Abstract
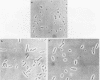
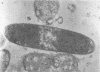
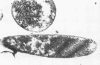
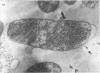
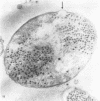
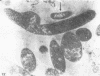
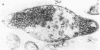
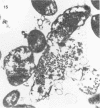
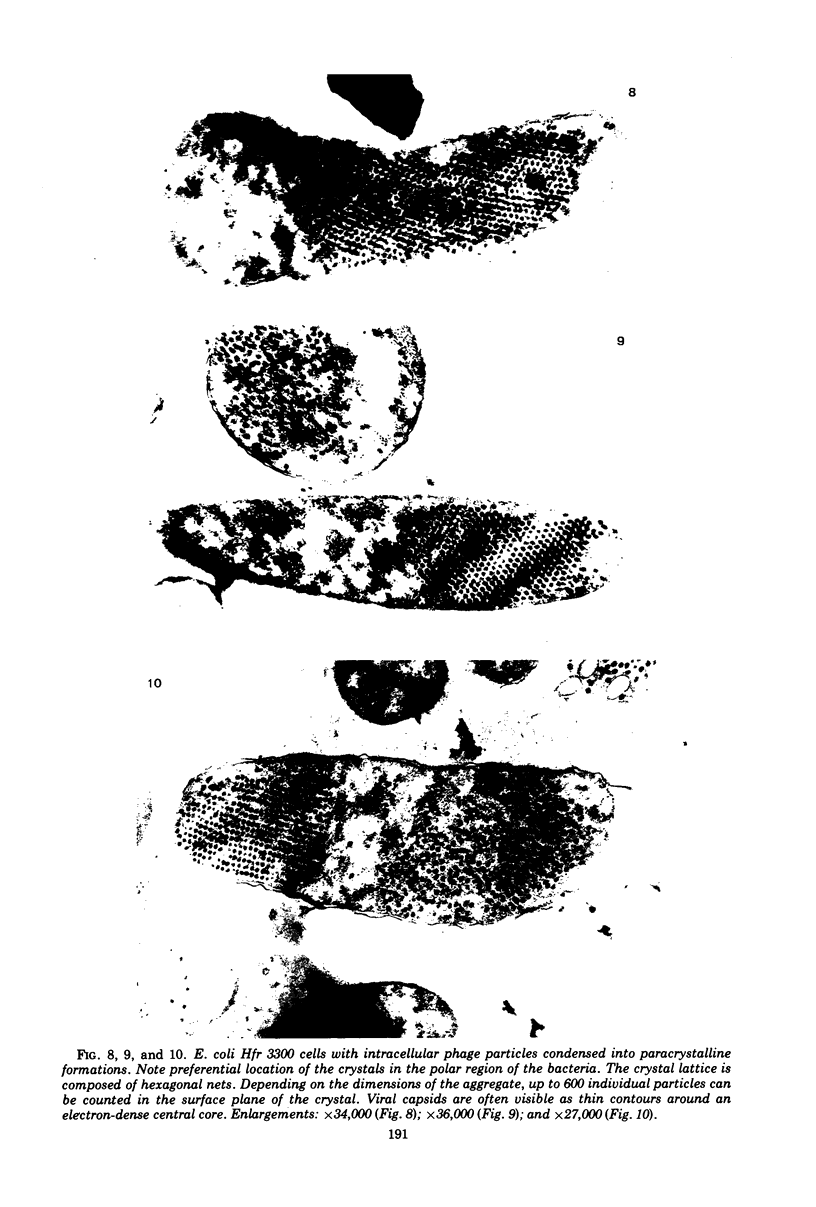
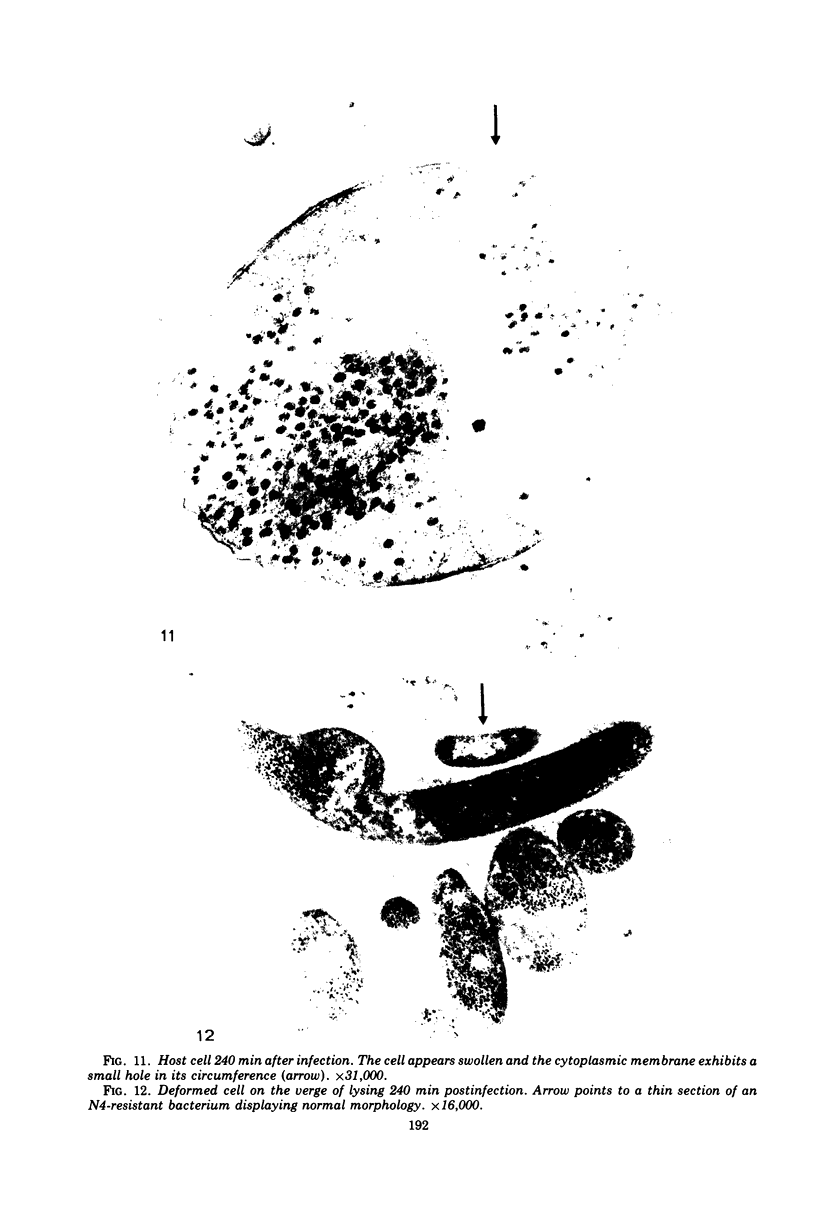
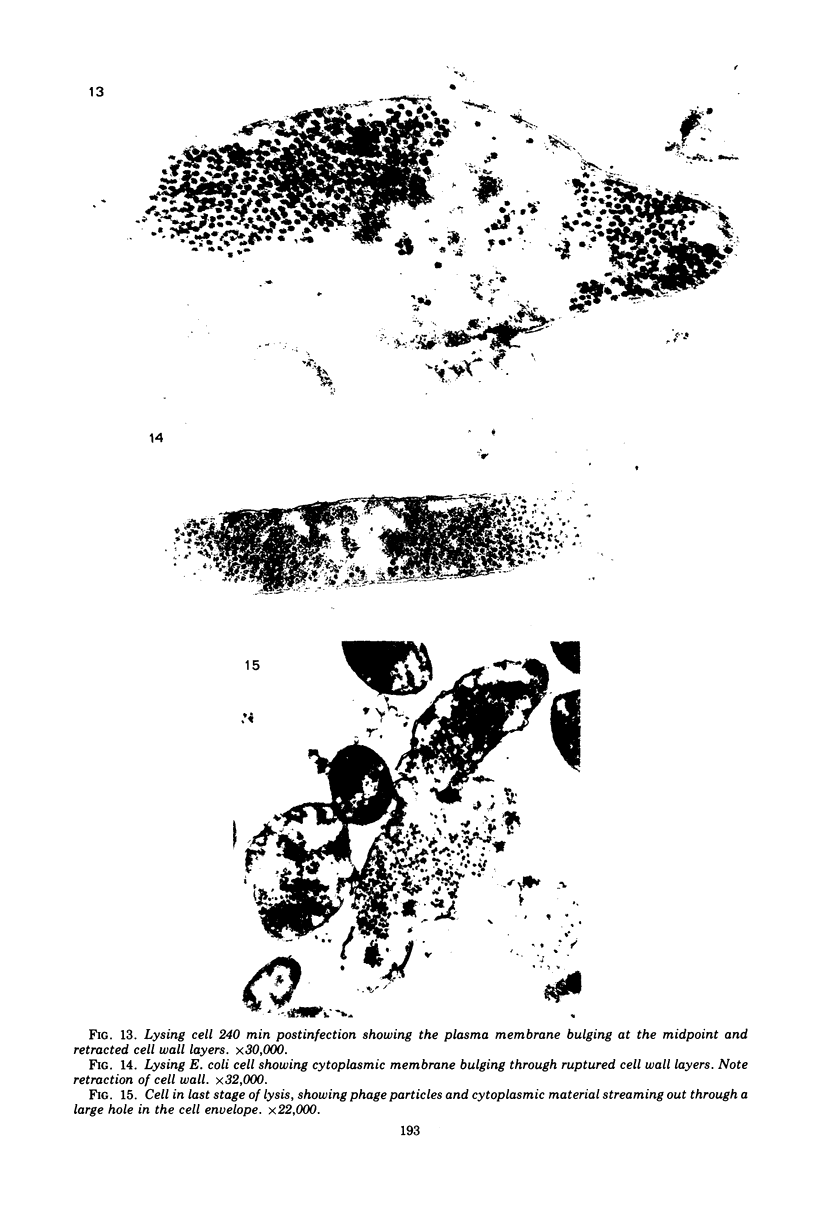
The basic properties of bacteriophage N4 development have been investigated in Escherichia coli Hfr 3300 under one-step growth and high cell density conditions. N4r+ -infected bacteria are lysis inhibited in mass culture, burst asynchronously starting 180 min postinfection, and release over 3,000 phage per cell. During lysis inhibition the bacteria continuously elongate, increase in girth, and undergo characteristic morphological changes represented by the appearance of dark spots located at the cell poles. In thin sections, during the late stages of replication and assembly, the phage particles are localized exclusively in restricted areas of the cytoplasm near the polar regions. Large paracrystalline arrays of virions are found in over 7% of the cells before lysis. The most common mechanism of lysis consists in the formation of bulges located at random in the cell circumference; these burst and, without extensive disruption of the cell wall, the phage progeny escapes into the medium.