Abstract
Diagnostic techniques in nephrology include clinical history, physical examination, laboratory tests, scintigraphy, diagnostic imaging techniques as well as renal biopsy. In kidney diseases, ultrasonography is used as a first-line imaging technique, and its role in medical nephropathy is to exclude urological pathologies, to differentiate between acute and chronic renal failure, to follow-up on the course of a disease, to guide needle biopsy, etc. Ultrasound images are useful at characterizing the pelvis, assessing renal dimensions and parenchymal echogenicity, sampling color–power Doppler signals and evaluating their characteristics and distribution as well as measuring parenchymal resistive index. Taken together, these data can provide useful clues to the diagnosis and help to reduce the number of possible differential diagnoses.
Keywords: Ultrasonography, Nephropathy, Resistive index
Sommario
La diagnostica in nefrologica comprende la storia clinica, l'esame fisico, gli esami di laboratorio, gli esami scintigrafici, la diagnostica per immagini e la biopsia renale. Nelle malattie renali l'ecografia rappresenta la tecnica per immagini di prima scelta e il suo ruolo nelle nefropatie mediche è quello di escludere una patologia urologica, differenziare fra un'insufficienza renale acuta e cronica, permettere il follow-up della malattia, guidare l'agobiopsia renale ecc. Le immagini ultrasonografiche permettono di caratterizzare la pelvi, di valutare le dimensioni renali e l'ecogenicità parenchimale, di campionare i segnali color–power Doppler e di valutarne caratteristiche e distribuzione, nonché di misurare gli indici di resistenza intraparenchimali. L'insieme di questi dati permette di ottenere importanti informazioni diagnostiche in molti casi, mentre in altri permette di ridurre le possibili diagnosi differenziali.
Introduction
In renal pathologies, diagnosis is based on the patient's clinical history, outcome of physical examination, laboratory tests, scintigraphy, diagnostic imaging and renal biopsy. Particularly diagnostic imaging is an important tool as it is essential to exclude urinary tract obstruction, differentiate between acute and chronic pathologies, follow-up on diagnosed diseases and guide biopsy.
Ultrasonography (US) and color Doppler are used in the initial evaluation as both are widely available, easy to perform, inexpensive and have no undesired side-effects. In urological pathologies, US frequently leads to a final diagnosis, but in kidney diseases its field of application still seems to be limited. The aim of this article is to define the present role of US and color Doppler in the study of medical nephropathy.
B-mode characteristics of medical nephropathy
The debate in connection with US examinations usually concerns the ability to identify a pathological condition, to distinguish between different histopathological lesions, and to identify patients with end-stage chronic renal failure [1]. The parameters are morphological (interpolar and anteroposterior diameter, parenchymal thickness, and echogenicity), pathological (lithiasis, cysts, hydronephrosis, neoplasm) and functional (vascularization, blood flow velocity, resistive index).
Renal dimensions
The mean right renal length is 10.74 ± 1.35 cm and the mean left renal length is 11.10 ± 1.15 cm, measured as the longest diameter obtained on a posterior oblique image [2,3], with a lower limit of normality generally indicated as 9 cm [4]. Renal length under 8 cm is definitely reduced and should be attributed to chronic renal failure (CRF), whereas a length between 8 and 9 cm should always be correlated to the patient's phenotype, particularly the height. In an attempt to improve differentiation of normal kidneys from those affected by chronic nephropathy, some authors have furthermore proposed the evaluation of renal volume using the ellipsoid formula (V = craniocaudal diameter × anteroposterior diameter × transverse diameter × 0.5233) [5] subsequently adjusted to the patient's body mass index (BMI) using the formula V/BMI × 25. This formula indicates the appropriate renal volume with mean values of 231 ± 50.5 ml [6]. Increased renal volume correlates with anatomic-pathological conditions implying kidney hypertrophy, protein deposits, fluid collections in the interstitial space or in the tubules, cellular infiltration, and neoplastic lesions with necrotic areas. In these conditions, the site of the lesion is most frequently the interstitial tubules, since the glomerular component accounts only for about 8% of the renal parenchyma in adults. Increased renal volume can therefore be found in neoplastic pathologies (both renal and systemic), in acute tubular necrosis (ATN), in acute interstitial nephritis, in acute tubulopathy, in accumulating diseases (glycogen, amyloid, lipid) and in other cases of nephromegalia (cirrhosis, diabetes mellitus, hyperalimentation) [7] (Fig. 1). Particularly ATN leads to a substantial increase in the anteroposterior diameter of both kidneys while the length is generally normal [8].
