Abstract
Development and aging are often mirror image processes and this may be equally true in the effects of estradiol, a potent endogenous steroid regulating brain development as well as a therapeutic used to relieve the negative components of perimenopause. Both the developing and perimenopausal brain are characterized by a sensitive period of hormone responsiveness, and in both cases, the neurotransmitters GABA and glutamate, as well as synaptogenesis and cell proliferation are major hormone targets. This review compares and contrasts the effects of estradiol on the developing and aging brain and highlights new avenues of exploration and therapeutic development.
Keywords: Estradiol, Neurogenesis, Synaptogenesis, GABA, Glutamate, Sex differences
1. Introduction
A discussion of the relative merits of hormone replacement therapy (HRT) for postmenopausal women rarely includes a discussion of the organizational actions of steroids on the developing brain. Yet, there can be striking and possibly informative similarities between these two bookends of life. The majority of what we know about steroid hormones on the developing brain is in the context of sexual differentiation, in particular masculinization and defeminization of the male brain, whereas steroid hormone effects in the menopausal period are by definition restricted only to females. But this discrepancy may be more apparent than real when considering that the female brain develops by default largely in the absence of gonadal hormones, yet the developing female is exquisitely sensitive to exogenous hormone therapy. Likewise, the postmenopausal female brain exists in the absence of endogenously derived hormones, but can be exquisitely sensitive to exogenous hormone therapy. Do these two phases of hormone sensitivity have any relation to each other? Development and aging are often mirror images and by comparing and contrasting what we know about how steroids act to build a brain, we might better understand the cellular processes initiated when the aging brain is deprived of steroids. The goal of this review is to provide a comparison of the two processes in the hope of identifying fruitful areas of exploration and therapeutic exploitation.
2. Both development and menopause have a sensitive period for steroid hormone effects
The hallmark of steroid-mediate sexual differentiation of the brain is that it occurs only during a restricted developmental window (Fig. 1). Before or after that window, there is no appreciable effect of steroid treatment. Indeed, the sensitive period is operationally defined by the natural onset of gonadal secretions in embryonic males and the lost of sensitivity to exogenous hormone treatment in females. The robust and enduring consequences of developmental steroid hormones on adult physiology and behavior are referred to as an organizational action which then determines the activational actions later on. The best characterized example is the sexual differentiation of sex behavior. Males exposed to testosterone developmentally will be more likely to display male sex behavior as adults when exposed to testosterone again. Females, in contrast, are considered the default phenotype and it is the absence of exposure to steroids developmentally that organizes the brain to respond to estrogens and progestins in adulthood with a lowering of the threshold to elicit female sexual receptivity. If the male is deprived of androgen action during the critical period he will not respond to androgen supplied in adulthood, and if the female is treated with androgen she will not respond to estradiol and progesterone with female sexual behavior but will respond to androgen with an increased tendency to display male sexual behavior. In rodents, the sensitive period begins during the last few days of gestation, with copious production of testosterone by the male testis which remains elevated until shortly after birth, and ends at varying periods of time depending on the endpoint in question but is generally concluded by postnatal day 10 (see for review McCarthy, 2008). In non-human primates the sensitive period appears to be entirely prenatal, during the last third of pregnancy, with postnatal exogenous steroid being ineffective in altering the psychosexual differentiation of females (see for review Wallen and Baum, 2002; Wallen, 2005). We cannot know with certainty the sensitive period for brain sexual differentiation in humans, for obvious reasons, but inferential evidence is consistent with a prenatal onset as elevated androgens during gestation, due to conditions such as congenital adrenal hyperplasia (CAH), has effects interpreted by some to be evidence of brain masculinization (see Iijima et al., 2001; Puts et al., 2008). More difficult is determining an ending of the critical period in humans. Gonadal steroid levels are known to be elevated for a period of months postnatally, but then decline to undetectable until the onset of adrenarch and puberty a decade or so later (Adeyemo, 1993; Forest, 1979). Regardless, it is evident that the human brain is altered by steroid hormone exposure developmentally, one need only look at the long list of sex differences in the human brain (Hines, 2002), not all of which can be explained as a consequence of environment of rearing.
Fig. 1.
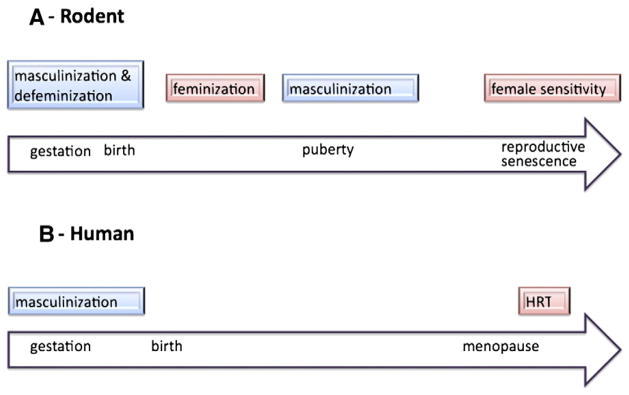
Sensitive periods of hormonal sensitivity across the lifespan. (A) In rodents, the developing brain is organized by steroid hormones during a perinatal sensitive window. The organizational actions of gonadal steroids derived from the testis in males serves to masculinize and defeminize the brain, which develops as female by default. In the absence of this early period of testicular secretions, there is a second active phase of brain feminization which involves estradiol derived from the later maturing ovary. Puberty serves as an additional finishing period for brain sexual differentiation, enhancing and maintaining the changes established during the organizational period of masculinization. Recently an additional late-in-life sensitive period has emerged that centers around reproductive senescence. During this time females remain sensitive to exogenous hormone, but outside of this window they become insensitive. (B) In humans two sensitive periods have been described, an organizational period that is largely prenatal and during which the brain is masculinized, and a perimenopausal window during which hormone replacement therapy provides largely beneficial effects as opposed to deleterious effects which can occur if therapy is initiated later. It is likely that humans also have additional sensitive periods for brain feminization and at puberty, but there is currently no experimental evidence to support or negate this view.
