Abstract
Tissue plasminogen activator (tPA) remains the only approved thrombolytic agent for the early treatment of ischemic stroke. However, treatment with tPA may lead to disruption of the blood–brain barrier and hemorrhagic transformation. 17β-estradiol (E2) has demonstrated efficacy in reduction of infarct volume in ischemic stroke models. The effects of acute administration of E2 on permeability of the blood–brain barrier and its ability to prevent hemorrhagic transformation in ischemic rats treated with tPA have not previously been studied. Here, we show that neurological deficits, brain water content, and Evan’s blue extravasation were increased in ovariectomized female Wistar rats treated with tPA and attenuated in rats receiving E2+ tPA. We also show that intracerebral hemoglobin and matrix metalloproteinase-9 activity were elevated with tPA treatment, and these increases were reduced by E2 treatment. Taken together, these data demonstrate that acute administration of E2 is capable of ameliorating some of the adverse effects of tPA administration, including the increase of matrix metalloproteinase-9 activity, blood–brain barrier permeability, and hemorrhagic transformation. These findings suggest a potential role for estrogen in thrombolytic treatment for ischemic stroke.
Keywords: Estrogen, Matrix metalloproteinase, Middle cerebral artery, Cerebral ischemia, Thrombolysis
Introduction
Tissue plasminogen activator (tPA) remains the only Food and Drug Administration (FDA) approved thrombolytic agent for ischemic stroke and requires administration within 3–4.5 h after ischemia onset (Adibhatla and Hatcher, 2008; Fujiwara et al., 2009). However, in addition to dissolving the intraluminal thrombus, tPA promotes activation of matrix metalloproteinases (MMPs) and degradation of extracellular matrix components, which has been implicated in breakdown of the blood vessel endothelium, resulting in edema, disruption of the blood–brain barrier (BBB), and markedly increased risk of symptomatic hemorrhage (Lapchak et al., 2000; Yang et al., 2007; Yepes et al., 2003). Mostly because of these side effects and the narrow therapeutic time window, the use of tPA in ischemic stroke remains limited (Adibhatla and Hatcher, 2008). There is an urgent clinical need to increase access to the potential benefits of thrombolytic therapy in stroke and reduce the treatment-related complications.
Although randomized trials failed to show significant improvement in outcome from ischemic stroke in women taking estrogen replacement therapy (Viscoli et al., 2001), and long-term hormone replacement therapy may have the adverse effect of increasing risk of venous and arterial thrombosis, coronary heart disease, ischemic stroke and breast cancer (Billeci et al., 2008; Damczyk and Gardner, 2000; Nelson et al., 2002), estrogen has been increasingly recognized to have neuroprotective properties in experimental ischemic stroke. Estrogen has been shown to inhibit neuronal apoptosis through multiple signaling pathways (Crosby et al., 2007; Jia et al., 2009; Liu et al., 2005; Rau et al., 2003; Won et al., 2006). Moreover, estrogen may protect the BBB in ischemia by down regulation of MMP expression (Kelly et al., 2006). As a result, estrogen attenuates brain edema and decreases infarct volume in both female and male animals without administration of tPA (Liu et al., 2005; Rusa et al., 1999; Saleh et al., 2001a, 2001b; Yang et al., 2000). Interestingly, acute administration of high doses of estrogen was shown to reduce infarct volume when combined with tPA in an animal model of focal ischemia–reperfusion injury (Liu et al., 2010). However, the possible role of estrogen in inhibiting the damage of BBB and intracerebral hemorrhagic transformation after administration of tPA in ischemic stroke remains unknown. In the present study, we investigated the potential for acute administration of 17β-estradiol to attenuate breakdown of blood–brain barrier and hemorrhagic transformation associated with tPA administration at reperfusion in a rat focal cerebral ischemia model.
Materials and methods
Animal preparation and transient middle cerebral artery occlusion model
This study was conducted in accordance with the National Institutes of Health guidelines for the care and use of animals in research, and protocols were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University. The surgical procedure is similar to that described previously (Rusa et al., 1999). In brief, anesthesia was induced with 5% isoflurane for adult female Wistar rats (250 to 280 g) with ovariectomy (OVX) two weeks earlier. When unconscious, the anesthetic was administered at a nominal concentration of 2% in 25% to 30% O2 via face mask with spontaneous ventilation for a brief period during incisions of the skin for vascular isolation. Anesthesia was then maintained with a nominal inspired concentration of 1% or 1.5% isoflurane for the remainder of the procedure. Rectal temperature was monitored with a thermometer (Monatherm model 6510, Mallinckrodt Medical, Inc., St. Louis, Missouri) and maintained at 37±0.5 °C with a warming plate. The right femoral artery was cannulated to monitor arterial blood pressure and arterial blood gasses. The right femoral vein was also exposed and cannulated for rtPA (Genentech, South San Francisco, CA) or saline administration. Middle cerebral artery (MCA) occlusion (MCAO) was induced by inserting a 4-0 monofilament nylon suture coated with silicon into the internal carotid artery (Toung et al., 2004). Briefly, the common and external carotid arteries were exposed and ligated. The occipital artery was cauterized, and the pterygopalatine artery was ligated. The internal carotid artery was transiently occluded with loosely tied 3-0 silk sutures; a silicon-coated 4-0 nylon thread was introduced into the internal carotid artery to occlude the proximal orifice of the MCA. To confirm MCAO, cerebral blood flow (CBF) in the MCA was measured from the temporal bone surface at a site 1 mm posterior to the bregma and 3 mm inferior to the temporal line with a laser-Doppler flow probe (Moor Instruments Inc., Wilmington, DE). After MCAO, the ipsilateral CBF decreased rapidly to 20% to 30% of baseline values in all animals and then remained at a stable level throughout (<40% baseline). Rats with successful MCAO consistently exhibited circling behavior, decreased resistance to lateral push, forelimb flexion, and shoulder adduction.