Fig. 1.
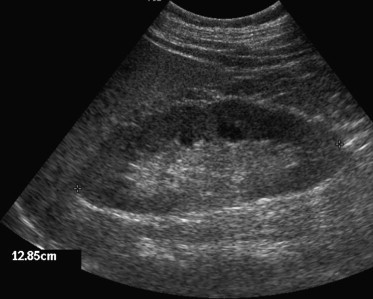
Kidney: maximum length in a 195 cm tall patient weighing 98 kg.
A reduced renal volume is a negative prognostic sign and correlates histopathologically with the degree of atrophy (after phlogosis caused by reflux), necrosis, fibrosis, congenital hypoplasia and hypoperfusion [7]. It can be caused by chronic glomerulonephritis, papillary necrosis, hereditary nephropathy, widespread nephrosclerosis, and end-stage chronic renal failure (Fig. 2).
Fig. 2.
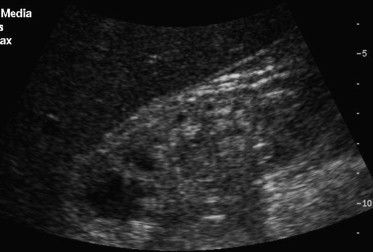
Patient undergoing dialysis for about 8 years: kidney of reduced length; poor corticomedullary differentiation, hyperechoic parenchyma and small cysts.
Parenchymal thickness
Parenchymal thickness is a US parameter used in the functional evaluation of the kidney, and a thickness ranging from 15 to 20 mm is considered normal. There are no established guidelines concerning the scanning plane and where measurement should be performed, i.e. if the thickness of the whole parenchyma should be evaluated or only the cortical parenchyma. Parenchymal thickness correlates with the longitudinal diameter of the kidney but not with prognosis and histopathology [1,9].
Parenchymal echogenicity
Parenchymal echogenicity is the most frequently used marker for evaluating the presence of nephropathy. It is evaluated by comparing the echogenicity of the renal cortex, medulla and pyelic sinus with that of the adjacent liver and spleen (assuming that the liver and spleen present normal echogenicity). Echogenicity is divided into four different grades from 0 to III (Table 1, Fig. 3) [10,11].
Table 1.
Classification of parenchymal echogenicity according to Hricak et al. [10]
| Grade 0: echogenicity poorer than that of the liver parenchyma (normal finding) |
| Grade I: echogenicity identical to that of the liver parenchyma (normal finding) |
| Grade II: echogenicity more intense than that of the liver parenchyma (pathological finding) |
| Grade III: echogenicity identical to that of the renal sinus (pathological finding) |
Fig. 3.
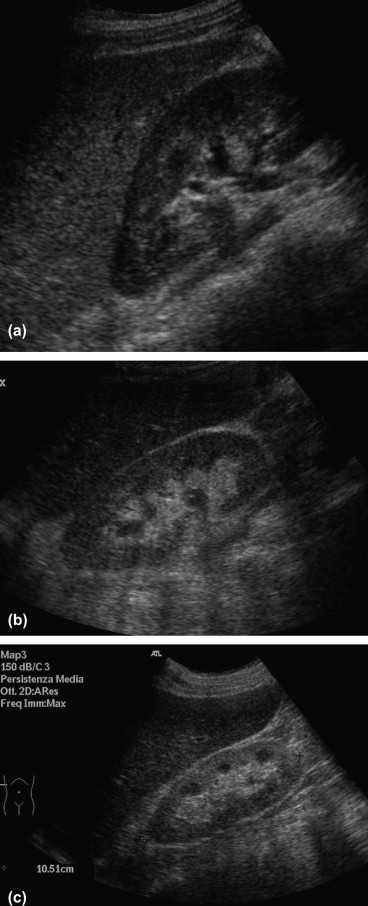
Kidney: (a) parenchyma appears hypoechoic when compared to the liver parenchyma; (b) parenchyma appears isoechoic when compared to the liver parenchyma; (c) parenchyma appears hyperechoic when compared to the liver parenchyma.