The concept of a developmental sensitive window for steroid hormone action has further expanded with the addition of two new phases; 1) a separate but later period for estradiol-induced feminization of the brain (Bakker and Baum, 2008), and 2) puberty as an additional organizational period of the male brain (Ahmed et al., 2008). Much less is known about these periods of development but what is emerging is consistent with the cellular changes known to occur during the perinatal sensitive period. Current parameters defining these and the original sensitive period are based on physiological and behavioral endpoints directly controlled by the hypothalamus and preoptic area. Assumptions that these same parameters apply to non-reproductive brain regions, such as the hippocampus, cortex and amygdala, have not been put to the test. Recent studies suggest an entirely different set of rules govern the effects of steroid hormones on the developing brain outside the context of reproduction, but it is still too early to elucidate clearly what those rules are. Nonetheless, since the primary interest in postmenopausal HRT is decidedly NOT related to reproductive capacity, but instead focuses on cognition, emotionality and neuroprotection, this brief review will focus on the relevant brain regions, most specifically the hippocampus as this is where the greatest amount of information is currently available.
Confusion and puzzlement following the negative results of the Women’s Health Initiative regarding neurological endpoints prompted a re-analyses and major rethinking of how steroid hormones, in particular estradiol, act on the aging brain. The startling contradiction between the overwhelmingly consistent preclinical data and prospective studies concluding that HRT was beneficial to cognitive function, emotionality and protection from Alzheimer’s disease seemed impossible to reconcile with the increased risks of stroke and dementia and no clear cognitive benefits observed in the largest double-blind placebo-controlled clinical trial of HRT ever attempted. But reconciliation is coming in the new concept that HRT must be initiated during a sensitive period during the early menopause. Increasing preclinical and clinical evidence suggests that a short period of hormone therapy can provide enduring benefits in both neuroprotection and enhanced cognition (Gibbs, 2010; Maki, 2006; Rodgers et al., 2010; Sherwin, 2007). The variables determining the onset and duration of this sensitive period are still being established, but changes in the amount of and sensitivity of the steroid receptors themselves appear to be a principal component (Rodgers et al., 2010). This may be partially a case of looking where the light is, as it is easy to quantify the amount of ER in a given brain region. Indeed, the study of all hormone sensitive critical periods has begun with an emphasis on the levels of the cognate receptors and ligands and only moved secondarily into discerning the signal transduction pathways which truly affect function. Commonalities between the basic parameters of the various sensitive periods may further provide insight into the detailed mechanisms. One domain in which it would be particularly useful to understand mechanism is the means by which the sensitive period ends, be it in the developing or aging brain. Why does the neural substrate of females become refractory to the masculinizing effects of estradiol around postnatal day 10, and similarly, why does the aging brain become refractory to the beneficial effects of estradiol if deprived for some period after menopause? At the moment we have no clear hypotheses for the loss of sensitivity during either time point, ideas about receptor downregulation notwithstanding.
3. Cellular changes induced by steroids are similar but also different during development and menopause
A primary interest in the actions of steroids during the postmenopausal period is to maintain or restore patterns of neurogenesis and synaptogenesis and to protect the brain in the event of injury. Among the principal actions of steroids on the developing brain are promotion of synaptogenesis, prevention of cell death, induction of neurogenesis and protection from injury (see for review McCarthy, 2008). But to simply highlight the similarities would be misleading, as the effects of steroids on the developing brain are complex, with opposing effects depending upon the brain region and or sex of the animal. Estradiol, the principal masculinizing hormone in rodents, both promotes and prevents cell death in the AVPV and SDN, respectively, and promotes and prevents synaptogenesis in the POA and ARC, respectively (Amateau and McCarthy, 2004; Davis et al., 1996; Mong et al., 1999; Waters and Simerly, 2009). In the hippocampus estradiol promotes neurogenesis by enhancing proliferation, but has no such effect in the hypothalamus or preoptic area (Zhang et al., 2008). Moreover, in our laboratory we find no evidence of an effect of estradiol on synaptogenesis in the neonatal hippocampus, but a principal action of estradiol in the mature and aging female hippocampus is promotion of new synapses (Woolley et al., 1990, 1996). The synaptogenic effects of estradiol in the hippocampus are at least in part dependent on an intact septohippocampal cholinergic innervation, suggesting that the steroid acts both within and outside the hippocampus to exert its effects (Hajszan et al., 2007; Leranth and Hajszan, 2007). Cholinergic input to the hippocampus is also critical to adult neurogenesis (Campbell et al., 2010) and this may also be true for the effects of estradiol on neurogenesis in the developing brain (Bowers et al., 2010). We are currently exploring this possibility. Interestingly, while estradiol promotes cell genesis in the adult hippocampus as well as the neonate, the sex of the animal in which the phenomenon is observed switches from young males to adult females. Thus there are many contradictions and similarities in estradiol action between the immature brain subject to organizational actions and the mature yet aging brain approaching reproductive senescence.
4. Estrogens modulate excitation and inhibition in the developing and aging brain
The appropriate balance of excitation and inhibition is critical to normal brain function and this is largely achieved by the opposing actions of two amino acid transmitters, excitatory glutamate and inhibitory GABA. Steroids, in particular estradiol, are potent regulators of both GABAergic and glutamatergic neurotransmission at multiple nodal points including synthesis and degradation of the ligand and the amount of receptor available for binding, ultimately enhancing or depressing the mode of action. The steroid sensitivity of the developing brain is greatly enhanced by higher levels of ER in both the cortex (Miranda and Toran-Allerand, 1992) and hippocampus (Solum and Handa, 2001), with maximal levels found shortly after birth and then declining precipitously and remaining low throughout adulthood.
