Abstract
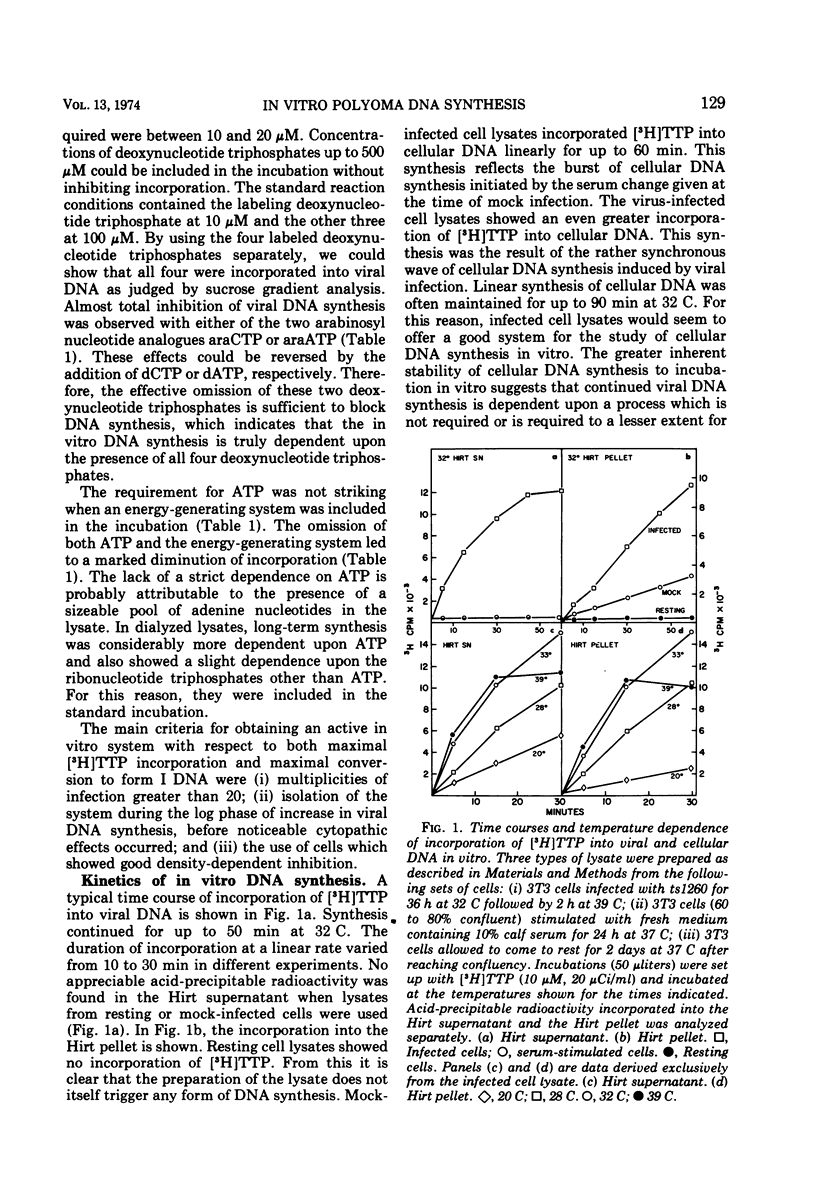
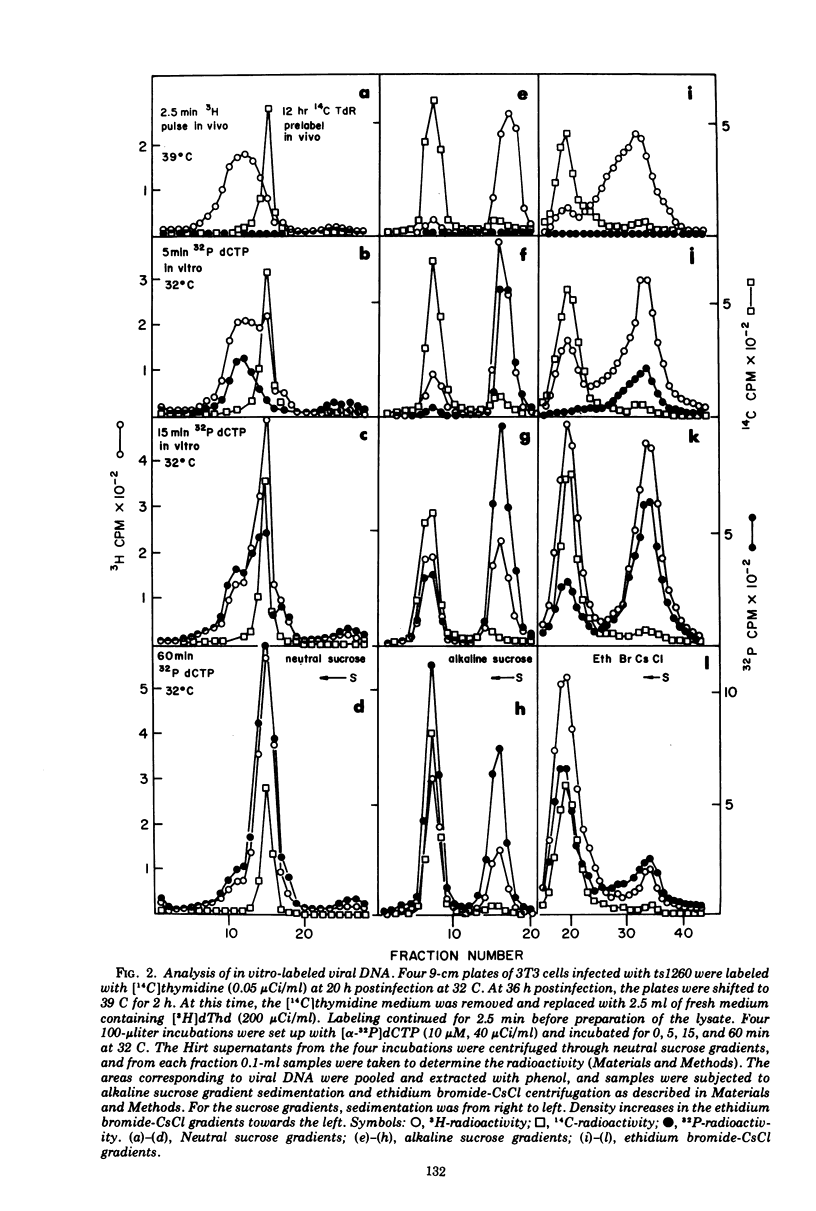
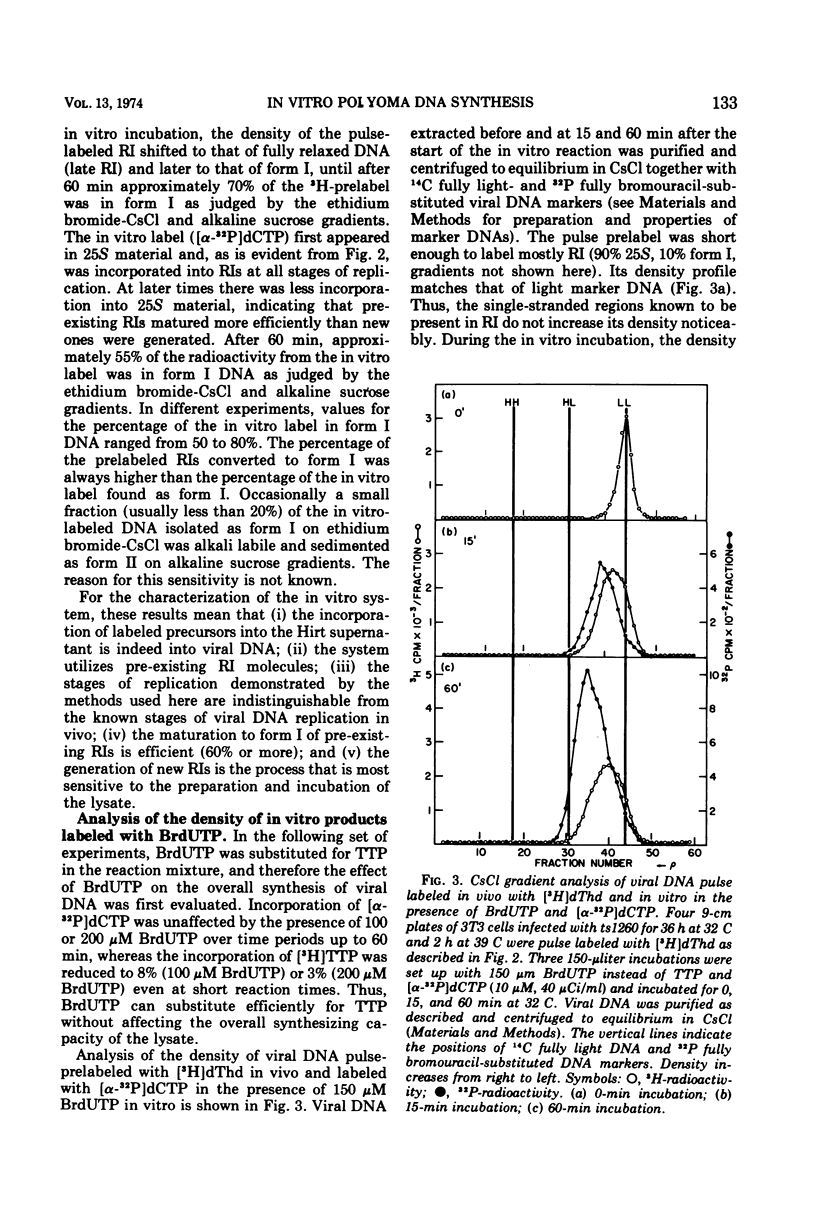
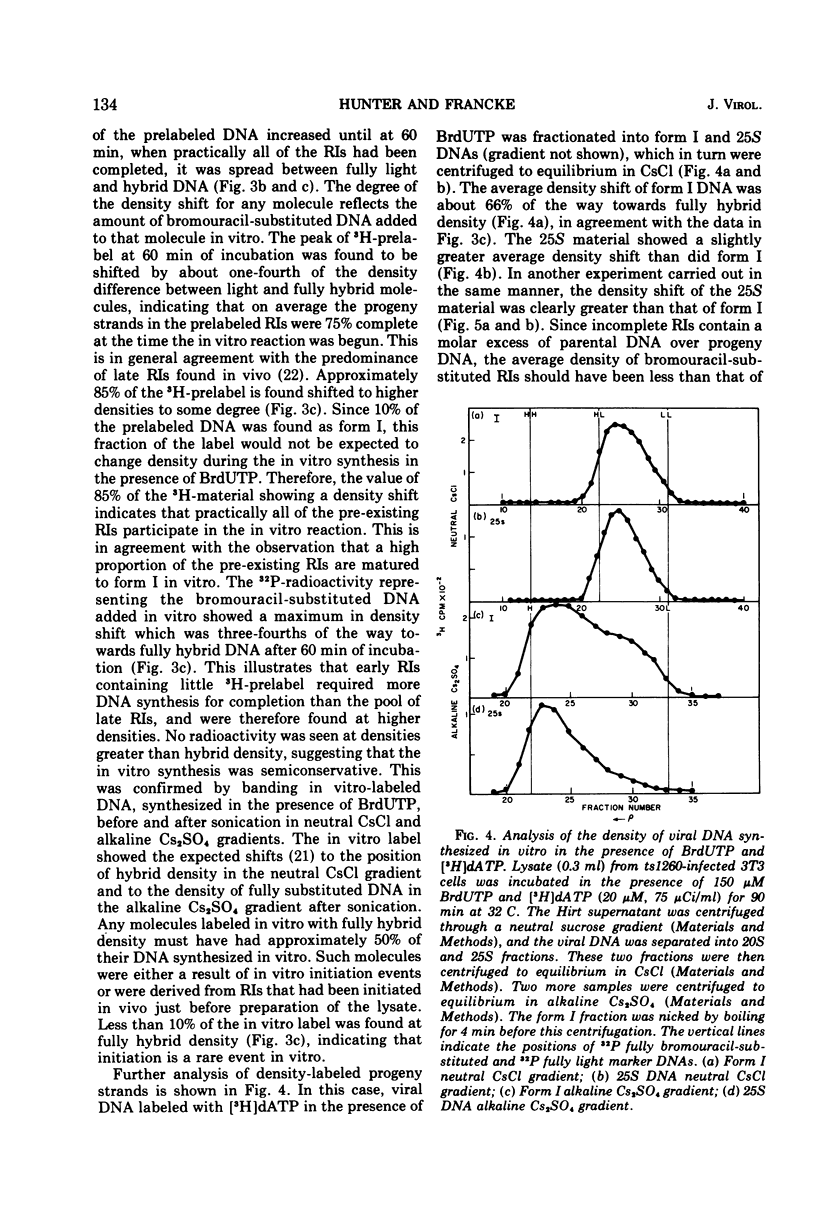
A lysate from hypotonically swollen polyoma-infected BALB/3T3 cells incorporated labeled deoxynucleotide triphosphates into both viral and cellular DNAs. The incorporation was stimulated by the presence of ATP, deoxynucleotide triphosphates, thiols, and magnesium ions. Strong inhibition of incorporation was observed with thiol reagents and arabinosyl nucleotide triphosphates. The rate of in vitro synthesis increased with the temperature of incubation as expected. Incorporation into cellular DNA for up to 2 h was observed in lysates from virus-infected and serum-stimulated cells but not from resting cells. Synthesis in the system, therefore, appeared to reflect the physiological state of the cells before preparation of the lysate. Incorporation into viral DNA stopped far sooner than that into cellular DNA. During the initial phase of the in vitro incubation, incorporation occurred into viral replicative intermediates (RI). These RIs had identical properties to those isolated after in vivo pulse labeling and a substantial proportion of them was matured to form I DNA at later times in the incubation through all the stages known to occur in vivo. Density labeling of the in vitro product showed that practically all of the RIs pre-existing in the infected cell took part in the in vitro reaction. Analysis of DNA labeled