Experimental groups
Two weeks after OVX, the rats were randomly divided into three groups: (i) control group (n=30) — rats underwent 2 h of MCAO and received intravenous saline (1 mL/kg body weight) immediately after reperfusion; (ii) tPA group (n=29) — rats were injected with 0.1 mL sesame oil subcutaneously (vehicle for E2) and 2 h of MCAO followed by intravenous infusion of tPA at reperfusion; and (iii) tPA and E2 group (n=29) — rats were treated with subcutaneous injection of E2 (100 μg/kg body weight(Liu et al., 2005), dissolved in 0.1 mL sesame oil) at the initiation of 2 h of MCAO and then received tPA immediately after reperfusion. For tPA treatment, rtPA was dissolved in saline (1 mL/kg body weight) and administered intravenously at a dose of 10 mg/kg body weight with 1 mg/kg given as an initial bolus over 1 min followed by continuous intravenous infusion of 9 mg/kg over 20 min starting at the onset of reperfusion.
Determination of E2 serum concentration
To assess serum concentrations of E2, 7 OVX animals were anesthetized with inhaled isoflurane, and blood samples were taken via the femoral vein before estradiol administration and at 5 min, 1, 6, and 24 h after estradiol administration. Serum estradiol levels were measured in duplicate by radioimmunoassay as previously described (Rusa et al., 1999).
Neurological deficit scoring
Twenty-four hours after MCAO, a neurologic examination was performed by a blinded investigator as previously described with modifications (Menzies et al., 1992). Briefly, the scores were 0 (no apparent deficits), 1 (contralateral forelimb flexion when suspended by the tail), 2 (decreased grip of the contralateral forelimb while tail pulled), 3 (spontaneous movement in all directions; contralateral circling only if pulled by the tail), and 4 (spontaneous contralateral circling).
Measurement of infarct volume
At 24 h after the induction of focal cerebral ischemia, the rats were euthanized. Their brains were removed immediately and brain tissue was cut into 7 serial 2-mm coronal sections. Sliced brain tissues were immersed in a 2% solution of 2,3,5-triphenyltetrazolium chloride (TTC) in phosphate-buffered saline (PBS) at 37 °C and then fixed in 10% formalin. The extent of ischemic infarction was traced and the infarction volume was calculated using SigmaScan Pro. Image Analysis version 5.0.0 software. For examination of Evan’s blue (EB) extravasation, intracerebral hemorrhage, and MMP-9 activity, animals were euthanized and transcardially perfused with cold PBS, and the brains were harvested and stored at −80 °C until use.
Determination of brain water content
Brain water content was determined by the dry–wet weight method on each group (n=4). Rats were decapitated under deep isoflurane anesthesia 24 h after reperfusion. Brains were immediately removed and weighed on pre-cooled aluminum foil to obtain wet weight. The tissue was then dried in a 100 °C oven for 48 h and reweighed to obtain the dry weight. Brain water content was calculated as 100%×(wet weight—dry weight)/wet weight.
Evaluation of blood–brain barrier permeability
Blood–brain barrier permeability was quantitatively assessed by fluorescent detection of extravasated Evan’s blue (EB) dye in 12 rats (n=4, per group) as previously described (Tang et al., 2009). Briefly, EB (2% in saline; 3 mL/kg) was administered intravenously 3 h before harvesting the brain. Via a thoracotomy under isoflurane anesthesia, intracardiac perfusion with 0.1% PBS was then performed through the left ventricle to remove intravascular EB dye, and continued until the fluid from the right atrium became colorless. Rats were then decapitated, and the brains were quickly removed, and dissected into right (ischemic) and left (nonischemic) hemispheres. Each hemisphere was weighed and homogenized in 4 mL of 50% trichloroacetic acid solution. After centrifugation at 10,000 g for 30 min, the supernatants were collected and diluted with ethanol (1:3), and EB concentration was determined with a spectrophotometer at 620 nm for absorbance against a standard curve. EB extravasation was expressed as the ratio of absorbance intensity in the ischemic hemisphere to that in the nonischemic hemisphere (EB extravasation index, EBI) (Chen et al., 2006a).
Spectrophotometric assay for intracerebral hemorrhage
Hemorrhagic transformation was quantified with a spectrophotometric assay of brain hemoglobin content (Qin et al., 2007). At 24 h after reperfusion, the animals were perfused transcardially with 0.1 mol/L PBS under deep anesthesia until the outflow fluid from the right atrium was colorless. The brain was rapidly removed and dissected into the left and right hemisphere. The hemispheric brain tissue was then homogenized in 0.1 mol/L PBS followed by 30-minute centrifugation (13,000 g). Then, 200 μL reagent (QuantiChrom™ Hemoglobin Assay Kit; BioAssay Systems) was added to 50 μL supernatant. After 15 min, optical density was measured at 400 nm with a spectrophotometer (Fisher Scientific Inc, Pittsburg, PA). Hemoglobin Content Index (HCI), which was calculated as hemoglobin content in ipsilateral divided by contralateral hemisphere, was compared among groups.