4.1. Estradiol modulates GABA in the developing and aging brain via diverse mechanisms
GABA is the dominant inhibitory neurotransmitter in the mature brain and as such plays a critical role in virtually all synaptic and network activity. Dysregulation of GABA is an underlying variable in numerous neurologic and psychiatric disorders, and a contributing factor to the extent of damage following injury. Estradiol acts on the GABA system both early in development and in adulthood via both similar and diverse mechanisms (Fig. 2). In some instances estradiol is observed to increase both inhibitory GABA and excitatory glutamate; this seemingly contradictory effect of the same hormone provides contrast enhancement by simultaneously maximizing the boundaries of excitatory and inhibitory input into a specific system (Smith and Chapin, 1996).
Fig. 2.
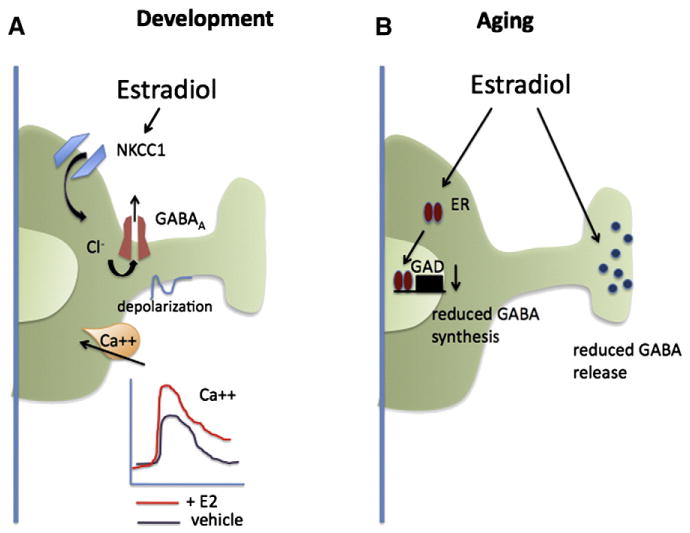
Estrogen modulation of GABAergic neurotransmission in developing and mature brain. The balance of excitation and inhibition is critical to both the developing and aging brain. Estradiol is a potent regulator of GABAergic neurotransmission and the manner of effects is largely consistent with increasing neuronal excitation, but this is achieved via distinct mechanisms in the developing versus mature brain. (A) In immature hippocampal neurons estradiol enhances the depolarizing actions of GABA by increasing the transmembrane chloride concentration gradient via increased activity of the ion co-transporter, NKCC1. The greater membrane depolarization achieved increases the magnitude of the calcium influx through voltage-gated calcium channels which impacts neuronal maturation and synaptogenesis. (B) In the adult hippocampus, estradiol downregulates GABA synthesis and retards vesicular trafficking to the synaptic terminal thereby reducing GABA release, except in CCK+ inhibitory interneurons where it has the opposite effect. The global impact of estradiol on adult hippocampal functioning is increased excitation, as evidenced by enhanced LTP and reduced seizure thresholds.
The role of GABA in the developing brain is fundamentally different from that in the mature and aging brain, being a major source of excitation instead of inhibition. Estradiol also enhances excitation in the developing brain by modulating GABA, but the effect is achieved indirectly via changes in the transmembrane chloride concentration gradient. The GABA-A receptor is a chloride ionophore which allows flux of chloride in either direction as a function of driving force and concentration. In mature neurons, extracellular chloride exceeds the levels inside the cell and the opening of the GABA-A channel results in chloride influx and hyperpolarization of the membrane, moving the cell away from the threshold for an action potential. The opposite situation exists in immature neurons, where intracellular chloride is sufficiently high that upon opening of the GABA-A channel this negative ion flows out of the cell and further depolarizes the membrane (see for review Sernagor et al., 2010). The change in membrane excitability is often sufficient to activate voltage-gated calcium channels, in particular the L-type, and initiate calcium influx. On occasion the membrane depolarization is strong enough to induce cell firing. The control of the chloride gradient is the function of two opposing transporters: NKCC1, which electroneutrally transports one Na+, one K+ and two Cl− ions into the cell, and KCC2 which transports an equal number of K+ and Cl− ions out of the cell. The relative activity of each transporter determines the amount of chloride allowed to accumulate inside the cell and thereby determines whether GABA action is excitatory or inhibitory upon binding to the GABA-A receptor. The expression of the two transporters is regulated developmentally such that NKCC1 is high in immature neurons and is gradually replaced by KCC2 as the neuron matures (Rivera et al., 1999; Stein et al., 2004). A few physiological regulators of the transporters have been identified, including injury (Kahle et al., 2008) and inflammation associated with chronic pain (Hasbargen et al., 2010), but estradiol has proved to be one of the most potent modulators of depolarizing GABA action. Both the magnitude of the calcium influx induced by depolarizing GABA and the developmental duration of depolarizing GABA responses are markedly enhanced by estradiol (Nunez et al., 2005; Nunez and McCarthy, 2008; Perrot-Sinal et al., 2001, 2003). The principal mechanism is enhanced activation of NKCC1, although effects on the voltage-gated calcium channel have also been identified.
