Abstract
Therapeutic treatment of large established tumors using immunotherapy has yielded few promising results. We investigated if adoptive transfer of tumor-specific CD8+ T cells together with tumor-specific CD4+ T cells would mediate regression of large established B16BL6-D5 (D5) melanomas in lymphopenic Rag1−/− recipients devoid of regulatory T cells. The combined adoptive transfer of subtherapeutic doses of both TRP1-specific TCR transgenic Rag1−/− CD4+ T cells and gp100-specific TCR transgenic Rag1−/− CD8+ T cells into lymphopenic recipients, that received vaccination, led to regression of large (100–400 mm2) melanomas. The same treatment strategy was ineffective in lymphoreplete wt mice. Twenty-five percent of mice (15/59) had tumors recur (15–180 days post regression). Recurrent tumors were depigmented and had decreased expression of gp100, the epitope targeted by the CD8+ T cells. Mice with recurrent melanoma had increased CD4+Foxp3+ TRP1-specific T cells compared to mice that did not show evidence of disease. Importantly, splenocytes from mice with recurrent tumor were able to suppress the in vivo therapeutic efficacy of splenocytes from tumor-free mice. These data demonstrate that large established tumors can be treated by a combination of tumor-specific CD8+ and CD4+ T cells. Additionally, recurrent tumors exhibited decreased antigen expression and were accompanied by conversion of the therapeutic tumor-specific CD4+ T cell population to a FoxP3+ CD4+ regulatory T cell population.
Introduction
Cancer immunosurveillance suggests that malignant cells are targeted and destroyed by the immune system (1). Preclinical mouse models have demonstrated that the presence of a functional immune system is critical to avoid the development of spontaneous tumors (2–4). Further, increased infiltration of human solid tumors with cytotoxic CD8+ T cells has correlated with prolonged survival (5–7). These studies show the protective role of the immune system against tumors, however, the immune system occasionally fails to completely eliminate potential malignant cells allowing tumors to escape and form larger, established tumors. As tumor becomes established it is often difficult to use the immune system to eradicate the tumor since the process of tumor escape selects for reduced immunogenicity of the tumor and/or the presence of immunosuppressive mechanisms to attenuate the anti-tumor immune response (8, 9). It is imperative to understand these interactions in order to develop more effective therapeutic approaches to treat cancer patients for whom cancer immunosurveillance has failed.
The presence of T cells specific for tumor antigens among a cancer patient’s T cell repertoire verifies the existence of tumor-specific T cell clones, and the presence of T cells at the tumor site has correlated with improved outcomes (10–12). However, the inability of these tumor-specific T cells to maintain immune surveillance implies that extrinsic factors limit the efficacy of these T cells and/or these T cells intrinsically lack the properties necessary to eliminate tumor cells. To prime a therapeutic anti-tumor immune response, tumor-specific T cells must have T-cell receptors (TCR) with sufficient affinity for tumor antigens to enable their activation (13–15). Strategies to enhance priming of these low-affinity, tumor-specific T cells would be beneficial. Seminal work by Mackall et al., provided evidence that T cells are more sensitive to T cell activation during immune reconstitution (16), primarily as a result of increased access to homeostatic cytokines (17, 18). Various groups have extended this observation demonstrating that anti-tumor immune responses are enhanced during immune reconstitution (19–21).
While initial interactions of tumor-specific T cells with their antigen dictate whether they will become activated, the tumor environment also influences the anti-tumor immune response (22). It has become increasingly clear that tumors can establish an immunosuppressive environment that blocks both the priming and the effector phase of the immune response. Factors such as TGF-β (23, 24), IL-10 (25), prostaglandins (26, 27) and IDO (28), secreted either by the tumor or by suppressive cell populations, have all been shown to mediate this effect. Regulatory T cells (Treg) are present in variety of tumors (29–31) and have been shown to attenuate graft-versus-host disease (32, 33) demonstrating their potent suppressive role in controlling the immune response. The Treg population can be subdivided into two groups: natural Treg cells, and peripherally-induced Treg cells both of which can contribute to immune suppression during the tumor-bearing state (34, 35). Importantly, various groups have shown that depletion of Treg cells results in enhanced anti-tumor immune responses that protect against tumor challenge or treat minimal tumor burden (36–40).
These data led us to investigate whether the adoptive transfer of tumor-specific T cells into a lymphopenic environment devoid of regulatory T cells would lead to regression of large established tumors. Previous work has examined the contribution of CD4+ T cells to the development, expression and maintenance of CD8+ T cells (41–44). Our studies have demonstrated that the adoptive transfer of activated CD8+ effector T cells could mediate therapeutic effects against 3-day experimental pulmonary metastases, but complete elimination and maintenance of long-term protection required CD4+ T cell help (45). These data provided the rationale to use both a tumor-specific CD4+ TCR transgenic T cell and tumor-specific CD8+ TCR transgenic T cell to examine if their adoptive transfer into lymphopenic hosts could regress large established tumors. To further insure that the observed effects were due to the transgenic TCR and not an endogenous TCR, both the CD4 and CD8 TCR transgenic mice were backcrossed to the Rag1−/− background.
Materials and Methods
Mice
Female Rag1−/− mice on a C57BL/6 background (B6.129S7-Rag1tm1Mom/J) were purchased from the Jackson Laboratory (Bar Harbor, ME). pmel-1 TCR transgenic mice were bred with Rag1tm1Mom (Rag1−/−) mice to generate pmel TCR X Rag1−/− mice. TRP1 TCR X tyrp-1bwRag1−/− transgenic mice express transgenic TCR specific for mtyrp1(113–127) peptide and are homozygous for the white-based brown mutation, Bw, that have a defect in exon 1 of tyrosinase-related protein-1 gene (46, 47). All mice were maintained in a specific pathogen-free environment. Recognized principles of laboratory animal care were followed (Guide for the Care and Use of Laboratory Animals, National Research Council, 1996), and all animal protocols were approved by the Earle A. Chiles Research Institute Animal Care and Use Committee.
Tumor cell lines
D5 (H2b) is a poorly immunogenic subclone of the B16 melanoma cell line, B16BL6, as vaccination with 107 irradiated D5 tumor fails to protect C57BL/6 mice from a subsequent minimal tumor challenge. D5-G6 is a clone generated by transduction of D5 with the MFG-mGM-CSF retroviral vector; it produces GM-CSF at 60ng/ml/106 cells/24 h. MCA-310 (H2b) is a chemically induced fibrosarcoma cell line and was used as an unrelated control for T-cell stimulation. D5-CIITA and MCA310-CIITA are stable cell lines of D5 and MCA-310 that express the human MHC class II transactivator leading to increased levels of MHC class II (I-Ab/I-Eb) and MHC class I (H-2Kb) expression on the cell surface. All cell lines have previously been described (21). D5-White and D5-Black were isolated from the depigmented and pigmented portions of a recurrent tumor, respectively. All cell lines were maintained in complete media (CM) comprising the following: RPMI 1640 (Cambrex, Walkersville, MD) containing 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 2 mM L-glutamine, and 50 μg/ml gentamicin sulfate. This was further supplemented with 50 μM 2-mercaptoethanol (Aldrich, Milwaukee, WI) and 10% (vol/vol) fetal calf serum (GIBCO BRL, Grand Island, NY).
