Abstract
Background
Rutin, a polyphenolic flavonoid, was investigated for its protective effects against the KBrO3 induced renal injuries in rat.
Methods
Group I was control (untreated), group II was given saline 0.5 ml/kg bw (0.9% NaCl), group III was administered KBrO3 (20 mg/kg bw) intragastric twice a week for four weeks. Rutin was administered to group VI (50 mg/kg bw) and Group V (70 mg/kg bw) along with KBrO3 (20 mg/kg bw) while group VI was given rutin (70 mg/kg bw) alone twice a week for four weeks. Protective effects of rutin on KBrO3-induced nephrotoxicity in rats were determined for biochemical parameter of urine, and serum, various antioxidant enzymes, DNA and histopathological damages in kidneys.
Results
The level of urinary red blood cells, leucocytes count, specific gravity, urea, creatinine and urobilinogen was increased (P<0.01) whereas creatinine clearance was reduced. Serum level of protein, albumin, globulin, nitrite, creatinine and blood urea nitrogen (BUN) was significantly increased (P<0.01) by KBrO3. Marked histopathological lesions, elevated DNA fragmentation and AgNORs count in renal tissues was determined. Activity of antioxidant enzymes; catalase, superoxide dismutase, glutathione peroxidase, glutathione-S-transferase, glutathione reductase, and reduced glutathione contents were decreased (P<0.01) while thiobarbituric acid reactive substances were increased (P<0.01) with KBrO3 treatment in kidneys. DNA ladder assay was intimately related with the DNA fragmentation assay. Telomerase activity was found positive in the KBrO3 treated kidneys. Treatment with rutin effectively ameliorated the alterations in the studied parameters of rat. Rutin administration alone to rats did not exhibit any significant change in any of the parameters studied.
Conclusion
These results suggest that rutin works as an antioxidant in vivo by scavenging reactive oxygen species and this serves to prevent oxidative renal damage in rat treated with KBrO3.
Keywords: Potassium bromate, Rutin, Histopathology, Renal function test, Antioxidant, DNA fragmentation, Telomerase
Background
Potassium bromate (KBrO3) has been used widely for water disinfection, hair-coloring solutions, cosmetics, and in food [1]. Toxicological studies have suggested that KBrO3 is: an oxidizing agent; causes hepatotoxicity, neurotoxicity, and thyroid toxicity; and induces the development of mesothelioma tumors in experimental animals as well as renal carcinomas in animals and humans [2,3]. Several studies have investigated the oxidative injuries and probable mechanism of KBrO3-induced carcinogenicity in experimental models [4-6]. KBrO3 induces mutations, base modification (8-oxodeoxyguanosine), chromosomal aberrations, and alters gene expression, leading to cancer [7-9]. KBrO3 increases lipid peroxidation, the creatinine concentration, and enzyme activity in the sera of rats. Cellular defense against oxidative stress is provided by several mechanisms. Antioxidant enzymes as well as non-enzymatic compounds such as reduced glutathione (GSH), ascorbic acid, and α-tocopherol all help to cope with the potential damage caused by oxidative stress [10]. Free radicals produced by KBrO3 are associated with the activation of telomerase activity. This activation induces neoplasia, hyperplasia and tumor formation [11,12], increases the number and size of argyrophilic nucleolar organizer regions (AgNORs) and can be utilized as an indicator of genotoxicity to complement other histological procedures [13,14].
Rutin (Figure 1) works as a scavenger of reactive oxidative species (ROS) by donating hydrogen atoms to peroxy radicals, superoxide anions, and singlet oxygen and hydroxyl radicals; it also functions as a terminator and chelator of metal ions that are capable of oxidizing lipid peroxidation [15-17]. Rutin has been shown to function as an anti-cancer, anti-viral, anti-bacterial and anti-inflammatory agent. It is also used to treat cardiovascular and neurodegenerative disorders because of its appreciable free radical-scavenging and anti-oxidant capacities [18-20]. Additionally, studies suggest that rutin alters signal transduction, causes activation of transcription factors and gene expression, and may also protect DNA by interacting with carcinogens that have escaped detoxification processes [21-23].
Figure 1.
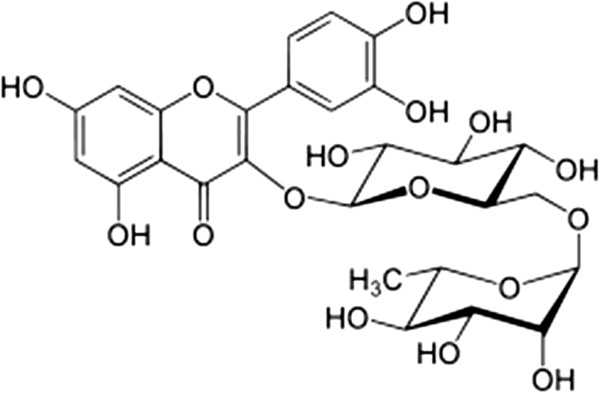
The structure of rutin.
The present study was therefore aimed at investigating the effect of rutin on KBrO3-induced nephrotoxicity in male Sprague–Dawley rats by the determination of biochemical parameters and by histological examination.
Methods
Chemicals
Reduced glutathione (GSH), oxidized glutathione (GSSG), glutathione reductase, gamma-glutamyl p-nitroanilide, glycylglycine, bovine serum albumin (BSA), 1,2-dithio-bis nitro benzoic acid (DTNB), 1-chloro-2,4-dinitrobenzene (CDNB), reduced nicotinamide adenine dinucleotide phosphate (NADPH), CCl4, flavine adenine dinucleotide (FAD), glucose-6-phosphate, Tween-20, 2,6-dichlorophenolindophenol, thiobarbituric acid (TBA), picric acid, sodium tungstate, sodium hydroxide, trichloroacetic acid (TCA) and perchloric acid (PCA) were purchased from Sigma Chemicals Co. St. Louis, USA.
Animals and treatment
Six-week-old male Sprague–Dawley rats weighing 180±10 g were provided with food and water ad libitum and kept at 20–22°C on a 12-h light–dark cycle. All experimental procedures involving animals were conducted in accordance with the guidelines of National Institutes of Health (NIH guidelines Islamabad, Pakistan). The study protocol was approved by Ethical Committee of Quaid-i-Azam University, Islamabad. The rats were acclimatized to laboratory condition for 7 days before commencement of experiment.
The following experimental groups (n = 06 rats per group) were studied.
Group I: (control); the animals were remained untreated.
Group II: animals were treated with saline (0.5 ml/kg bw; 0.9% NaCl) intragastric twice a week for four weeks.
Group III: was treated with KBrO3 (20 mg/kg bw; aqueous) intragastric twice a week for four weeks.
Group IV: was treated with KBrO3 (20 mg/kg bw; aqueous) intragastric and rutin (50 mg/kg bw) intragastric twice a week for four weeks.
Group V: rats were treated with KBrO3 (20 mg/kg bw; aqueous) intragastric and rutin (70 mg/kg bw) intragastric twice a week for four weeks.
Group VI: was treated with rutin (70 mg/kg bw) intragastric twice a week for four weeks.
In addition all the rats were provided free access of drinking water and food. After the completion of dosages rats were kept individually in metabolic cages for 24 h; collect the urine and volume was determined. All the animals were sacrificed; blood was drawn prior to the excision of organ. The serum was stored at −80°C after separation until it was assayed as described below. Half of kidney tissues were treated with liquid nitrogen and stored at −80°C for further enzymatic analysis while the other portion was processed for histology.
