Abstract
Purpose
Intramuscular injection of botulinum toxin A (BTX-A) is a common treatment for iliopsoas muscle spasticity, but it is not easy to position the needle in this muscle without guidance. In this paper we describe an ultrasound-guided technique for the intramuscular injection of BTX-A to treat spasticity of the iliopsoas muscle. Its effectiveness was assessed in 10 patients.
Method and materials
The ultrasound-guided technique for BTX-A injection was used on 10 patients. The needle was inserted into the muscle belly at an angle of 45° along the longitudinal axis of the muscle when allowed by patient's condition.
Results
In all cases, the iliopsoas muscle was easily identified and both the iliac and psoas components were assessed. Introduction of the needle and drug injection were entirely carried out under ultrasonographic guidance. The procedure was successful in all patients, even in those with a high-grade spasticity, and general anesthesia was not required.
Conclusions
This ultrasound-guided technique allows accurate guidance for the injection of BTX-A, and it can be considered as an alternate supportive therapy in patients with spasticity and dystonia.
Keywords: Botulinum A toxin, Interventional ultrasonography, Injection, Iliopsoas muscle, Cerebral palsy
Sommario
Obiettivi
L'iniezione intramuscolare di tossina botulinica A (BTX-A) per il trattamento della spasticità del muscolo ileopsoas è tecnica consolidata, ma è gravata da un'alta difficoltà nel preciso posizionamento dell'ago. Scopo del nostro lavoro è descrivere una tecnica ecoguidata per l'introduzione intramuscolare di tossina botulinica nel trattamento della spasticità del muscolo ileopsoas e valutare l'efficacia terapeutica di questa terapia.
Materiali e metodi
Dieci pazienti con spasticità cronica del muscolo ileopsoas sono stati trattati mediante inoculazione ecoguidata intramuscolare di BTX-A. L'ago è stato introdotto in sede intramuscolare nel contesto del muscolo con un'inclinazione di 45° sull'asse longitudinale del muscolo stesso.
Risultati
In tutti i casi trattati, il muscolo ileopsoas è stato facilmente identificato e distinto nelle sue componenti iliaca e psoas. L'intera procedura, sia di introduzione dell'ago sia di Do not break this word. (iniezione) del farmaco nel sito corretto è stata monitorata ecograficamente. La procedura è stata eseguita con successo in tutti i casi, anche in quelli con intensa spasticità, senza ricorrere ad alcuna anestesia generale.
Conclusioni
La tecnica ecografica descritta consente una guida precisa alla corretta inoculazione del farmaco. L'iniezione ecoguidata di BTX-A può essere considerata una terapia alternativa di supporto nei pazienti con spasticità e distonia.
Introduction
Botulinum toxin is a protein produced by Clostridium botulinum, which inhibits muscle contraction by transiently blocking the release of acetylcholine at the neuromuscular junction. In nature, there are 7 botulinum toxins identified with capital letters from A to G. Only 3 of them (toxins A, B, and E) are capable of causing the botulinum intoxication syndrome, also known as botulism. This condition is characterized by flaccid paralysis of the striated muscles and blockade of the cholinergic vegetative functions. At a neuromuscular junction, the toxin inactivates some of the fusion proteins, such as SNAP-25, syntaxin or synaptobrevin, which are essential for cellular function. This process involves the temporary inhibition of presynaptic acetylcholine release; consequently, its effects are restricted to motor neurons that depend on cholinergic transmission (muscular plate, gland innervating cells) [1,2]. Injections of botulinum toxin A (BTX-A) have been shown to be effective [3–6] in the treatment of etiologically diverse types of muscle spasms. Correct placement of the needle within the muscular structure is essential to avoid adverse effects. Moreover, unless one is certain that the toxin has indeed been injected into the muscle poor clinical outcomes cannot be reliably attributed to a lack of response to BTX-A [6].
The purpose of this paper was to describe an ultrasound (US)-guided technique for the accurate injection of BTX-A and to assess the results achieved with this technique in patients with iliopsoas muscle spasticity.