Western blot of murine gp100
Tumor cell lines were lysed in cell lysis buffer (150mM NaCl, 50 mM Tris-HCl, 1% NP-40, freshly added protease inhibitors). Samples were run on SDS gel and western blotted using anti-mgp100 antibody (Labvision, Fremont, CA), anti-tyrp1 (Santa Cruz) or anti-β-Actin antibody (I-19, Santa Cruz Biotechnology, Santa Cruz, CA). Western blots were developed using chemiluminscent Pierce SuperSignal West Substrate (Pierce, Rockford, IL).
Intracellular tyrp1 protein staining
Tumor cell lines were washed in FACs buffer (2% FBS, HBSS) and permeabilized by incubation with Cytofix/Cytoperm solution (BD-PharMingen, San Diego, CA), for 20 minutes at 4° C. After washing and resuspension in Perm/wash solution (BD-PharMingen, San Diego, CA), intracellular staining with PE-labeled anti-Tyrp1 antibody (M-19, Santa Cruz Biotechnology, Santa Cruz, CA) was performed 20 minutes at 4° C. Cells were washed twice with perm/wash solution, resuspended in FACS-buffer, and analyzed on a BD Bioscience FACSCalibur.
Adoptive Transfer of transgenic T cells
C57BL/6 mice or Rag1−/− mice were injected subcutaneously in the hind flank with 106 tumor cells (TD100=104 cells). Tumors were allowed to establish and become sizeable (50 – 400 mm2). In our experience all mice with tumor in this size range remained mobile and did not experience clinical signs of distress or cachexia. Animals were euthanized at signs of distress and/or cachexia. Nine to 16 days after tumor challenge, indicated numbers of naïve TRP1 X Rag1−/−splenocytes and/or pmel X Rag1−/− splenocytes were injected intravenously into tumor-bearing mice. Recipient mice also received a subcutaneous injection in the contralateral flank with 107 irradiated (10,000 Rads) D5-G6 cells. In some experiments TRP1 X Rag1−/− splenocytes and pmel X Rag1−/− splenocytes were plated at 2 × 106 cells per ml in CM and cultured in 24 well plates with 5 μg/ml anti-CD3 antibody (2C11). After 48 hours of activation, the cells were harvested and expanded in CM containing 60 IU per ml of rhIL-2 (Chiron, Emeryville, CA) at a starting cell density of 1.50 × 105 cells per ml in 6 well plate. After 72 hours, ‘effector’ T cells were harvested and adoptively transferred into tumor-bearing Rag1−/− recipients.
CFSE-staining
Rag1−/− mice were injected subcutaneously with 5×105 D5 tumor cells. Splenocytes from pmel TCR transgenic mice and TRP1 TCR transgenic mice were labeled with 5 μM of CFSE according to manufacturer’s protocol (Invitrogen). Twenty days after tumor challenge 3×106 CFSE-labeled pmel splenocytes and 3×106 CFSE-labeled TRP1 splenocytes were adoptively transferred into the tumor-bearing mice. Some mice were vaccinated subcutaneously with 107 irradiated D5-G6. Four days after adoptive transfer splenocytes were collected and stained with anti-CD4 PE, anti-CD3 PE-Cy7, and anti-CD8 APC. Cells were analyzed on a FACSCalibur (BD Biosciences).
Intracellular Foxp3 Staining
Intracellular staining for FoxP3 was performed using the manufacturer’s protocol (eBioscience). Briefly, cells were stained for surface molecules with anti-CD3 PE-Cy7, anti-CD4 FITC. Cells were permeabilized using freshly prepared Fix/Perm solution and incubated for 1 to 2 hours at 4°C. Cells were washed once with permeabilization buffer. Cells were blocked with purified anti-mouse Fc-receptor for 15 minutes. Cells were then stained intracellularly with PE-labeled anti-FoxP3 at 0.5 ug per 106 cells and incubated at 4°C for 30 minutes. Cells were washed and resuspended in 1% paraformaldehyde and analyzed on a FACSCalibur (BD Biosciences).
ELISA
Spleens from either pmel TCR transgenic or TRP1 TCR transgenic mice were collected and processed to yield single cell suspensions. 106 splenocytes were cultured with 105 D5, MCA-310, D5-CIITA, MCA310-CIITA, or D5-White tumor cells. Splenocytes stimulated with plate-bound anti-CD3 antibody (10 μg/ml) or no stimulation were used as positive and negative controls, respectively. After culture for 20 hours, supernatants were harvested and IFN-γ concentration determined by ELISA using commercially available reagents (anti-IFN-γ, BD Biosciences). The concentration of IFN-γ was determined by regression analysis.
Statistical analysis
Statistical analysis of the data was performed using Prism (GraphPad Software). A two-tailed p value less than 0.05 was considered significant for ELISA data and frequency of FoxP3+ TRP1 T cells. Statistical significance for survival studies was determined by using the Gehan-Breslow-Wilcoxon Test, a p value less than 0.05 was considered significant.
Results
gp100-specific TCR transgenic CD8+ T cells and TRP1-specific TCR transgenic CD4+ T cells can respond to gp100 and tyrp1 antigens expressed by the B16BL6-D5 melanoma cell line
The availability of both CD4+ and CD8+ TCR transgenic mice that are specific to endogenous proteins expressed by melanoma provided an opportunity to examine if adoptive transfer of tumor-specific CD8+ T cells together with tumor-specific CD4+ T cells could mediate regression of established tumors. The T cell compartment of the TRP1 TCR transgenic X Rag1−/− mouse contains only CD4+ TCR transgenic T cells that recognizes the tyrp1(109–130) peptide (46, 47). Likewise, we bred the pmel TCR transgenic mouse with the Rag1−/− mouse to obtain the pmel TCR transgenic X Rag1−/− mouse that contains only CD8+ TCR transgenic T cells that recognize gp10025–33 of the endogenous murine gp100 protein (48). Both of these strains of mice will be devoid of endogenous TCR rearrangement since they lack the Rag1 protein. We verified that D5, a poorly-immunogenic subclone of B16BL6, expresses both gp100 and tyrp1 (Fig. 1A). Additionally, splenocytes from pmel mice secreted significantly increased amounts of IFN-γ when cultured with D5, but not MCA-310, verifying that processing and presentation of gp10025–35 on MHC class I molecules was occurring in D5 (Fig. 1B). In order to assay whether D5 expresses sufficient tyrp1 protein to stimulate TRP1 TCR CD4+ transgenic T cells, we transduced D5 with CIITA to upregulate MHC class II on the surface of this cell line. Splenocytes from TRP1 TCR transgenic mice secreted significantly increased amounts of IFN-γ when stimulated with D5-CIITA compared to the irrelevant sarcoma cell line, MCA310-CIITA (Fig. 1C) that does not express tyrp1 protein (Fig. 1A).