The functional significance of estradiol-mediated enhancement of depolarizing GABA remains unclear. There is a role for depolarizing GABA in synaptic maturation (Ganguly et al., 2001), but this has not been tied to the effects of estradiol. In the adult hippocampus, depolarizing GABA is a component of neurogenesis by promoting proliferation and integration of newly born neurons into the neural network (Tozuka et al., 2005), but again this connection has not been made in the neonatal hippocampus (see further below). What has emerged regarding estradiol enhancement of depolarizing GABA is the potential for deleterious effects. Hypoxia/ischemia induces cell death via calcium-mediated excitotoxicity, and this is usually a consequence of elevated extrasynaptic glutamate. But GABA is also released during an anoxic event, and the magnitude of the calcium transient induced in a neonatal brain is sufficiently augmented in the presence of elevated estradiol to initiate excitotoxicity (Nunez et al., 2003a,b). The cell death induced is via classic apoptotic mechanisms and is prevented if the animal is pretreated with blockers of the voltage-gated calcium channel, the source of the excitotoxic calcium (Nunez and McCarthy, 2003, 2004). Thus, rather than being neuroprotective, in this paradigm estradiol is neurodamaging.
In the mature brain estradiol also modulates hippocampal GABAergic neurotransmission and in general the result is again enhanced excitation but in this instance it is due to disinhibition, via downregulation of either GAD (Murphy et al., 1998) or GABA release (Ledoux and Woolley, 2005; Rudick and Woolley, 2001). But the actions of estradiol on the mature hippocampus are mixed, with estradiol increasing GAD, GABA release and depolarizing GABA actions in some circumstances (Hart et al., 2007; Ikeda et al., 2006; Nakamura et al., 2004). Estrogen receptors in the dorsal hippocampus are predominantly expressed in a subset of GABAergic interneurons, whereas in the ventral hippocampus ER is found in both interneurons and principal cells (Yankova et al., 2001). There is even greater subdivision within the population of interneurons in the dorsal hippocampus where some inhibitory axon terminals contain vesicles co-localized with ER-alpha and which respond to estradiol by moving towards the readily releasable pool (Hart et al., 2007). This at first seems contradictory to the earlier observation that estradiol reduces GABA release but reflects a high degree of specialization as this response is observed only in a subset of inhibitory presynaptic boutons, those expressing cholecystokinin. These interneurons play specific roles in information processing by the hippocampus and the selective modulation by estradiol may underlie sex differences in cognition, stress responding or addiction.
4.2. Estradiol enhances glutamate action in the developing and mature brain
Compared to GABA, glutamatergic neurotransmission matures much later. Some forms of glutamate receptor are functional embryonically, but they do not reach full functionality until after the first week of postnatal life. There are two principal forms of glutamate receptor, the ionotropic and metabotropic, and each of these is further subdivided as a function of calcium permeability, G-protein coupling and intracellular localization. In the very young brain, glutamate-mediated excitotoxicity does not involve the NMDA receptor, as it does in the adult brain, but instead involves activation of mGluR and release of calcium from internal stores. Estradiol protects against this excitotoxicity by downregulating the mGluR and attenuating the amount of calcium entering the cell following glutamate (Hilton et al., 2006). The level of estradiol-mediated neuroprotection in a model system of cultured hippocampal neurons is profound, with a reduction in cell death of 100% by pretreatment with estradiol, far greater than that observed in adult models of estradiol neuroprotection. Nonetheless, estradiol is neuroprotective in many adult models of stroke and many other cellular mechanisms have been invoked and reviewed elsewhere (see for review Wise, 2003).
The most obvious of estradiol effects on glutamatergic transmission in the mature brain is the induction of new dendritic spine synapses, which are almost exclusively exacitatory (Woolley, 1999, 2007). The induction of these new synapses is part a consequence of downregulation of GABA and thus disinhibition (Murphy et al., 1998), but there are also direct effects of estradiol on the cytoskeleton of the dendritic spine itself (Kramar et al., 2009a,b). Estradiol also modulates glutamate transmission in the adult hippocampus via a complex relationship involving direct interactions between the subtype of ER and subtypes of mGluR (Boulware et al., 2005). These are membrane ER mediated effects, akin to the effects of ER on vesicle mobilization observed by Woolley and colleagues and highlight the critical role of non-classical steroid hormone action in the adult hippocampus and cortex. To date there is no evidence either in support of or against similar rapid effects of estradiol on the developing hippocampus. There may be a critical developmental shift from earlier dependence on classic genomic transcriptionally mediated effects of estradiol to later rapid membrane-associated signal transduction pathways. Strategies to recapitulate some of the earlier transcriptional effects may offer therapeutic benefits to the aging brain.
5. Estrogens enhance cell proliferation in the developing and mature hippocampus
Among the first sex differences observed in the brain were those in which a region was found to be larger in one sex versus the other. Shortly thereafter it was determined that robust volumetric sex differences, such as the song control nuclei in birds, or the spinal nucleus housing the motor neurons innervating the penis, where the result of differential cell death between males and females. Put simply, both sexes begin life with the same number of neurons, but they selectively die in one sex (usually females), due to a lack of the requisite steroid hormone (usually estradiol) required for survival (see for review McCarthy, 2008). Attempts generally failed to find sex differences in cell proliferation in brain regions with high degrees of sexual dimorphism in overall volume, at least that is until the search went outside the brain regions most closely tied to reproduction (Fig. 3). In the developing hippocampus, the rate of cell genesis is almost twice as high in males as females (Zhang et al., 2008), and the rate in females is raised to the level of males by exogenous treatment with estradiol. Moreover, the rate in males is substantially reduced, to even below that of females, by treatment with either an aromatase inhibitor or estrogen receptor (ER) antagonist (Bowers et al., 2010) When the cells born in response to estradiol are tracked out to 3 weeks of age, the majority of them have become neurons. Thus estradiol is a critical determinant of a sex difference in neurogenesis in the developing hippocampus. It is worth noting that the hippocampus is not twice as big in males, and so precisely what role these new neurons are playing in males remains unclear.
Fig. 3.
