Abstract
Introduction
Vascularity influences the characteristics of gynecologic tumors observed with direct imaging techniques that reveal the macrovascular component of these lesions (color and power Doppler) and with indirect imaging involving the administration of contrast agents to examine the microcirculation and interstitial perfusion (contrast-enhanced computed tomography [CT] and magnetic resonance [MR] imaging). The purpose of this study was to determine whether contrast-enhanced ultrasonography (CEUS) of ovarian lesions provides useful information that cannot be obtained with conventional US.
Materials and methods
We used CEUS to assess 72 nonspecific adnexal lesions in 61 patients. CEUS was performed with a 4.8-ml bolus of a second-generation ultrasonographic contrast agent and dedicated imaging algorithms. For each lesion, B-mode morphology, CEUS morphology, and time/intensity curves were evaluated.
Results
In 8/61 cases (13.1%) CEUS offered no additional morphovascular information. In 38/61 cases (62.3%), it provided additional information that did not modify the management of the lesion, and in 15/61 cases (24.6%) it gave additional information that modified the management of the lesion. Malignant lesions were characterized by significantly shorter times to peak enhancement (11.9 ± 3.1 s vs 19.8 ± 4.0 s p < 0.01) and significantly higher peak intensity (24.7 ± 4.2 dB vs 17.8 ± 3.3 dB p < 0.01) compared with benign lesions.
Conclusions
CEUS improves diagnostic confidence in the characterization of liquid-corpuscular lesions where conventional US is inconclusive. CEUS can be proposed as a valid alternative to CT and MR. However, information obtained by CEUS influences the therapy in a limited percentage of cases (24.6%).
Keywords: Adnexal masses, Contrast-enhanced ultrasound, Ovarian cancer
Sommario
Introduzione
La ricchezza della componente vascolare dei tumori ginecologici influenza le caratteristiche dell'imaging diretto, utilizzando metodiche che evidenziano la componente macrovascolare delle lesioni (color e power Doppler), e di quello indiretto, mediante somministrazione di mezzi di contrasto (MdC) per lo studio del microcircolo e della perfusione interstiziale. Lo scopo di questo lavoro è di valutare l'aggiunta di informazioni diagnostiche fornite dalla valutazione ecografica con MdC nello studio delle lesioni ovariche.
Materiali e metodi
Abbiamo valutato 72 lesioni annessiali in 61 pazienti con lesioni ovariche di incerta interpretazione mediante somministrazione di 4,8 ml di MdC ecoamplificatore di II generazione. Per ogni lesione, abbiamo valutato la morfologia basale, quella contrastografica e le curve intensità/tempo.
Risultati
La valutazione post-contrasto confrontata con la basale non ha apportato informazioni aggiuntive morfovascolari in 8 pazienti (13,1%); in 38 pazienti (62,3%) ha apportato informazioni senza modifiche del comportamento clinico; in 15 soggetti (24,6%) ha apportato elementi che hanno modificato il comportamento clinico. Le lesioni maligne presentavano valori di tempo massimo di enhancement significativamente minori (11,9 ± 3,1 s vs 19,8 ± 4,0 s p < 0,01) e intensità di picco massimo significativamente maggiore (24,7 ± 4,2 dB vs 17,8 ± 3,3 dB p < 0,01) rispetto alle lesioni benigne.
Conclusioni
La CEUS consente di migliorare la confidenza diagnostica nelle lesioni liquide corpuscolate in cui l'indagine convenzionale non risulta dirimente, proponendosi nella diagnosi differenziale di un limitato numero di lesioni complesse, in alternativa a TC ed RM. Le informazioni ottenute influenzano tuttavia il successivo iter diagnostico e terapeutico in una limitata percentuale di casi (24,6%).