However, the grading of parenchymal echogenicity does not differentiate between different histopathological conditions, and a normal renal echogenicity does not exclude that the patient's kidney is damaged. In the evaluation of renal pathologies, sensitivity and specificity of echogenicity in grades I and II are 62% and 58%, respectively, whereas sensitivity and specificity of echogenicity in grade III are 20% and 96%, respectively [12]. Parenchymal echogenicity varies also with the patient's age (it is increased in newborn babies due to elevated cellularity and in elderly patients due to fibrosis). Increased cortical echogenicity has proved to correlate significantly with the prevalence of glomerular sclerosis, tubular atrophy, focal leukocyte infiltration, fluid retention, arteriosclerosis and with the presence of hyaline cylinders [13,14]. These lesions are generally tubulointerstitial since the glomerular component accounts only for 8% of the renal parenchyma. Also the presence of diffused calcifications (e.g. Pneumocystis carinii infection) or precipitation of the calcium salts (e.g. hyperoxaluria) can increase the cortical echogenicity. The renal medulla can also appear hyperechoic, correlating histopathologically with the grade of medullary nephrocalcinosis, medullary tubular ectasia, medullary fibrosis, vascular congestion, and urate or protein deposits. The main conditions causing these anomalies are gout, medullary sponge kidney (Fig. 4), primary hyperaldosteronism, hyperparathyroidism, glycogenosis and Wilson's disease. The presence of hyperechogenic areas in the corticomedullary junction (Fig. 5) is not a specific diagnostic sign, but it can be idiopathic or associated with vascular lesions (diabetes, pseudoxanthoma elasticum) and arterial hypertension [15,16]. The kidneys can also appear diffusely hypoechoic in cases of acute pyelonephritis, lymphoma and nephroblastomatosis (Fig. 6).
Fig. 4.
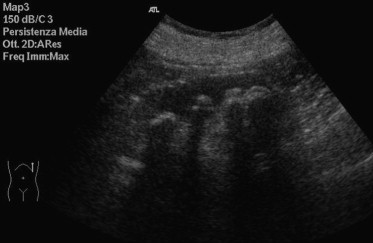
Medullary sponge kidney: calcifications at the level of the corticomedullary junction with an associated acoustical shadow.
Fig. 5.
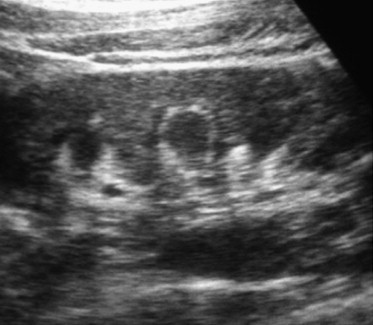
Hyperechogenicity of the corticomedullary junction; incidental finding in patient with hypertension.
Fig. 6.
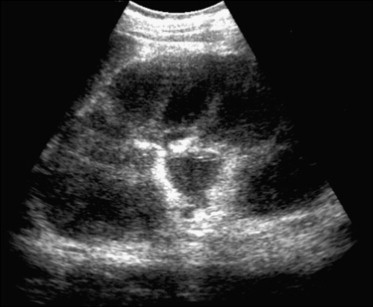
Diffusely hypoechoic kidney developing lymphoma (courtesy: Carlo Martinoli).
Utility of color and power Doppler
Color and power Doppler can provide an accurate morphological and functional evaluation of the intraparenchymal vascularity and detect reduced or no blood flow in the kidney or in a portion of the kidney. In this case, there will be color signals from the undamaged part of the kidney but not from the ischemic part. The use of contrast agent increases diagnostic confidence in this type of lesions [17].
Intrarenal resistive index (RI) is a more sensitive parameter measured on the renal interlobar arteries, which provides physiopathological information about medical nephropathy. RI is easy to calculate manually, but it is often calculated automatically by the equipment, and it expresses the following relationship:
RI is commonly used for evaluating renal arterial resistance, and a significant correlation between RI and renal vascular resistance is repeatedly reported in the literature [18].