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bode W. Lysis inhibition in Escherichia coli infected with bacteriophage T4. J Virol. 1967 Oct;1(5):948–955. doi: 10.1128/jvi.1.5.948-955.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller C. S., Astrachan L. Replication of T4rII bacteriophage in Escherichia coli K-12 (lambda). J Virol. 1968 Apr;2(4):298–307. doi: 10.1128/jvi.2.4.298-307.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse N. L. Control of lysis of T4-infected Escherichia coli. J Virol. 1968 Mar;2(3):198–207. doi: 10.1128/jvi.2.3.198-207.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doermann A. H. Lysis and Lysis Inhibition with Escherichia coli Bacteriophage. J Bacteriol. 1948 Feb;55(2):257–276. doi: 10.1128/jb.55.2.257-276.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M., Granboulan N. Ultrastructure of Escherichia coli cells infected with bacteriophage R17. J Bacteriol. 1966 Feb;91(2):834–848. doi: 10.1128/jb.91.2.834-848.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. L., Krisch R. E. Enlargement of Escherichia coli after bacteriophage infection. II. Proposed mechanism. J Virol. 1971 Jul;8(1):95–102. doi: 10.1128/jvi.8.1.95-102.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman N. B., Suzuki G. Effect of spermine on lysis and reproduction by bacteriophages phi-X174, lambda, and f2. J Bacteriol. 