Abstract
Secondary cone degeneration in the transgenic rats carrying the S334ter rhodopsin mutation (S334ter-3 rats) starts at the peak of rod degeneration (PD12) and progresses with age. An early sign of cone degeneration is the loss of cone outer segments (COS) distributed in many small patches throughout the retina. Cone cell death occurs about 2 months later. When treated with CNTF (ciliary neurotrophic factor), impaired cones regenerate COS. Sustained delivery of CNTF prevents cones from degeneration and helps to maintain COS and function. These results indicate that cone degeneration is reversible at early stages, and supports a therapeutic strategy of sustained delivery of CNTF to prevent cone degeneration.
13.1. Introduction
Hereditary retinal degenerations are a major cause of blindness and effective treatments are still being sought (Hartong et al. 2006). Among these conditions, the worldwide prevalence of retinitis pigmentosa, a group of inherited retinal degenerations, is about 1 in 4000 for a total of more than 1 million affected individuals with well over 100 genes implicated. Although most mutations responsible for RP only affect rod photoreceptors directly, cones undergo a secondary degeneration (Delyfer et al. 2004; Hartong et al. 2006). Since cones are responsible for our central and color vision, rescue of the cones has become a major research challenge for therapeutic discovery and development.
We investigated the secondary cone degeneration in a retinal degeneration model, the S334ter-3 rats. In these rats, rod degeneration starts at postnatal day (PD) 8, peaks at PD 12 to 13, and most rods are degenerated by PD 20 (Liu et al. 1999). Cone degeneration starts with an early sign of COS loss, which in these animals occurs in many round or irregular areas across the retina (Li et al. 2010). When treated with CNTF, degenerating cones regenerate COS (Li et al. 2010). These results provide evidence that in early stages, CNTF reverses secondary cone degeneration. Sustained delivery of CNTF helps cones to maintain COS and their functions.
13.2 Materials and Methods
Homozygous S334ter rats and Sprague-Dawley rats were used. Intravitreal injections were carried out with 33-gauge needles. The left eyes were injected with recombinant human CNTF protein (10μg/5μl), the right ones with phosphate buffered saline (PBS) (5μl) and served as control. CNTF was injected intravitreally at a given time point and retinas were collected 10 days later. CNTF-secreting microdevices were implanted in the vitreous at postnatal day (PD) 20 and eyes were collected 140 days later. Flat-mounted retinas were stained with fluorescent conjugated peanut agglutinin (PNA) or antibodies against cone arrestin (CAR) and examined by confocal microscopy. Photopic ERG responses were elicited by 1 ms white flashes of 2.5 log cd·s/m2 in the Ganzfeld sphere with white background illumination of 30 cd·s/m2.
13.3 Results
13.3.1 Loss of COS in Early Stages of Cone Degeneration
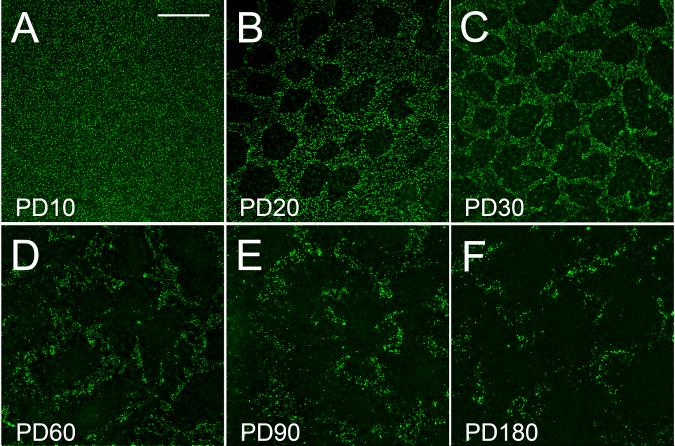
At PD 10, PNA staining is evenly distributed across the retina (Fig. 13.1A). Loss of PNA staining is detected at PD12 when rod degeneration is at its peak (not shown). By PD 20, loss of COS appears in many small round or irregularly shaped PNA-negative areas (Fig. 13.1A). PNA staining outside those areas is similar to that of PD 10. Loss of COS progresses with age (Fig. 13.1A-1F).
Fig. 13.1.
Loss of COS. Flat-mounted retinas of different ages were stained with PNA. Loss of PNA staining is concentrated in small round or irregularly shaped areas and progressive with age. Scale bar: 200 μm. Modified from Li et al. (2010).