Gelatinase zymography
After removing the cerebellum and brain stem, brains in each group were divided into ipsilateral and contralateral hemisphere. Each hemisphere was homogenized in 10× volumes of ice-cold lysis buffer (50 mmol/L Tris–HCl [pH 7.4] containing 150 mmol/L NaCl, 1% NP-40 and 0.25% deoxycholic acid) including EDTA-free cocktail protease inhibitors (Roche) on ice with the use of a Teflon glass homogenizer (AS ONE, Osaka, Japan). After centrifugation (12,000 rpm) for 7 min at 4 °C, the supernatant fluid was collected. The total protein concentration of each sample was determined by the bovine serum albumin protein assay (Bio-Rad Laboratories, Hercules, CA). Equal volumes of total protein extracts normalized for protein concentration (30 μg/10 μL) were prepared. Then 10 μL of×2 sample buffer (126 mmol/L Tris–HCl, 20% glycerol, 4% sodium dodecyl sulfate [SDS], 0.005% bromophenol blue) was added to each protein extract. Protein samples were loaded on and separated by 10% Tris-glycine gel with 0.1% gelatin as substrate. After separation by electrophoresis, the gel was incubated in renaturing buffer (2.7% Triton X-100 in distilled water) at room temperature for 30 min with gentle agitation. Then the renaturing buffer was decanted and replaced with developing buffer (50 mmol/L Tris base, 40 mmol/L HCl, 200 mmol/L NaCl, 5 mmol/L CaCl2, 0.2% Brij35). After 30 min of equilibration by the developing buffer, the gel was incubated with fresh developing buffer at 37 °C for 24 h. After developing, the gel was stained with 0.5% Coomassie blue R-250 for 30 min and then destained appropriately. Human MMP-9 and MMP-2 standard (92 kDa, 62 kDa) was purchased from Chemicon International, Inc. After destaining, the presence of gelatinases was identified as clear bands on a blue background. Zymograms were scanned and images were analyzed using NIH image J software. The relative density of an individual sample was normalized by the sham sample.
Statistical analysis
All data were expressed as mean±SEM. SPSS for Windows 16.0 software package was used to analyze the data. Differences among groups were determined by one-way ANOVA test. The t-test was used to compare the difference between two groups. Differences were considered significant at P values less than 0.05.
Results
Concentration of serum estrogen
Before E2 administration, the average serum concentration of estradiol was 9±2 pg/mL. After subcutaneous administration of E2, the serum concentration was increased to 3319±998 pg/mL as early as 5 min later, peaked to 5823±2430 pg/mL at 1 h and decreased to 2048±441 pg/mL at 6 h. By 24 h, the serum concentration was 41±17 pg/mL, a value that was higher than the baseline value (Fig. 1). This response in serum levels after subcutaneous administration is comparable to that attained in Sprague–Dawley rats (Yang et al., 2000).
Fig. 1.
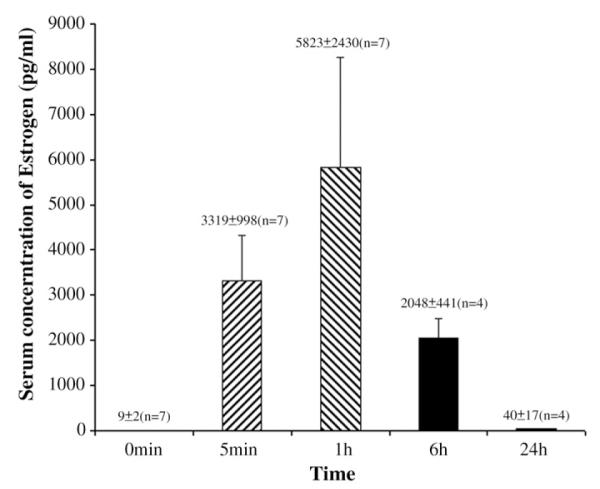
Serum concentration of 17β-estradiol in OVX rats at different times after subcutaneous administration of 100 μg/kg of 17β-estradiol.
Mortality rate, physiological data and neurological assessment
Mortality rate in control group, tPA group and tPA plus E2 group was 13.3% (4 of 30 rats), 17.2% (5 of 29 rats) and 6.9% (2 of 29 rats), respectively. There was no significant difference among the groups. Physiological parameters, including mean arterial blood pressure (MABP), blood gasses and body temperatures were measured immediately before the initiation of MCAO, 1 h after MCAO and right after reperfusion. There were no significant differences among the three groups (one-way ANOVA test) (Table 1). There was also no difference in average CBF among the groups. The average CBF (% of baseline) was 30±2%, 29±1% and 29±1% in MCAO group, tPA group and tPA plus E2 group, respectively. However, neurological deficit score in tPA-treated mice was higher than that of control mice (P<0.01), and combined treatment with E2 decreased the neurological deficit score (Fig. 2).
Table 1.
Comparison of physiological data.
| Variables (mean±SD) |
Controls, n=30 |
tPA group, n=29 |
tPA plus E2 group, n=29 |
|---|---|---|---|
| Blood pressure, mm Hg |
113.73±10.57 | 114.61±9.8 | 115.38±7.03 |
| Temperature, °C | 36.89±0.27 | 36.8±0.25 | 36.75±0.21 |
| pH | 7.41±0.03 | 7.42±0.04 | 7.41±0.04 |
| pO2, mm Hg | 40±5.72 | 38.34±4.98 | 37.76±5.30 |
| pCO2, mm Hg | 118.5±17.24 | 120.07±16.57 | 125.14±15.29 |
| HCO3–, mmol/L | 24.61±2.73 | 24.05±2.79 | 23.82±2.13 |
| Glucose, mg/dL | 216.97±43.54 | 213.1±32.43 | 206.9±23.97 |
Fig. 2.

Neurological deficit score on a 0–4 scale at 1 day after MCAO in groups with no treatment (control, n=30), tPA treatment (n=29), and tPA+E2 treatment (n=29). *P<0.01.