Introduction
In industrialized countries, particularly those of Europe and North America, ovarian carcinoma is extremely common. They represent the sixth most frequent type of cancer in terms of incidence and are seventh on the list of cancers causing death. The total number of cases in the world has been estimated at approximately 192,000. These cancers represent around 4% of the tumors diagnosed in females and the second most common gynecologic tumor (after endometrial cancer) [1]. These malignancies are characterized by a paucity of symptoms, which appear late in the disease. For these reasons, the diagnosis is frequently made when the tumor has already reached an advanced stage. No other gynecologic tumor is associated with a higher mortality rate [2]. A positive family history is the main risk factor for ovarian cancer, and an estimated 10% of all cases have a hereditary basis. Other risk factors include nulliparity, early menarche, and delayed menopause [3]. Transvaginal ultrasonography is currently the most effective method for early diagnosis of ovarian tumors, but it does not allow precise characterization of the nature of the lesion [4].
The role of lesional vascularity studies in identifying malignant pelvic lesions has been the subject of numerous studies, some dating back to the late 1990s. The use of this approach is based on histopathological findings of intense neoplastic angiogenesis mediated by various types of blood factors that are necessary for primary tumor growth. Malignant tumors present several characteristics, including rich neovascularization, vessels with irregular course, arteriovenous shunts that can increase blood flow velocity, tumor lacunae, and lower flow resistance secondary to the incompletely developed muscle layer of the tumor vessels [4]. Color Doppler ultrasonography has been used to document the characteristics of ovarian vascularization [5,6]. Power Doppler is a useful technique for mapping ovarian vessels, including those with characteristics suggestive of malignancy, and pulsed Doppler techniques are used to measure blood flow velocities [7,8].
Vascularity influences the characteristics of gynecologic tumors observed with direct imaging techniques that reveal the macrovascular component of these lesions (color and power Doppler), but it also has an impact during indirect imaging involving the administration of contrast agents to examine the microcirculation and interstitial perfusion (contrast-enhanced computed tomography and magnetic resonance imaging). As early as 1996, Buy [9] demonstrated the value of integrating conventional B-mode sonography with color Doppler data on vessel morphology, an approach that significantly improved sensitivity in the differentiation of benign and malignant tumors. In fact, for distinguishing benign from malignant tumors, conventional transvaginal ultrasonography has displayed 91% sensitivity and 84% specificity, and these results can be improved by the addition of color Doppler studies during the sonographic examination, which increases the sensitivity to 95% and the specificity to 86% [10]. Parameters like contrast agent wash-in and wash-out times could be useful for differentiating benign and malignant tumors [11], although the results obtained with this approach are characterized by wide variability [4,7,11].
The aim of the present study was to assess the efficacy of contrast-enhanced ultrasonography (CEUS) in the differentiation of ovarian lesions and the potential impact of this approach on subsequent clinical management.
Materials and methods
This prospective study was approved by the hospital ethics committee, and informed consent was obtained from all participants. We prospectively enrolled 61 women who were consecutively referred to our department for the evaluation of 71 adnexal lesions of unknown nature. The CEUS protocol provided for the administration of a 4.8-cc bolus of Sonovue (Bracco, Italy), a second-generation ultrasound contrast agent, in the basilic or cephalic vein. Sonography was performed transabdominally (TA) in 23/61 patients, transvaginally (TV) in 55/61, and with a combined approach in 22/61. The entire examination, which lasted 180 s, was recorded on the hard disk and later reviewed by an experienced radiologist and a gynecologist. The following parameters were analyzed:
-
A)Morphological assessment with and without contrast enhancement
-
1)No additional information compared with that provided by conventional sonography;
-
2)Information that does not modify management of the lesion;
-
3)Information that modifies management of the lesion (wait-and-see versus surgery, timing of surgery, surgical technique to be used, aspiration versus surgery).
-
1)
-
B)Time/intensity curve
-
1)Time of maximum enhancement;
-
2)Intensity of peak enhancement expressed as the mean of values measured at three different points in the lesion.
-
1)
The time/intensity curves were evaluated starting from the first appearance of contrast bubbles in the field of view.