Figure 1. B16BL/6-D5 (D5) expresses gp100 and tyrp1 protein, and is recognized by pmel TCR transgenic T cells and TRP1 TCR transgenic T cells.
(A) Western blot analysis of gp100, tyrp1, and β-Actin expression from D5 or MCA310 cell lysates. 106 splenocytes from pmel TCR transgenic mice (B) or TRP1 TCR transgenic mice (C) were stimulated in vitro with 105 D5, MCA310, D5CIITA, MCA310CIITA, or no tumor. Supernatants were harvested and IFN-γ concentration was determined by ELISA. Data are mean of two independent experiments (±SE).
Adoptive transfer of CD4+ TCR transgenic T cells together with CD8+ TCR transgenic T cells into a lymphopenic mouse with D5 tumor causes the regression of D5
D5 is classified as poorly immunogenic since vaccination with irradiated D5 does not protect mice against subsequent D5 tumor challenge. Previous work in our lab has demonstrated the ability of a polyclonal anti-tumor T cell response to reproducibly treat 3-day experimental pulmonary metastases. However, adoptive transfer of these tumor-specific T cells has not been effective against established subcutaneous tumors. Since we had never observed effective treatment of these tumors with adoptive transfer of polyclonal tumor-specific effector T cells, in the first experiment we combined multiple strategies (Fig. 2A). Specifically, wt C57BL/6 mice were injected subcutaneously with 106 D5 tumor cells, followed nine days later with an adoptive transfer of 106 pmel “naïve” splenocytes and 5×105 TRP1 “naïve” splenocytes. These mice were then vaccinated with irradiated GM-CSF-secreting D5 (D5-G6). On day 12 after tumor challenge, the mice received 2 × 106 in vitro-activated “effector” pmel splenocytes together with 2×105 in vitro-activated “effector” TRP1 splenocytes. IL-2 (90,000 IU/day) was given for 5 days separated by 2 days of rest for up to 5 weeks. Amazingly, this combination vaccine/adoptive immunotherapy strategy had absolutely no impact on tumor growth in lymphoreplete wt C57BL/6 mice (Fig. 2 B&D). In the same experiment we also tested the same combination immunotherapy strategy in lymphopenic Rag1−/− recipients. D5 tumors in lymphopenic conditions grew similar to the tumors in lymphoreplete mice (Fig. 2 B&C). In striking contrast to wt mice, tumor-bearing Rag1−/− mice that received the same combination immunotherapy specified above began to regress tumor on day 16 (Fig. 2E) when the tumors had reached an average size of 76 mm2. By day 60 all of the lymphopenic mice that had received pmel and TRP1 T cells did not have palpable tumors.
Figure 2. The adoptive transfer of TRP1 and pmel T cells combined with systemic IL-2 and vaccination causes the regression of established D5 tumor in lymphopenic mice.
(A) 106 D5 tumor cells were injected subcutaneously into wt C57BL/6 mice (B&D) or Rag1−/− mice (C&E). Nine days later, these mice received 106 pmel splenocytes and 5 × 105 TRP1 splenocytes (D&E) and a subcutaneous vaccination with lethally-irradiated D5-G6 (B–E). On day 12 after tumor challenge, the mice also received 2 × 106 in vitro-activated pmel splenocytes together with 2 × 105 in vitro-activated TRP1 splenocytes (D&E). All mice received IL-2 (90,000 IU/day) for 5 days for up to 5 cycles separated by 2 days. Data is representative of four independent experiments. (F) 106 D5 tumor cells were injected subcutaneously into Rag1−/− mice. Twelve days later, these mice received 7.5×105 pmel splenocytes and 5×105 TRP1 splenocytes and a subcutaneous vaccination with 107 lethally-irradiated D5-G6 (G&H). On day 17 after tumor challenge, the mice also received 106 in vitro-activated pmel splenocytes together with 2 × 105 in vitro-activated TRP1 splenocytes (H). Data are representative of two independent experiments
The striking result that adoptive transfer of tumor-specific “naïve” and “effector” CD4 and CD8 T cells, IL-2 administration and vaccination in a lymphopenic environment mediated regression of established tumors led us to evaluate if regression required all elements of the therapeutic strategy. It was clear that the lymphopenic environment was required so all further in vivo experiments were performed in lymphopenic hosts (Rag1−/− mice). We wanted to determine if the adoptive transfer of naïve tumor-specific TCR transgenic T cells together with vaccination would be sufficient to mediate tumor regression (Fig. 2F). As shown previously, Rag1−/− recipient mice challenged with D5 tumor followed by naïve TCR transgenic CD4 and CD8 T cells on day 12 and in vitro-activated ‘effector’ TCR transgenic CD4 and CD8 T cells on day 16 regressed large established tumor averaging 273 mm2 (Fig. 2G). However, the regression of tumor was not dependent on the ex vivo-activation of T cells, as the adoptive transfer of naïve pmel and naïve TRP1 cells together with the D5-G6 vaccination was sufficient to cause tumor regression (Fig. 2H).
Previous work has demonstrated that the adoptive transfer of TRP1 CD4+ TCR transgenic cells can mediate regression of B16BL6 tumors (46, 47, 49). Since we were interested in the cooperative effect of the CD4+ TCR transgenic T cells combined with the CD8+ TCR transgenic T cells, we titrated the dose of naïve TRP1 splenocytes to find a dose that did not cause complete regression of tumor in all vaccinated tumor-bearing Rag1−/− mice. The adoptive transfer of 5×105 TRP1 splenocytes led to the regression of D5 tumor in a majority of Rag1−/− mice (Fig 3 A&B) and 85% survival 100 days after tumor challenge (Fig. 3E). A majority of tumor-bearing mice receiving 1.5×105 TRP1 splenocytes also exhibited tumor regression (Fig. 3C), however only 33% of mice survived until day 100 (Fig. 3E). The adoptive transfer of 5×104 TRP1 splenocytes caused tumor regression in less than 40% of the treated mice (Fig. 3D), and none of the mice that received this dose of TRP1 splenocytes survived to day 100 (Fig. 3E). This lower dose of TRP1 splenocytes did not provide a statistically significant increase in survival versus no T cell transfer. Based on these data we adoptively transferred into tumor-bearing mice 2.5×104 TRP1 splenocytes combined with 7.5×105 of pmel splenocytes, a dose that was 10-fold less than the therapeutic dose determined by other groups (46–49) The combined adoptive transfer of pmel and TRP1 splenocytes led to regression of large established D5 tumors in a majority of mice (Fig. 3 F&G) and significantly enhanced survival versus the transfer of TRP1 splenocytes alone (Fig. 3H).