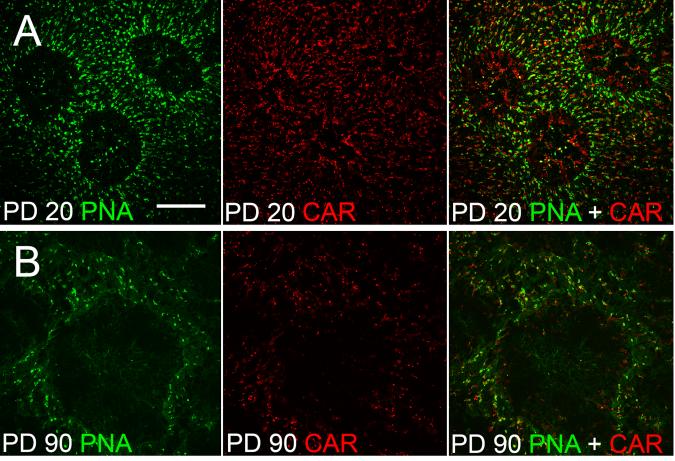
Since PNA only stains COS, antibodies against CAR were used as a marker to identify cone cells (Zhu et al. 2002). At PD 20, cells inside PNA-negative areas are positive of CAR, indicating they are cones (Fig. 13.2A). By PD 90, however, only a few CAR-positive cells remained in the PNA-negative areas (Fig. 13.2B). Thus, by PD 90, most of the cones in the PNA-negative areas were degenerated.
Fig. 13.2.
Loss of cones. Flat-mounted retinas of S334ter-3 rats were double stained with PNA and antibodies against cone arrestin (CAR). Many CAR-positive cells were found in the PNA-negative areas at PD 20 (A), but only a few remained in the PNA-negative areas at PD 90 (B). Scale bars: 100 μm. Modified from Li et al. (2010).
13.3.2 CNTF Promotes COS Regeneration
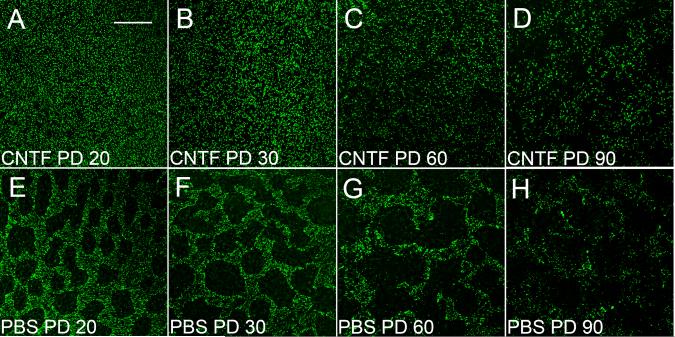
Retinas treated with CNTF at PD 10 (endpoint PD 20) had no obvious PNA-negative areas (Fig. 13.3A), unlike PBS-treated ones in which many PNA-negative areas appeared (Fig. 13.3E). In most cases, PNA-negative areas completely disappeared in CNTF-treated retinas between PD 20 to PD 50 (endpoints PD 30 to PD 60) (Fig. 13.3B and C). In contrast, PBS-treated fellow retinas had many PNA-negative areas (Fig. 3F and G). This effect became less dramatic at PD 80 (endpoint PD 90, Fig. 13.3D) and almost disappeared in retinas of PD 170 (endpoint PD 180, not shown).
Fig. 13.3.
Effect of CNTF on PNA-positive cells. Flat-mounted retinas of different ages were stained with PNA. After CNTF treatment, no PNA-negative areas were seen at PD 20 (A), whereas many PNA-negative areas appeared in PBS-treated retinas (E). The PNA-negative areas completely disappeared in PD 30 and 60 CNTF-treated retinas (B and C). This effect became less dramatic in PD 60 and retinas (D). Scale bar: 200 μm. Modified from Li et al. (2010).
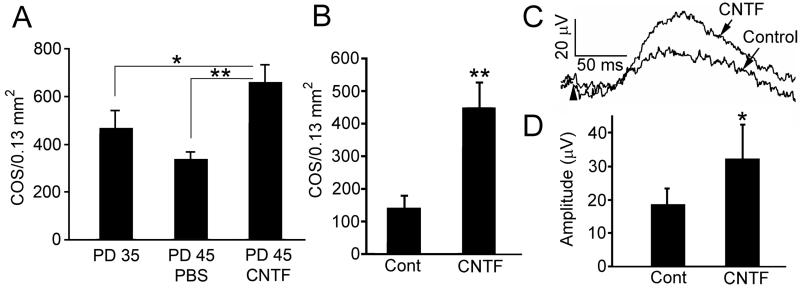
CNTF-induced reappearance of PNA-positive cells (Fig. 13.3A and B) suggests COS regeneration. To confirm this observation, eyes were treated with CNTF at PD 35 and retinas were examined at PD 45. Indeed, the density of PNA-positive cells in CNTF-treated retinas of PD 45 was significantly more than in the PD 35 controls before the treatment started (Fig. 13.4A).