Analysis of urine
Urine samples were assayed for pH, specific gravity, urea, creatinine, protein, albumin, urobilinogen, red blood cells (RBCs) and white blood cells (WBCs) count by using standard diagnostic kits (MediScreen Urine Strips, Orgenics, France) and standard AMP diagnostic kits (Stattogger Strasse 31b 8045 Graz, Austria) . Urinary creatinine clearance was estimated by using the formula:
Where U: concentration of creatinine in urine
P: concentration of creatinine in plasma
V: 24 h of urinary volume
T: Time in minutes
Analysis of serum
Analysis of serum for blood urea nitrogen (BUN), nitrite, creatinine, total protein, globulin, and albumin was estimated by using standard AMP diagnostic kits (Stattogger Strasse 31b 8045 Graz, Austria).
Assessment of antioxidant profile
Renal tissue was homogenized in 10 volume of 100 mmol KH2PO4 buffer containing 1 mmol EDTA (pH 7.4) and centrifuged at 12,000 × g for 30 min at 4°C. The supernatant was collected and used for enzymatic studies. Protein concentration of kidney tissue supernatant was determined by the method of Lowry et al. [24] using crystalline BSA as standard.
Catalase assay (CAT)
CAT activities were determined by the method of Chance and Maehly [25] with some modifications. The reaction solution of CAT activities contained: 2.5 ml of 50 mmol phosphate buffer (pH 5.0), 0.4 ml of 5.9 mmol H2O2 and 0.1 ml enzyme extract. Changes in absorbance of the reaction solution at 240 nm were determined after one min. One unit of CAT activity was defined as an absorbance change of 0.01 as units/min.
Superoxide dismutase assay (SOD)
SOD activity of lung tissues was estimated by the method of Kakkar et al. [26]. Reaction mixture of this method contained: 0.1 ml of phenazine methosulphate (186 μmol), 1.2 ml of sodium pyrophosphate buffer (0.052 mmol; pH 7.0), 0.3 ml of supernatant after centrifugation (1500 × g for 10 min followed by 10000 × g for 15 min) of lung homogenate was added to the reaction mixture. Enzyme reaction was initiated by adding 0.2 ml of NADH (780 μmol) and stopped after 1 min by adding 1 ml of glacial acetic acid. Amount of chromogen formed was measured by recording color intensity at 560 nm. Results are expressed in units/mg protein.
Estimation of lipid peroxidation assay (TBARS)
The assay for lipid peroxidation was carried out with modified method of Iqbal et al. [27]. The reaction mixture in a total volume of 1.0 ml contained: 0.58 ml phosphate buffer (0.1 mol; pH 7.4), 0.2 ml homogenate sample, 0.2 ml ascorbic acid (100 mmol), and 0.02 ml ferric chloride (100 mmol). The reaction mixture was incubated at 37°C in a shaking water bath for 1 h. The reaction was stopped by addition of 1.0 ml 10% trichloroacetic acid. Following addition of 1.0 ml 0.67% thiobarbituric acid, all the tubes were placed in boiling water bath for 20 min and then shifted to crushed ice-bath before centrifuging at 2500 × g for 10 min. The amount of malonaldehyde formed in each of the samples was assessed by measuring optical density of the supernatant at 535 nm using spectrophotometer against a reagent blank. Tetramethoxypropane was used as an external standard. The results were expressed as nmol of TBARS/min/mg tissue protein.
Glutathione-S-transferase assay (GST)
The reaction mixture of glutathione-S-transferase activity consisted of 1.475 ml phosphate buffer (0.1 mol, pH 6.5), 0.2 ml reduced glutathione (1 mmol), 0.025 ml (CDNB; 1 mmol) and 0.3 ml of tissue homogenate in a total volume of 2.0 ml. The changes in the absorbance were recorded at 340 nm and enzymes activity was calculated as nmol CDNB conjugate formed/min/mg protein using a molar extinction coefficient of 9.6 × 103M-1cm-1[28].
Glutathione reductase assay (GSR)
Glutathione reductase activity was determined with the protocol of Carlberg and Mannervik [29]. The reaction mixture consisted of 1.65 ml phosphate buffer: (0.1 mol; pH 7.6), 0.1 ml EDTA (0.5 mmol), 0.05 ml oxidized glutathione (1 mmol), 0.1 ml NADPH (0.1 mmol) and 0.1 ml of homogenate in a total volume of 2 ml. Enzyme activity was quantitated at 25°C by measuring disappearance of NADPH at 340 nm and was calculated as nmol NADPH oxidized/min/mg protein using molar extinction coefficient of 6.22 × 103 M-1cm-1.
Glutathione peroxidase assay (GSH-px)
Glutathione peroxidase activity was assayed by the method of Mohandas et al. [30]. The reaction mixture consisted of 1.49 ml phosphate buffer (0.1 mol; pH 7.4), 0.1 ml EDTA (1 mmol), 0.1 ml sodium azide (1 mmol), 0.05 ml glutathione reductase (1 IU/ml), 0.05 ml GSH (1 mmol), 0.1 ml NADPH (0.2 mmol), 0.01 ml H2O2 (0.25 mmol) and 0.1 ml of homogenate in a total volume of 2 ml. The disappearance of NADPH at 340 nm was recorded at 25°C. Enzyme activity was calculated as nmol NADPH oxidized/min/mg protein using molar extinction coefficient of 6.22 × 103 M-1cm-1.
Reduced glutathione assay (GSH)
1.0 ml sample of homogenate was precipitated with 1.0 ml of (4%) sulfosalicylic acid. The samples were kept at 4°C for 1 h and then centrifuged at 1200 × g for 20 min at 4°C. The total volume of 3.0 ml assay mixture contained: 0.1 ml filtered aliquot, 2.7 ml phosphate buffer (0.1 mol; pH 7.4) and 0.2 ml DTNB (100 mmol). The yellow color developed was read immediately at 412 nm on a SmartSpecTM plus Spectrophotometer. It was expressed as μmol GSH/g tissue [31].
DNA fragmentation assay
DNA fragmentation assay was conducted using the procedure of Wu et al. [32] with some modifications. The tissue (50 mg) was homogenized in 10 volumes of a TE solution pH 8.0 (5 mmol Tris–HCl, 20 mmol EDTA) and 0.2% triton X-100. 1.0 ml aliquot of each sample was centrifuged at 27,000 × g for 20 min to separate the intact chromatin (pellet, B) from the fragmented DNA (supernatant, T). The pellet and supernatant fractions were assayed for DNA content using a freshly prepared DPA (Diphenylamine) solution for reaction. Optical density was read at 620 nm with (SmartSpecTM Plus Spectrophotometer catalog # 170–2525) spectrophotometer. The results were expressed as amount of % fragmented DNA by the following formula;
AgNORs count
Silver staining technique was used according to the Trere et al. [33]. The AgNORs technique was performed on dried slides as follows; unstained fixed slides were dewaxed by dipping for 3 minutes in xylene. After complete removal of wax the slides were hydrated in decrease ethanol concentration (90, 70 and 50%) and washed in distilled water for 10 min and dried in an oven. After drying slides were treated with one drop of colloidal solution (2% gelatin and 1% formic acid) and two drops of 50% AgNO3 solution onto the slide and incubated at 35°C for about 8–12 min. The progressive staining was followed under microscope to get golden colored nuclei and brown/black NORs. Then, the slide was washed in distilled water, treated for 1 min with 1% sodium thiosulphate at room temperature to stop the reaction, and washed in tap water. The cells were examined under light microscope at 100 × magnification and number of AgNORs was counted per cell.
DNA ladder assay
DNA was isolated by using the methods of Gilbert et al. [34]. To estimate DNA damages. 5 μg of rat DNA was separately loaded in 1.5% agarose gel containing 1.0 μg/ml ethidium bromide including DNA standards (0.5 μg per well). Electrophoresis was performed for 45 min at 100 Volt. After electrophoresis gel was studied under gel doc system and was photographed through digital camera.