Materials and methods
We treated 10 patients with spasticity of the iliopsoas muscle caused by infantile cerebral palsy (CP) in 4 cases (2 hemiplegic patients who were ambulatory and 2 nonambulatory patients, 1 diplegic and 1 paraparetic; mean age 13 ± 3.56 years); multiple sclerosis (MS) in 4 cases (all nonambulatory; mean age 44.75 ± 8.85 years); or spinal-cord trauma in 2 cases (both nonambulatory patients; mean age 32.50 ± 2.12 years). Spasticity was assessed with a Visual Analogue Scale (VAS) before and 40 days after the procedure [7].
After the neuromuscular blocking, patients underwent a 4-week rehabilitative treatment.
We used a 22-gauge spinal needle to inoculate 150 units of BTX-A with a single injection (Dysport®, Ipsen SA, France). The toxin was injected only when the needle was located in the correct position.
Technique
The iliopsoas is a deep muscle that is closely associated with the neurovascular bundle of the lower limb. For this reason, US guidance is mandatory for correct placement of the needle prior to the injection of BTX-A. Using the femoral head and the articular capsule as markers, the sonographer can clearly locate the distal portion of the iliopsoas and the neurovascular bundle, which lies medial to the muscle.
In this study, we used an anterior approach, with the patient in a supine position. The preferred site for BTX-A injections is the pre-insertional segment of the distal portion of the iliopsoas muscle, proximal to the myotendinous junction, beneath the inguinal ligament.
The needle is introduced under US guidance in a caudocranial direction at a 45° angle until its tip is correctly positioned in the muscle. No needle guide was needed. Nevertheless, in patients with severe spasticity, this approach is precluded by the strong hyperflexion of the thigh. In these cases, the drug should be injected into the most distal portion of the muscle possible; the needle is inserted in the axial plane with a lateromedial orientation.
US was performed with a Philips iU22 scanner (Koninklijke Philips Electronics N.V., The Netherlands) equipped with a 5–2 MHz convex transducer and a 12–5 MHz high-resolution linear broadband array transducer.
The study was conducted according to the principles of the Declaration of Helsinki. Informed consent was obtained from all patients.
Statistical analysis
Post-treatment variations in VAS scores were assessed with a non-parametric Mann–Whitney U test. We considered p values < 0.05 to be statistically significant.
Results
In all patients, the US examination allowed easy identification of the iliopsoas muscle and reliable guidance for needle insertion and BTX-A injection. All patients had good responses to the therapy. No peri- or post-procedural complications occurred (Figs. 1 and 2).
Fig. 1.
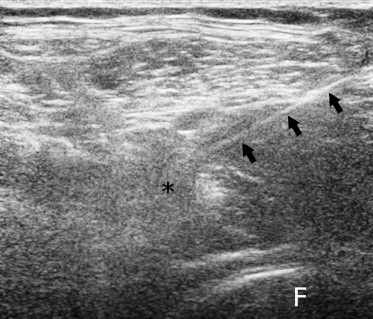
Ultrasonographic image obtained with a linear probe (12–5 MHz). The needle (arrows) has been inserted into the psoas muscle (asterisk). F = femoral head.
Fig. 2.
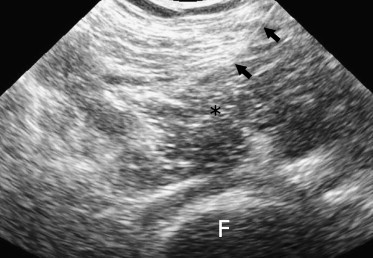
Ultrasonographic image obtained with a convex probe (5–2 MHz). The needle (arrows) has been inserted into the psoas muscle (asterisk). F = femoral head.
The patients who were ambulatory prior to treatment presented significantly improved hip kinetics and spatiotemporal walking parameters. The nonambulatory MS patients with postural alterations before the treatment showed significant improvement in the posture that facilitated nursing care.
Patients with spinal lesions experienced remarkable reductions in muscle spasticity, which translated into less pain, better sleep (which was no longer interrupted by spastic contractions), and a better quality of life.
All patients presented significantly reduced muscle tone with significant decreases (p < 0.01) in the VAS score (Table 1), increases in the passive range of motion of the hip, and a reduction of spasms.
Table 1.