Statistical analysis
The χ2 test was used to measure the amount of clinical information provided by the administration of the microbubble contrast agent (no additional information compared with that provided by conventional sonography, information that did not modify management of the lesion, or information that modified management of the lesion). The Mann–Whitney U test was used to identify significant differences between peak enhancement times and intensities for malignant lesions and those of benign lesions.
Results
Compared with the baseline evaluation, the injection of contrast enhancement provided no additional information on the vascular morphology of lesions in 8 patients (13.1%). In 38 patients (62.3%) it provided additional information that did not modify clinical management, and in 15 patients (24.6%) the contrast-enhanced examination provided useful data that changed clinical management (Figs. 1 and 3–5).
Fig. 1.
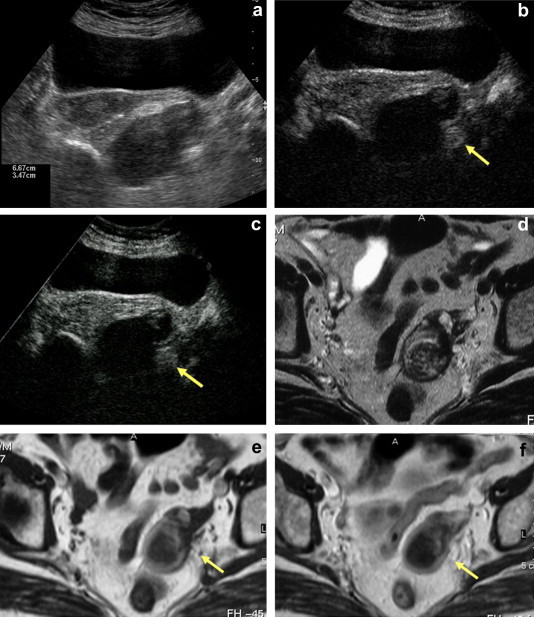
An 82-year-old woman with cachexia. (a) Left adnexal mass that appears solid and hypoechoic. (b) Early arterial phase of CEUS (c) Late phase of CEUS. Absence of enhancement. (d) T2-weighted MRI shows inhomogeneous signal intensity that is not clearly fluid. (e) T1-weighted sequence. (f) T1-weighted post-gadolinium sequence. Total absence of enhancement. Final diagnosis: chronic salpingeal abscess.
Fig. 3.
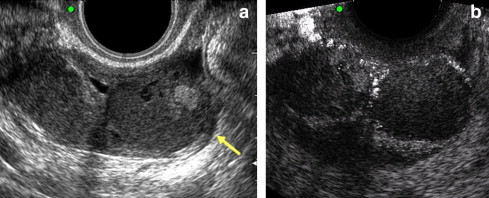
(a) Presence of a complex ovarian mass of uncertain origin in an asymptomatic patient. (b) Contrast-enhancement reveals 2 highly corpuscular avascular fluid collections within the lesion. Final diagnosis (confirmed by laparoscopy): burned-out nonbleeding endometriosis.
Fig. 4.
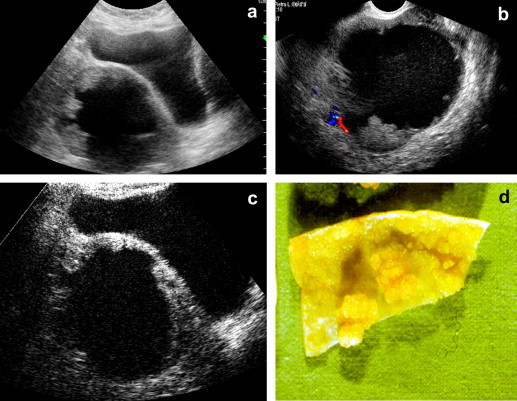
Pelvic hematoma 6 months earlier following colpohysterectomy. (a) Complex pelvic mass with suspected papillae: differential diagnosis chronic hematoma vs neoplastic lesion. (b) Absence of color Doppler signal. (c) Vascularization of the papillary component at CEUS. Histologic diagnosis: seropapilliferous tumor of the ovary. (d) Surgical specimen.
