Abstract
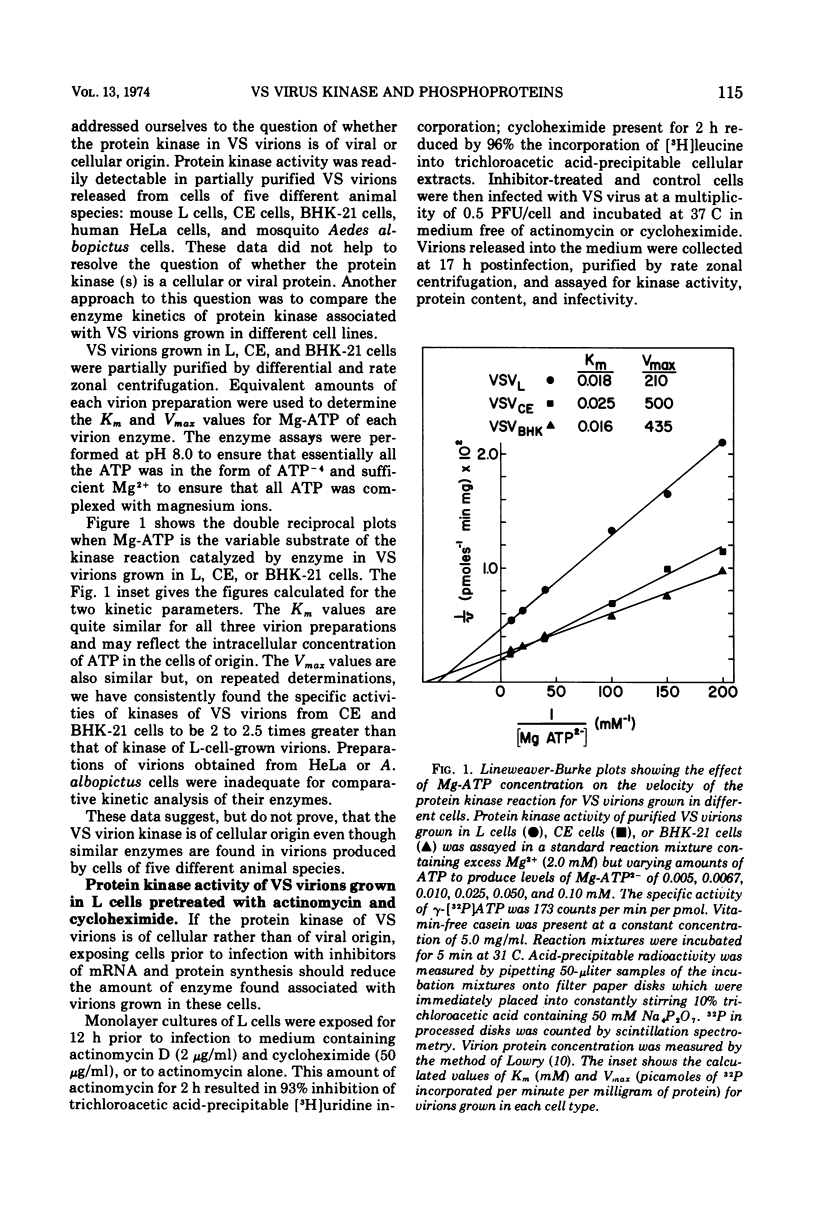
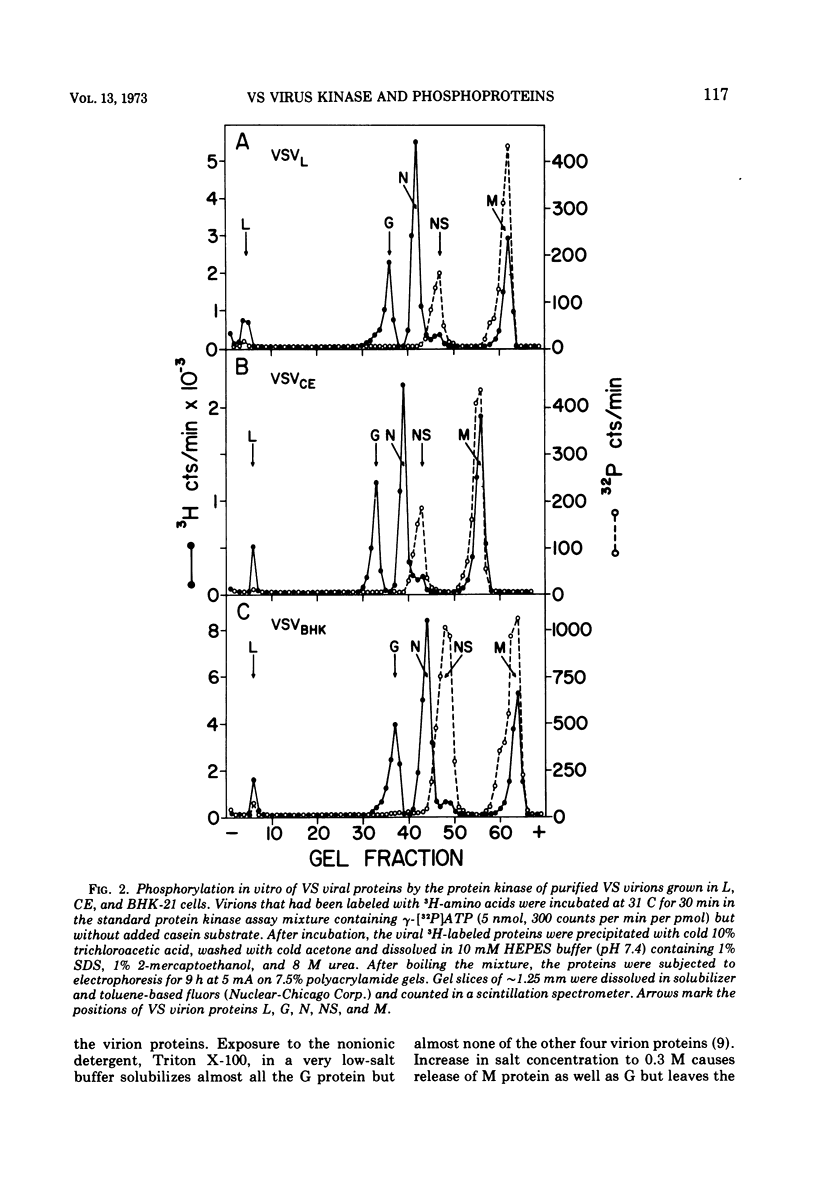
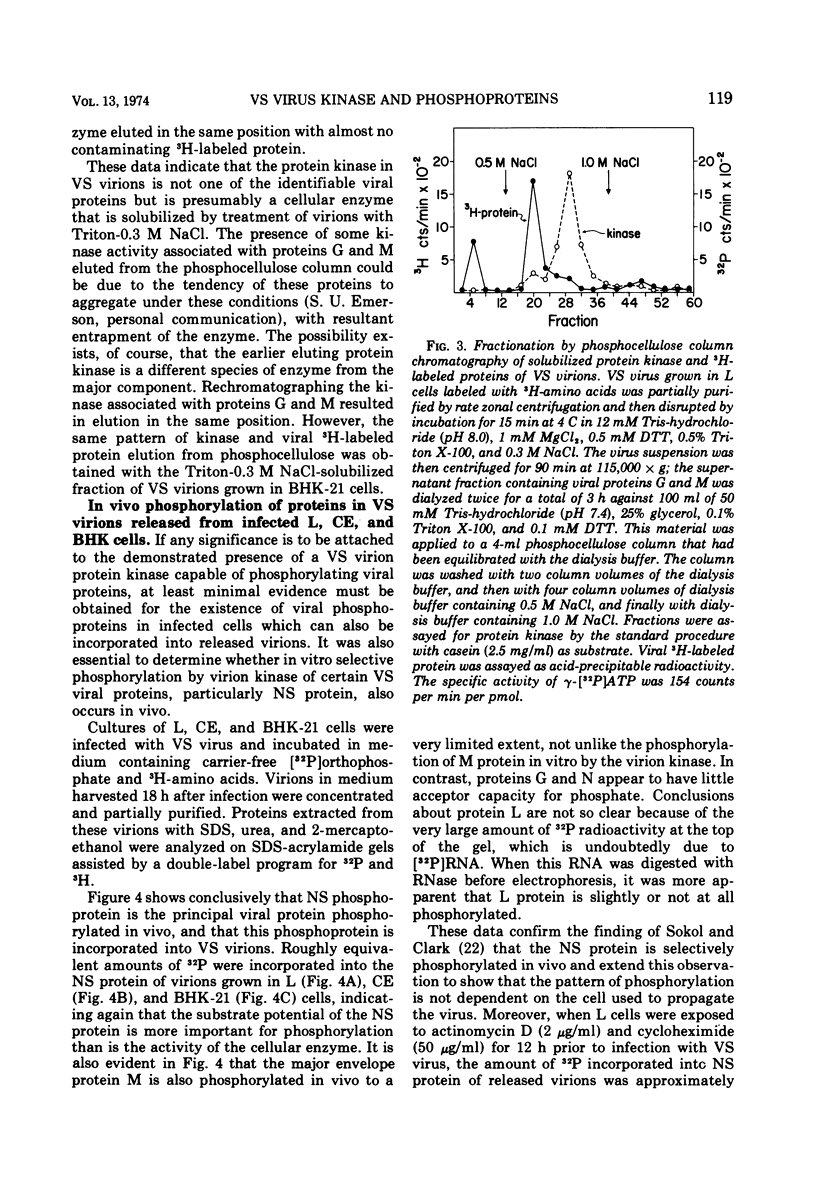
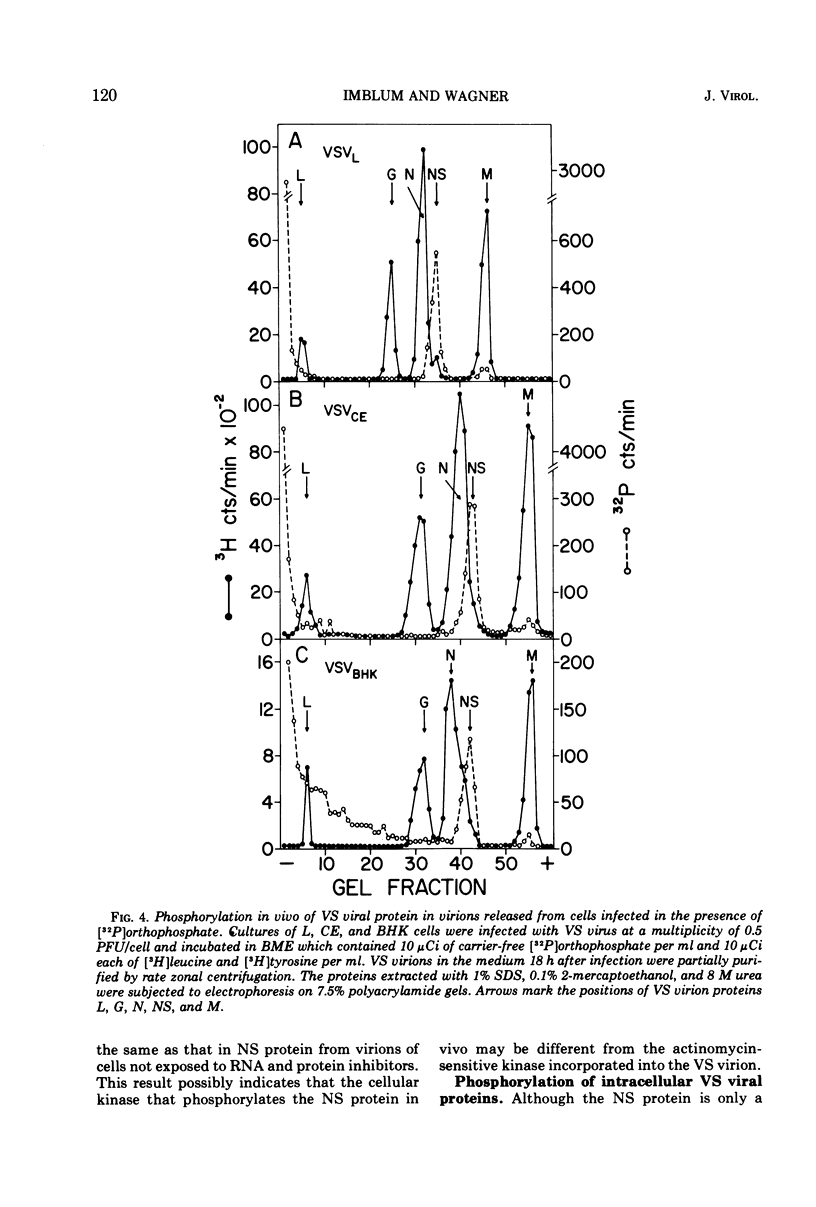
Protein kinases of similar but not identical activity were found associated with vesicular stomatitis (VS) virions grown in mouse L cells, primary chicken embryo (CE) cells, and BHK-21 cells, as well as being present in VS virions grown in HeLa and Aedes albopictus cells. The virion kinase preferentially phosphorylated the nucleocapsid NS protein in vitro and to a lesser extent the envelope M protein. Other virion proteins were phosphorylated in vitro only after drastic detergent treatment. Partial evidence that the virion kinase is of cellular origin was obtained by finding reduced enzyme activity in virions released from cells pretreated with actinomycin D and cycloheximide. Selective detergent and detergent-salt fractionation of VS virions revealed that the kinase activity was present in the envelope but not the spikes. The virion kinase activity in a Triton-salt-solubilized envelope fraction could be separated from M and G proteins and partially purified by phosphocellulose column chromatography. Virions released from L, CE, and BHK-21 cells infected in the presence of [32P]orthophosphate were labeled almost exclusively in the NS protein. Both soluble and nucleocapsid-associated NS phosphoprotein were present in cytoplasmic extracts of VS viral-infected L cells. The origin and function of the NS phosphoprotein remain to be elucidated.