Fig. 13.4.
Regeneration of COS and long-term effects. To demonstrate regeneration of COS, retinas were treated with either PBS or CNTF at PD 35 and collected by PD45 (A). PNA-positive cell counts in CNTF-treated retinas are 657±76 (n=4), significantly more than in PBS treated (334±33, n=3), or untreated at PD 35 before the treatment (467±73, n=3) (A, ANOVA and Bonferroni test). To see the long-term effects of CNTF, CNTF secreting microdevices were implanted at PD 20 and retinas were collected by PD 160 (B). PNA-positive cells are significantly more in CNTF-treated retinas (446±80) than the controls (141±40) (B, mean ± SD, n=3, Student t-test). Cone ERGs were recorded at PD 135 from eyes implanted at PD 30. Representative cone b-waves are shown in panel C (arrowhead indicates the onset of flash). The amplitude of cone b-wave in CNTF-treated eyes (32.46±10.02 μV, mean ± SD, n=3) is significantly higher than the control (17.70±4.78 μV, n=3) (D, Student t-test) Asterisk: P<0.05; double-asterisk: P<0.01. Modified from Li et al. (2010).
Long-term effect of CNTF was achieved by using CNTF-secreting microdevices (Tao 2006; Tao et al. 2006; Tao and Wen 2007), which were implanted in the left eyes and control devices in the right eyes at PD 20, and retinas were collected at PD160. The densities of PNA-positive cells in CNTF-treated retinas are significantly more than in the control retinas (Fig. 13.4B). In another experiment, CNTF-secreting devices were implanted in the left eyes and control devices in the right eyes at PD 30. Cone ERGs were recorded at PD 135. The cone b-wave amplitude in CNTF-treated eyes is significantly higher than in control eyes (Fig. 13.4C and D).
13.4 Discussion
Loss of COS constitutes a prelude to cone degeneration, followed by cone cell death with a delay. In the S334ter-3 rats, the delay of cone death is about two month. PNA-negative patches are readily observable at PD20, but the cones in the PNA-negative areas are visibly alive. In contrast, cones are scarce to nonexistent in the PNA-negative areas in retinas by PD 90. This is also reflected in the CNTF induced COS regeneration. Although partially responsive up to PD 60, regenerative potential has dramatically decreased in retinas by PD 80. A two-month window is rather large considering the rapid rate of rod degeneration (10 days) in these animals. Although the time frame shifts, a strong parallel exists in patients with RP. In most clinical cases, rod degeneration in RP patients is much slower, and cone death should occur much later.
It is rather surprising that degenerating cones remain capable of regenerating COS, indicating that to a certain degree cone degeneration is reversible. COS are functional organelles that initiate the conversion of photons into neuronal signals. Regeneration COS should restore the light-sensing function of cones. This is very significant since it is possible that in RP patients, degenerating cones may also be capable of regenerating COS. In that case, it is possible to stimulate those cones to regenerate COS and to restore vision to some extent.
Reports from CNTF clinical trials in patients with RP are consistent with our preclinical observations. In a Phase I clinical trial, 3 patients with late-stage RP had significant improvement of vision after implantation of a device that delivers CNTF to the retina (Sieving et al. 2006). Recently, adaptive optics scanning laser ophthalmoscopy was used to observe 3 patients with retinal degeneration over a two-year interval (Talcott et al. 2010). While cone density significantly decreased in the sham-treated eyes, no decrease in cone density occurred in the CNTF-treated eye (Talcott et al. 2010). Together, these findings challenge us to rethink our goals in treating retinal degenerations. As a useful first step we may want to emphasize early cone protection while continuing efforts to find ways to restore vision in late stage RP patients.
CNTF has been shown to protect rod photoreceptors in different animal models (LaVail et al. 1992; Cayouette and Gravel 1997; Cayouette et al. 1998; LaVail et al. 1998; Chong et al. 1999; Caffe et al. 2001; Liang et al. 2001; Bok et al. 2002; Tao et al. 2002). Our data support long-term sustained delivery CNTF as a therapy to benefit RP patients by preserving cone function and useful vision.