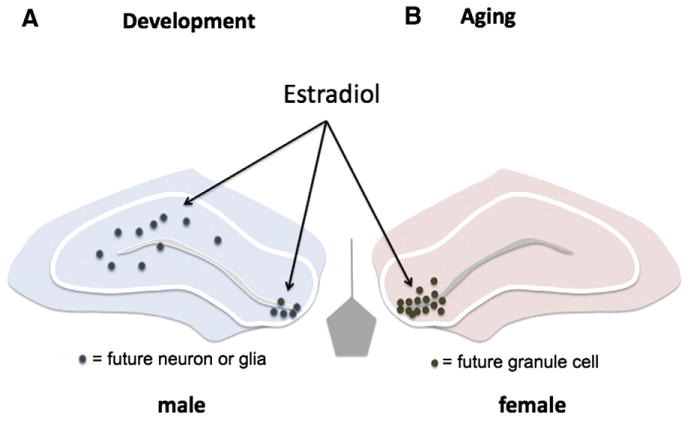
Estrogen promotes neurogenesis in the developing and adult brain. Active cell proliferation continues in the neuropil of the developing hippocampus during the early postnatal period but this is not true in the adult where neuronal birth is limited to the proliferative zone of the subgranular layer in the dentate gyrus. Estradiol promotes cell proliferation in both the developing and adult hippocampus, but the dominant effect is in males developmentally, whereas females are the responsive sex in adulthood. Whether there are different mechanisms regulating the two periods of neurogenesis in each sex is unknown.
Estradiol also modulates neurogenesis in the adult hippocampus, one of the few brain regions where cells continue to be born. The effects of estradiol are similar to that in development in that the primary effect is to promote proliferation, as opposed to effects on survival or differentiation (see for review Pawluski et al., 2009), but there are two important differences between the neurogenic effects of estradiol in the adult versus immature hippocampus. First is the location where new cells are being born. In the adult hippocampus new cells are born only in the proliferative zone of the granule cell layer in the dentate gyrus, and this is where estradiol exerts its effects (Barha et al., 2009). By contrast, in the developing hippocampus a sex difference in neurogenesis is observed within the neuropil proper of CA1 and CA3 as well as the dentate gyrus, and estradiol stimulates new cell genesis in all these areas. Thus there is potential for a far greater diversity of new cell types induced by estradiol in the developing as opposed to aging brain. The second difference in neurogenic estradiol between neonates and adults is the principal sex being affected; endogenous estradiol promotes cell proliferation in the developing male hippocampus but the mature female hippocampus. This may be a simple matter of when the ligand, estradiol, is available, being derived from testicular androgens in the neonate and ovarian secretions in the adult. Or, it may reflect a changing sensitivity to estradiol across the lifespan. Developing and adult females are highly sensitive to exogenous estradiol treatment, including the induction of neurogenesis in the young and synaptogenesis in the old, whereas mature males are insensitive to both the neurogenic (Spritzer and Galea, 2007) and synaptogenic effects of estradiol (Hajszan et al., 2008). An understanding of the cellular events organizing the male hippocampus could provide insight into the loss of sensitivity of aging female brains to exogenous estradiol after the close of the proposed perimenopausal sensitive period. In both the developing and mature hippocampus the effects of estradiol are ER mediated. Additional evidence in the adult supports a neuroproliferative role for the alpha-isoform of estradiol, 17α-estradiol (Barha et al., 2009), which is largely inactive at canonical ER but proposed to be the endogenous ligand for ER-X (Toran-Allerand, 2004), a putative plasma membrane-associated receptor expressed at high levels in the early postnatal cortex but not the adult. Injury to the adult female brain recapitulates development by upregulating both ER-α and ER-X expression, suggesting a potential therapeutic point of entry to protect the aging female brain.
The mechanism(s) underlying estradiol-induced neurogenesis in either the developing or mature brain is unknown yet of profound importance. Are the mechanism(s) the same or different? Excitation was identified as a critical component of successful adult neurogenesis over a decade ago (Cameron et al., 1998a, 1998b) and confirmed many times since. The many variables that regulate neurogenesis, including exercise, an enriched environment, singing (birds), pregnancy and others have all been mechanistically linked to excitation. The early emphasis was on glutamate, with a clear role for NMDA receptor activation (Deisseroth et al., 2004), but more recent studies have identified a critical contribution of depolarizing GABA and the opening of L-type voltage-gated calcium channels (Liu et al., 2005; Tozuka et al., 2005). In the adult there is a two-step phase of cell genesis, the radial glial-like cells which have stem cell-like properties which give way to transiently amplifying neuronal progenitor cells with robust proliferative activity (Fukuda et al., 2003; Namba et al., 2005). It is the latter cell type that is the primary target of depolarizing GABA. Depolarization of these proliferating cells induces expression of the pro-neuronal differentiation protein, NeuroD, and suppresses pro-glial differentiation genes (Deisseroth et al., 2004). However, unlike in the embryonic or neonatal brain, GABA activation of these cells in the adult is not via tonic release but is instead the result of innervation of the progenitor cells from the surrounding neural circuitry (Tozuka et al., 2005). As discussed above, estradiol enhances depolarizing GABA action in the developing hippocampus, raising the intriguing possibility that this may be a primary mechanism by which neurogenesis is increased in the neonatal male versus female hippocampus. Moreover, it is possible estradiol also promotes local depolarizing GABA action in proliferative cells of the mature hippocampus, thereby increasing both the rate and survival of new neurons. To our knowledge there have been no attempts to link the actions of estradiol on neurogenesis in the adult hippocampus to enhanced depolarizing GABA but this seems like a potential worth exploring.