1966 Dec;92(6):1735–1740. doi: 10.1128/jb.92.6.1735-1740.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., SECHAUD J., RYTER A. Electron microscopical studies of phage multiplication. IV. The establishment of the DNA pool of vegetative phage and the maturation of phage particles. Virology. 1959 Aug;8:478–498. doi: 10.1016/0042-6822(59)90050-9. [DOI] [PubMed] [Google Scholar]
- LURIA S. E., HUMAN M. L. Chromatin staining of bacteria during bacteriophage infection. J Bacteriol. 1950 Apr;59(4):551–560. doi: 10.1128/jb.59.4.551-560.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaretten W., Morgan C., Rosenkranz H. S., Rose H. M. Effect of hydroxyurea on virus development. I. Electron microscopic study of the effect on the development of bacteriophage T4. J Bacteriol. 1966 Feb;91(2):823–833. doi: 10.1128/jb.91.2.823-833.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai F., Streisinger G., Miller B. The mechanism of lysis in phage T4-infected cells. Virology. 1967 Nov;33(3):398–404. doi: 10.1016/0042-6822(67)90115-8. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., LEE H. H. Mechanism of cell wall penetration by viruses. I. An increase in host cell permeability induced by bacteriophage infection. J Exp Med. 1954 May 1;99(5):481–494. doi: 10.1084/jem.99.