Characteristics of the 10 patients with iliopsoas spasticity
| Age | Cause of spasticity | VAS (T = 0) | VAS = 40 days | |
|---|---|---|---|---|
| 1 | 8 | CP | 7.5 | 3.1 |
| 2 | 16 | CP | 5.4 | 2.1 |
| 3 | 13 | CP | 6.5 | 3.4 |
| 4 | 15 | CP | 6.5 | 2.9 |
| 5 | 57 | MS | 7.6 | 3.9 |
| 6 | 42 | MS | 7.4 | 3.4 |
| 7 | 36 | MS | 5.2 | 2.2 |
| 8 | 44 | MS | 7.9 | 2.8 |
| 9 | 34 | Spinal lesion | 6.9 | 2.8 |
| 10 | 31 | Spinal lesion | 5.9 | 1.9 |
VAS = Visual Analogue Scale, CP = cerebral palsy, MS = multiple sclerosis.
None of the patients required a second injection to achieve full therapeutic effects.
Rehabilitative treatments proved to be easier to administer after this treatment.
We did not perform any ultrasound assessments of the maximum level of efficacy of the injection, which was achieved 20–29 days after the injection (mean 24.1 ± 1.21 days). All patients were followed for 6 months after the treatment by monthly US scans. In all cases, satisfactory muscle relaxation was maintained for 3 months; in 4 patients the effects lasted 4 months, and 2 experienced beneficial effects for 6 months.
Discussion
Clostridium botulinum was discovered by Emile Pierre van Ermengem in 1895. The therapeutic use of botulinum toxin was first proposed in 1973 by Scott et al., who used the serotype A toxin to correct strabismus [8]. Injections of botulinum toxin are currently used to treat primary and secondary dystonia, post-stroke and post-traumatic spasticity, and cerebral palsy [4,5,9,10]. More recent applications include neuromuscular blockade of inappropriately contracted sphincters (e.g., associated with spasmodic dysphonia, anal spasms, vaginism, achalasia, and altered ocular movements such as those of nystagmus) [11–13] and of exocrine glands affected by autonomic hyperactivity (as in hyperhidrosis or sialorrhea) [14,15]. The toxin has also been successfully used in the treatment of pain associated with the myofascial pain syndrome, lumbar back pain, migraines, and tension headaches [16]. It is also widely used to eliminate facial wrinkles [17].
Though botulinum toxin is considered the most powerful poison in nature (a few nanograms are enough to kill an adult man), the therapeutic window is definitely safe: doses used in daily practice are 50–100 times lower than the lethal dose. Moreover, central nervous system side effects are unlikely since the toxin does not cross the brain-blood barrier. The widespread use and large-scale application of this toxin are basically justified by the absence of reports of significant side effects, the reversibility of its action, and the fact that the treatment can be repeated if necessary. BTX-A is preferred over surgical approaches because it can be used to produce calibrated states of muscle relaxation, and it does not produce permanent lesions. Patient satisfaction is usually high, and the cost of treatment is considerably lower than that based on conventional drug therapy [18]. In most cases, the BTX-A therapy was effective 2 or 3 days after the injection, and the maximum effect is observed after about 3 weeks. They last up to 3–4 months. After this period, the chemical denervation induced by the toxin is hindered by spontaneous neo-innervation. Some patients (2–3%) do not respond to the therapy, and 5–7% develop an antibody response to the A serotype of the toxin. In these cases, serotype B toxin is used. Its clinical effects are the same as those of BTX-A, but the doses used are different [19].
Spasticity of the iliopsoas muscle can cause several problems in patients undergoing rehabilitative therapy. In patients who are ambulatory, it alters hip kinematics during walking, hindering the physiologic extension that occurs during the central-late terminal period of the step. In non-ambulatory patients, iliopsoas spasticity can cause them to assume an incorrect posture in the wheelchair, and this can lead to myotendinous retraction that is difficult to reduce. These problems are even more serious if the spasticity also affects other muscle groups or both lower limbs.
Different techniques for the treatment of iliopsoas spasticity with BTX-A have been proposed. Ward [20] used palpation alone to position the needle (with an anterior or posterior approach). Willenborg et al. [21] used US to locate the muscle and verified the correct position of the needle by electrostimulation. The computed tomography-guided technique described in 2000 by Porta et al. had several disadvantages, including high costs, long examination time, use of ionizing radiation, and the need for sedation especially in young patients [22]. In 2003, Westhoff et al. [6] described a US-guided technique with an anterior approach, which allowed the needle to be positioned quickly, safely, and without the use of ionizing radiation.