6. Estrogens may be synthesized de novo in both the developing and mature hippocampus
Steroid hormones are synthesized on demand by two principal endocrine organs, the gonads and the adrenals. Cholesterol is the universal precursor and sequential enzymatic modifications generally reduce the size of the molecule while retaining the basic 3-hexagonal and 1 pentagon ring structure. Aromatization of androgens to estrogens is a penultimate step prior to further modifications which render the molecules largely inactive, water soluble and readily secreted from the body. That the brain is a major site for aromatization of androgens to estrogens has been long established, playing a critical role in the process of developmental masculinization in rodents (McEwen et al., 1977; Whalen and Olsen, 1981). Lower rates of aromatization continue in the adult male brain, with local estradiol production contributing to the control of male sexual behavior (Vagell and McGinnis, 1997). Levels of the aromatase enzyme and its end product estradiol are exceedingly low in both the developing and adult hippocampus compared to reproductively relevant brain regions (Lauber and Lichtensteiger, 1994; Lephart et al., 1992; Maclusky et al., 1994; Roselli et al., 1984; Wagner and Morrell, 1995). There is an emerging consensus that at least some of the estradiol detected in the hippocampus is locally synthesized de novo from cholesterol. All of the necessary synthetic enzymes have been detected (it requires at least 7 reactions to convert estradiol into cholesterol), and aromatase activity is responsive to physiological changes (Hojo et al., 2004). Most compellingly, antagonizing either ER or aromatase in culture dishes of hippocampal neurons has effects that are consistent with reducing estradiol action, this when all potential non-neuronal sources of estradiol have been eliminated (Nunez and McCarthy, 2008; Prange-Kiel et al., 2003). The majority of research emphasis has been on the significance of de novo estradiol synthesis to the adult brain, yet the use of neuronal cultures is de facto a study of development; thus, it seems likely this phenomenon occurs in both the immature and mature hippocampus. However, it is also evident that not all estradiol-mediated effects on the hippocampus are the result of de novo synthesis, unless one proposes that profound changes following ovariectomy are secondary to a loss of local estradiol production in the hippocampus. There is no evidence that would currently support or negate this view and a future challenge to the field is a reconciliation of the various roles played by gonadal versus neuronal estradiol synthesis on hippocampal functioning.
7. Conclusions
The brain is a major target organ of steroid hormone action, but the role and effectiveness of steroids varies dramatically across the lifespan and between males and females. Common to each phase of life is the occurrence of a sensitive window during which steroid effects are particularly pronounced. This may be due to increased sensitivity, elevated levels of the steroid or increased numbers of receptors. In general steroids, in particular estradiol, are potent trophic factors which increase neurogenesis, cell survival, neurite outgrowth and synaptogenesis. Many of the deleterious consequences of hormone deprivation during the postmenopausal period may be due to the lack of these beneficial actions of estradiol. By understanding in greater detail the mechanisms of estradiol action on the developing brain, we may reveal new methods by which designer steroids can be exploited to provide the beneficial effects without incurring the deleterious costs of extensive hormone exposure. Moreover, the greater neuroprotective effect of estradiol observed in the developing compared to injured mature brain may also offer new insights into how this naturally occurring endogenous and potent compound can be exploited for greater benefit.
References
- Adeyemo OaJH. Plasma progesterone, estradiol 17-beta and tesosterone in maternal and cord blood, and maternal human chorionic gonadotropin at parturition. Afr J Med Med Sci. 1993;22:55–60. [PubMed] [Google Scholar]
- Ahmed EI, Zehr JL, Schulz KM, Lorenz BH, Doncarlos LL, Sisk CL. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amateau SK, McCarthy MM. Induction of PGE(2) by estradiol mediates developmental masculinization of sex behavior. Nat Neurosci. 2004;7:643–650. doi: 10.1038/nn1254. [DOI] [PubMed] [Google Scholar]
- Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Galea LA. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. J Neuroendocrinol. 2009;21:155–166. doi: 10.1111/j.1365-2826.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers J, Waddell J, McCarthy M. A developmental sex difference in hippocampal neurogenesis is mediated by endogenous estradiol. Bio Sex Differ. 2010;1:8. doi: 10.1186/2042-6410-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998a;36:287–306. [PubMed] [Google Scholar]
- Cameron HA, Tanapat P, Gould E. Adrenal steroids and N-methyl-D-aspartate receptor activation regulate neurogenesis in the dentate gyrus of adult rats through a common pathway. Neuroscience. 1998b;82:349–354. doi: 10.1016/s0306-4522(97)00303-5. [DOI] [PubMed] [Google Scholar]
- Campbell NR, Fernandes CC, Halff AW, Berg DK. Endogenous signaling through alpha7-containing nicotinic receptors promotes maturation and integration of adult-born neurons in the hippocampus. J Neurosci. 2010;30:8734–8744. doi: 10.1523/JNEUROSCI.0931-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, Malenka RC. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- Forest MG. Plasma androgens (testosterone and 4-androstenedione) and 17-hydroxyprogesterone in the neonatal, prepubertal and peripubertal periods in the human and the rat: differences between species. J Steroid Biochem. 1979;11:543–548. doi: 10.1016/0022-4731(79)90080-3. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Kato F, Tozuka Y, Yamaguchi M, Miyamoto Y, Hisatsune T. Two distinct subpopulations of nestin-positive cells in adult mouse dentate gyrus. J Neurosci. 2003;23:9357–9366. doi: 10.1523/JNEUROSCI.23-28-09357.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Does short-term estrogen therapy produce lasting benefits in brain? Endocrinology. 2010;151:843–845. doi: 10.1210/en.2009-1453. [DOI] [PubMed] [Google Scholar]