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Haselkorn R., Schito G. C. Selective shutoff of catabolite-sensitive host syntheses by bacroxyurea pharmaco mutation genes coliphages growth ł virus replication escherichia coli growth ł lysogeny crosses genetic coliteriophage N4. Virology. 1972 Oct;50(1):95–102. doi: 10.1016/0042-6822(72)90349-2. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ F. M., ZINDER N. D. CRYSTALLINE AGGREGATES IN BACTERIAL CELLS INFECTED WITH THE RNA BACTERIOPHAGE F2. Virology. 1963 Oct;21:276–278. doi: 10.1016/0042-6822(63)90272-1. [DOI] [PubMed] [Google Scholar]
- Schito G. C. A rapid procedure for the purification of bacterial viruses. Virology. 1966 Sep;30(1):157–159. doi: 10.1016/s0042-6822(66)81023-1. [DOI] [PubMed] [Google Scholar]
- Schito G. C., Rialdi G., Pesce A. Biophysical properties of N4 coliphage. Biochim Biophys Acta. 1966 Dec 21;129(3):482–490. doi: 10.1016/0005-2787(66)90063-3. [DOI] [PubMed] [Google Scholar]
- Schito G. C., Rialdi G., Pesce A. The physical properties of the deoxyribonucleic acid from N4 coliphage. Biochim Biophys Acta. 1966 Dec 21;129(3):491–501. doi: 10.1016/0005-2787(66)90064-5. [DOI] [PubMed] [Google Scholar]
- Schito G. C. The genetics and physiology of coliphage N4. Virology. 1973 Sep;55(1):254–265. doi: 10.1016/s0042-6822(73)81028-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]