Figure 3. The addition of pmel T cells to a suboptimal dose of TRP1 T cells significantly enhances survival.
106 D5 tumor cells were injected subcutaneously into Rag1−/− mice. On day 13 after tumor challenge, mice received titrated doses of TRP1 splenocytes (A–D). Mice receiving 5×104 TRP1 splenocytes had no surviving mice at day 100 (E). 106 D5 tumor cells were injected subcutaneously into Rag1−/− mice. On day 13 after tumor challenge, mice received either 2.5×104 TRP1 splenocytes (F) or 2.5×104 TRP1 splenocytes and 7.5×105 pmel splenocytes (G). (H) The addition of 7.5×105 pmel splenocytes to 2.5×104 TRP1 splenocytes led to a significant enhancement of survival versus 2.5×104 TRP1 splenocytes alone. Data are summary of two independent experiments.
GM-CSF secreting tumor vaccination induces pmel T cell proliferation and tumor regression
Since homeostatic proliferation can lead to the expansion of T cells in the lymphopenic environment, it is possible that the lymphopenic environment might activate both pmel and TRP1 T cells independent of the D5-G6 vaccine. Approximately 20% of both pmel CD8+ transgenic T cells and TRP1 CD4+ transgenic T cells undergo homeostatic proliferation when they are transferred into lymphopenic mice that are tumor-free and not vaccinated (Fig. 4A). The presence of subcutaneous D5 tumor caused the TRP1 T cells to undergo strong proliferation, whereas there was minimal enhancement of proliferation of the pmel T cells (Fig. 4A). Vaccination with the GM-CSF-secreting tumor vaccine induced 2-fold more pmel T cells to proliferate (Fig. 4A), and importantly sustained tumor regression was observed only with this D5-G6 vaccination (Fig. 4B). Taken together these data indicate that, at the doses used in the adoptive transfer, three components are needed for tumor regression (TRP1 CD4+ T cells, pmel CD8+ T cells, and D5-G6 vaccination).
Figure 4. Tumor regression is dependent on tumor vaccination.
(A) 5×105 D5 tumor cells were injected subcutaneously into Rag1−/− mice. Twenty days after tumor challenge 3×106 CFSE-labeled pmel splenocytes and 3×106 CFSE-labeled TRP1 splenocytes were adoptively transferred into the tumor-bearing Rag1−/− mice. Some mice were vaccinated subcutaneously with 107 irradiated D5-G6. Four days after adoptive transfer splenocytes were analyzed by flow cytometry. Numbers represents the percent of CD3+CD8+ cells (left column) or CD3+CD4+ cells (right column) that have proliferated one cycle or more. (B) 5×105 D5 tumor cells were injected subcutaneously into Rag1−/− mice. On day 13, 5×105 pmel splenocytes and 2.5×104 TRP1 splenocytes were adoptively transferred into tumor-bearing Rag1−/− mice and subcutaneously vaccinated with 107 irradiated D5-G6 or not vaccinated at all. Data are representative of two independent experiments.
Tumor recurrence is associated with tumor depigmentation and loss of antigen expression
The adoptive transfer of pmel and TRP1 T cells caused tumor regression in Rag1−/− recipient mice, however twenty-five percent of mice had a recurrent tumor that grew out after the initial tumor regression (15 mice had tumor recurrence/59 treated animals – 10 experiments). Interestingly, all recurrent tumors displayed some degree of depigmentation (Fig. 5A). Since both tyrp1 and gp100 are proteins involved in the pigmentation of this melanoma, we were interested to determine if the adoptive transfer of pmel and TRP1 TCR transgenic cells selected for an antigen-loss variant. Two cells lines were derived from a tumor that upon resection had both pigmented (D5-Black) and depigmented (D5-White) portions of the tumor. As expected the parental D5, as well as the D5-Black cell lines expressed tyrp1 protein while the methylcholanthrene-induced sarcoma cell line, MCA-310, did not express tyrp1 protein (Fig. 5B). The depigmented D5-White cell line continued to express tyrp1, demonstrating that it was not the loss of tyrp1 that resulted in depigmentation. D5-Black retained expression of gp100 whereas the depigmented tumor cell line, D5-White, did not express detectable amounts of gp100 protein by Western blot analysis (Fig. 5C). We also observed that D5-White had increased expression of MHC class I compared to the parental D5 tumor cell line (Fig. 5D). Interestingly, pmel T cells cultured with D5-White tumor cells secreted higher amounts of IFN-γ than pmel T cells cultured alone (without stimulation), and statistically greater amounts compared to pmel T cells cultured with the irrelevant MCA-310 sarcoma cell line (p=0.05, Fig. 5E). Although gp100 expression was undetectable by western blot analysis, it appeared that low levels of gp100 were present to stimulate pmel T cells. However, the reduction in gp100 expression in D5-White tumors resulted in less IFN-γ secretion compared to that secreted by pmel T cells cultured with the parental D5 cell line. Culturing TRP1 T cells with the D5-White cell line resulted in significant (p<0.05) IFN-γ secretion when compared to TRP1 T cells stimulated with the MCA-310 cell line. Since the D5-White cell line had undetectable levels of MHC class II at the start of the cytokine-release experiment, we analyzed class II expression again at the end of the assay. While D5 or D5-White cells cultured alone had no detectable levels of class II, D5-White cultured with TRP1 T cells for 20 hrs had more than 50% of the tumor cells expressing class II (data not shown). In contrast, D5 had less than 25% of the tumor cells expressing class II (data not shown), which may explain the reduced amount of IFN-γ secreted following stimulation with D5 (Fig. 5E)
Figure 5. Decreased expression of gp100 in depigmented recurrent tumor.