- Hajszan T, Milner TA, Leranth C. Sex steroids and the dentate gyrus. Prog Brain Res. 2007;163:399–415. doi: 10.1016/S0079-6123(07)63023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Role of androgens and the androgen receptor in remodeling of spine synapses in limbic brain areas. Horm Behav. 2008;53:638–646. doi: 10.1016/j.yhbeh.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbargen T, Ahmed MM, Miranpuri G, Li L, Kahle KT, Resnick D, Sun D. Role of NKCC1 and KCC2 in the development of chronic neuropathic pain following spinal cord injury. Ann NY Acad Sci. 2010;1198:168–172. doi: 10.1111/j.1749-6632.2010.05462.x. [DOI] [PubMed] [Google Scholar]
- Hilton GD, Nunez JL, Bambrick L, Thompson SM, McCarthy MM. Glutamate-mediated excitotoxicity in neonatal hippocampal neurons is mediated by mGluR-induced release of Ca++ from intracellular stores and is prevented by estradiol. Eur J Neurosci. 2006;24:3008–3016. doi: 10.1111/j.1460-9568.2006.05189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M. Sexual differentiation of human brain and behavior. In: Pfaff D, editor. Hormones, Brain and Behavior. Vol. 5. Academic Press; London, UK: 2002. pp. 425–462. [Google Scholar]
- Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P4. 5017a. lpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima M, Arisaka O, Minamoto F, Arai Y. Sex differences in children’s free drawings: a study on girls with congenital adrenal hyperplasia. Horm Behav. 2001;40:99–104. doi: 10.1006/hbeh.2001.1670. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Matsuki N, Yamada MK. Estrogen produced in cultured hippocampal neurons is a functional regulator of a GABAergic machinery. J Neurosci Res. 2006;84:1771–1777. doi: 10.1002/jnr.21083. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4:490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009a;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramar EA, Chen LY, Rex CS, Gall CM, Lynch G. Estrogen’s place in the family of synaptic modulators. Mol Cell Pharmacol. 2009b;1:258–262. [PMC free article] [PubMed] [Google Scholar]
- Lauber ME, Lichtensteiger W. Pre-and postnatal ontogeny of aromatase cytochrome p450 messenger ribonucleic acid expression in the male rat brain studied by in situ hybridization. Endocrinology. 1994;135:1661–1668. doi: 10.1210/endo.135.4.7925130. [DOI] [PubMed] [Google Scholar]
- Ledoux VA, Woolley CS. Evidence that disinhibition is associated with a decrease in number of vesicles available for release at inhibitory synapses. J Neurosci. 2005;25:971–976. doi: 10.1523/JNEUROSCI.3489-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lephart ED, Simpson ER, McPhaul MJ, Kilgore MW, Wilson JD, Ojeda SR. Brain aromatase cytochrome P-450 messenger RNA levels and enzyme activity during prenatal and perinatal development in the rat. Brain Res Mol Brain Res. 1992;16:187–192. doi: 10.1016/0169-328x(92)90224-y. [DOI] [PubMed] [Google Scholar]
- Leranth C, Hajszan T. Extrinsic afferent systems to the dentate gyrus. Prog Brain Res. 2007;163:63–84. doi: 10.1016/S0079-6123(07)63004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang Q, Haydar TF, Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclusky NJ, Walters MJ, Clark AS, Toran-Allerand CD. Aromatase in the cerebral cortex, hippocampus, and mid-brain: ontogeny and developmental implications. Mol Cell Neurosci. 1994;5:691–698. doi: 10.1006/mcne.1994.1083. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and cognitive function: is there a critical period for benefit? Neuroscience. 2006;138:1027–1030. doi: 10.1016/j.neuroscience.2006.01.001. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9:249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Miranda RC, Toran-Allerand CD. Developmental expression of estrogen receptor mRNA in the rat cerebral cortex: a nonisotopic in situ hybridization histochemistry study. Cereb Cortex. 1992;2:1–15. doi: 10.1093/cercor/2.1.1. [DOI] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura NH, Rosell DR, Akama KT, McEwen BS. Estrogen and ovariectomy regulate mRNA and protein of glutamic acid decarboxylases and cation-chloride cotransporters in the adult rat hippocampus. Neuroendocrinology. 2004;80:308–323. doi: 10.1159/000083657. [DOI] [PubMed] [Google Scholar]
- Namba T, Mochizuki H, Onodera M, Mizuno Y, Namiki H, Seki T. The fate of neural progenitor cells expressing astrocytic and radial glial markers in the postnatal rat dentate gyrus. Eur J Neurosci. 2005;22:1928–1941. doi: 10.1111/j.1460-9568.2005.04396.x. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Alt J, McCarthy MM. A new model for prenatal brain damage. I GABAA receptor activation induces cell death in developing rat hippocampus. Exp Neurol. 2003a;181:258–269. doi: 10.3201/eid0906.030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, Alt J, McCarthy MM. A new model for prenatal brain damage. II Long-term deficits in hippocampal cell number and hippocampal dependent behavior following neonatal GABAA receptor activation. Exp Neurol. 2003b;181:270–280. doi: 10.3201/eid0906.020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Estradiol exacerbates hippocampal damage in a model of preterm brain injury. Endocrinology. 2003;144:2350–2359. doi: 10.1210/en.2002-220840. [DOI] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Cell death in the rat hippocampus in a model of prenatal brain injury: time course and expression of death related proteins. Neuroscience. 2004;129:393–402. doi: 10.1016/j.neuroscience.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Nunez JL, Bambrick LL, Krueger BK, McCarthy MM. Prolongation and enhancement of gamma-aminobutyric acid receptor mediated excitation by chronic treatment with estradiol in developing rat hippocampal neurons. Eur J Neurosci. 2005;21:3251–3261. doi: 10.1111/j.1460-9568.2005.04175.x. [DOI] [PubMed] [Google Scholar]