Infarct volume
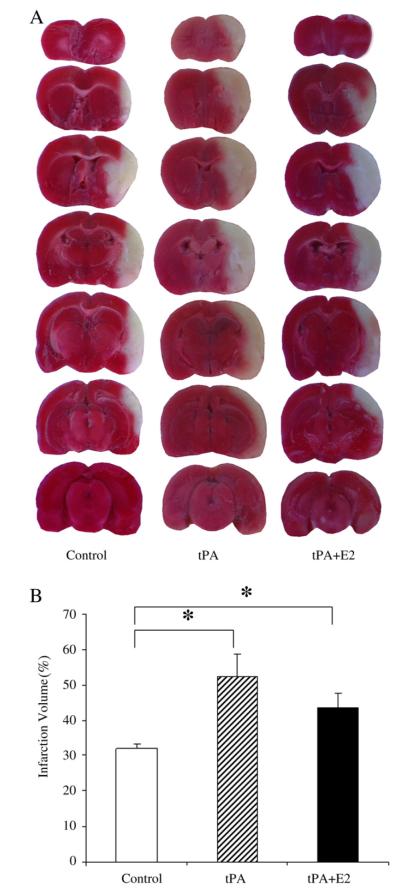
Administration of tPA significantly increased infarct volume (Fig. 3). Average infarct volume in the control group was 31.9±1.4% of the contralateral hemisphere. In the tPA-treated animals, the infarct volume was significantly increased to 52.2±6.3% (P<0.01). After administration of tPA combined with E2, infarct volume (43.5±4.1%) was intermediate between the control group and the tPA group, and was not significantly different from the tPA-only treated group.
Fig. 3.
(A). Representative images of TTC-stained brain slices from the control, tPA, and tPA+E2 groups. (B) Quantitative analysis of infarct volume in the control group (n=6), tPA treatment group (n=6), and tPA+E2 treatment group (n=6). *P<0.05 from control group.
Water content
As compared to control animals, rats given post-MCAO treatment with tPA had a greater brain water content as measured at the 24-hour time point. The edema index (EI), which normalizes water content by the individual contralateral value, was greater with tPA treatment (P<0.01) than in the control group. The combined administration of E2 before tPA significantly decreased the EI (P<0.05), but the EI remained significantly higher than that of the control group (P<0.05) (Fig. 4).
Fig. 4.
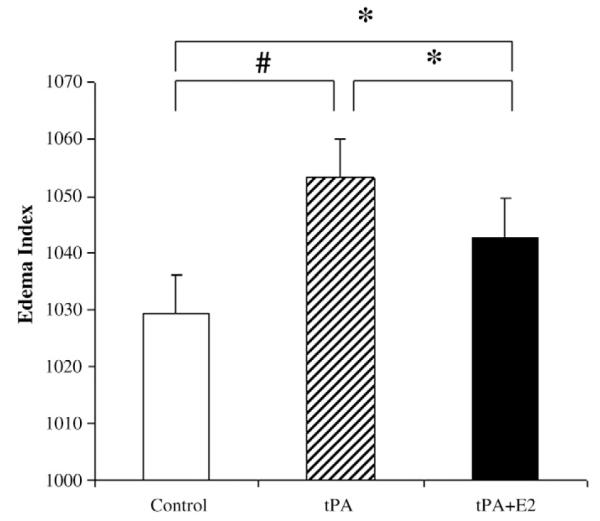
Edema Index (EI) (ratio of ipsilateral to contralateral hemisphere water content) in the control group (n=4), tPA treatment group (n=4), and tPA+E2 treatment group (n=4). *P<0.05; #P<0.01.
Evaluation of blood–brain barrier permeability
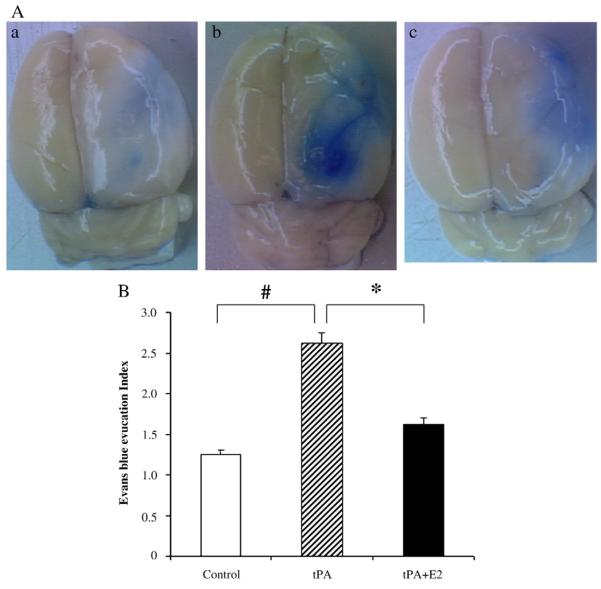
At 24 h after reperfusion, EB dye leakage appeared to be concentrated mostly in the regions of ischemic hemisphere. In the control group, the EB index (EBI), which normalizes EB tissue content by EB content in the hemisphere contralateral to the ischemia, was significantly greater than one (Fig. 5), thereby indicating that ischemia–reperfusion-induced BBB leakage to albumin (to which EB primarily binds). In tPA-treated rats, the EBI was markedly augmented (P<0.01). With E2 treatment before tPA administration, EBI was significantly lower than that of tPA-treated group (P<0.05) and not different from the value in the control group.
Fig. 5.
(A) Representative images of brain surface showing Evan’s blue dye extravasation. a, control; b, tPA; c, tPA plus E2; (B) Evans Blue Index (EBI) was calculated as the ratio of ipsilateral to contralateral hemisphere content of Evan’s blue dye in the control group (n=4), tPA treatment group (n=4), and tPA+E2 treatment group (n =). *P<0.05; #P<0.01.