RT-PCR (telomerase assay)
Telomerase activity was determined by the protocol of Wen et al. [35] with some modifications.100 mg kidney was washed in ice-cold wash buffer (10 mmol Hepes-KOH pH 7.5, 1.5 mmol MgCl2, 10 mM KCl, 1 mM dithiothreitol, 20 μl RNAs inhitors), and homogenised in 200 μl ice cold lysis buffer. The homogenate was incubated on ice for 30 min and then centrifuged at 10,000 ×g for 30 min at 4°C. PCR reaction mixture (total 48 μl) consisted of 36.6 μl DEPC treated water, 2 μl (6 μg protein) extract, 5 μl 10x TRAP reaction solution, 2 μl (50 μmol) each dNTP, 0.4 μl (2 U) Taq DNA polymerase, and 2 μl (0.1 μg) of TS primer sequence (5'-AATCCGTCGAGCAGAGTT-3'). The PCR reaction mixture was incubated at 25°C in water bath for 30 min for extension of TS primer. CX primer sequence (5'-CCCTTACCCTTACCCTTACCCTAA-3') 2 μl (0.1 μg) was added. The reaction mixture (total 50 μl) was subjected to PCR cycles (25) at 94°C for 30s, 55°C for 30s, and 72°C for 90s (then 10 min for the final step). After amplification 5μl of loading dye (0.25% bromophenol blue, 0.25% xylenocyanol and 50% glycerol) was mixed to each PCR product and 25 μl of each sample were loaded onto a 12.5% non-denaturing polyacrylamide gel. After complete running of gel was fixed in fixing solution (0.5% acetic acid, 10% ethanol) and stained with 0.2% AgNO3 for 10 min, followed by 15 min incubation in developing solution (0.1% formaldehyde and 3% NaOH) and then photographed.
Histopathalogical determination
For microscopic evaluation tissues were fixed in a fixative (absolute ethanol 60%, formaldehyde 30%, and glacial acetic acid 10%) and embedded in paraffin, sectioned at 4 μm and subsequently stained with hematoxylin/eosin. Sections were studied under light microscope (DIALUX 20 EB) at 40 and 100 magnifications. Slides of all the treated groups were studied and photographed. A minimum 12 fields of each section were studied and approved by pathologist without saying of its treatment nature.
Statistical analysis
The values were expressed as the mean ± SEM for the 06 rats in each group. Differences between groups were assessed by one-way analysis of variance (ANOVA) using the Statistical Package for Social Sciences (SPSS) software package for Windows (version 13.0). Post hoc testing was performed for intergroup comparisons using the least significant difference (LSD) test. A value corresponding to P<0.05 was deemed to be statistically significant.
Results
Effects of rutin on urine profile
Reactive oxygen species (ROS) especially nephrotoxic chemicals effects the urinary profile of kidney. Table 1 shows the changes in renal profile including pH, specific gravity, RBCs and WBCs count, creatinine, creatinine clearance, urea, urobilinogen, protein, and albumin level. Administration of nephrotoxic KBrO3 treatment significantly (P<0.01) increased the level of specific gravity, RBCs and WBCs count, creatinine, urea, urobilinogen, protein, and albumin level whereas decreased (P<0.01) the pH and creatinine clearance as compared to the control group. Treatment of rutin attenuated the KBrO3 intoxication, dose dependently, and decreased (P<0.01) the specific gravity, RBCs and WBCs count, urea, creatinine, urobilinogen, protein and albumin while increased the pH and creatinine clearance of urine. Rats treated with rutin (70 mg/kg bw) alone did not statistically (P>0.05) change the parameters studied to that of the control group.
Table 1.
Effects of rutin on physical and biochemical parameters of urine
| Treatment | pH | Specific gravity | Urea (mg/dl) | Creatinine (mg/dl) | Creatinine clearance (ml/min) |
|---|---|---|---|---|---|
| Control |
7.2±0.07++ |
1.2±0.02++ |
93.0±2.0++ |
38.0±1.34++ |
3.4±1.3++ |
| Saline (0.5 ml/kg bw; 0.9% NaCl) |
7.1±0.04++ |
1.1±0.01++ |
91.0±3.25++ |
36.2±0.09++ |
3.3±0.8++ |
| KBrO3 (20 mg/kg bw) |
6.3±0.08** |
1.8±0.05 ** |
195.30±3.98++ |
86.7±3.89** |
1.1±1.9** |
| KBrO3+Rutin (50 mg/kg bw) |
6.7±0.10++ |
1.2±0.07++ |
110.0±2.01*++ |
53.5±1.52++ |
2.7±1.6*++ |
| KBrO3+Rutin (70 mg/kg bw) |
7.0±0.03++ |
1.2±0.04++ |
103.1±3.5++ |
46.3±2.31++ |
3.1±1.3++ |
| Rutin alone (70 mg/kg bw) |
7.1±0.04++ |
1.2±0.06++ |
92.2±3.0++ |
39.1±1.03++ |
3.5±0.3++ |
|
Treatment |
RBC/μl |
WBC/μl |
Protein (mg/dl) |
Albumin (mg/dl) |
Urobilinogen (mg/dl) |
| Control |
0.02±0.00++ |
16.0±1.81++ |
42.8±2.3 ++ |
18.2±2.76++ |
2.47±0.22++ |
| Saline (0.5 ml/kg bw; 0.9% NaCl) |
0.03±0.05++ |
19.6±1.15++ |
43.0±3.2++ |
19.0±3.9++ |
2.43±0.21++ |
| KBrO3 (20 mg/kg bw) |
25.7±1.84** |
68.0±5.0** |
100.0±1.2** |
34.7±1.86** |
15.7±1.89** |
| KBrO3+Rutin (50 mg/kg bw) |
8.8±0.15**++ |
41.00±2.25**++ |
55.0±1.2++ |
24.7±1.86++ |
5.67±1.89++ |
| KBrO3+Rutin (70 mg/kg bw) |
2.3±0.12**++ |
22.5±1.3++ |
45.7±2.1++ |
20.6±1.25++ |
3.62±1.23++ |
| Rutin alone (70 mg/kg bw) | 2.0±0.95**++ | 18.66±0.95++ | 39.0±2.3++ | 17.33±3.71++ | 2.933±0.25++ |
Mean ± SEM (n=6 number).
*, ** indicate significance from the control group at P<0.05 and P<0.01 probability level.
++ indicate significance from the KBrO3 group at P<0.01 probability level.
Effects of rutin on serum profile
Table 2 shows the assessment of serum biomarkers i.e. concentration of nitrite, creatinine, BUN, total protein; albumin and globulin indicate the functional integrity of the kidneys. KBrO3 administration significantly (P<0.01) increased the level of nitrite, creatinine while significantly (P<0.01) decreased the total protein, albumin and globulin as compared to control group. Serum level of these parameters were significantly (P<0.01) improved by administration of rutin as compared to KBrO3 treated rats. However, more restoration effects on studied parameters were determined at the higher dose (70 mg/kg bw) of rutin. These parameters were statistically (P>0.05) remained unchanged with the treatment of rutin (70 mg/kg bw) alone as compared to the control group of rat.
Table 2.
Effects of rutin on serum profile
| Treatment | Nitrite (μmol/ml) | Creatinine (mg/dl) | BUN (mg/dl) | Protein (mg/dl) | Globulin (mg/dl) | Albumin (mg/dl) |
|---|---|---|---|---|---|---|
| Control |
25.7±1.4++ |
36.55±0.84++ |
41.0±2.04++ |
42.83±2.26 ++ |
24.67±1.33++ |
18.17±2.76++ |
| Saline (0.5 ml/kg bw; 0.9% NaCl) |
24.9±1.7++ |
34.9±0.24++ |
43.0±2.56++ |
43.6±2.0 ++ |
23.07±1.83++ |
17.10±2.25++ |
| KBrO3 (20 mg/kg bw) |
72.12±3.4** |
90.0±7.06** |
125.9±3.5** |
23.00±1.18** |
12.33±1.54** |
10.67±1.86** |
| KBrO3+Rutin (50 mg/kg bw) |
32.0±2.6++ |
50.3±1.58++ |
65.8±5.58++ |
33.33±1.54**++ |
16.17±1.14**++ |
13.16±1.42*++ |
| KBrO3+Rutin (70 mg/kg bw) |
25.3±2.3++ |
43.6±2.15++ |
52.6±4.62++ |
37.65±2.16++ |
20.42±1.64++ |
16.53±1.26++ |
| Rutin alone (70 mg/kg bw) | 22.3±1.9++ | 35.17±1.9++ | 40.17±3.75++ | 38.00±2.27++ | 19.67±2.01++ | 17.33±3.71++ |
Mean ±SEM (n=6 number).