Fig. 5.
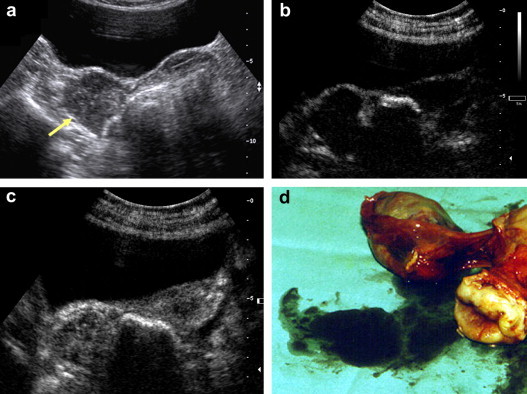
Vascularized lesion (a) conventional ultrasound (b) and (c) CEUS. Final diagnosis Brenner tumor, borderline. (d) Surgical specimen.
Peak enhancement times for malignant tumors were significantly shorter than those observed for benign lesions (11.9 ± 3.1 s vs 19.8 ± 4.0 s, p < 0.01; Fig. 2), and peak enhancement intensity was significantly greater in these lesions (24.7 ± 4.2 dB vs 17.8 ± 3.3 dB in benign lesions; p < 0.01; Fig. 2).
Fig. 2.
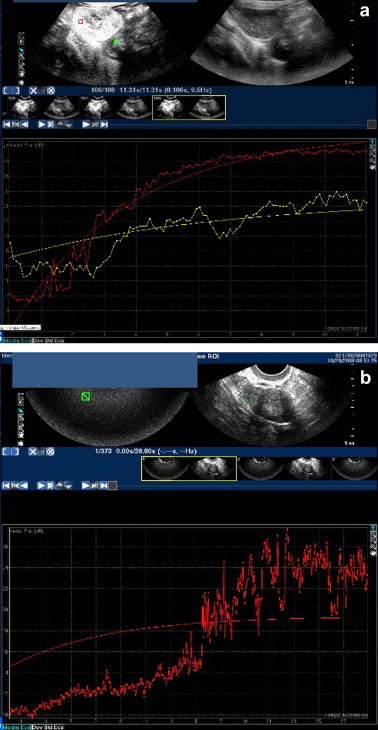
Time/intensity curve. (a) Small benign tumor of the ovarian stroma. Time to peak enhancement 21 s, peak enhancement intensity 19 dB. (b) Malignant granulosa cell tumor (red curve) and pedunculated uterine myoma (yellow): Time to peak enhancement 11 s vs 14 s, peak enhancement intensity 27 dB vs 16 dB.
Discussion
This prospective study evaluated the efficacy of CEUS in distinguishing benign from malignant ovarian lesions and the possible impact of this approach on subsequent case management. Our findings indicate that malignant adnexal lesions are characterized by significantly shorter times to maximal contrast enhancement and significantly greater peak enhancement intensity than benign lesions. In 86.9% of the patients, CEUS provided information that had not been obtained with the conventional sonographic morphological study, but this information modified case management only in 24.6%.
The standard treatment for ovarian lesions is surgical removal since around 25% of ovarian masses prove to be malignant. In many centers, however, the decision to use laparotomy or laparoscopy is conditioned by the estimated probability of malignancy, which is based largely on the results of diagnostic imaging studies. The final diagnosis of ovarian masses is based on histology, but preoperative differentiation of benign and malignant lesions is essential for decisions on the timing and technical characteristics of the surgery.