However, it should be pointed out that RI is only a marker of renal vascular resistance and not an indicator of renal function. In some diseases, elevated renal arterial resistance is associated with impaired renal function, while other renal pathologies can cause significantly impaired renal function despite little or no changes in renal vascular resistance.
The real value of echo color Doppler analysis of RI in native kidneys can be its predictive use in particular clinical situations. In the literature, RI 0.6 ± 0.2 is considered normal [19,20], but most studies agree that RI 0.70 should be the upper limit of normal intrarenal vascular resistance [21,22]. In addition to renal diseases, also other conditions can cause increased RI values, such as very low blood pressure, particularly fast or slow heart rate, and subcapsular or perirenal fluid collections. In newborn babies and children, RI values are often higher than 0.70 but this finding should not necessarily be considered pathological [19,21,22]. RI values are higher in interstitial pathologies (≥0.70) compared to purely glomerular pathologies in which RI values exceed 0.70 only in the advanced stage of the disease [20]. The literature reports a positive correlation between RI values and vascular-interstitial pathologies, glomerular sclerosis, fluid retention, focal fibrosis, arteriosclerosis and arteriolar sclerosis, whereas correlation with plasma creatinine levels and renal echogenicity is poor [13,23–25]. In patients with chronic renal failure, RI > 0.80 predicts progression of nephropathy more accurately than creatinine clearance and proteinuria, showing a sensitivity and specificity of 64% and 98%, respectively [26].
Diabetic nephropathy
In the initial phase of nephropathy (diabetes mellitus type 1 and 2), glomerular filtration and renal volume are increased, whereas kidney volume is progressively reduced in the chronic phase. In diabetic patients with normal renal function, 65% of those affected by type 1 and 25% of those affected by type 2 have RI values ≥ 0.70. Mean RI is higher in patients affected by diabetes type 2 (0.71 vs. 0.65; p < 0.001) and can to some extent be correlated with the difference in the patients' age [27]. In these patients, RI values correlate with macroangiopathy, more frequent in patients affected by diabetes mellitus type 2 and in patients with nephroangiosclerotic damage, whereas RI does not correlate with microalbuminuria, which is an indicator of glomerular microangiopathy [28]. Diabetic patients with chronic renal failure and RI values ≥ 0.70 are generally older (62 vs. 44 years old), have higher proteinuria (3.3 vs. 1.1 mg/dl), higher serum creatinine level (3.2 vs. 1.1 mg/dl), longer duration of diabetes (20 vs. 11 years) and higher blood pressure, and present a higher rate of renal failure requiring dialysis (71% con RI = 1.0) [29,30]. All these clinical data correlate with the level of renal arteriolosclerosis (nephroangiosclerosis) (Fig. 7).
Fig. 7.
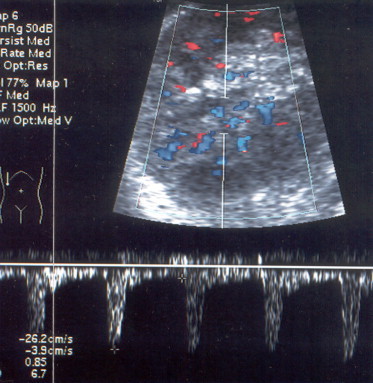
Patient affected by IDDM: increased intraparenchymal RI (0.85); renal structure and vascularity are normal.