(A) Twenty-five percent (15/59 mice 10 experiments) of Rag1−/− mice that had D5 tumors regress had tumors recur at later time points. Recurred tumors were depigmented or exhibited a mixture of depigmented and pigmented portions. Tumor cell lines were created from depigmented (D5-White) and pigmented (D5-Black) portions of the recurrent tumor. (B) The parental D5, D5-Black, D5-White, and MCA310 (sarcoma) were stained intracellularly with anti-tyrp1 protein mAb. (C) D5-Black, D5-Black, and MCA310 were analyzed for expression of gp100 protein by Western blot. (D) D5 (left panel) and D5-White (right panel) were stained with anti-H-2Kb mAb (MHC class I). (E) 106 pmel splenocytes (left panel) or 106 TRP1 splenocytes (right panel) were cultured with 105 tumor targets for 20 hours. Supernatants were collected and assayed for IFN-γ by ELISA. (F) Rag1−/− mice were injected subcutaneously with either 105 D5 tumor cells or 2×105 D5-White tumor cells. On day 15 after tumor challenge, 5×105 pmel splenocytes and 2.5×104 TRP1 splenocytes were adoptively transferred into the tumor-bearing Rag1−/− mice and subcutaneously vaccinated with 107 irradiated D5-G6. Data are representative of two independent experiments.
The reduced expression of gp100 in D5-White raised the possibility that tumor recurrence was due to the inability of pmel T cells to sufficiently recognize the tumor in vivo. We investigated this possibility by determining if naïve pmel and TRP1 cells could impact the growth of D5-White tumor in vivo. Rag1−/− mice were challenged with D5-White tumor followed fifteen days later with an adoptive transfer of naïve pmel and TRP1 cells together with an irradiated D5-G6 vaccine. The D5-White cell line grew slightly slower than the parental D5 tumor in Rag1−/−recipient mice, but Rag1−/− mice that received the adoptive transfer of pmel and TRP1 T cells, combined with vaccination, showed regression of their D5-White tumors (Fig. 5F). This shows that the reduced expression of gp100 expression alone did not protect tumors from rejection and suggests that gp100 loss alone was not sufficient for tumor recurrence. These data are consistent with tumor recurrence being the result of multiple mechanisms dampening the immune response and facilitating immune escape of the depigmented tumor.
Immune responses from mice with recurrent tumor exhibit suppressive function
Since pmel and TRP1 T cells could regress depigmented D5-White tumors we examined if mice with recurrent tumors had dysfunctional immune responses. The adoptive transfer of splenocytes from mice that regressed tumor and remained tumor-free maintained the ability to cause parental D5 tumor regression (Fig. 6B). In contrast, splenocytes from mice that recurred tumor were not therapeutic against D5, even though splenocytes from those mice contained similar frequencies of pmel and TRP1 T cells as splenocytes from tumor-free mice (data not shown). The inability of TCR transgenic CD4+ and CD8+ T cells from mice that recurred tumor to provide therapeutic impact against parental D5 tumor (gp100+) demonstrates that mechanisms other than antigen loss are responsible for the tumor recurrence.
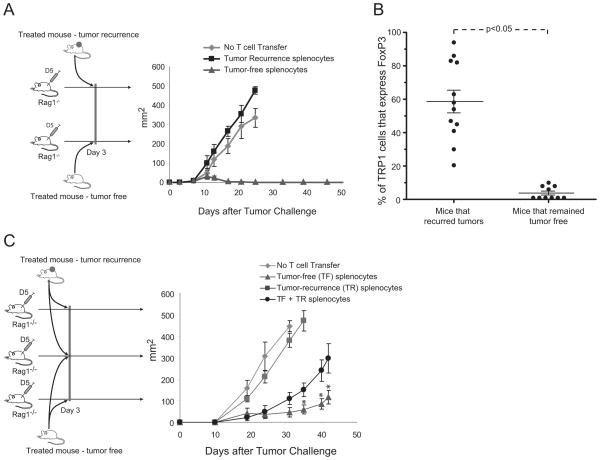
Figure 6. Mice with recurrent tumors have increased frequency of FoxP3+ TRP1 T cells and mediate immunosuppression.
(A) 106 D5 tumor cells were injected subcutaneously into Rag1−/− mice. On day 3 after tumor challenge, mice received either 106 splenocytes from a mouse that recurred tumor or 106 splenocytes from a mouse that remained tumor free. All mice were vaccinated subcutaneously with 107 irradiated D5-G6. Data are representative of two independent experiments. (B) Splenocytes from mice that either recurred tumor or remained tumor-free were analyzed by flow cytometry for the expression of CD3+, CD4+, and FoxP3+. Data are frequency of CD3+CD4+FoxP3+ of CD3+CD4+ cells. (C) 106 D5 tumor cells were injected subcutaneously into Rag1−/− mice. On day 3 after tumor challenge, mice received: 106 splenocytes from a mouse that recurred tumor; or 106 splenocytes from a mouse that remained tumor free; or 106 splenocytes from a mouse that remained tumor free combined with 106 splenocytes from a mouse that recurred tumor. All mice were vaccinated subcutaneously with 107 irradiated D5-G6. * p<0.05. Data are representative of two independent experiments (C).
We have previously reported in a separate model that D5 tumors can sensitize regulatory T cells that inhibit the generation of a productive anti-tumor immune response (23, 36). Interestingly, in the studies reported here, mice with recurrent tumor had a significant increase in the frequency of FoxP3+ TRP1 T cells, with a majority of animals having greater than 40% of their TRP1 T cells expressing FoxP3 (Fig. 6B). In contrast, mice that regressed tumor and remained tumor-free maintained frequencies of FoxP3+ TRP1 T cells similar to frequencies that exist in naïve TRP1 transgenic mice. The increase in frequency of FoxP3+ TRP1 T cells raised the question of whether these cells could exert suppressive effects. The co-transfer of splenocytes from mice that recurred tumor mixed with splenocytes from mice that regressed tumor and remained tumor-free showed a statistically (p<0.05) impaired therapeutic function versus the transfer of splenocytes from tumor-free mice alone (Fig. 6C). These data demonstrate that the splenocytes from mice that recur tumor exert immunosuppressive function and have a large frequency of TRP1 T cells expressing a regulatory T cell phenotype.