Transvaginal ultrasonography is the method most widely used to study the adnexae, and meta-analyses have shown that ovarian lesions can be optimally characterized with a combination of B-mode ultrasonography and Doppler studies [12]. A multicenter study by the International Ovarian Tumor Analysis (IOTA) group has generated algorithms for characterizing these lesions, and on the basis of such findings studies were later conducted to identify the ultrasound features and vascular characteristics of different types of tumors defined by specific clinical features and tumor-marker profiles [7,13–17]. On the basis of these criteria, the main lesion characteristics that are suggestive of malignancy have recently been summarized: the presence of intense vascularization at color Doppler, the presence of ascites, masses with irregular shapes, or solid multilocular masses. Findings suggestive of benign masses include the absence of vascularization at color Doppler, the presence of a unilocular cyst, the presence of a solid component measuring <7 mm or a multilocular cystic component with a maximum diameter <100 mm [18].
However, even in the hands of an expert examiner, ultrasonography alone is not sufficiently reliable for distinguishing benign from malignant lesions, especially those in the early stage.
Since increased vascularization is one of the criteria that define neoplastic lesions [12,19,20], so assessment of this parameter needs to be facilitated with a contrast enhancer that improves visualization of vascular structures, especially those of the microcirculation [7,21].
A previous study [4] demonstrated that use of a contrast agent improves the clarity of the power Doppler signal and aids identification of vascularized areas of a tumor. Malignant lesions were also found to contain a significantly higher number of identifiable vessels than their benign counterparts, before and after administration of the contrast agent. In the present study, we used a sonographic contrast agent to improve visualization of tumor vascularization and to evaluate the possibility that diagnostic performance in differentiating benign and malignant lesions could be increased by perfusional assessment of the lesion. We found that malignant lesions were characterized by significantly higher peak enhancement intensity and significantly shorter peak enhancement times than benign tumors. This phenomenon might be related to the high-velocity flow through the arteriovenous shunts that are typical of malignant neovascularization and seems to be more pronounced in carcinomas, where the increased vascularization is thought to decrease the arrival time of the contrast agent [4]. These findings appear to be similar to those previously reported by Maija-Riitta Ordén et al. [4] and Marret et al. [5] although in the latter study the findings did not prove to be statistically significant. However, the literature on this subject is still fairly limited. The use of contrast agents in the study of adnexal masses was described for the first time in 1994 by Suren et al. [22]. In a study of 45 women, the use of 3-dimensional ultrasonography with contrast enhancement increased diagnosis of malignancy by increasing the recognition of vascular structures [23]. Another study revealed differences in wash-out times between benign and malignant lesions [24]. In our series, the use of CEUS provided additional morphovascular data, compared with that obtained with conventional sonography, in 88% of the patients, but this information changed the management strategy in only 24.6% of the cases. Our findings suggest therefore that CEUS can be proposed as a method capable of furnishing additional morphological information not obtainable with conventional ultrasound and that analysis of the contrast-enhancement features of the lesions can furnish new valid criteria for discriminating between benign and malignant lesions and for improving the preoperative diagnosis. However, use of this approach seems to have less impact on subsequent management of the patient.
The main limitation of this study is the relatively small number of cases analyzed. However, this number seems sufficient to provide important preliminary evidence of the potential value of time/intensity curve analysis in the study of ovarian lesions. There are, however, limitations regarding the use of CEUS, a technique that is not used routinely in clinical practice: approximately 60% of the ultrasound scanners in western countries lack the software needed for contrast-enhanced studies. In addition, studies of this type require a skilled, experienced operator. It is also important to note that contrast-enhancement parameters are dependent on morphological criteria and on the vascularization characteristics seen on Doppler studies, so these parameters must be integrated into the analysis.
In conclusion, in our study, analysis of peak intensities and time/intensity curves revealed significant differences between benign and malignant lesions. CEUS improved diagnostic confidence in the diagnosis of lesions with liquid-corpuscular structures, which is difficult with the conventional technique. CEUS proved to be particularly useful in hemorrhagic lesions (particularly hemorrhagic corpus luteum), hydrosalpinx, and solid lesions with pseudoliquid features. Use of this method could be an alternative to other contrast-enhanced techniques like CT or MRI for the differential diagnosis of a limited number of complex lesions, orienting the clinical management of the lesion. Time/intensity curves and evaluation of peak enhancement levels can increase the chances of high-accuracy differential diagnosis. CEUS is therefore efficacious for the differential diagnosis of ovarian masses. The information it provides, however, influence subsequent management (diagnosis and therapeutic) in a limited percentage of cases (24.6%). Therefore, the efficacy of this technique needs to be investigated in a larger, randomized clinical trial.