Acute renal failure (ARF)
B-mode US provides a fast, inexpensive, non-invasive and repeatable morphological evaluation of the kidneys and urinary tract making it clear if ARF is caused by a urological or nephrological pathology. Also color Doppler is useful for defining the origin of ARF. Non-obstructive ARF can be caused by numerous pathologies, but intraparenchymal renal RI values are always elevated in ATN, and also in 50% of cases which cannot be diagnosed as ATN (acute glomerulonephritis, interstitial nephritis, lupus nephritis, lymphoma, etc.) [31]. However, these pathologies mainly involve the tubulointerstitial part of the kidney [32]. Color Doppler cannot establish the origin of ATN, as increased intrarenal RI values are found in hypovolemia, rhabdomyolysis, sepsis, nephrotoxic substances, and multiple organ failure. In experimental studies carried out on animals, reversible ARF was induced in rabbits, causing a significantly reduced renal plasma flow due to intense intrarenal vasoconstriction, particularly in the early phase [33]. RI values rise very early, before serum creatinine level, and reach maximum levels within the first 12 h (serum creatinine level after 24 h) returning to normal values about one week after the onset of ARF, much before the serum creatinine level, which takes about two weeks. Both humoral and neurogenic mechanisms are thought to be responsible for the increased arteriolar resistance [34]. Only 11% of patients affected by ARF present morphological changes at B-mode US, whereas 69% of patients present renal blood flow changes and increased intraparenchymal RI values [31]. In patients affected by prerenal ARF, 80% have normal or only slightly impaired parenchymal flow, and RI values are always below 0.75. In contrast, color Doppler performed on the renal vessels in patients affected by ARF caused by ATN shows increased pulsatility and reduced telediastolic flow, with RI values ≥ 0.75 in 91% of patients. When prerenal ARF is serious and of long duration, resulting in ATN, RI values are constantly higher than 0.75 [32]. Color Doppler sonography also allows long-term monitoring of the severity and evolution of ATN, detecting a particularly protracted evolution of ARF requiring dialysis in patients with very high RI values [28]. Color Doppler monitoring during the phase of recovery of renal function after ARF is particularly important to detect improved plasma flow and improved intraparenchymal RI values before recovery of renal function and the drop in serum creatinine levels (Fig. 8) [33,35].
Fig. 8.
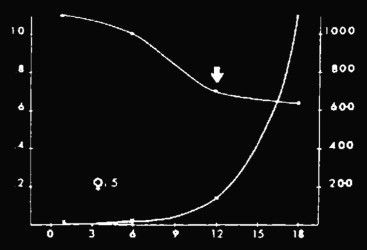
Left ordinate: RI; x-axis: days; right ordinate: diuresis (ml). Patient with ARF due to interstitial nephritis; intraparenchymal RI values are substantially reduced despite anuria.
Monitoring response to therapy
Evaluation of intraparenchymal RI values can provide early signals of nephropathy and/or predict impaired renal function before alteration of clinical-biochemical values in a number of pathologies, such as hemolytic–uremic syndrome [36], ATN [31], and hepatic–renal syndrome [37]. Repeated evaluations are obviously required in order to achieve early detection of possible variations in RI values.
Cirrhosis
In cirrhotic patients without ascites and normal renal function (evaluated by assessment of serum creatinine level) mean RI values are almost normal (0.61 ± 0.05), whereas patients with ascites present substantially elevated RI values (0.70 ± 0.04); in fact, 53% of patients with RI ≥ 0.70 have ascites. Patients without evidence of esophageal varices present mean RI = 0.65 ± 0.08, whereas patients with esophageal varices present mean RI = 0.70 ± 0.07. Likewise, cirrhotic patients without portosystemic shunt present mean RI = 0.66 ± 0.07, whereas hepatopathic patients with portosystemic shunt present mean RI = 0.70 ± 0.07. In all patients with advanced cirrhosis (Child–Pugh B and C), elevated RI values show renal vasoconstriction, also in the absence of hepatorenal syndrome, although the rise in renal RI values always correlates to the severity of portal hypertension [38–40].
Hepatorenal syndrome
Hepatorenal syndrome is irreversible ARF, a frequent complication in liver diseases which generally occurs suddenly in patients whose renal function used to be normal. The only effective therapy is liver transplantation. In all patients with hepatorenal syndrome, RI values are elevated (mean RI = 0.79 ± 0.06) in line with the values obtained in patients with advanced cirrhosis. These blood flow alterations are very early signs which can usually be detected long before ARF is clinically evident. Early detection and subsequent monitoring of increased parenchymal RI values in cirrhotic patients permit identification of patients at a high risk of developing ARF and hepatorenal syndrome. Hepatopathic patients with RI > 70 face a 26-fold greater risk of developing hepatorenal syndrome than hepatopathic patients with RI ≤ 0.70. After liver transplant, renal RI values are rapidly reduced despite unvaried serum creatinine levels. Pre-transplant RI values > 0.70 correlate with higher post-transplant morbidity, and persistent post-transplant RI > 0.70 is a negative prognostic finding [31,37–40].