Discussion
Our previous work using the D5 melanoma cell line demonstrated that complete and sustained elimination of experimental pulmonary metastases required both CD4+ and CD8+ T cell subsets (45). These studies guided our rationale to use both CD4+ TCR transgenic T cells and CD8+ TCR transgenic T cells to investigate the ability of the immune system to regress large established tumors. Both the pmel CD8+ TCR transgenic T cell and the TRP1 CD4+ TCR transgenic T cell have been used separately to treat tumor-bearing mice (46–49). The triple combination of 107 freshly isolated pmel splenocytes with a recombinant fowlpox vaccine encoding human gp10025–33 and IL-2 administration caused the regression of B16 tumors (<100 mm2) (48). Reducing the dose to 106 transferred pmel cells was deemed suboptimal and led to resurgent tumor growth. In our studies we transferred 0.5–1×106 freshly isolated pmel splenocytes (containing approximately 1–2×105 CD8+ pmel T cells), which minimally impacted tumor growth on their own (data not shown). The TRP1 TCR transgenic T cell has been reported to cause tumor regression through CD4+ dependent cytotoxicity (47, 49). However, in a non-transgenic system we have never observed significant therapeutic effects mediated by adoptive transfer of bulk populations of wt CD4+ T cells, from vaccinated recipients, which contained tumor-specific IFN-γ secreting CD4+ T cells (B. Fox unpublished observation). The failure to mediate significant therapeutic effect may reflect the low frequency of tumor-specific CD4+T cells that are present in bulk populations. While the frequency of tumor-specific CD4+ T cells may be too low to mediate significant regression of tumor when only CD4+ T cells are transferred, when combined with tumor-specific CD8+ T cells they can mediate long-term cure of mice bearing 3-day established pulmonary metastases (45). In contrast, tumor-specific CD8+ T cells can mediate initial tumor regression in the absence of CD4 T cells, but all mice recur tumor at later time points (45). On this basis we designed a model that used sub-therapeutic doses of both TRP1 CD4+ TCR transgenic T cells and pmel CD8+ TCR transgenic T cells. The suboptimal dose of 2.5×104 TRP1 splenocytes used in these studies equates to 3.75×103 TRP1 TCR transgenic T cells since they comprise approximately 15% of freshly isolated TRP1 TCR transgenic mouse splenocytes. This dose was 13-fold less than the dose used by Quezada et al., who transferred 5×104 enriched CD4+ TRP1 T cells (49). The combined adoptive transfer of both of these suboptimal doses of TRP1 T cells and pmel T cells led to tumor regression and a statistically significant increase in survival of mice. It is not clear from these studies if this was due to the additive anti-tumor activities from each of these two cell subsets or whether it results from the increased efficacy of pmel T cells helped by TRP1 T cells. Although it is most likely a combination of both, recent data from our lab supports the latter explanation (Church, S., manuscript in preparation).
Prophylactic vaccination prior to tumor challenge provides an advantage to the immune system. Activated T cells are primed and ready to eradicate the minimal number of tumor cells that are injected into the environment. In contrast, the number of tumor cells proliferating in a large established tumor mass raises the question whether the immune-mediated tumor destruction can eliminate tumor cells faster than they are being produced. Regression of tumors as large as 400 mm2 after adoptive transfer of pmel and TRP1 T cells clearly demonstrates that the immune system can outpace tumor growth. Although naïve TRP1 T cells proliferated when they were transferred into tumor-bearing hosts (Fig. 4A), they were unable on their own to mediate sustained tumor regression. In addition to the TRP1 T cells, tumor regression required vaccination with the GM-CSF secreting tumor vaccine that promoted proliferation and differentiation of the pmel T cells (Fig. 4 A&B). These data document that CD8+ pmel T cells contribute to tumor regression in this model. It also supports the concept that mechanisms that reduce function of either CD4+ or CD8+ T cells could impact the efficacy of tumor elimination. These studies further demonstrate that properly activated tumor-specific T cells can mount a potent anti-tumor immune response that can eradicate a large tumor mass. A caveat of these studies is that the frequency of tumor-specific T cells achieved using transgenic TCR T cells might be difficult to meet with polyclonal T cell populations. However, redirecting T cell specificity using tumor-specific chimeric antigen receptors (CAR) or TCR gene transfer affords strategies that could be used to increase the frequency and avidity of tumor-specific T cells (15, 50). Our data indicates that these strategies would benefit from adoptive transfer of both CD4+ and CD8+ redirected T cell subsets in the lymphopenic setting.
A sizeable amount of data demonstrates that immunosuppressive environments established by tumors can subvert the ability of the immune system to mount a therapeutic anti-tumor immune response. Mechanisms of immunosuppression are varied, including both tumor-mediated as well as immune-mediated. Our studies demonstrated that a delineator of therapeutic efficacy was the difference between lymphopenic and lymphoreplete environments. The lymphopenic environment may release adoptively transferred T cells from the constraints of immunoregulation. Treg cells have been shown to prevent homeostasis-driven proliferation of T cells displaying either low affinity or high affinity for self-antigens (51). The absence of a Treg cell population in lymphopenic Rag1−/− mice may be sufficient to allow the adoptively transferred T cells to become activated and functionally therapeutic. Previous work in our lab has demonstrated that D5 tumor-bearing lymphoreplete mice generate a population of Treg cells that block the generation of a therapeutic immune response. Removal of this tumor-sensitized Treg population released the immune system from immunosuppression that blocked the activation of a therapeutic immune response (23, 36). Splenocytes from mice that had recurrent tumors exhibited immunosuppressive properties as demonstrated by their ability to suppress therapeutic efficacy in adoptive transfer studies. The ability to adoptively transfer the immune suppression is reminiscent of previous work demonstrating T-cell mediated suppression from tumor-bearing mice (52). All of the mice in this study that had recurrent tumors had an increase in the frequency of FoxP3-expressing TRP1 T cells in the spleen. Efforts were made to analyze the frequency of FoxP3+ Trp1 T cells in the tumor; however few T cells were recoverable from the tumor (data not shown). While these data are supportive of FoxP3+CD4+ Treg cells playing a critical role in mediating the observed suppression of therapeutic efficacy, additional studies are necessary to clarify their role in tumor recurrence and in the observed suppression of therapeutic efficacy. Quezada et al., showed that administration of αCTLA-4 with the transfer of TRP1 T cells increased the number of effector TRP1 T cells and reduced the number of CD4+ FoxP3+ TRP1 T cells (49). This treatment strategy blocked tumor recurrence, but it is unclear if this was a result of complete tumor elimination due to the increased number of effector TRP1 T cells or the lack of immunosuppression due to the reduced number of TRP1 FoxP3+ Treg cells. In our studies all recurrent tumors exhibited depigmentation most likely as a result of the decreased expression of gp100, a protein important for melanosome biogenesis. The appearance of these depigmented tumors clearly demonstrated the immunoselective force mounted by the pmel T cells. The presence of tyrp1 protein in these depigmented tumors suggests that direct cytotoxic effects from TRP1 T cells were less important, at least under the conditions used in this model. The gp100 protein was not detected in the D5-White tumor by Western blot, however D5-White tumor cells could stimulate pmel T cells to secrete low levels of IFN-γ. This response was lower than the response of pmel T cells to the parental D5 tumor supporting reduced but not complete loss of gp100 expression. TRP1 T cells also secreted IFN-γ when stimulated with D5-White tumor. Although D5-White tumor did not constitutively express levels of MHC class II that were detectable by flow cytometry, co-culturing TRP1 T cells with D5-White or D5 induced upregulation of MHC class II on these tumors. It is unclear what mechanism led to upregulation of MHC class II. We know that IFN-γ can upregulate expression of both MHC class I and class II on these tumor cell lines (data not shown), raising the possibility that low level secretion of IFN-γ, either as background secretion by TRP1 T cells or as a TRP1 T cell response to very low levels of MHC class II (undetectable by flow cytometry), resulted in increased expression of MHC. In turn, this increase could be further amplified when TRP1 T cells recognized the low levels of MHC class II induced by this background secretion.