Conflict of interest statement
The authors have no conflict of interest.
Footnotes
Award for the best Poster presented at the 19th SIUMB Congress.
References
- 1.Parkin D.M., Bray F., Ferlay J., Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Vergote I., Amant F., Ameye L., Timmerman D. Screening for ovarian carcinoma: not quite there yet. Lancet Oncol. 2009 Apr;10(4):308–309. doi: 10.1016/S1470-2045(09)70072-5. [DOI] [PubMed] [Google Scholar]
- 3.Shaaban A., Rezvani M. Ovarian cancer: detection and radiologic staging. Clin Obstet Gynecol. 2009;52(1):73–93. doi: 10.1097/GRF.0b013e3181961625. [DOI] [PubMed] [Google Scholar]
- 4.Ordén M.R., Jurvelin J.S., Kirkinen P.P. Kinetics of US contrast agents in benign and malignant adnexal tumors. Radiology. 2003;226(2):405–410. doi: 10.1148/radiol.2262011450. [DOI] [PubMed] [Google Scholar]
- 5.Marret H., Tranquart F., Sauget S., Lansac J. Apport du Doppler pour le diagnostic des tumeurs ovariennes. J Radiol. 2003;84:1725–1731. [PubMed] [Google Scholar]
- 6.Zanetta G., Vergani P., Lissoni A. Color Doppler ultrasound in the preoperative assessment of adnexal masses. Acta Obstet Gynecol Scand. 1994;73(8):637–641. doi: 10.3109/00016349409013458. [DOI] [PubMed] [Google Scholar]
- 7.Timmerman D., Valentin L., Bourne T.H., Collins W.P., Verrelst H., Vergote I. Terms, definitions and measurements to describe the sonographic features of adnexal tumors: a consensus opinion from the International Ovarian Tumor Analysis (IOTA) Group. Ultrasound Obstet Gynecol. 2000;16(5):500–505. doi: 10.1046/j.1469-0705.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 8.Tailor A., Jurkovic D., Bourne T.H., Natucci M., Collins W.P., Campbell S. Comparison of transvaginal color Doppler imaging and color Doppler energy for assessment of intraovarian blood flow. Obstet Gynecol. 1998;91(4):561–567. doi: 10.1016/s0029-7844(98)00037-4. [DOI] [PubMed] [Google Scholar]
- 9.Buy J.N., Ghossain M.A., Hugol D. Characterization of adnexal masses: combination of color Doppler and conventional sonography compared with spectral Doppler analysis alone and conventional sonography alone. AJR Am J Roentgenol. 1996 Feb;166(2):385–393. doi: 10.2214/ajr.166.2.8553953. [DOI] [PubMed] [Google Scholar]
- 10.Reles A., Wein U., Lichtenegger W. Transvaginal color Doppler sonography and conventional sonography in the preoperative assessment of adnexal masses. J Clin Ultrasound. 1997 Jun;25(5):217–225. doi: 10.1002/(sici)1097-0096(199706)25:5<217::aid-jcu1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Ordén M.R., Gudmundsson S., Kirkinen P. Contrast-enhanced sonography in the examination of benign and malignant adnexal masses. J Ultrasound Med. 2000;19(11):783–788. doi: 10.7863/jum.2000.19.11.783. [DOI] [PubMed] [Google Scholar]
- 12.Kinkel K., Hricak H., Lu Y., Tsuda K., Filly R.A. US characterization of ovarian masses: a meta analysis. Radiology. 2000;217(3):803–811. doi: 10.1148/radiology.217.3.r00dc20803. [DOI] [PubMed] [Google Scholar]