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cartwright B., Smale C. J., Brown F., Hull R. Model for vesicular stomatitis virus. J Virol. 1972 Aug;10(2):256–260. doi: 10.1128/jvi.10.2.256-260.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972 Aug;10(2):297–309. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. L protein requirement for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1973 Dec;12(6):1325–1335. doi: 10.1128/jvi.12.6.1325-1335.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravell M., Cromeans T. L. Viron-associated protein kinase and its involvement in nongenetic reactivation of frog polyhedral cytoplasmic deoxyribovirus. Virology. 1972 Jun;48(3):847–851. doi: 10.1016/0042-6822(72)90167-5. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Twiddy E., Gilden R. V. Protein kinase associated with RNA tumor viruses and other budding RNA viruses. Virology. 1972 Feb;47(2):536–538. doi: 10.1016/0042-6822(72)90297-8. [DOI] [PubMed] [Google Scholar]
- Heine J. W., Schnaitman C. A. Entry of vesicular stomatitis virus into L cells. J Virol. 1971 Nov;8(5):786–795. doi: 10.1128/jvi.8.5.786-795.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley J. M., Emerson S. U., Wagner R. R. The glycoprotein of vesicular stomatitis virus is the antigen that gives rise to and reacts with neutralizing antibody. J Virol. 1972 Dec;10(6):1231–1235. doi: 10.1128/jvi.10.6.1231-1235.