The observation that combination immunotherapy with naïve pmel T cells, naïve TRP1 T cells and vaccination could mediate regression of D5-White in vivo demonstrated that even though pmel T cells respond less well against D5-White in vitro, the diminution of gp100 did not prevent complete regression of established tumor. Thus, we consider it unlikely that the reduction in gp100 alone was solely responsible for tumor recurrence (Fig. 5E). We consider that these data fit the proposed cancer immunoediting model (8, 9). During the initial elimination phase of the immunoediting model, pmel T cells and TRP1 T cells cooperate to cause the regression of large established tumors. This is followed by an equilibrium period during which the continued presence of pmel T cells select for tumor cells with decreased expression of gp100. Decreased expression of gp100 reduces the therapeutic effectiveness of pmel T cells, but is not solely responsible for tumor escape. In all cases, tumors recurred in mice that had depigmented tumors coupled with increased frequencies of FoxP3-expressing TRP1 T cells. These data reveal that crippling both CD4 and CD8 T cells through separate mechanisms correlated with progression to the tumor escape phase of the immunoediting model.
We consider this preclinical system could be a relevant model for what occurs in patients with cancer. Specifically, the observation that patients with a T cell infiltrate at the invading margin of their primary colon cancer do significantly better than patients without this infiltrate (12, 53). Even colon cancer patients with metastatic disease that have T cell infiltrate have a better response to chemotherapeutic intervention than those lacking the T cell infiltrate (53). Presumably, patients that loose the T cell infiltrate (which may be secondary to antigen loss and/or development of a suppressive environment) mimic what happens in our D5-White tumor model, where there appears to be a correlation between tumor recurrence and relative antigen loss coupled with an immunosuppressive environment. While additional studies are underway to validate these findings in the clinic, a preponderance of evidence supports the concept that intra- and peritumorial T cell infiltrates have both a prognostic and predictive impact (54, 55).
While it may not be possible to prevent the immune selection that leads to antigen-loss tumor variants, current research efforts are examining the immunosuppressive function of Treg cells and their ability to decrease the efficiency of immunotherapy. This has led to a number of clinical trials that aim to downmodulate or remove the Treg population prior to and/or during immunotherapy with the intent of maximizing the anti-cancer immune response (56). However, the resurgence of the Treg population after immunotherapy and the resulting suppression of the anti-cancer immune response at later time points require us to study what drives this increase in Treg cells. While our current focus is to understand the kinetics and cause of Treg cell expansion during the equilibrium phase, the results of the studies reported above underscore the complexity of cancer. This complexity has recently been identified as a major hurdle for cancer immunotherapy and it is becoming increasingly clear that the successful development of effective immunotherapy for cancer necessitates a better understanding of that complexity (57). In our opinion, that understanding will enable the design and preclinical testing of combination immunotherapy strategies that enhance tumor elimination, block increases in Treg cells and reduce immune escape. The translation of these strategies to patients with cancer will not be easy. The complexity present in the spectrum of known tumor escape mechanisms will likely require development of immune assessments that will allow pairing of a specific strategy, or strategies, aimed at incapacitating a specific tumor escape mechanism in patients that have a tumor that employs that same escape mechanism. This type of evidence-based approach to determine combination immunotherapy strategies for patients with advanced cancer represents a “next generation” of personalized cancer immunotherapy.
Acknowledgments
The authors thank Tacy Hedge, Amanda Lyons, and Carol Oteham for their help in maintaining the mouse colonies. The authors also thank Lisa Said and Vanessa Lee for their technical assistance.
This work was supported by NIH grant CA080964 (BAF), Earle M. Chiles and The Chiles Foundation, Robert W. Franz, Elsie Franz Finley, Lynn and Jack Loacker, Wes and Nancy Lematta, the Safeway Foundation, and the Providence Portland Medical Foundation.
References
- 1.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 2.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 5.Eerola AK, Soini Y, Pääkkö P. A high number of tumor-infiltrating lymphocytes are associated with a small tumor size, low tumor stage, and a favorable prognosis in operated small cell lung carcinoma. Clin Cancer Res. 2000;6:1875–1881. [PubMed] [Google Scholar]
- 6.Galon J. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 7.Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10:4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 9.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. Journal of Clinical Oncology. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 13.Schmid DA, Irving MB, Posevitz V, Hebeisen M, Posevitz-Fejfar A, Sarria JCF, Gomez-Eerland R, Thome M, Schumacher TNM, Romero P, Speiser DE, Zoete V, Michielin O, Rufer N. Evidence for a TCR affinity threshold delimiting maximal CD8 T cell function. J Immunol. 2010;184:4936–4946. doi: 10.4049/jimmunol.1000173. [DOI] [PubMed] [Google Scholar]
- 14.Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–270. doi: 10.1016/s1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 15.McKee MD, Roszkowski JJ, Nishimura MI. T cell avidity and tumor recognition: implications and therapeutic strategies. J Transl Med. 2005;3:35. doi: 10.1186/1479-5876-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackall CL, Bare CV, Granger LA, Sharrow SO, Titus JA, Gress RE. Thymic-independent T cell regeneration occurs via antigen-driven expansion of peripheral T cells resulting in a repertoire that is limited in diversity and prone to skewing. J Immunol. 1996;156:4609–4616. [PubMed] [Google Scholar]
- 17.Baccala R, Gonzalez-Quintial R, Dummer W, Theofilopoulos AN. Tumor immunity via homeostatic T cell proliferation: mechanistic aspects and clinical perspectives. Springer Semin Immunopathol. 2005;27:75–85. doi: 10.1007/s00281-004-0196-9. [DOI] [PubMed] [Google Scholar]
- 18.Guimond M, Fry TJ, Mackall CL. Cytokine signals in T-cell homeostasis. J Immunother. 2005;28:289–294. doi: 10.1097/01.cji.0000165356.03924.e7. [DOI] [PubMed] [Google Scholar]