Conclusions
US and color Doppler are the diagnostic methods of choice in the morphological evaluation of renal diseases. Particularly in nephrological pathologies, the combined use of B-mode US and color Doppler including measurement of intraparenchymal RI values, is important in the initial evaluation of nephropathic patients, as these examinations combined with other diagnostic clues can provide useful information about the origin of the pathology, thus allowing an orientation of the diagnosis. Repeated evaluations can in some cases provide information about renal functionality before other traditional parameters. This method requires highly skilled operators and expertise in assessing RI values which must be measured on the interlobar and/or arcuate arteries in order to obtain reliable and repeatable data.
References
- 1.Webb J.A. The role of ultrasonography in the diagnosis of intrinsic renal disease. Clin Radiol. 1994;49:589–591. doi: 10.1016/s0009-9260(05)81872-0. [DOI] [PubMed] [Google Scholar]
- 2.Brandt T.D., Neiman H.L., Dragowski M.J., Bulawa W., Claykamp G. Ultrasound assessment of normal renal dimensions. J Ultrasound Med. 1982;1:49–52. doi: 10.7863/jum.1982.1.2.49. [DOI] [PubMed] [Google Scholar]
- 3.Emamian S.A., Nielsen M.B., Pedersen J.F. Tenth percentiles of kidney length in adult volunteers. AJR Am J Roentgenol. 1994;163:748. doi: 10.2214/ajr.163.3.7980828. [DOI] [PubMed] [Google Scholar]
- 4.Webb J.A., Reznek R.H., White F.E., Cattell W.R., Fry I.K., Baker L.R. Can ultrasound and computed tomography replace high-dose urography in patients with impaired renal function? Q J Med. 1984;53:411–425. [PubMed] [Google Scholar]
- 5.Han B.K., Babcock D.S. Sonographic measurement and appearance of normal kidneys in children. AJR Am J Roentgenol. 1985;145:611–616. doi: 10.2214/ajr.145.3.611. [DOI] [PubMed] [Google Scholar]
- 6.Derchi L.E., Martinoli C., Saffioti S., Pontremoli R., De Micheli A., Bordone C. Ultrasonographic imaging and Doppler analysis of renal changes in non-insulin-dependent diabetes mellitus. Acad Radiol. 1994;1:100–105. doi: 10.1016/s1076-6332(05)80826-8. [DOI] [PubMed] [Google Scholar]
- 7.Davidson A.J. Diagnostic set: large smooth, bilateral. In: Davidson A.J., Hartmann D.S., editors. Radiology of the kidney and urinary tract. 2nd ed. Saunders; Philadelphia: 1994. [Google Scholar]
- 8.Nomura G., Kinoshita E., Yamagata Y., Koga N. Usefulness of renal ultrasonography for assessment of severity and cause of acute tubular necrosis. J Clin Ultrasound. 1984;12:135–139. doi: 10.1002/jcu.1870120304. [DOI] [PubMed] [Google Scholar]
- 9.Roger S.D., Beale A.M., Catteil W.R., Webb J.A. What is the value of measuring renal parenchymal thickness before renal biopsy? Clin Radiol. 1994;49:45–49. doi: 10.1016/s0009-9260(05)82913-7. [DOI] [PubMed] [Google Scholar]
- 10.Hricak H., Cruz C., Romanski R., Uniewski M.H., Levin N.W., Madrazo B.L. Renal parenchymal disease: sonographic–histologic correlation. Radiology. 1982;144:141–147. doi: 10.1148/radiology.144.1.7089245. [DOI] [PubMed] [Google Scholar]
- 11.Barozzi L., Pavlica P., Napoli V. Anatomia-aspetti ecografici. In: Barozzi L., Pavlica P., Santoro A., editors. Ecografia e color Doppler in nefrologia. Poletto Editore; 1999. [Google Scholar]