Acknowledgments
We thank Dr. Cheryl Craft for anti cone-arrestin antibodies; Dr. Ying Song, Dr. Lian Zhao, and Yun Liu for technical assistance. Supported by Grants from NIH: R01EY015289 (RW), R01EY018586 (RW), R01EY001919 (MML), R01EY006842 (MML), Hope for Vision (RW), the James and Esther King Biomedical Research Program of the State of Florida (YL), the Department of Defense (W81XWH-09-1-0674, RW), and NIH Core Grants P30EY14801, P30EY002162, the Foundation Fighting Blindness (MML), and unrestricted grants from Research to Prevent Blindness Inc., New York, NY to Bascom Palmer Eye Institute, Beckman Vision Center, and Scheie Eye Institute.
References
- Bok D, Yasumura D, Matthes MT, et al. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res. 2002;74:719–735. doi: 10.1006/exer.2002.1176. [DOI] [PubMed] [Google Scholar]
- Caffe AR, Soderpalm AK, Holmqvist I, et al. A combination of CNTF and BDNF rescues rd photoreceptors but changes rod differentiation in the presence of RPE in retinal explants. Invest Ophthalmol Vis Sci. 2001;42:275–282. [PubMed] [Google Scholar]
- Cayouette M, Gravel C. Adenovirus-mediated gene transfer of ciliary neurotrophic factor can prevent photoreceptor degeneration in the retinal degeneration (rd) mouse. Hum Gene Ther. 1997;8:423–430. doi: 10.1089/hum.1997.8.4-423. [DOI] [PubMed] [Google Scholar]
- Cayouette M, Behn D, Sendtner M, et al. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci. 1998;18:9282–9293. doi: 10.1523/JNEUROSCI.18-22-09282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong NH, Alexander RA, Waters L, et al. Repeated injections of a ciliary neurotrophic factor analogue leading to long-term photoreceptor survival in hereditary retinal degeneration. Invest Ophthalmol Vis Sci. 1999;40:1298–1305. [PubMed] [Google Scholar]
- Delyfer MN, Leveillard T, Mohand-Said S, et al. Inherited retinal degenerations: therapeutic prospects. Biol Cell. 2004;96:261–269. doi: 10.1016/j.biolcel.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- LaVail MM, Unoki K, Yasumura D, et al. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci U S A. 1992;89:11249–11253. doi: 10.1073/pnas.89.23.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail MM, Yasumura D, Matthes MT, et al. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602. [PubMed] [Google Scholar]
- Li Y, Tao W, Luo L, et al. CNTF induces regeneration of cone outer segments in a rat model of retinal degeneration. PLoS One. 2010;5:e9495. doi: 10.1371/journal.pone.0009495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FQ, Aleman TS, Dejneka NS, et al. Long-term protection of retinal structure but not function using RAAV.CNTF in animal models of retinitis pigmentosa. Mol Ther. 2001;4:461–472. doi: 10.1006/mthe.2001.0473. [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Peng M, et al. Activation of caspase-3 in the retina of transgenic rats with the rhodopsin mutation s334ter during photoreceptor degeneration. J Neurosci. 1999;19:4778–4785. doi: 10.1523/JNEUROSCI.19-12-04778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talcott K, E., Ratnam K, Sundquist SM, et al. Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.10-6479. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W. Application of encapsulated cell technology for retinal degenerative diseases. Expert Opin Biol Ther. 2006;6:717–726. doi: 10.1517/14712598.6.7.717. [DOI] [PubMed] [Google Scholar]
- Tao W, Wen R. Application of encapsulated eell technology for retinal degenerative diseases. In: Tombran-Tink J, Barnstable CJ, editors. Retinal Degenerations. Humana Press; 2007. [Google Scholar]
- Tao W, Wen R, Laties AM. Cell-based delivery systems: Development of encapsulated cell technology for ophthalmic applications. In: Jaffe JJ, Ashton P, Pearson PA, editors. Intraocular drug delivery. Humana Press; New York, NY: 2006. [Google Scholar]
- Tao W, Wen R, Goddard MB, et al. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002;43:3292–3298. [PubMed] [Google Scholar]
- Zhu X, Li A, Brown B, et al. Mouse cone arrestin expression pattern: light induced translocation in cone photoreceptors. Mol Vis. 2002;8:462–471. [PubMed] [Google Scholar]