Tissue hemoglobin content
Hemorrhage in ischemic brain tissue was evaluated by hemoglobin content index (HCI), which was calculated by dividing hemoglobin content in ipsilateral hemisphere by that in the contralateral hemisphere. HCI was significantly higher in the tPA-treated group than in the control group (P<0.01). HCI was significantly reduced in rats treated with both E2 and tPA (P<0.01), although the difference of HCI between the combination group and the control group remained significant (Fig. 6).
Fig. 6.
Hemoglobin Content Index (ratio of ipsilateral to contralateral hemisphere hemoglobin content) in the control group (n=4), tPA treatment group (n=4), and tPA+E2 treatment group (n=4). *P<0.05; #Pb0.01.
MMP-9 and MMP-2 activity
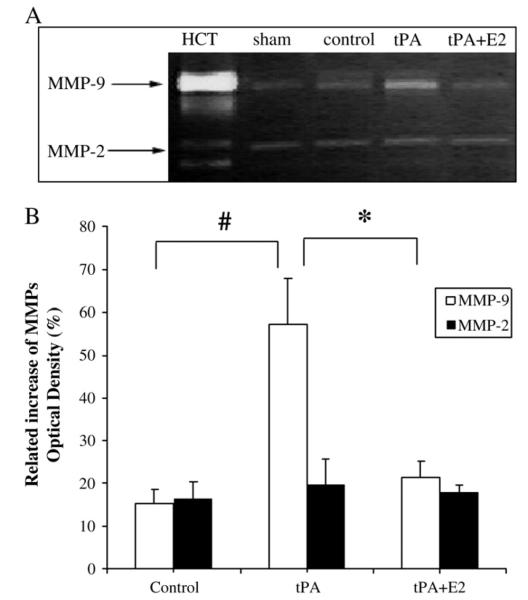
The effect of estrogen on activity of MMP-2 and MMP-9 is shown in Fig. 7. In the ischemic brain, the activity of MMP-9 was substantially augmented by tPA (P<0.01). In contrast, when MCAO rats were treated with E2 and tPA, MMP-9 activity was significantly decreased (P<0.01). MMP-2 activity was moderately increased in all MCAO groups relative to that in sham-operated rats, but activity was not significantly different among the MCAO groups. These findings suggest that estrogen suppressed tPA-induced intracerebral hemorrhage by reducing the activity of MMP-9.
Fig. 7.
Representative zymogram and quantitation of relative increase of MMP optical density (normalized to sham) at 24 h after ischemia. (A) Representative zymogram showed activity of MMPs. HCT indicates human MMP-9 and MMP-2 standards; Sham, OVX without MCAO; Control, OVX with MCAO; tPA, OVX with MCAO and tPA; E2, OVX rats with MCAO, tPA+E2. (B) Quantitation of MMP-9 and MMP-2 activity (normalized by the value in sham-operated rats) in the control group (n=5), tPA treatment group (n=5), and tPA+E2 treatment group (n=4). *P<0.05; #P<0.01.
Discussion
In the present study, we found that administration of 17β-estradiol at the onset of ischemia diminished the deleterious effects of tPA on neurobehavioral outcome. In addition, we observed that E2 countered another major side effect of tPA, i.e., its augmentation of ischemic-induced damage to the BBB. By maintaining a functional BBB, E2 inhibited tPA-associated intraparenchymal hemorrhage. The latter advantage may largely result from E2’s suppression of proteolysis by MMP-9. These benefits were independent of changes in core temperature, CBF, and other physiologic parameters, none of which differed much among the control and treated groups.
When tPA is administered shortly after the onset of ischemia, it stimulates thrombolysis. As this process restores blood flow, it can reduce the loss of brain tissue. In some cases, tPA-treated stroke victims have recovered without any symptoms (Alexandrov et al., 2004). Administration of tPA in the aftermath of ischemic stroke, however, increases the risk of hemorrhagic transformation and neurotoxicity, which may aggravate the outcome of ischemic stroke (Audebert et al., 2006; Cocho et al., 2005).
Several studies have investigated the effect co-administration of agents that may decrease tPA-associated complications following ischemic stroke (Zhu et al., 2010). For example, supplementing tPA with the free radical scavenger edaravone (Kano et al., 2005; Yagi et al., 2009; Zhang et al., 2004), melatonin (Chen et al., 2006b), or statins (Liu et al., 2006) was found to decrease infarct volume and lower the risk of intracerebral hemorrhage.
Estrogen is known to be neuroprotective in experimental ischemic stroke (Dubal and Wise, 2001; Fan et al., 2003; Liu et al., 2005; Rusa et al., 1999; Toung et al., 1998; Yang et al., 2000) and is known to preserve BBB integrity with consequent reduction in brain edema (Crosby et al., 2007; O’Donnell et al., 2006; Rusa et al., 1999; Saleh et al., 2001a; Toung et al., 1998). However, hormone replacement therapy was not found to be beneficial for reducing clinical stroke outcome (Wassertheil-Smoller et al., 2003). Nevertheless, acute administration of a large dose of estrogen may be beneficial. Pretreatment with E2 has been reported to counter disruption of the BBB and decrease brain edema in transient focal cerebral ischemia without tPA (Liu et al., 2005). Recently, combined use of estrogen with tPA has been reported to significantly reduce infarct volume after 1 h of MCAO (Liu et al., 2010). The present study adds to our knowledge of the potential for acute estrogen therapy by demonstrating that estrogen ameliorates the adverse effects of tPA on MMP-9 activation, EB extravasation, brain edema, tissue hemoglobin content, and neurological deficits after 2 h of MCAO. Thus to our knowledge, the present study is the first to report that E2 counters tPA’s effects of weakening the BBB and augmenting hemorrhagic transformation.