in vitro in the presence of 5-bromodeoxyuridine triphosphate showed that synthesis occurred on RIs at all stages of replication and that the progeny strands were elongated by up to 80% of unit viral DNA length. Pre-existing RIs, pulse labeled in vivo, showed evidence of a pool at a late stage of replication which required elongation of their progeny strands by approximately 25% during conversion to form I molecules. From density-labeling experiments, we were also able to show that viral DNA synthesis in vitro was semiconservative. The major reason for cessation of viral DNA synthesis in vitro was the very limited ability of the lysate to initiate new rounds of viral DNA synthesis.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgaux P., Bourgaux-Ramoisy D. A symmetrical model for polyoma virus DNA replication. J Mol Biol. 1971 Dec 28;62(3):513–524. doi: 10.1016/0022-2836(71)90152-5. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D. Unwinding of replicating polyoma virus DNA. J Mol Biol. 1972 Oct 14;70(3):399–413. doi: 10.1016/0022-2836(72)90548-7. [DOI] [PubMed] [Google Scholar]
- Branton P. E., Cheevers W. P., Sheinin R. The effect of cycloheximide on DNA synthesis in cells productively-infected with polyoma virus. Virology. 1970 Dec;42(4):979–992. doi: 10.1016/0042-6822(70)90346-6. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. MECHANISM OF RNA POLYMERASE ACTION: FORMATION OF DNA-RNA HYBRIDS WITH SINGLE-STRANDED TEMPLATES. J Mol Biol. 1964 Feb;8:297–313. doi: 10.1016/s0022-2836(64)80139-x. [DOI] [PubMed] [Google Scholar]