Our experience indicates that the choice of whether to use a convex or linear US probe depends exclusively on the biometrical features of the patient. Although many difficulties can arise during the treatment of spastic patients, we did not use a needle guide for any of the procedures we performed. However, this aid may be helpful for less experienced operators. The pre- and post-treatment clinical evaluations included a preliminary physiatric examination, VAS assessment [7], evaluation of muscle tone (modified Ashworth scale) [23], assessments of the active and passive ranges of movement of the hip and of autonomy in activities of daily living (Barthel ADL index) [24,25]. The Ashworth and Barthel indexes are more relevant within the context of a broader program of rehabilitation. We preferred to limit our evaluation to the general improvement perceived by the patients and assessed with the VAS.
Conclusions
The US-guided technique for BTX-A treatment of iliopsoas spasticity allows the operator to verify the position of the needle prior to injection of the toxin. In this way, incorrect needle placement can be excluded as possible causes of nonresponse. This procedure is safe, rapid, and economical and it can be repeated, if needed, without the risk of overexposure to ionizing radiation. The position of the needle can also be continuously monitored during the injection, thus eliminating the risk of damage to the neurovascular bundle.
However, it must be stressed that this procedure should be performed only by expert radiologists who have received adequate training.
The US-guided intramuscular injection of BTX-A is an effective treatment for iliopsoas spasticity. It can be considered an effective form of supporting therapy in patients with spasticity and/or dystonia of the lower limbs. This treatment considerably reduces the discomfort associated with iliopsoas spasticity, and it can be used in association with conventional rehabilitative therapy.
Acknowledgements
The authors thank Mr. Gianluca Di Cara for his kind contribution to the translation of this paper.
Conflict of interest
The authors have no conflict of interest.
References
- 1.Erbguth F.J. From poison to remedy: the chequered history of botulinum toxin. J Neural Transm. 2008;115(4):559–565. doi: 10.1007/s00702-007-0728-2. [DOI] [PubMed] [Google Scholar]
- 2.Erbguth F.J. Historical notes on botulism, Clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin. Mov Disord. 2004;19(Suppl. 8):S2–S6. doi: 10.1002/mds.20003. [DOI] [PubMed] [Google Scholar]
- 3.Kessler K.R., Skutta M., Benecke R. Long-term treatment of cervical dystonia with botulinum toxin A: efficacy, safety, and antibody frequency. J Neurol. 1999;246:265–274. doi: 10.1007/s004150050345. [DOI] [PubMed] [Google Scholar]
- 4.Bakheit A.M., Severa S., Cosgrove A. Safety profile and efficacy of botulinum toxin A (Dysport) in children with muscle spasticity. Dev Med Child Neurol. 2001;43:234–238. doi: 10.1017/s0012162201000445. [DOI] [PubMed] [Google Scholar]
- 5.Kirschner J., Berweck S., Mall V., Korinthenbeg R., Heinen F. Botulinum toxin treatment in cerebral palsy: evidence for a new treatment option. J Neurol. 2001;248(Suppl. 1):28–30. doi: 10.1007/pl00007815. [DOI] [PubMed] [Google Scholar]
- 6.Westhoff B., Seller K., Wild A., Jaeger M., Krauspe R. Ultrasound-guided botulinum toxin injection technique for the iliopsoas muscle. Dev Med Child Neurol. 2003 Dec;45(12):829–832. doi: 10.1017/s0012162203001531. [DOI] [PubMed] [Google Scholar]
- 7.Vles G.F., de Louw A.J., Speth L.A. Visual Analogue Scale to score the effects of Botulinum Toxin A treatment in children with cerebral palsy in daily clinical practice. Eur J Paediatr Neurol. 2008;12(3):231–238. doi: 10.1016/j.ejpn.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Scott A.B., Rosenbaum A., Collins C.C. Pharmacologic weakening of extraocular muscles. Invest Ophthalmol. 1973;12:924–927. [PubMed] [Google Scholar]