The disruption of the BBB is a complex process, one thought to be orchestrated by several molecular mechanisms. Among the most important is the activation of MMPs (Sumii and Lo, 2002; Tsuji et al., 2005). Once activated, MMPs degrade vascular basal lamina and other constituent structures of the BBB with consequent edema and vascular rupture (Kelly et al., 2006; Lo et al., 2004). Studies in knockout mice have identified MMP-9 to be one of the principal MMPs activated after ischemia (Kelly et al., 2006; Tsuji et al., 2005). Moreover, administration of tPA following ischemic stroke was found to augment the activation of MMPs. An equivalent phenomenon has been observed in clinical practice: patients who suffer hemorrhagic conversion following treatment with tPA have unusually high levels of plasma MMP-9 activity (Montaner et al., 2003). In vitro studies indicate that MMP-9 is upregulated in cerebral microvascular endothelial cells by tPA (Wang et al., 2003), and that estrogen reduces cerebrovascular inflammation(Razmara et al., 2005) and protects endothelial cells from stress (Guo et al., 2010; Razandi et al., 2000; Razmara et al., 2008). MMP-9 is thought to be the key molecule in tPA induced injury. In the present study, we also found that tPA-treatment of ischemic rats increased activation of MMP-9, whereas tPA did not augment the increase in MMP-2 activity. These findings are in agreement with others (Tsuji et al., 2005). Moreover, we found that co-administering E2 caused a significant blunting of MMP-9 activity following MCAO with tPA treatment. These results suggest that E2 diminishes the damage to the BBB incurred by tPA by suppressing MMP-9 activity.
The adverse effects of tPA in stroke are augmented when tPA administration is delayed. In our protocol, E2 was administered subcutaneously at the onset of MCAO, and MCAO persisted for 2 h before administering tPA at reperfusion. Although we observed a trend for a reduction in infarct volume with E2 treatment, the difference was not significant. A recent paper reported that E2 treatment 2 h before MCAO decreased infarct volume after 1 h of MCAO with tPA treatment at reperfusion (Liu et al., 2010). However, in that study tPA treatment alone at 1 h did not augment infarct volume, whereas we observed a significant augmentation of infarct volume when tPA was administered alone at 2 h. Together, these findings suggest that the reduction in infarct volume with acute treatment of E2 is more robust with a shorter ischemic duration when the severity of the injury is less severe and the adverse effects of tPA are less prominent. Alternatively, the 2-hour pretreatment used by Liu et al. (2010) may have allowed more time to affect changes in the neurovascular unit before the onset of ischemia. Therefore, the duration of MCAO and the timing of E2 and tPA administration are likely important in determining the impact of treatment on infarct volume (Yang et al., 2000). Further work is required to define the therapeutic window for E2 administration, whether E2 would be efficacious when administered after tPA, and whether E2 efficacy would be lost when tPA administration is delayed beyond 2 h and BBB damage is further augmented. Nevertheless, the present study together with the work of Liu et al. (2010) provides a proof-of-concept that E2 can be added to the list of agents that can protect the neurovascular unit from the adverse effects of tPA.
In conclusion, we found that administration of 17β-estradiol reduces tPA-induced augmentation of MMP-9 activity, BBB permeability, cerebral edema, cerebral hemorrhage and neurological deficits after reperfusion from 2 h of MCAO in rats. Thus, acute administration of E2 has the potential to serve as a prophylactic therapy against tPA’s life-threatening side effect of cerebral hemorrhage.
Acknowledgments
This work was supported by the Clinician Scientist Award of the Johns Hopkins University School of Medicine to Dr. Judy Huang. Dr. Raymond C. Koehler was supported by grants from the National Institutes of Health (NS038684 and NS067525). The authors would like to thank Dr. Ethan Shimony and Dr. Ning Lin of Harvard Medical School for editing the manuscript.
Footnotes
Financial disclosures The authors have no relevant financial disclosures.