- Nunez JL, McCarthy MM. Resting intracellular calcium concentration, depolarizing GABA and possible role of local estradiol synthesis in the developing male and female hippocampus. Neuroscience. 2008;158:623–634. doi: 10.1016/j.neuroscience.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009;30:343–357. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Auger AP, McCarthy MM. Excitatory actions of GABA in developing brain are mediated by L-type Ca2+ channels and dependent on age, sex and brain region. Neuroscience. 2003;116:995–1003. doi: 10.1016/s0306-4522(02)00794-7. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal TS, Davis AM, Gregerson KA, Kao JPY, McCarthy MM. Estradiol enhances excitatory gamma-aminobutyric acid-mediated calcium signaling in neonatal hypothalamic neurons. Endocrinology. 2001;143:2238–2243. doi: 10.1210/endo.142.6.8180. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Wehrenberg U, Jarry H, Rune GM. Para/Autocrine regulation of estrogen receptors in hippocampal neurons. Hippocampus. 2003;13:226–234. doi: 10.1002/hipo.10075. [DOI] [PubMed] [Google Scholar]
- Puts DA, McDaniel MA, Jordan CL, Breedlove SM. Spatial ability and prenatal androgens: meta-analyses of congenital adrenal hyperplasia and digit ratio (2D:4D) studies. Arch Sex Behav. 2008;37:100–111. doi: 10.1007/s10508-007-9271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation [see comments] Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rodgers SP, Bohacek J, Daniel JM. Transient estradiol exposure during middle age in ovariectomized rats exerts lasting effects on cognitive function and the hippocampus. Endocrinology. 2010;151:1194–1203. doi: 10.1210/en.2009-1245. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Ellinwood WE, Resko JA. Regulation of brain aromatase activity in rats. Endocrinology. 1984;114:192–200. doi: 10.1210/endo-114-1-192. [DOI] [PubMed] [Google Scholar]
- Rudick CN, Woolley CS. Estrogen regulates functional inhibition of hippocampal CA1 pyramidal cells in the adult female rat. J Neurosci. 2001;21:6532–6543. doi: 10.1523/JNEUROSCI.21-17-06532.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sernagor E, Chabrol F, Bony G, Cancedda L. GABAergic control of neurite outgrowth and remodeling during development and adult neurogenesis: general rules and differences in diverse systems. Front Cell Neurosci. 2010;4:11. doi: 10.3389/fncel.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. The critical period hypothesis: can it explain discrepancies in the oestrogen-cognition literature? J Neuroendocrinol. 2007;19:77–81. doi: 10.1111/j.1365-2826.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- Smith SS, Chapin JK. The estrous cycle and the olivo-cerebellar circuit. I Contrast enhancement of sensorimotor-correlated cerebellar discharge. Exp Brain Res. 1996;111:371–384. doi: 10.1007/BF00228726. [DOI] [PubMed] [Google Scholar]
- Solum DT, Handa RJ. Localization of estrogen receptor alpha (ER alpha) in pyramidal neurons of the developing rat hippocampus. Brain Res Dev Brain Res. 2001;128:165–175. doi: 10.1016/s0165-3806(01)00171-7. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Galea LA. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol. 2007;67:1321–1333. doi: 10.1002/dneu.20457. [DOI] [PubMed] [Google Scholar]
- Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KCl cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J Comp Neurol. 2004;468:57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand CD. Minireview: A plethora of estrogen receptors in the brain: where will it end? Endocrinology. 2004;145:1069–1074. doi: 10.1210/en.2003-1462. [DOI] [PubMed] [Google Scholar]
- Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Vagell ME, McGinnis MY. The role of aromatization in the restoration of male rat reproductive behavior. J Neuroendocrinol. 1997;9:415–421. doi: 10.1046/j.1365-2826.1997.00598.x. [DOI] [PubMed] [Google Scholar]
- Wagner C, Morrell JI. Distribution and steroid hormone regulation of aromatase MRNA expression in the forebrain of adult male and female rats: a cellular -level analysis using in situ hybridization. J Comp Neurol. 1995;370:71–84. doi: 10.1002/(SICI)1096-9861(19960617)370:1<71::AID-CNE7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wallen K, Baum MJ. Masculinization and defeminization in altricial and precocial mammals: comparative aspects of steroid hormone action. In: Pfaff D, editor. Hormones Brain and Behavior. Vol. 5. Academic Press; London, UK: 2002. pp. 385–424. [Google Scholar]
- Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Waters EM, Simerly RB. Estrogen induces caspase-dependent cell death during hypothalamic development. J Neurosci. 2009;29:9714–9718. doi: 10.1523/JNEUROSCI.0135-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen RE, Olsen KL. Role of aromatization in sexual differentiation: effects of prenatal ATD treatment and neonatal castration. Horm Behav. 1981;15:107–122. doi: 10.1016/0018-506x(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Wise PM. Estrogens: protective or risk factors in brain function? Prog Neurobiol. 2003;69:181–191. doi: 10.1016/s0301-0082(03)00035-2. [DOI] [PubMed] [Google Scholar]
- Woolley C, Wenzel HJ, Schawartzkroin PA. Estradiol increases the frequency of multiple synapse boutons in the hippocampal CA1 region of the adult female rat. J Comp Neurol. 1996;373:108–117. doi: 10.1002/(SICI)1096-9861(19960909)373:1<108::AID-CNE9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS. Effects of estrogen in the CNS. Curr Opin Neurobiol. 1999;9:349–354. doi: 10.1016/s0959-4388(99)80051-8. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci USA. 2001;98:3525–3530. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JM, Konkle ATM, Zup SL, McCarthy MM. Impact of sex and hormones on new cells in the developing rat hippocampus: a novel source of sex dimorphism? Eur J Neurosci. 2008;27:791–800. doi: 10.1111/j.1460-9568.2008.06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