- 9.Deleplanque B., Lagueny A., Flurin V. Botulinum toxin in the management of spastic hip adductors in non-ambulatory cerebral palsy children. Rev Chir Orthop Reparatrice Appar Mot. 2002;88:279–285. [PubMed] [Google Scholar]
- 10.Molenaers G., Eyssen M., Desloovere K., Jonkers I., De Cock P. A multilevel approach to botulinum toxin type A treatment of the (ilio)psoas in spasticity in cerebral palsy. Eur J Neurol. 1999;6(Suppl. 4):59–62. [Google Scholar]
- 11.Dutton J.J., Fowler A.M. Botulinum toxin in ophthalmology. Surv Ophtalmol. 2007;52(1):13–31. doi: 10.1016/j.survophthal.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Jones O.M. Towards safer treatments for benign anorectal disease: the pharmacological manipulation of the internal anal sphincter. Ann R Coll Surg Engl. 2007;89(6):574–579. doi: 10.1308/003588407X205576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Watts C.R., Truong D., Nye C. Evidence for the effectiveness of botulinum toxin for spasmodic dysphonia from high-quality research designs. J Neural Transm. 2008;115(4):625–630. doi: 10.1007/s00702-007-0757-x. [DOI] [PubMed] [Google Scholar]
- 14.Schnider P., Binder M., Kittler H., Steinhoff N., Auff E. Uses of botulinum toxin. Lancet. 1997;349:953. doi: 10.1016/S0140-6736(05)62729-8. [DOI] [PubMed] [Google Scholar]
- 15.Raval T.H., Elliott C.A. Botulinum toxin injection to the salivary glands for the treatment of sialorrhea with chronic aspiration. Ann Otol Rhinol Laryngol. 2008 Feb;117(2):118–122. doi: 10.1177/000348940811700209. [DOI] [PubMed] [Google Scholar]
- 16.Lew H.L., Lee E.H., Castaneda A., Klima R., Date E. Therapeutic use of botulinum toxin type A in treating neck and upper-back pain of myofascial origin: a pilot study. Arch Phys Med Rehabil. 2008 Jan;89(1):75–80. doi: 10.1016/j.apmr.2007.08.133. [DOI] [PubMed] [Google Scholar]
- 17.Sator P.G. Skin treatments and dermatological procedures to promote youthful skin. Clin Interv Aging. 2006;1(1):51–56. doi: 10.2147/ciia.2006.1.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinnet D. Botulinum toxin A injections in children: technique and dosing issues. Am J Phys Med Rehabil. 2004;83(Suppl. 10):s59–s64. doi: 10.1097/01.phm.0000141131.66648.e9. [DOI] [PubMed] [Google Scholar]
- 19.Costa J., Espírito-Santo C., Borges A. Botulinum toxin type B for cervical dystonia. Cochrane Database Syst Rev. 2005;(1) doi: 10.1002/14651858.CD004315.pub2. CD004315. [DOI] [PubMed] [Google Scholar]
- 20.Ward A.B. Botulinum toxin type A treatment of hip and thigh spasticity: a technique for injection of psoas major muscle. Eur J Neurol. 1999;6(Suppl. 4):91–93. [Google Scholar]
- 21.Willenborg M.J., Shilt J.S., Smitz B.P., Estrada R.L., Castle J.A., Koman L.A. Technique for iliopsoas ultrasound-guided active electromyography-directed botulinum A toxin injection in cerebral palsy. J Pediatr Orthop. 2002;22:165–168. [PubMed] [Google Scholar]
- 22.Porta M. A comparative trial of botulinum toxin type A and methylprednisolone for the treatment of myofascial pain syndrome and pain from chronic muscle spasm. Pain. 2000;85:101–105. doi: 10.1016/s0304-3959(99)00264-x. [DOI] [PubMed] [Google Scholar]
- 23.Bohannon R.W., Smith M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–207. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 24.Mahoney F.D., Barthel D.W. Functional evaluation: the Barthel index. MD State Med J. 1965;145:61–63. [PubMed] [Google Scholar]
- 25.Katz S., Ford A.B., Moskovitz R.W., Jackson B.A., Jaffe M.W. Studies of illness in the aged. The Index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]