** indicate significance from the control group at P<0.01 probability level.
++ indicate significance from the KBrO3 group at P<0.01 probability level.
Effects of rutin on antioxidant profile
The results regarding the protective effects of rutin against the toxic affect of KBrO3 in rat on kidney protein and activities of antioxidant enzymes such as CAT, SOD, GSH-Px, GSR and GST are shown in Table 3. Activities of antioxidant enzymes such as CAT, SOD, GSH-Px, GSR and GST were reduced (P<0.01) by treatment of KBrO3 as compared to control group. This reduction in enzymes activity was reversed significantly (P<0.01), in a concentration dependent way, by the treatment of rutin as compared to the KBrO3 group. Alteration in the renal enzyme activities with the treatment of rutin (70 mg/kg bw) alone was statistically (P>0.05) remained unchanged as compared to the control group of rat.
Table 3.
Effects of rutin on renal antioxidant profile
| Treatment | CAT (U/min) | SOD (U/mg protein) | GSH-Px (nmol/mg protein) | GSR (nmol/min /mg protein) | GST (nmol/min/mg protein) |
|---|---|---|---|---|---|
| Control |
18.2±2.2++ |
19.05±1.54++ |
45.33±1.65++ |
203.3±10.3 ++ |
123.8±13.3++ |
| Saline (0.5 ml/kg bw; 0.9% NaCl) |
18.5±2.6++ |
19.75±1.4++ |
43.5±1.35++ |
200.6±8.3 ++ |
121.8±10.3++ |
| KBrO3 (20 mg/kg bw) |
7.5±0.85** |
7.58±2.08** |
23.35±3.08 ** |
114.5±20.7** |
53.8±26.1** |
| KBrO3+Rutin (50 mg/kg bw) |
11.5±2.2*++ |
12.83±1.87*++ |
34.91±5.54 *++ |
158.0±10.5*++ |
104.8±11.5 ++ |
| KBrO3+Rutin (70 mg/kg bw) |
16.5±2.6++ |
17.62±2.14++ |
41.26±4.6++ |
184.5±11.3++ |
116.5±14.3++ |
| Rutin alone (70 mg/kg bw) | 17.37±1.17++ | 20.72±1.72++ | 46.06±2.12++ | 207.3±13.2++ | 126.2±21.5++ |
Mean ± SEM (n=6 number).
*, ** indicate significance from the control group at P<0.05 and P<0.01 probability level.
++ indicate significance from the KBrO3 group P<0.01 probability level.
Effects of rutin on TBARS, GSH, DNA fragmentation and AgNORs
Table 4 shows the changes of protection by rutin versus the KBrO3 intoxication of rat on TBARS, GSH, DNA fragmentation and AgNORs count in kidney of rat. Treatment of KBrO3 significantly (P<0.01) increased the contents of TBARS, DNA fragmentation and AgNORs count while significantly (P<0.01) decreased the content of GSH in kidneys as compared to control group. Concentration of TBARS, AgNORs count, and DNA injuries was significantly decreased (P<0.01) by administration of rutin, in a dose dependent way, in rat treated with KBrO3. Level of renal GSH was significantly (P<0.01) increased by administration of rutin as compared to the KBrO3 treated group. However these effects were more pronounced at the higher dose of rutin (70 mg/kg bw). Statistically nonsignificant (P>0.05) alterations on TBARS, GSH, DNA fragmentation and AgNORs count in kidney of rat was determined with rutin (70 mg/kg bw) alone as compared to the control group.
Table 4.
Effects of rutin on renal level of TBARS, GSH, AgNORs and DNA fragmentation
| Treatment | TBARS (nmol/min/mg protein) | GSH (μmol/g tissue) | DNA fragmentation% | AgNORs (NORs/cell) |
|---|---|---|---|---|
| Control |
8.17±0.93++ |
1.72±0.157++ |
7.75±0.834++ |
1.86±0.10++ |
| Saline (0.5 ml/kg bw; 0.9% NaCl) |
8.2±0.73++ |
1.68±0.17++ |
7.65±0.94++ |
1.81±0.12++ |
| KBrO3 (20 mg/kg bw) |
20.90±1.91** |
0.73±0.15** |
54.42±1.02** |
8.08±0.52** |
| KBrO3+Rutin (50 mg/kg bw) |
15.17±1.20++ |
1.28±0.19*++ |
27.85±1.26++ |
2.23±0.09++ |
| KBrO3+Rutin (70 mg/kg bw) |
10.25±1.35++ |
1.58±0.16++ |
9.25±1.35++ |
2.12±0.06++ |
| Rutin alone (70 mg/kg bw) | 9.17±1.06++ | 1.760±0.0817++ | 8.08±1.17++ | 1.95±0.13++ |
Mean ±SE M (n=6 number).
** indicate significance from the control group at P<0.01 probability level.
++ indicate significance from the KBrO3 group at P<0.01 probability level.
Effects of rutin on percent increase in body weight, absolute kidney weight and relative kidney weight
Protective effects of different dosages of rutin against KBrO3 administration to rat on percent increase in body weight, absolute kidney weight and relative kidney weight are shown in Table 5. Administration of KBrO3 to rats significantly increased (P<0.01) the absolute kidney weight, relative kidney weight while significantly decreased (P<0.01) the percent increase in body weight for four experimental weeks as compared to the control group. Administration of rutin consistently restored (P<0.01) the percent increase in body weight, kidney weight and relative kidney weight as compared to the KBrO3 group. More protective effects of rutin for the percent increase in body weight, kidney weight and relative kidney weight against the KBrO3 intoxication to rats were determined at the higher concentration of rutin (70 mg/kg bw). Administration of rutin alone did not cause any significant (P>0.05) change in the percent increase in body weight, absolute kidney weight and relative kidney weight as compared to the control group.
Table 5.
Effects of rutin on body weight, kidney weight and relative kidney weight of rat
| Treatment |
Percent increase in body weight (g) after |
Absolute kidney weight (g) | Relative kidney weight (% to bw) | |||
|---|---|---|---|---|---|---|
| Istweek | 2ndweek | 3rdweek | 4rthweek | |||
| Control |
6.3±0.6++ |
13.4±1.3++ |
21.3±2.3++ |
28.2±3.4++ |
4.0±0.08++ |
0.040±0.00072++ |
| Saline (0.5 ml/kg bw; 0.9% NaCl) |
6.6±0.5++ |
13.8±2.1++ |
22.4±2.6++ |
27.6±3.2++ |
4.0±0.08++ |
0.040±0.00072++ |
| KBrO3 (20 mg/kg bw) |
3.4±0.3** |
7.6±1.5** |
10.2±1.6** |
14.4±2.6** |
4.9±0.60** |
0.049±0.00125** |
| KBrO3+Rutin (50 mg/kg bw) |
4.4±0.3*++ |
10.4±1.2*++ |
15.3±1.4*++ |
19.6±2.4++ |
4.50±0.12++ |
0.045±0.00079++ |
| KBrO3+Rutin (70 mg/kg bw) |
4.9±0.4++ |
12.6±1.4++ |
19.6±1.5++ |
23.4±3.2++ |
4.23±0.14++ |
0.042±0.0014++ |
| Rutin alone (70 mg/kg bw) | 6.2±0.4++ | 14.8±1.3++ | 22.7±1.6++ | 27.8±2.4++ | 4.12±0.06++ | 0.041±0.00049++ |
Mean ±SEM (n=6 number).