- Chang L. M., Bollum F. J. Low molecular weight deoxyribonucleic acid polymerase in mammalian cells. J Biol Chem. 1971 Sep 25;246(18):5835–5837. [PubMed] [Google Scholar]
- Cohen S. S. Introduction to the biochemistry of D-arabinosyl nucleosides. Prog Nucleic Acid Res Mol Biol. 1966;5:1–88. doi: 10.1016/s0079-6603(08)60231-7. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Complementation and transformation by temperature-sensitive mutants of polyoma virus. Virology. 1969 May;38(1):120–125. doi: 10.1016/0042-6822(69)90133-0. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., McKerlie M. L., Salzman N. P. Characterization of Simian virus 40 DNA component II during viral DNA replication. J Mol Biol. 1973 Feb 25;74(2):95–111. doi: 10.1016/0022-2836(73)90101-0. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., Salzman N. P. Intermediate in SV40 DNA chain growth. Nat New Biol. 1972 Aug 30;238(87):274–277. doi: 10.1038/newbio238274a0. [DOI] [PubMed] [Google Scholar]
- Friedman D. L., Mueller G. C. A nuclear system for DNA replication from synchronized HeLa cells. Biochim Biophys Acta. 1968 Jul 23;161(2):455–468. doi: 10.1016/0005-2787(68)90122-6. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Mayer A., Levine A. Replicating SV40 molecules containing closed circular template DNA strands. Nat New Biol. 1971 Sep 15;233(37):72–75. doi: 10.1038/newbio233072a0. [DOI] [PubMed] [Google Scholar]