- 12.Platt J.F., Rubin J.M., Bowerman R.A., Marn C.S. The inability to detect kidney disease on the basis of echogenicity. AJR Am J Roentgenol. 1988;151:317–319. doi: 10.2214/ajr.151.2.317. [DOI] [PubMed] [Google Scholar]
- 13.Mostbeck G.H., Kain R., Mallek R., Derfler K., Walter R., Havelec L. Duplex Doppler sonography in renal parenchymal disease. Histopathologic correlation. J Ultrasound Med. 1991;10:189–194. doi: 10.7863/jum.1991.10.4.189. [DOI] [PubMed] [Google Scholar]
- 14.Rosenfield A.T., Siegel N.J. Renal parenchymal disease: histopathologic–sonographic correlation. AJR Am J Roentgenol. 1981;137:793–798. doi: 10.2214/ajr.137.4.793. [DOI] [PubMed] [Google Scholar]
- 15.Crespi G., Derchi L.E., Saffioti S. Sonographic detection of renal changes in pseudoxantoma elasticum. Urol Radiol. 1992;13:223–225. doi: 10.1007/BF02924627. [DOI] [PubMed] [Google Scholar]
- 16.Cressa C., Bazzocchi M., Bendini M., Delendi M. Renal echography in diabetes mellitus. Radiol Med. 1992;84:79–84. [PubMed] [Google Scholar]
- 17.Yucel C., Ozdemir H., Akpek S., Gurel K., Kapucu L.O., Arac M. Renal infarct: contrast-enhanced power Doppler sonographic findings. J Clin Ultrasound. 2001;29:237–242. doi: 10.1002/jcu.1026. [DOI] [PubMed] [Google Scholar]
- 18.Sauvain J.L., Bourscheid D. Duplex Doppler sonography of intrarenal arteries. Normal and pathological aspects. Ann Radiol. 1991;34:237–247. [PubMed] [Google Scholar]
- 19.Petersen L.J., Petersen J.R., Ladefoged S.D. The pulsatility index and the resistive index in renal arteries in patients with hypertension and chronic renal failure. Nephrol Dial Transplant. 1995;10:2060–2064. [PubMed] [Google Scholar]
- 20.Keogan M.T., Kliewer M.A., Hertzberg B.S., Delong D.M., Tupler R.H., Carrol B.A. Renal resistive indexes: variability in Doppler US measurement in healthy population. Radiology. 1996;199(1):165–169. doi: 10.1148/radiology.199.1.8633141. [DOI] [PubMed] [Google Scholar]
- 21.Platt J.F., Rubin J.M., Ellis J.H. Distinction between obstructive and nonobstructive pyelocaliectasis with duplex Doppler sonography. AJR Am J Roentgenol. 1989;153:997–1000. doi: 10.2214/ajr.153.5.997. [DOI] [PubMed] [Google Scholar]
- 22.Brkljacic B., Drinkovic I. Intrarenal duplex Doppler sonographic evaluation of unilateral native kidney obstruction. J Ultrasound Med. 1994;13:197–204. doi: 10.7863/jum.1994.13.3.197. [DOI] [PubMed] [Google Scholar]
- 23.Platt J.F., Ellis J.H., Rubin J.M., DiPietro M.A., Sedman A.B. Intrarenal arterial Doppler sonography in patients with nonobstructive renal disease: correlation of resistive index with biopsy findings. AJR Am J Roentgenol. 1990;154:1223–1227. doi: 10.2214/ajr.154.6.2110732. [DOI] [PubMed] [Google Scholar]
- 24.Platt J.F. Duplex Doppler evaluation of native kidney dysfunction: obstructive and non obstructive disease. AJR Am J Roentgenol. 1992;158:1035–1042. doi: 10.2214/ajr.158.5.1566663. [DOI] [PubMed] [Google Scholar]
- 25.Platt J.F., Rubin J.M., Ellis J.H., DiPietro M.A. Dupplex Doppler US of the kidney: differentiation of obstructive from non obstructive dilatation. Radiology. 1989;171:515–517. doi: 10.1148/radiology.171.2.2649925. [DOI] [PubMed] [Google Scholar]
- 26.Radermacher J., Ellis S., Haller H. Renal resistance index and progression of renal disease. Hypertension. 2002;39:699–703. doi: 10.1161/hy0202.103782. [DOI] [PubMed] [Google Scholar]