*, ** indicate significance from the control group at P<0.05 and P<0.01 probability level, respectively.
++ indicate significance from the KBrO3 group at P<0.01 probability level.
Effect of rutin on DNA damages (DNA ladder assay)
KBrO3 forming DNA-free radical adduct, induces DNA damages in the kidneys of rats. DNA ladder assay of kidneys showed the severe DNA damages in the KBrO3 treated group of rat. However, administration of rutin to KBrO3 treated animals reduced the DNA damages, dose dependently, as shown by DNA banding patterns of different groups of rutin (50 and 70 mg/kg bw) as compared to KBrO3 group (Figure 2).
Figure 2.
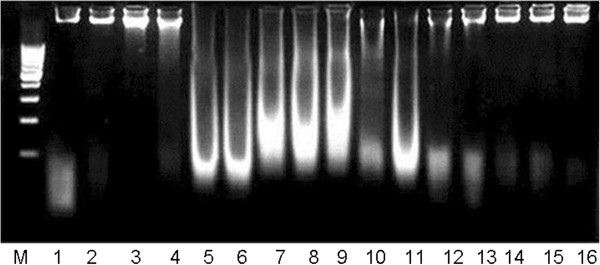
Agarose gel showing KBrO3-induced DNA damages in kidneys and preventive effects of rutin in various experimental groups of rat. Lanes (from left) DNA marker (M), Control (1–2), Saline (0.9% NaCl) 0.5 ml/kg bw (3–4), KBrO3 20 mg/kg bw (5–8), KBrO3 (20 mg/kg bw) + rutin 50 mg/kg bw (9–11), KBrO3 (20 mg/kg bw) + rutin 70 mg/kg bw (12–13), rutin alone 70 mg/kg bw (14–16).
RT-PCR (telomerase enzyme assay)
Telomerase enzyme assay was used to find out the telomerase enzyme activity in various groups of this study (Figure 3). Presence of a single band (primer) in lane (1–2; control group) and in lane (3–6; KBrO3 + rutin groups) showed that amplification was absent, and indicated the protective effects of rutin while KBrO3 treated group showed positive telomerase activity (lane 7–8) having two or more DNA bands.
Figure 3.
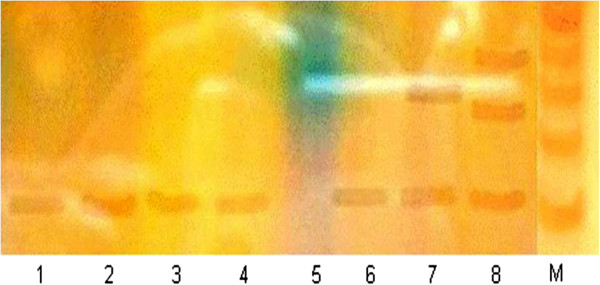
Polyacrylamide gel shows the telomerase enzyme activity in various groups of the study. Lane (1–2) control group, Lane (3–4) KBrO3 (20 mg/kg bw) + rutin (50 mg/kg bw) group, Lane (5–6) KBrO3 (20 mg/kg bw) + rutin (70 mg/kg bw), Lane (7–8) KBrO3 (20 mg/kg bw) group.
Effects of rutin on histological changes of kidneys
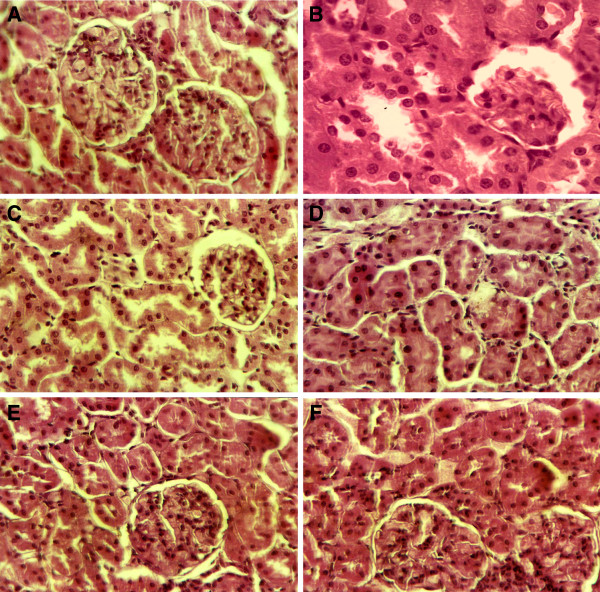
The histological changes were graded and summarized in Table 6. The sections of control group showed normal histology including normal glomerulus, bowman capsule, distal and proximal convoluted tubules. Marked histological changes were observed in cortex of kidneys in KBrO3-treated rats. The cross section showed tubular degeneration, tubular congestion, tubular dilatation and glomerular injuries in KBrO3-treated rats (Figure 4). Treatment of rutin to KBrO3 treated rats markedly recovered the toxic changes near to the control rat. Histology of the kidneys showed normal glomerulus, bowman capsule, reversed the tubular degeneration, congestion and dilatation and prevented interstitial edema and capillary congestion in a dose dependent way. However, treatment of rutin (70 mg/kg bw) alone did not induce histopathological changes in kidneys.
Table 6.
Effects of rutin on renal histopathology of rat
| Treatment | Tubular dilatation | Tubular necrosis | Tubular cell swelling | Tubular congestion | Glomerular injuries |
|---|---|---|---|---|---|
| Control |
- |
- |
- |
- |
- |
| Saline (0.5 ml/kg bw; 0.9% NaCl) |
- |
- |
- |
- |
- |
| KBrO3 (20 mg/kg bw) |
++ |
++ |
++ |
−/+ |
−/+ |
| KBrO3+Rutin (50 mg/kg bw) |
−/+ |
−/+ |
−/+ |
- |
- |
| KBrO3+Rutin (70 mg/kg bw) |
- |
- |
- |
- |
- |
| Rutin alone (70 mg/kg bw) | - | - | - | - | - |
-, normal; −/+, mild; ++, medium damaged.
Figure 4.
H & E stain. Representative section of kidney, Control group; normal tubules, glomerulus and bowman capsule (A), KBrO3 (20 mg/kg bw) group; injuries in glomeulus and bowman capsule (B), KBrO3 (20 mg/kg bw) group, tubular necrosis, tubular cell swelling, tubular dilatation (C), KBrO3 (20 mg/kg bw) group; tubular congestion (D), KBrO3 (20 mg/kg bw) + rutin (50 mg/kg bw) group (E), KBrO3 (20 mg/kg bw) + rutin (70 mg/kg bw) group (F).
Discussion
Rutin modulates several biological functions and exhibits anti-cancer, anti-viral, anti-bacterial and anti-inflammatory activities due to its appreciable free radical-scavenging and anti-oxidant capacities [18-20]. At the end of the study period, KBrO3 treatment was found to have decreased the percent increase in body weight but increased the absolute kidney weight and relative kidney weight as compared with the control group. Similar alterations for these parameters with KBrO3 have been determined previously [36-38]. In the present study, the observations made with respect to percent increase in body weight, absolute kidney weight and relative kidney weight in male Sprague–Dawley rats appeared to suggest that rutin can alleviate KBrO3-induced toxicity in these animals.