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martelo O. J., Woo S. L., Reimann E. M., Davie E. W. Effect of protein kinase on ribonucleic acid polymerase. Biochemistry. 1970 Nov 24;9(24):4807–4813. doi: 10.1021/bi00826a027. [DOI] [PubMed] [Google Scholar]
- McSharry J. J., Wagner R. R. Lipid composition of purified vesicular stomatitis viruses. J Virol. 1971 Jan;7(1):59–70. doi: 10.1128/jvi.7.1.59-70.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A., Summers D. F. Protein synthesis in vesicular stomatitis virus-infected HeLa cells. Virology. 1970 Oct;42(2):328–340. doi: 10.1016/0042-6822(70)90277-1. [DOI] [PubMed] [Google Scholar]
- Paoletti E., Moss B. Protein kinase and specific phosphate acceptor proteins associated with vaccinia virus cores. J Virol. 1972 Sep;10(3):417–424. doi: 10.1128/jvi.10.3.417-424.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penman S., Scherrer K., Becker Y., Darnell J. E. POLYRIBOSOMES IN NORMAL AND POLIOVIRUS-INFECTED HELA CELLS AND THEIR RELATIONSHIP TO MESSENGER-RNA. Proc Natl Acad Sci U S A. 1963 May;49(5):654–662. doi: 10.1073/pnas.49.5.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall C. C., Rogers H. W., Downer D. N., Gentry G. A. Protein kinase activity in equine herpesvirus. J Virol. 1972 Feb;9(2):216–222. doi: 10.1128/jvi.9.2.216-222.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann E. M., Walsh D. A., Krebs E. G. Purification and properties of rabbit skeletal muscle adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1986–1995. [PubMed] [Google Scholar]