- 27.Derchi L.E., Martinoli C., Saffioti S., Pontremoli R., De Micheli A., Bordone C. US imaging and Doppler analysis of renal changes in non-insulin-dependent diabetes mellitus. Acad Radiol. 1994;1:100–105. doi: 10.1016/s1076-6332(05)80826-8. [DOI] [PubMed] [Google Scholar]
- 28.Boeri D., Derchi L.E., Martinoli C., Saffioti S., Bordone C. Intrarenal arteriosclerosis and impairment of kidney function in NIDDM subjects. Diabetologia. 1988;41:121–124. doi: 10.1007/s001250050877. [DOI] [PubMed] [Google Scholar]
- 29.Platt J., Rubin J., Ellis J. Diabetic nephropathy: evaluation with renal duplex Doppler US. Radiology. 1994;190:343–346. doi: 10.1148/radiology.190.2.8284379. [DOI] [PubMed] [Google Scholar]
- 30.Brkljacić B., Mrzljak V., Drinković I., Soldo D., Sabljar-Matovinović M., Hebrang A. Renal vascular resistance in diabetic nephropathy: duplex Doppler US evaluation. Radiology. 1994;192:549–554. doi: 10.1148/radiology.192.2.8029430. [DOI] [PubMed] [Google Scholar]
- 31.Platt J.F., Rubin J.M., Ellis J.H. Acute renal failure: possible role of duplex Doppler US in distinction between acute prerenal failure and acute tubular necrosis. Radiology. 1991;179:419–423. doi: 10.1148/radiology.179.2.2014284. [DOI] [PubMed] [Google Scholar]
- 32.Platt J.F. Doppler ultrasound of the kidney. Semin Ultrasound CT MRI. 1997;18:22–32. doi: 10.1016/s0887-2171(97)90035-4. [DOI] [PubMed] [Google Scholar]
- 33.Yoon D.Y., Kim S.H., Kim H.D., Na D.G., Goo J.M., Choi H.J. Doppler sonography in experimentally induced acute renal failure in rabbits. Resistive index versus serum creatinine levels. Invest Radiol. 1995;30:168–172. doi: 10.1097/00004424-199503000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Rose D.B. Acute renal failure. Prerenal disease versus acute tubular necrosis. In: Rose D.B., editor. Pathophysiology of renal disease. 2nd ed. McGraw-Hill; New York: 1987. pp. 63–117. [Google Scholar]
- 35.Stevens P.E., Gwyther S.J., Hanson M.E., Boultbee J.E., Kox W.J., Phillips M.E. Noninvasive monitoring of renal blood flow characteristics during acute renal failure in man. Intensive Care Med. 1990;16:153–158. doi: 10.1007/BF01724793. [DOI] [PubMed] [Google Scholar]
- 36.Patriquin H.B., O'Regan S., Robitaille P., Paltiel H. Hemolytic–uremic syndrome: intrarenal arterial Doppler patterns as a useful guide to therapy. Radiology. 1989;172:625–628. doi: 10.1148/radiology.172.3.2672090. [DOI] [PubMed] [Google Scholar]
- 37.Pompili M., Rapaccini G.L., De Luca F., Agnes S., Avolio A.W., Covino M. Doppler ultrasonographic evaluation of the early changes in renal resistive index in cirrhotic patients undergoing liver transplantation. J Ultrasound Med. 1999;18:497–502. doi: 10.7863/jum.1999.18.7.497. [DOI] [PubMed] [Google Scholar]
- 38.Platt J.F., Ellis J.H., Rubin J.M., Merion R.M., Lucey M.R. Renal duplex Doppler ultrasonography: a noninvasive predictor of kidney dysfunction and hepatorenal failure in liver disease. Hepatology. 1994;20:362–369. [PubMed] [Google Scholar]
- 39.Maroto A., Gines A., Salo J., Claria J., Gines P., Anibarro L. Diagnosis of functional kidney failure of cirrhosis with Doppler sonography: prognostic value of resistive index. Hepatology. 1994;20:839–844. doi: 10.1002/hep.1840200411. [DOI] [PubMed] [Google Scholar]
- 40.Peraldi M.N., Kanfer A. Insuffisance rénale aigue. Editions Techniques – Encycl Méd Chir (Paris-France) Néphrologie Urologie. 1994;18:42–100. [Google Scholar]