Urinalysis provides important clues about acid–base balance and kidney function [39]. Urobilinogen is a conjugated product of bilirubin, which passes through the bile duct and is metabolized in the intestine [40,41]. High levels of urobilinogen, urea, creatinine, protein and albumin in urine reflect the kidney dysfunction and renal injuries induced by KBrO3 treatment [42,43]. Increased specific gravity is a basic symptom of dehydration, renal artery steatosis, necrosis, and decreased blood flow to the kidneys. In addition, increased RBC and WBC counts in the urine of KBrO3-treated rats suggested severe injuries to renal tissues. Higher levels of protein and the number of RBCs and WBCs might have also contributed to the values of specific gravity obtained in the present study. This increase in specific gravity consequently reflects the high degree of damage to kidney tissue. The hematuria and proteinuria observed in the present study could be related to necrosis and kidney dysfunction [44]. Ogawa et al. [45] also found that the glomerular capillary wall is permeable to low-molecular-weight (LMW) proteins. Therefore, an appreciably high level of proteinuria indicates the leakage of LMW proteins. The oxidative stress induced by KBrO3 might promote the formation of various vasoactive mediators that can affect renal function directly by initiating renal vasoconstriction or decreasing the glomerular capillary ultrafiltration coefficient. This action will reduce the glomerular filtration rate, leading to proteinuria. Rutin administration to rats treated with KBrO3 ameliorated the toxicity of KBrO3 in kidneys to restore the level of the studied parameters in a concentration-dependent manner. These results suggest that rutin can be used as renoprotective agent against KBrO3 induced toxicity.
The present study revealed that KBrO3 administration caused marked increases in the serum levels of creatinine, urobilinogen, BUN, total bilirubin and direct bilirubin, as reported previously [23,46]. In addition, elevated levels of urinary albumin and reduced levels of serum albumin in KBrO3-treated rats might have resulted from considerable leakage due to injuries to glomeruli and tubules. The results suggested that rutin prevented KBrO3-induced toxicity, and that the levels of creatinine, urobilinogen, BUN, total bilirubin, and direct bilirubin could be altered to those seen in the control group. Other studies have shown that different plant extracts can significantly reduce the renal injuries induced through KBrO3 intoxication [12,23,43]. There is a large body of evidence implicating oxidative stress and ROS in the mechanism of KBrO3-induced toxicity in animal models [10]. In the present study, the mean activity of the antioxidant enzymes CAT, SOD, GSH-Px, GST and GSR were found to be significantly lowered in the KBrO3-treated group compared with that of the control group. Lowered activities of these antioxidant enzymes with KBrO3 in in-vivo experimental models have been reported [47]. However, the treatment of rutin with KbrO3 modified the biochemical changes caused by KBrO3 in rat. In the present study, the mean activities of antioxidant enzymes were significantly higher compared with those of the KBrO3-treated group and thus had a potential protective effect.
GSH is a vital extracellular and intracellular protective antioxidant against oxidative stress. It reduces hydrogen peroxides and hydroperoxides by its redox and detoxification reactions, and protects protein thiol groups from oxidation. This tripeptide is present in high concentrations in kidney cells. In the present study, the mean level of GSH upon KBrO3 treatment was depleted in the kidneys compared with that seen in the control group. Following decreases in the level of GSH, oxidative stress increases and, thereafter, cell damage occurs [47]. In the present study, the animals receiving KBrO3 showed glomerular injuries, tubular necrosis, tubular dilatation, tubular cell swelling and tubular brush border loss. Endogenous levels of GSH were found to be increased with an accompanying increase in the mean activities of GSH-Px, GST and GSR with rutin to that of the KBrO3-treated group. By the catalyses of GSH-Px, GSH is oxidized to GSSG, which can then be reduced back to GSH by GSR. GSH is also a cofactor for the phase-II enzyme GST, which confers protection against toxic chemicals by catalyzing the formation of GSH-electrophile conjugates [48].
Free radicals and reactive oxygen species mediate the propagation of peroxidation of polyunsaturated fatty acids, this cascade can be prevented through enzymatic and non-enzymatic antioxidants. Increased TBARS concentration of renal tissues in KBrO3 treated rat may be result of increased oxidative stress. TBARS, the final metabolite of peroxidized polyunsatured fatty acids [49], considered as a late biomarker of oxidative stress [50-52], not only translate reactive oxygen species into active chemicals but also magnifies the function of reactive oxygen species through the chain reaction, inducing alterations in cellular and functional impairment [53], and serves to indicate the presence of free radicals, lipid peroxide formation [54,55]. The increment in lipid peroxidation as assessed by the elevated levels of TBARS following KBrO3 administration has been well documented in other studies [39,56]. This may be the consequence of an increment in the formation of oxygen free radicals (generated by KBrO3) since antioxidant defense systems are compromised [10]. Lower concentration of TBARS with rutin in KBrO3 treated animals obtained in this study indicates the ameliorating effects of rutin against the oxidative stress induced with KBrO3 in kidneys.
Oxidative damage to DNA is a result of interaction of DNA with reactive oxygen species (ROS), in particular the hydroxyl radical, produces a multiplicity of modifications in DNA; generating strand breaks with various sugar modifications, and release of free bases from nucleic acid. The present study showed increased percentage of DNA fragmentation in the KBrO3 treated rat kidneys. This reveals that KBrO3 induces oxidative stress to the cells thus causing damage to DNA. Increase in oxidative status with KBrO3 in kidneys can permit further oxidation of cellular DNA resulting in the formation of the mutagenic lesion, 8-oxodeoxyguanosine [6,49]. GSH and other sulfhydryls play a major role in the excretion of bromate in the rat kidney [3]. Thiol-mediated oxidation of DNA by bromine oxides and bromine radicals has been well characterized in vitro and are thought to play a role in DNA damage in vivo[5], while extra cellular pools of GSH may be important in protecting target organs from bromate uptake and oxidative DNA damage [39], intracellular GSH may facilitate the formation of DNA reactive metabolites [57].
Telomeres are specialized structures present at the ends of chromosomes possessing highly conserved G rich sequence TTAGGG in all eukaryotic cells that are gradually decreases during cell division of cell cycle. Telomerase is a key enzyme for synthesis of telomeric repeats and is used as a diagnostic tool for malignant tumors [58,59] and in cell lines [60]. Activity of telomerase was found positive in KBrO3 treated kidneys indicating the involvement of oxidative stress in the activation of telomerase. However, telomerase activity was not determined in control as well as in rutin treated groups, which might be due to the presence of various telomerase and cancer inhibitor compounds [61,62]. Similar results have been documented in different studies [63,64]. Renal dysfunction induced by KBrO3 in experimental animals characterized by tubular damage, loss of brush border, tubular necrosis, tubular dilatation, tubular cell swelling and glomerular injuries [44,65]. It is believed that these histopathological changes can alter the capacity of tubular absorption, thus bringing about functional overload of nephrons with subsequent renal dysfunction [12,23], which was normalized by co-treatment with rutin. Similar observations were also observed by co-treatment with caffeic acid phenyl ester [43].
Conclusion
The protective role of rutin at different levels was evaluated in this manuscript. It may contribute its protective effects by erasing the damaging action of potassium bromate at various metabolic cycles, and the repair of DNA damage. The protective potential may involve scavenging potential and antioxidant capacity to ameliorate the KBrO3 induced toxicity. This study substantiated the scientific evidence in favors of its pharmacological use in renal injuries.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RAK made a significant contribution to acquisition of data, analysis, and revising the manuscript for intellectual content. MRK and SS help in data analysis and drafting. The authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Rahmat Ali Khan, Email: rahmatgul_81@yahoo.com.
Muhamad Rashid Khan, Email: mrkhaqau@yahoo.com.
Sumaira Sahreen, Email: Sumaira@gmail.com.