References
- 1.Adibhatla RM, Hatcher JF. Tissue plasminogen activator (tPA) and matrix metalloproteinases in the pathogenesis of stroke: therapeutic strategies. CNS Neurol Disord Drug Targets. 2008;7:243–253. doi: 10.2174/187152708784936608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexandrov AV, Hall CE, Labiche LA, Wojner AW, Grotta JC. Ischemic stunning of the brain: early recanalization without immediate clinical improvement in acute ischemic stroke. Stroke. 2004;35:449–452. doi: 10.1161/01.STR.0000113737.58014.B4. [DOI] [PubMed] [Google Scholar]
- 3.Audebert HJ, Kukla C, Vatankhah B, Gotzler B, Schenkel J, Hofer S, Furst A, Haberl RL. Comparison of tissue plasminogen activator administration management between Telestroke Network hospitals and academic stroke centers: the Telemedical Pilot Project for Integrative Stroke Care in Bavaria/Germany. Stroke. 2006;37:1822–1827. doi: 10.1161/01.STR.0000226741.20629.b2. [DOI] [PubMed] [Google Scholar]
- 4.Billeci AM, Paciaroni M, Caso V, Agnelli G. Hormone replacement therapy and stroke. Curr. Vasc. Pharmacol. 2008;6:112–123. doi: 10.2174/157016108783955338. [DOI] [PubMed] [Google Scholar]
- 5.Chen CH, Toung TJ, Sapirstein A, Bhardwaj A. Effect of duration of osmotherapy on blood–brain barrier disruption and regional cerebral edema after experimental stroke. J. Cereb. Blood Flow Metab. 2006a;26:951–958. doi: 10.1038/sj.jcbfm.9600248. [DOI] [PubMed] [Google Scholar]
- 6.Chen TY, Lee MY, Chen HY, Kuo YL, Lin SC, Wu TS, Lee EJ. Melatonin attenuates the postischemic increase in blood–brain barrier permeability and decreases hemorrhagic transformation of tissue-plasminogen activator therapy following ischemic stroke in mice. J. Pineal Res. 2006b;40:242–250. doi: 10.1111/j.1600-079X.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 7.Cocho D, Belvis R, Marti-Fabregas J, Molina-Porcel L, Diaz-Manera J, Aleu A, Pagonabarraga J, Garcia-Bargo D, Mauri A, Marti-Vilalta JL. Reasons for exclusion from thrombolytic therapy following acute ischemic stroke. Neurology. 2005;64:719–720. doi: 10.1212/01.WNL.0000152041.20486.2F. [DOI] [PubMed] [Google Scholar]
- 8.Crosby KM, Connell BJ, Saleh TM. Estrogen limits ischemic cell death by modulating caspase-12-mediated apoptotic pathways following middle cerebral artery occlusion. Neuroscience. 2007;146:1524–1535. doi: 10.1016/j.neuroscience.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Damczyk MP, Gardner DM. Risk of hormone replacement therapy in stroke patients. J. Clin. Pharm. Ther. 2000;25:239–241. doi: 10.1046/j.1365-2710.2000.00280.x. [DOI] [PubMed] [Google Scholar]
- 10.Dubal DB, Wise PM. Neuroprotective effects of estradiol in middle-aged female rats. Endocrinology. 2001;142:43–48. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- 11.Fan T, Yang SH, Johnson E, Osteen B, Hayes R, Day AL, Simpkins JW. 17beta-estradiol extends ischemic thresholds and exerts neuroprotective effects in cerebral subcortex against transient focal cerebral ischemia in rats. Brain Res. 2003;993:10–17. doi: 10.1016/j.brainres.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara N, Murata Y, Arai K, Egi Y, Lu J, Wu O, Singhal AB, Lo EH. Combination therapy with normobaric oxygen (NBO) plus thrombolysis in experimental ischemic stroke. BMC Neurosci. 2009;10:79. doi: 10.1186/1471-2202-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo J, Krause DN, Horne J, Weiss JH, Li X, Duckles SP. Estrogen-receptor-mediated protection of cerebral endothelial cell viability and mitochondrial function after ischemic insult in vitro. J. Cereb. Blood Flow Metab. 2010;30:545–554. doi: 10.1038/jcbfm.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia J, Guan D, Zhu W, Alkayed NJ, Wang MM, Hua Z, Xu Y. Estrogen inhibits Fas-mediated apoptosis in experimental stroke. Exp. Neurol. 2009;215:48–52. doi: 10.1016/j.expneurol.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kano T, Harada T, Katayama Y. Attenuation of extravasation of tissue plasminogen activator by the free radical scavenger, edaravone: evaluation in a rat thromboembolic stroke model. Neurol. Res. 2005;27:499–502. doi: 10.1179/016164105X17387. [DOI] [PubMed] [Google Scholar]
- 16.Kelly MA, Shuaib A, Todd KG. Matrix metalloproteinase activation and blood–brain barrier breakdown following thrombolysis. Exp. Neurol. 2006;200:38–49. doi: 10.1016/j.expneurol.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke. 2000;31:3034–3040. doi: 10.1161/01.str.31.12.3034. [DOI] [PubMed] [Google Scholar]
- 18.Liu R, Wen Y, Perez E, Wang X, Day AL, Simpkins JW, Yang SH. 17beta-estradiol attenuates blood–brain barrier disruption induced by cerebral ischemia–reperfusion injury in female rats. Brain Res. 2005;1060:55–61. doi: 10.1016/j.brainres.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 19.Liu R, Liu Q, He S, Simpkins JW, Yang SH. Combination therapy of 17beta-estradiol and recombinant tissue plasminogen activator for experimental ischemic stroke. J. Pharmacol. Exp. Ther. 2010;332:1006–1012. doi: 10.1124/jpet.109.160937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35:354–356. doi: 10.1161/01.STR.0000115164.80010.8A. [DOI] [PubMed] [Google Scholar]
- 21.Liu XS, Zhang ZG, Zhang L, Morris DC, Kapke A, Lu M, Chopp M. Atorvastatin downregulates tissue plasminogen activator-aggravated genes mediating coagulation and vascular permeability in single cerebral endothelial cells captured by laser microdissection. J Cereb Blood Flow Metab. 2006;26(6):787–796. doi: 10.1038/sj.jcbfm.9600227. [DOI] [PubMed] [Google Scholar]
- 22.Menzies SA, Hoff JT, Betz AL. Middle cerebral artery occlusion in rats: a neurological and pathological evaluation of a reproducible model. Neurosurgery. 1992;31:100–106. doi: 10.1227/00006123-199207000-00014. discussion 106–107. [DOI] [PubMed] [Google Scholar]