- 19.Asavaroengchai W, Kotera Y, Mulé JJ. Tumor lysate-pulsed dendritic cells can elicit an effective antitumor immune response during early lymphoid recovery. Proc Natl Acad Sci USA. 2002;99:931–936. doi: 10.1073/pnas.022634999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, Surh CD, Rosenberg SA, Restifo NP. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu HM, Poehlein CH, Urba WJ, Fox BA. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Research. 2002;62:3914–3919. [PubMed] [Google Scholar]
- 22.Ahmad M, Rees RC, Ali SA. Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother. 2004;53:844–854. doi: 10.1007/s00262-004-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petrausch U, Jensen SM, Twitty C, Poehlein CH, Haley DP, Walker EB, Fox BA. Disruption of TGF-beta signaling prevents the generation of tumor-sensitized regulatory T cells and facilitates therapeutic antitumor immunity. J Immunol. 2009;183:3682–3689. doi: 10.4049/jimmunol.0900560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas DA, Massagué J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Díaz-Valdés N, Basagoiti M, Dotor J, Aranda F, Monreal I, Riezu-Boj JI, Borras-Cuesta F, Sarobe P, Feijoó E. Induction of monocyte chemoattractant protein-1 and interleukin-10 by TGFbeta1 in melanoma enhances tumor infiltration and immunosuppression. Cancer Research. 2011;71:812–821. doi: 10.1158/0008-5472.CAN-10-2698. [DOI] [PubMed] [Google Scholar]
- 26.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor cyclooxygenase-2/prostaglandin E2-dependent promotion of FOXP3 expression and CD4+ CD25+ T regulatory cell activities in lung cancer. Cancer Research. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 27.Lee SY, Choi HK, Lee KJ, Jung JY, Hur GY, Jung KH, Kim JH, Shin C, Shim JJ, In KH, Kang KH, Yoo SH. The immune tolerance of cancer is mediated by IDO that is inhibited by COX-2 inhibitors through regulatory T cells. J Immunother. 2009;32:22–28. doi: 10.1097/CJI.0b013e31818ac2f7. [DOI] [PubMed] [Google Scholar]
- 28.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 30.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, Xu Y, Li YW, Tang ZY. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. Journal of Clinical Oncology. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 31.Petersen RP, Campa MJ, Sperlazza J, Conlon D, Joshi MB, Harpole DH, Patz EF. Tumor infiltrating Foxp3+ regulatory T-cells are associated with recurrence in pathologic stage I NSCLC patients. Cancer. 2006;107:2866–2872. doi: 10.1002/cncr.22282. [DOI] [PubMed] [Google Scholar]
- 32.Negrin RS. Role of regulatory T cell populations in controlling graft vs host disease. Best Pract Res Clin Haematol. 2011;24:453–457. doi: 10.1016/j.beha.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493–3499. doi: 10.1182/blood.v99.10.3493. [DOI] [PubMed] [Google Scholar]
- 34.Zhou G, Levitsky H. Natural Regulatory T Cells and De Novo-Induced Regulatory T Cells Contribute Independently to Tumor-Specific Tolerance. J Immunol. 2007;178:2155–2162. doi: 10.4049/jimmunol.178.4.2155. [DOI] [PubMed] [Google Scholar]
- 35.Ai WZ, Hou JZ, Zeiser R, Czerwinski D, Negrin RS, Levy R. Follicular lymphoma B cells induce the conversion of conventional CD4+ T cells to T-regulatory cells. Int J Cancer. 2009;124:239–244. doi: 10.1002/ijc.23881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poehlein CH, Haley DP, Walker EB, Fox BA. Depletion of tumor-induced Treg prior to reconstitution rescues enhanced priming of tumor-specific, therapeutic effector T cells in lymphopenic hosts. Eur J Immunol. 2009;39:3121–3133. doi: 10.1002/eji.200939453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lacelle MG, Jensen SM, Fox BA. Partial CD4 depletion reduces regulatory T cells induced by multiple vaccinations and restores therapeutic efficacy. Clin Cancer Res. 2009;15:6881–6890. doi: 10.1158/1078-0432.CCR-09-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Research. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 39.Turk MJ, Guevara-Patiño JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jing W, Yan X, Hallett WHD, Gershan JA, Johnson BD. Depletion of CD25+ T cells from hematopoietic stem cell grafts increases posttransplantation vaccine-induced immunity to neuroblastoma. Blood. 2011;117:6952–6962. doi: 10.1182/blood-2010-12-326108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 42.Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–144. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 43.Sacks JA, Bevan MJ. TRAIL deficiency does not rescue impaired CD8+ T cell memory generated in the absence of CD4+ T cell help. J Immunol. 2008;180:4570–4576. doi: 10.4049/jimmunol.180.7.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu HM, Winter H, Urba WJ, Fox BA. Divergent roles for CD4+ T cells in the priming and effector/memory phases of adoptive immunotherapy. J Immunol. 2000;165:4246–4253. doi: 10.4049/jimmunol.165.8.4246. [DOI] [PubMed] [Google Scholar]
- 46.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EKM, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. Journal of Experimental Medicine. 2010;207:651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, Restifo NP, Allison JP. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. Journal of Experimental Medicine. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jena B, Dotti G, Cooper LJN. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen S. Control of homeostatic proliferation by regulatory T cells. Journal of Clinical Investigation. 2005;115:3517–3526. doi: 10.1172/JCI25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berendt MJ, North RJ. T-cell-mediated suppression of anti-tumor immunity. An explanation for progressive growth of an immunogenic tumor. J Exp Med. 1980;151:69–80. doi: 10.1084/jem.151.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G, Luber B, Feyen N, Martens UM, Beckhove P, Gnjatic S, Schirmacher P, Herpel E, Weitz J, Grabe N, Jaeger D. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Research. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- 54.Galon J, Pagès F, Marincola FM, Thurin M, Trinchieri G, Fox BA, Gajewski TF, Ascierto PA. The immune score as a new possible approach for the classification of cancer. J Transl Med. 2012;10:1. doi: 10.1186/1479-5876-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fridman WH, Galon J, Pagès F, Tartour E, Sautès-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Research. 2011;71:5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 56.Peng DJ, Liu R, Zou W. Regulatory T Cells in Human Ovarian Cancer. J Oncol. 2012;2012:345164. doi: 10.1155/2012/345164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fox BA, Schendel DJ, Butterfield LH, Aamdal S, Allison JP, et al. Defining the critical hurdles in cancer immunotherapy. J Transl Med. 2011;9:214. doi: 10.1186/1479-5876-9-214. [DOI] [PMC free article] [PubMed] [Google Scholar]