References
- IARC monographs on the evaluation of the carcinogenic risk of chemicals to humans. Some naturally occurring and synthetic food components, furocoumarins and ultraviolet radiation. Lyon, France: IARC Publication No. 40, World Health Organization/IARC; 1986. pp. 207–220. [PubMed] [Google Scholar]
- Deangelo A, George M, Kilburn S, Moore T, Wolf D. Carcinogenicity of potassium bromate administered in the drinking water to male B6C3F1 mice and F344/N rats. Toxicol Pathol. 1998;26:724–729. doi: 10.1177/019262339802600602. [DOI] [PubMed] [Google Scholar]
- Kurokawa Y, Takamura N, Matsuoka C, Imazawa T, Matsushima Y, Onodera H, Hayashi Y. Comparative studies on lipid peroxidation in the kidney of rats, mice, and hamsters and on the effect of cysteine, glutathione, and diethyl maleate treatment on mortality and nephrotoxicity after administration of potassium bromate. J Am Coll Toxicol. 1987;6:489–501. doi: 10.3109/10915818709075694. [DOI] [Google Scholar]
- Chipman J, Davies J, Parsons J, Nair G, O’Neill J, Fawell J. DNA oxidation by potassium bromate; a direct mechanism or linked to lipid peroxidation? Toxicology. 1998;126:93–102. doi: 10.1016/S0300-483X(97)00174-1. [DOI] [PubMed] [Google Scholar]
- Murata M, Bansho Y, Inoue S, Ito K, Ohnishi S, Midorikawa K, Kawanashi S. Requirement of glutathione and cysteine in guanine-specific oxidation of DNA by carcinogenic potassium bromated. Chem Res Toxicol. 2001;14:678–685. doi: 10.1021/tx000209q. [DOI] [PubMed] [Google Scholar]
- Umemura T, Takagi A, Sai K, Hasegawa R, Kurokawa Y. Oxidative DNA damage and cell proliferation in kidneys of male and female rats during 13-weeks exposure to potassium bromate (KBrO3) Arch Toxicol. 1998;72:264–269. doi: 10.1007/s002040050500. [DOI] [PubMed] [Google Scholar]
- Ishidate M, Sofuni T, Yoshikawa K, Hayashi M, Nohmi T, Sawada M, Matsuoka A. Primary mutagenicity screening of food additives currently used in Japan. Food Chem Toxicol. 1994;22:623–636. doi: 10.1016/0278-6915(84)90271-0. [DOI] [PubMed] [Google Scholar]
- Ballmaier B, Epe B. OxidativeDNAdamage induced by potassium bromate under cell-free conditions and in mammalian cells. Carcinogenesis. 1995;16:335–342. doi: 10.1093/carcin/16.2.335. [DOI] [PubMed] [Google Scholar]
- Crosby L, Hyder K, DeAngelo A, Kepler T, Gaskill B, Benavides G, Yoon L, Morgan K. Morphologic analysis correlates with gene expression changes in cultured F344 rat mesothelial cells. Toxicol Appl Pharmacol. 2000;169:205–221. doi: 10.1006/taap.2000.9049. [DOI] [PubMed] [Google Scholar]
- Farombi EO, Alabi MC, Akuru TO. Kolaviron modulates cellular redox status and impairment of membrane protein activities induced by potassium bromate (KBrO3) in rats. Pharmacol Res. 2003;45:63–68. doi: 10.1006/phrs.2001.0907. [DOI] [PubMed] [Google Scholar]
- Grace A, Mabruk M, Leader M, Kay E. Telomerase: does it have an application in tumour pathology? Curr Diagn Pathol. 2006;6:282–285. [Google Scholar]
- Khan RA, Khan MR, Sahreen S. Evaluation of Launaea procumbens use in renal disorders: a rat model. J Ethnopharmacol. 2010;128:452–461. doi: 10.1016/j.jep.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Bocking A, Motherby H, Rohn BL, Bucksteege B, Knops K, Pomjanski N. Early diagnosis of mesothelioma in serous effusions using AgNOR analysis. Annals of Q Cytol Histol. 2001;23:151–160. [PubMed] [Google Scholar]
- Khanna AK, Ansari MA, Kumar M, Khanna A. Correlation between AgNOR quantity in needle biopsy specimens of prostatic adenocarcinomas: correlation with proliferation state, Gleason score, clinical stage, and DNA content. Clinic Mol Pathol. 2001;49:209–213. doi: 10.1136/mp.49.4.m209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry F, Danoux L, Pauly G, Charrouf Z. A plant extract and its pharmaceutical and cosmetic use. Patent Applied WO; 2005. 039610 AI 0506. [Google Scholar]
- Noroozi M, Angerson WJ, Lean ME. Effects of flavonoids and vitamin C on oxidative DNA damage to human lymphocytes. Am J Clin Nutrit. 1998;67:1210–1218. doi: 10.1093/ajcn/67.6.1210. [DOI] [PubMed] [Google Scholar]
- Middleton JE, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharm Reviews. 2000;52:673–751. [PubMed] [Google Scholar]
- Wattenberg LW. Inhibition of carcinogenesis by minor dietary constituents. Cancer Res. 1992;52:2085–2091. [PubMed] [Google Scholar]
- Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Rad Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Kampkotter A, Nkwonkam CG, Zurawski RF, Timpel C, Chovolou Y, Watjen W, Kahl R. Investigations of protective effects of the flavonoids quercetin and rutin on stress resistance in the model organism Caenorhabditis elegans. Toxicology. 2007;234:113–123. doi: 10.1016/j.tox.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Hanasaki Y, Ogawa S, Fukui S. The correlation between active oxygen scavenging and antioxidative effects of Flavonoids. Free Radic Biol Med. 1994;16:845–850. doi: 10.1016/0891-5849(94)90202-X. [DOI] [PubMed] [Google Scholar]
- Srinivasan M, Rukkumani R, Sudheer AR, Menon VP. Ferulic acid, a natural protector against carbon tetrachloride-induced toxicity. Fund Clinic Pharmacol. 2005;19:491–496. doi: 10.1111/j.1472-8206.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- Adewole SO, Salako AA, Doherty OW, Naicker T. Effect of melatonin on carbon tetrachloride-induced kidney injury in Wistar rats. African J Biomed Res. 2007;10:153–164. [Google Scholar]
- Lowry OH, Rosenberg NJ, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Chance B, Maehly AC. Assay of catalase and peroxidases. Methods Enzymol. 1955;24:764–775. [Google Scholar]
- Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- Iqbal SD, Sharma M, Zadeh HR, Hasan N, Abdulla M, Athar M. Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate (Fe-NTA) mediated hepatic injury. Redox Rep. 1996;2:385–391. doi: 10.1080/13510002.1996.11747079. [DOI] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- Carlberg I, Mannervik EB. Glutathione level in rat brain. J Biol Chem. 1975;250:4475–4480. [PubMed] [Google Scholar]
- Mohandas J, Marshal JJ, Duggin GG, Horvath JS, Tiller DJ. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney, possible implications in analgesic nephropathy. Biochem Pharmacol. 1984;33:801–1807. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- Jollow DJ, Mitchell JR, Zampaglione N, Gillete JR. Bromobenzene induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as a hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- Wu B, Ootani A, Iwakiri R, Sakata Y, Fujise T, Amemori S, Yokoyama F, Tsunada S, Fujimoto K. T cell deficiency leads to liver carcinogenesis in Azoxymethane-treated rats. Experimental Biol Med. 2005;231:91–98. doi: 10.1177/153537020623100111. [DOI] [PubMed] [Google Scholar]