- Rosemond H., Moss B. Phosphoprotein component of vaccinia virions. J Virol. 1973 Jun;11(6):961–970. doi: 10.1128/jvi.11.6.961-970.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Bishop D. H. Nucleoside triphosphate phosphotransferase. A new enzyme activity of oncogenic and non-oncogenic "budding" viruses. Biochim Biophys Acta. 1971 Apr 14;235(1):191–206. doi: 10.1016/0005-2744(71)90047-7. [DOI] [PubMed] [Google Scholar]
- Rubenstein A. S., Gravell M., Darlington R. Protein kinase in enveloped herpes simplex virions. Virology. 1972 Oct;50(1):287–290. doi: 10.1016/0042-6822(72)90374-1. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Shekel J. J., Machado R., Pereira H. G. Phosphorylated polypeptides in adenovirus-infected cells. Virology. 1972 Dec;50(3):931–934. doi: 10.1016/0042-6822(72)90450-3. [DOI] [PubMed] [Google Scholar]
- Sokol F., Clark H. F. Phosphoproteins, structural components of rhabdoviruses. Virology. 1973 Mar;52(1):246–263. doi: 10.1016/0042-6822(73)90413-3. [DOI] [PubMed] [Google Scholar]
- Strand M., August J. T. Protein kinase and phosphate acceptor proteins in Rauscher murine leukaemia virus. Nat New Biol. 1971 Sep 29;233(39):137–140. doi: 10.1038/newbio233137a0. [DOI] [PubMed] [Google Scholar]