- Krakoff I. H., Brown N. C., Reichard P. Inhibition of ribonucleoside diphosphate reductase by hydroxyurea. Cancer Res. 1968 Aug;28(8):1559–1565. [PubMed] [Google Scholar]
- Kumar K. V., Friedman D. L. Initiation of DNA synthesis in HeLa cell-free system. Nat New Biol. 1972 Sep 20;239(90):74–76. doi: 10.1038/newbio239074a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Lynch W. E., Brown R. F., Umeda T., Langreth S. G., Lieberman I. Synthesis of deoxyribonucleic acid by isolated liver nuclei. J Biol Chem. 1970 Aug 10;245(15):3911–3916. [PubMed] [Google Scholar]
- Magnusson G., Pigiet V., Winnacker E. L., Abrams R., Reichard P. RNA-linked short DNA fragments during polyoma replication. Proc Natl Acad Sci U S A. 1973 Feb;70(2):412–415. doi: 10.1073/pnas.70.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson G., Winnacker E. L., Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. II. Evidence for semi-conservative replication. J Mol Biol. 1972 Dec 30;72(3):539–552. doi: 10.1016/0022-2836(72)90173-8. [DOI] [PubMed] [Google Scholar]
- Mayer A., Levine A. J. DNA replication in SV40-infected cells. 8. The distribution of replicating molecules at different stages of replication in SV40-infected cells. Virology. 1972 Nov;50(2):328–338. doi: 10.1016/0042-6822(72)90384-4. [DOI] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D., Danna K. J. Specific origin in SV40 DNA replication. Nat New Biol. 1972 Apr 19;236(68):200–202. doi: 10.1038/newbio236200a0. [DOI] [PubMed] [Google Scholar]
