S. A. 1990;87(15):5744–5748. doi: 10.1073/pnas.87.15.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ.Res. 2005;96(3):327–336. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- [4].Zhang H, Kusunose J, Kheirolomoom A, Seo JW, Qi JY, Watson KD, Lindfors HA, Ruoslahti E, Sutcliffe JL, Ferrara KW. Dynamic imaging of arginine-rich heart-targeted vehicles in a mouse model. Biomaterials. 2008;29(12):1976–1988. doi: 10.1016/j.biomaterials.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380(6572):364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- [6].Fan XM, Venegas R, Fey R, van der Heyde H, Bernard MA, Lazarides E, Woods CM. An in vivo approach to structure activity relationship analysis of peptide ligands. Pharm. Res. 2007;24(5):868–879. doi: 10.1007/s11095-007-9238-z. [DOI] [PubMed] [Google Scholar]
- [7].Rajotte D, Ruoslahti E. Membrane dipeptidase is the receptor for a lung-targeting peptide identified by in vivo phage display. J. Biol. Chem. 1999;274(17):11593–11598. doi: 10.1074/jbc.274.17.11593. [DOI] [PubMed] [Google Scholar]
- [8].Zhang LL, Hoffman JA, Ruoslahti E. Molecular profiling of heart endothelial cells. Circulation. 2005;112(11):1601–1611. doi: 10.1161/CIRCULATIONAHA.104.529537. [DOI] [PubMed] [Google Scholar]
- [9].Arap W, Haedicke W, Bernasconi M, Kain R, Rajotte D, Krajewski S, Ellerby HM, Bredesen DE, Pasqualini R, Ruoslahti E. Targeting the prostate for destruction through a vascular address. Proc. Natl. Acad. Sci. U. S. A. 2002;99(3):1527–1531. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Essler M, Ruoslahti E. Molecular specialization of breast vasculature: A breast-homing phage-displayed peptide binds to aminopeptidase P in breast vasculature. Proc. Natl. Acad. Sci. U. S. A. 2002;99(4):2252–2257. doi: 10.1073/pnas.251687998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. U. S. A. 2009;106(38):16157–16162. doi: 10.1073/pnas.0908201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Coadministration of a Tumor-Penetrating Peptide Enhances the Efficacy of Cancer Drugs. Science. 2010;328(5981):1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Torchilin VP, Narula J, Halpern E, Khaw BA. Poly(ethylene glycol)-coated anti-cardiac myosin immunoliposomes: Factors influencing targeted accumulation in the infarcted myocardium. Biochim. Biophys. Acta-Biomembr. 1996;1279(1):75–83. doi: 10.1016/0005-2736(95)00248-0. [DOI] [PubMed] [Google Scholar]
- [14].Dvir T, Bauer M, Schroeder A, Tsui JH, Anderson DG, Langer R, Liao R, Kohane DS. Nanoparticles Targeting the Infarcted Heart. Nano Letters. 2011;11(10):4411–4414. doi: 10.1021/nl2025882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kanki S, Jaalouk DE, Lee S, Yu AYC, Gannon J, Lee RT. Identification of targeting peptides for ischemic myocardium by in vivo phage display. J. Mol. Cell. Cardiol. 2011;50(5):841–848. doi: 10.1016/j.yjmcc.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279(5349):377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- [17].Porkka K, Laakkonen P, Hoffman JA, Bernasconi M, Ruoslahti E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc. Natl. Acad. Sci. U. S. A. 2002;99(11):7444–7449. doi: 10.1073/pnas.062189599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Corti A, Curnis F, Arap W, Pasqualini R. The neovasculature homing motif NGR: more than meets the eye. Blood. 2008;112(7):2628–2635. doi: 10.1182/blood-2008-04-150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ruoslahti E. Vascular zip codes in angiogenesis and metastasis. Biochem. Soc. Trans. 2004;32:397–402. doi: 10.1042/BST0320397. [DOI] [PubMed] [Google Scholar]
- [20].Abu Lila AS, Ishida T, Kiwada H. Recent advances in tumor vasculature targeting using liposomal drug delivery systems. Expert Opin. Drug Deliv. 2009;6(12):1297–1309. doi: 10.1517/17425240903289928. [DOI] [PubMed] [Google Scholar]
- [21].Neri D, Bicknell R. Tumour vascular targeting. Nat. Rev. Cancer. 2005;5(6):436–446. doi: 10.1038/nrc1627. [DOI] [PubMed] [Google Scholar]