- 13.Testa A.C., Ferrandina G., Timmerman D. Imaging in gynecological disease (1): ultrasound features of metastases in the ovaries differ depending on the origin of the primary tumor. Ultrasound Obstet Gynecol. 2007;29:505–511. doi: 10.1002/uog.4020. [DOI] [PubMed] [Google Scholar]
- 14.Demidov V.N., Lipatenkova J., Vikhareva O. Imaging of gynecological disease (2): clinical and ultrasound characteristics of Sertoli cell tumors, Sertoli–Leydig cell tumors and Leydig cell tumors. Ultrasound Obstet Gynecol. 2008;31:85–91. doi: 10.1002/uog.5227. [DOI] [PubMed] [Google Scholar]
- 15.Van Holsbeke C., Domali E., Holland T.K. Imaging of gynecological disease (3): clinical and ultrasound characteristics of granulosa cell tumors of the ovary. Ultrasound Obstet Gynecol. 2008;31:450–456. doi: 10.1002/uog.5279. [DOI] [PubMed] [Google Scholar]
- 16.Savelli L., Testa A.C., Timmerman D. Imaging of gynecological disease (4): clinical and ultrasound characteristics of struma ovarii. Ultrasound Obstet Gynecol. 2008;32:210–219. doi: 10.1002/uog.5396. [DOI] [PubMed] [Google Scholar]
- 17.Paladini D., Testa A., Van Holsbeke C. Imaging of gynecological disease (5): clinical and ultrasound characteristics in fibroma and fibrotechoma of the ovary. Ultrasound Obstet Gynecol. 2009;34:188–195. doi: 10.1002/uog.6394. [DOI] [PubMed] [Google Scholar]
- 18.Timmerman D., Testa A.C., Bourn T. Simple ultrasound-based rules for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2008;31:681–690. doi: 10.1002/uog.5365. [DOI] [PubMed] [Google Scholar]
- 19.Mercè L.T., Caballero R.A., Barco M.J., Bau S., Lopez G. B-mode, utero-ovarian and intratumoral transvaginal colour Doppler ultrasonography for differential diagnosis of ovarian tumours. Eur J Obstet Gynecol Reprod Biol. 1998;76:97–107. doi: 10.1016/s0301-2115(97)00167-x. [DOI] [PubMed] [Google Scholar]
- 20.Marret H., Ecochard R., Giraudeau B., Golfier F., Raudrant D., Lansac J. Color Doppler energy prediction of malignancy in adnexal masses using logistic regression models. Ultrasound Obstet Gynecol. 2002;20(6):597–604. doi: 10.1046/j.1469-0705.2002.00853.x. [DOI] [PubMed] [Google Scholar]
- 21.Valentin L. Prospective cross-validation of Doppler ultrasound examination and gray-scale ultrasound imaging for discrimination of benign and malignant pelvic masses. Ultrasound Obstet Gynecol. 1999;14(4):273–283. doi: 10.1046/j.1469-0705.1999.14040273.x. [DOI] [PubMed] [Google Scholar]
- 22.Suren A., Osmers R., Kulenkampff D., Kuhn W. Visualization of blood flow in small ovarian tumor vessels by transvaginal color Doppler sonography after echo enhancement with injection of Levovist. Gynecol Obstet Invest. 1994;38(3):210–212. doi: 10.1159/000292481. [DOI] [PubMed] [Google Scholar]
- 23.Kupesic S., Kurjak A. Contrast-enhanced, three-dimensional power Doppler sonography for differentiation of adnexal masses. Obstet Gynecol. 2000;96(3):452–458. doi: 10.1016/s0029-7844(00)00923-6. [DOI] [PubMed] [Google Scholar]
- 24.Szymanski M., Szymanski W., Grabiec M., Korenkiewicz J. Evaluation of using Levovist in the differential diagnosis of ovarian tumors. Ginekol Pol. 1999;70(6):444–449. [PubMed] [Google Scholar]