- Tan K. B., Sokol F. Structural proteins of simian virus 40: phosphoproteins. J Virol. 1972 Nov;10(5):985–994. doi: 10.1128/jvi.10.5.985-994.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng C. S., Teng C. T., Allfrey V. G. Studies of nuclear acidic proteins. Evidence for their phosphorylation, tissue specificity, selective binding to deoxyribonucleic acid, and stimulation effects on transcription. J Biol Chem. 1971 Jun 10;246(11):3597–3609. [PubMed] [Google Scholar]
- Wagner R. R., Kiley M. P., Snyder R. M., Schnaitman C. A. Cytoplasmic compartmentalization of the protein and ribonucleic acid species of vesicular stomatitis virus. J Virol. 1972 Apr;9(4):672–683. doi: 10.1128/jvi.9.4.672-683.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Prevec L., Brown F., Summers D. F., Sokol F., MacLeod R. Classification of rhabdovirus proteins: a proposal. J Virol. 1972 Dec;10(6):1228–1230. doi: 10.1128/jvi.10.6.1228-1230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Schnaitman T. C., Snyder R. M., Schnaitman C. A. Protein composition of the structural components of vesicular stomatitis virus. J Virol. 1969 Jun;3(6):611–618. doi: 10.1128/jvi.3.6.611-618.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. R., Snyder R. M., Yamazaki S. Proteins of vesicular stomatitis virus: kinetics and cellular sites of synthesis. J Virol. 1970 May;5(5):548–558. doi: 10.1128/jvi.5.5.548-558.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Wagner R. R. Action of interferon: kinetics and differential effects on viral functions. J Virol. 1970 Oct;6(4):421–429. doi: 10.1128/jvi.6.4.421-429.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