- Pisetsky D., Berkower I., Wickner R., Hurwitz J. Role of ATP in DNA synthesis in Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):557–571. doi: 10.1016/s0022-2836(72)80023-8. [DOI] [PubMed] [Google Scholar]
- Schaller H., Otto B., Nüsslein V., Huf J., Herrmann R., Bonhoeffer F. Deoxyribonucleic acid replication in vitro. J Mol Biol. 1972 Jan 28;63(2):183–200. doi: 10.1016/0022-2836(72)90369-5. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog K. L., Nordenskjöld B. A., Bjursell K. G. Deoxyribonucleoside-triphosphate pools and DNA synthesis in synchronized hamster cells. Eur J Biochem. 1973 Mar 15;33(3):428–432. doi: 10.1111/j.1432-1033.1973.tb02699.x. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M. J. Drugs which affect the structure and function of DNA. Nature. 1968 Sep 28;219(5161):1320–1325. doi: 10.1038/2191320a0. [DOI] [PubMed] [Google Scholar]
- Weber M. J., Edlin G. Phosphate transport, nucleotide pools, and ribonucleic acid synthesis in growing and in density-inhibited 3T3 cells. J Biol Chem. 1971 Mar 25;246(6):1828–1833. [PubMed] [Google Scholar]
- Weissbach A., Schlabach A., Fridlender B., Bolden A. DNA polymerases from human cells. Nat New Biol. 1971 Jun 9;231(23):167–170. doi: 10.1038/newbio231167a0. [DOI] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Replication of polyoma DNA in isolated nuclei. I. Characterization of the system from mouse fibroblast 3T6 cells. J Mol Biol. 1972 Dec 30;72(3):523–537. doi: 10.1016/0022-2836(72)90172-6. [DOI] [PubMed] [Google Scholar]