- [22].Kelly KA, Nahrendorf M, Yu AM, Reynolds F, Weissleder R. In vivo phage display selection yields atherosclerotic plaque targeted peptides for imaging. Mol. Imaging. Biol. 2006;8(4):201–207. doi: 10.1007/s11307-006-0043-6. [DOI] [PubMed] [Google Scholar]
- [23].Lindner JR. Molecular Imaging of Vascular Phenotype in Cardiovascular Disease: New Diagnostic Opportunities on the Horizon. J. Am. Soc. Echocardiogr. 2010;23(4):343–350. doi: 10.1016/j.echo.2010.01.025. [DOI] [PubMed] [Google Scholar]
- [24].Godin B, Sakamoto JH, Serda RE, Grattoni A, Bouamrani A, Ferrari M. Emerging applications of nanomedicine for the diagnosis and treatment of cardiovascular diseases. Trends Pharmacol. Sci. 2010;31(5):199–205. doi: 10.1016/j.tips.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Mulder WJM, Fayad ZA. Nanomedicine captures cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2008;28(5):801–802. doi: 10.1161/ATVBAHA.108.165332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Greig JA, Shirley R, Graham D, Denby L, Dominiczak AF, Work LM, Baker AH. Vascular-Targeting Antioxidant Therapy in a Model of Hypertension and Stroke. J. Cardiovasc. Pharmacol. 2010;56(6):642–650. doi: 10.1097/FJC.0b013e3181f8f19f. [DOI] [PubMed] [Google Scholar]
- [27].Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M. Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Cancer Res. 2003;63(21):7400–7409. [PubMed] [Google Scholar]
- [28].Maeda N, Takeuchi Y, Takada M, Sadzuka Y, Namba Y, Oku N. Anti-neovascular therapy by use of tumor neovasculature-targeted long-circulating liposome. Journal of Controlled Release. 2004;100(1):41–52. doi: 10.1016/j.jconrel.2004.07.033. [DOI] [PubMed] [Google Scholar]
- [29].Oh P, Borgstrom P, Witkiewicz H, Li Y, Borgstrom BJ, Chrastina A, Iwata K, Zinn KR, Baldwin R, Testa JE, Schnitzer JE. Live dynamic imaging of caveolae pumping targeted antibody rapidly and specifically across endothelium in the lung. Nat. Biotechnol. 2007;25(3):327–337. doi: 10.1038/nbt1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Inai T, Mancuso M, Hashizume H, Baffert F, Haskell A, Baluk P, Hu-Lowe DD, Shalinsky DR, Thurston G, Yancopoulos GD, McDonald DM. Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am. J. Pathol. 2004;165(1):35–52. doi: 10.1016/S0002-9440(10)63273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Borden MA, Zhang H, Gillies RJ, Dayton PA, Ferrara KW. A stimulus-responsive contrast agent for ultrasound molecular imaging. Biomaterials. 2008;29(5):597–606. doi: 10.1016/j.biomaterials.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang JF, Bo HB, Meng XY, Wu Y, Bao YL, Li YX. A simple and fast experimental model of myocardial infarction in the mouse. Tex. Heart Inst. J. 2006;33(3):290–293. [PMC free article] [PubMed] [Google Scholar]
- [33].Camus P, Martin WJ, Rosenow EC. Amiodarone pulmonary toxicity. Clin. Chest Med. 2004;25(1):65. doi: 10.1016/S0272-5231(03)00144-8. + [DOI] [PubMed] [Google Scholar]
- [34].Naccarelli GV, Wolbrette DL, Patel HM, Luck JC. Amiodarone: clinical trials. Curr. Opin. Cardiol. 2000;15(1):64–72. doi: 10.1097/00001573-200001000-00009. [DOI] [PubMed] [Google Scholar]
- [35].Schmidt N, Mishra A, Lai GH, Wong GCL. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010;584(9):1806–1813. doi: 10.1016/j.febslet.2009.11.046. [DOI] [PubMed] [Google Scholar]
- [36].Di Marco M, Shamsuddin S, Razak KA, Aziz AA, Devaux C, Borghi E, Levy L, Sadun C. Overview of the main methods used to combine proteins with nanosystems: absorption, bioconjugation, and encapsulation. Int. J. Nanomed. 2010;5:37–49. [PMC free article] [PubMed] [Google Scholar]
- [37].Tahara K, Miyazaki Y, Kawashima Y, Kreuter J, Yamamoto H. Brain targeting with surface-modified poly(D,L-lactic-co-glycolic acid) nanoparticles delivered via carotid artery administration. European Journal of Pharmaceutics and Biopharmaceutics. 2011;77(1):84–88. doi: 10.1016/j.ejpb.2010.11.002. [DOI] [PubMed] [Google Scholar]
- [38].Liehn EA, Postea O, Curaj A, Marx N. Repair After Myocardial Infarction, Between Fantasy and Reality. J. Am. Coll. Cardiol. 2011;58(23):2357–2362. doi: 10.1016/j.jacc.2011.08.034. [DOI] [PubMed] [Google Scholar]