- 23.Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribo M, Quintana M, Alvarez-Sabin J. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003;107:598–603. doi: 10.1161/01.cir.0000046451.38849.90. [DOI] [PubMed] [Google Scholar]
- 24.Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- 25.O’Donnell ME, Lam TI, Tran LQ, Foroutan S, Anderson SE. Estradiol reduces activity of the blood–brain barrier Na–K–Cl cotransporter and decreases edema formation in permanent middle cerebral artery occlusion. J. Cereb. Blood Flow Metab. 2006;26:1234–1249. doi: 10.1038/sj.jcbfm.9600278. [DOI] [PubMed] [Google Scholar]
- 26.Qin Z, Karabiyikoglu M, Hua Y, Silbergleit R, He Y, Keep RF, Xi G. Hyperbaric oxygen-induced attenuation of hemorrhagic transformation after experimental focal transient cerebral ischemia. Stroke. 2007;38:1362–1367. doi: 10.1161/01.STR.0000259660.62865.eb. [DOI] [PubMed] [Google Scholar]
- 27.Rau SW, Dubal DB, Bottner M, Gerhold LM, Wise PM. Estradiol attenuates programmed cell death after stroke-like injury. J. Neurosci. 2003;23:11420–11426. doi: 10.1523/JNEUROSCI.23-36-11420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Razandi M, Pedram A, Levin ER. Estrogen signals to the preservation of endothelial cell form and function. J. Biol. Chem. 2000;275:38540–38546. doi: 10.1074/jbc.M007555200. [DOI] [PubMed] [Google Scholar]
- 29.Razmara A, Krause DN, Duckles SP. Testosterone augments endotoxin-mediated cerebrovascular inflammation in male rats. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H1843–H1850. doi: 10.1152/ajpheart.00465.2005. [DOI] [PubMed] [Google Scholar]
- 30.Razmara A, Sunday L, Stirone C, Wang XB, Krause DN, Duckles SP, Procaccio V. Mitochondrial effects of estrogen are mediated by estrogen receptor alpha in brain endothelial cells. J. Pharmacol. Exp. Ther. 2008;325:782–790. doi: 10.1124/jpet.107.134072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rusa R, Alkayed NJ, Crain BJ, Traystman RJ, Kimes AS, London ED, Klaus JA, Hurn PD. 17beta-estradiol reduces stroke injury in estrogen-deficient female animals. Stroke. 1999;30:1665–1670. doi: 10.1161/01.str.30.8.1665. [DOI] [PubMed] [Google Scholar]
- 32.Saleh TM, Cribb AE, Connell BJ. Estrogen-induced recovery of autonomic function after middle cerebral artery occlusion in male rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001a;281:R1531–R1539. doi: 10.1152/ajpregu.2001.281.5.R1531. [DOI] [PubMed] [Google Scholar]
- 33.Saleh TM, Cribb AE, Connell BJ. Reduction in infarct size by local estrogen does not prevent autonomic dysfunction after stroke. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001b;281:R2088–R2095. doi: 10.1152/ajpregu.2001.281.6.R2088. [DOI] [PubMed] [Google Scholar]
- 34.Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33:831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- 35.Tang J, Li YJ, Mu J, Li Q, Yang DY, Xie P. Albumin ameliorates tissue plasminogen activator-mediated blood–brain barrier permeability and ischemic brain injury in rats. Neurol. Res. 2009;31:189–194. doi: 10.1179/174313209X393898. [DOI] [PubMed] [Google Scholar]
- 36.Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- 37.Toung TJ, Chen TY, Littleton-Kearney MT, Hurn PD, Murphy SJ. Effects of combined estrogen and progesterone on brain infarction in reproductively senescent female rats. J. Cereb. Blood Flow Metab. 2004;24:1160–1166. doi: 10.1097/01.WCB.0000135594.13576.D2. [DOI] [PubMed] [Google Scholar]
- 38.Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN, Huang PL, Wang X, Montaner J, Lo EH. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke. 2005;36:1954–1959. doi: 10.1161/01.STR.0000177517.01203.eb. [DOI] [PubMed] [Google Scholar]
- 39.Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. N. Engl. J. Med. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Lee SR, Arai K, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat. Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 41.Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 42.Won CK, Kim MO, Koh PO. Estrogen modulates Bcl-2 family proteins in ischemic brain injury. J. Vet. Med. Sci. 2006;68:277–280. doi: 10.1292/jvms.68.277. [DOI] [PubMed] [Google Scholar]
- 43.Yagi K, Kitazato KT, Uno M, Tada Y, Kinouchi T, Shimada K, Nagahiro S. Edaravone, a free radical scavenger, inhibits MMP-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke. 2009;40:626–631. doi: 10.1161/STROKEAHA.108.520262. [DOI] [PubMed] [Google Scholar]
- 44.Yang SH, Shi J, Day AL, Simpkins JW. Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke. 2000;31:745–749. doi: 10.1161/01.str.31.3.745. discussion 749–750. [DOI] [PubMed] [Google Scholar]
- 45.Yang DY, Pan HC, Chen CJ, Cheng FC, Wang YC. Effects of tissue plasminogen activator on cerebral microvessels of rats during focal cerebral ischemia and reperfusion. Neurol. Res. 2007;29:274–282. doi: 10.1179/016164107X159171. [DOI] [PubMed] [Google Scholar]
- 46.Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood–brain barrier via the LDL receptor-related protein. J. Clin. Invest. 2003;112:1533–1540. doi: 10.1172/JCI19212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Sato K, Hayashi T, Omori N, Nagano I, Kato S, Horiuchi S, Abe K. Extension of ischemic therapeutic time window by a free radical scavenger, Edaravone, reperfused with tPA in rat brain. Neurol. Res. 2004;26:342–348. doi: 10.1179/016164104225014058. [DOI] [PubMed] [Google Scholar]
- 48.Zhu H, Fan X, Yu Z, Liu J, Murata Y, Lu J, Zhao S, Hajjar KA, Lo EH, Wang X. Annexin A2 combined with low-dose tPA improves thrombolytic therapy in a rat model of focal embolic stroke. J. Cereb. Blood Flow Metab. 2010;30(6):1137–1146. doi: 10.1038/jcbfm.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]