- Trere D, Zilbering A, Dittus D, Kim P, Ginsberg PC, Daskal I. AgNOR quantity in needle biopsy specimens of prostatic adenocarcinomas: correlation with proliferation state, Gleason score, clinical stage, and DNA content. Clinic Mol Pathol. 1996;49:209–213. doi: 10.1136/mp.49.4.M209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MT, Haselkorn T, Bunce M, Sanchez JJ, Lucas SB, Jewell LD, Van Marck E, Worobey M. The isolation of nucleic acids from fixed, paraffin- embedded tissues, which methods are useful when? PloS ONE. 2007. p. e537. [DOI] [PMC free article] [PubMed]
- Wen JM, Zhang M, Zheng MH. A non isotopic method for the detection of telomerase activity in tumor tissue: TRAP Silver Staining Assay. J Clinic Mol Pathol. 1998;51:110–112. doi: 10.1136/mp.51.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa Y, Maekawa A, Takahashi M, Hayashi Y. Toxicity and carcinogenicity of potassium bromate-a new renal carcinogen. Environ Health Perspective. 1990;87:309–35. doi: 10.1289/ehp.9087309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas S, Barja G. Resveratrol, Melatonin, Vitamine, and PBN protect against renal oxidative DNA damage induced by kidney carcinogen KBrO3. Free Rad Biol Med. 1999;26:1531–1537. doi: 10.1016/S0891-5849(99)00019-2. [DOI] [PubMed] [Google Scholar]
- Sai K, Umemura T, Takagi A, Hasegawa R, Kurokawa Y. The protective role of glutathione, cysteine and Vitamin C against oxidative DNA damage induced in rat kidney by potassium bromate. Japan J Cancer Res. 1992;83:45–51. doi: 10.1111/j.1349-7006.1992.tb02350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free AH, Free HM. Urine analysis, critical discipline of clinical science. Critical Review Clinic Lab Sci. 2002;3:481–531. doi: 10.3109/10408367209151553. [DOI] [PubMed] [Google Scholar]
- Pels RJ, Bor DH, Woolhandler S, Himmelstein DU, Lawrence RS. Dip sticks urinalysis screening of asymptomatic adults for urinary tract disorders. J Am Med Assoc. 1989;262:1221–1224. doi: 10.1001/jama.262.9.1221. [DOI] [PubMed] [Google Scholar]
- Simerville JA, Maxted WC, Pahira JJ. Urinalysis: A Comprehensive Review. Am. Family Physician. 2005;71:1153–1162. [PubMed] [Google Scholar]
- Ogeturk M, Kus I, Colakoglu N, Zararsiz I, Ilhan N, Sarsilmaz M. Caffeic acid phenyl ester protects kidney against carbon tetrachloride toxicity in rats. J Ethnopharmcol. 2005;97:273–280. doi: 10.1016/j.jep.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Ozturk F, Ucar M, Ozturk IC, Vardi N, Batcioglu K. Carbon tetrachloride induced nephrotoxicity and protective effect of betaine in Sprague dawley rats. Urology. 2003;62:353–356. doi: 10.1016/S0090-4295(03)00255-3. [DOI] [PubMed] [Google Scholar]
- Bhattacharya H, Lun L, Gomez R. Biochemical effects to toxicity of CCl4 on rosy barbs (Puntius conchonius) Our Nature. 2005;3:20–25. [Google Scholar]
- Ogawa M, Mori T, Mori Y, Ueda S, Azemoto R, Makino Y, Wakashin Y, Ohto M, Wakashin M, Yoshida H. Study on chronic renal injuries induced by carbon tetrachloride: selective inhibition of the nephrotoxicity by irradiation. Nephron. 1992;60:68–73. doi: 10.1159/000186707. [DOI] [PubMed] [Google Scholar]
- Bhadauria M, Nirala KS, Shukla S. Multiple treatment of Propolis ameliorates carbon tetrachloide induced liver injuries in rats. Food Chem Toxicol. 2008;46:2703–2712. doi: 10.1016/j.fct.2008.04.025. [DOI] [PubMed] [Google Scholar]
- Khan N, Sharma S, Sultana S. Nigella sativa (black cumin) ameliorates potassium bromate-induced early events of carcinogenesis: diminution of oxidative stress. Human Exp Toxicol. 2003;23:193–203. doi: 10.1191/0960327103ht349oa. [DOI] [PubMed] [Google Scholar]
- Umemura T, Kitamura Y, Kanki K, Maruyama S, Okazaki K, Imazawa T, Nishimura T, Hasegawa R, Nishikawa A, Hirose M. Dose-related changes of oxidative stress and cell proliferation in kidneys of male and female F344 rats exposed to potassium bromate. Cancer Sciences. 2004;95:393–398. doi: 10.1111/j.1349-7006.2004.tb03221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yogi K. Assay for lipid peroxidation in animal tissues by thiobarbituric acid reaction. Analytical Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Draper HH, Squires EJ, Mahmoodi H, Agarwal J, Wu S, Hadley MA. Comparative evaluation of thiobarbituric acid methods for the determination of malondialdehyde in biological materials. Free Radic Biol Med. 1993;15:353–363. doi: 10.1016/0891-5849(93)90035-S. [DOI] [PubMed] [Google Scholar]
- Kim YH, Mun KC, Lee SS, Seo SH, Kwak CS, Park SB, Kim HC. Oxidative damage in renal transplant patients. Transplant Proc. 2000;32:1777–1778. doi: 10.1016/S0041-1345(00)01380-4. [DOI] [PubMed] [Google Scholar]
- Dotan Y, Lichtenberg D, Pinchuk I. Lipid peroxidation cannot be used as a universal criterion of oxidative stress. Prog Lipid Res. 2004;43:200–227. doi: 10.1016/j.plipres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Cheeseman KH. Mechanisms and effects of lipid peroxidation. Mol Aspects Med. 1993;14:191–197. doi: 10.1016/0098-2997(93)90005-X. [DOI] [PubMed] [Google Scholar]
- Banerjee SK, Sood S, Dinda AK, Das TK, Maulik SK. Chronic oral administration of raw garlic protects against isoproterenol-induced myocardial necrosis in rat. Comp Biochem Physiol Part C. 2003;136:377–86. doi: 10.1016/j.cca.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Hazarika A, Sarkar SN, Hajare S, Kataria M, Malik JK. Influence of malathion pretreatment on the toxicity of anilofos in male rats: a biochemical interaction study. Toxicology. 2003;185:1–8. doi: 10.1016/S0300-483X(02)00574-7. [DOI] [PubMed] [Google Scholar]
- Khan N, Sharma S, Alam A, Saleem M, Sultana S. Tephrosia purpurea ameliorates N-diethylnitrosamine and potassium bromate-mediated renal oxidative stress and toxicity in Wistar rats. Pharmacol Toxicol. 2001;88:294–299. doi: 10.1034/j.1600-0773.2001.880602.x. [DOI] [PubMed] [Google Scholar]
- Parsons JL, Chipman JK. The role of glutathione in DNA damage by potassium bromate in vitro. Mutagenesis. 2000;15:311–316. doi: 10.1093/mutage/15.4.311. [DOI] [PubMed] [Google Scholar]
- Lin Y, Uemura H, Fujinami K, Hosaka M, Harada M, Kubota Y. Telomerase activity in primary prostate cancer. Urology. 1997;157:1161–1165. doi: 10.1016/S0022-5347(01)65160-7. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Sugino T, Tahara H, Woodman A, Bolodeoku J, Nargund V, Fellows G, Goodison S, Tahara E, Tarin D. Telomerase activity in bladder carcinoma and its implication for noninvasive diagnosis by detection of exfoliated cancer cells in urine. Cancer. 1997;79:362–369. doi: 10.1002/(SICI)1097-0142(19970115)79:2<362::AID-CNCR20>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Ghoshb U, Bhattacharyya NP, Bhattacharya RK, Roy M. Inhibition of telomerase activity and induction of apoptosis by curcumin in K-562 cells. Mutat Res. 2006;596:81–90. doi: 10.1016/j.mrfmmm.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Sammon AM. Protease Inhibitors and Carcinoma of the Esophagus. Cancer. 1998;83:405–408. doi: 10.1002/(SICI)1097-0142(19980801)83:3<405::AID-CNCR6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Thomson B, Shaw IA. Comparison of risk and protective factors for colorectal cancer in the diet of New Zealand maori and non-maori. Asian Pacific J Cancer Prevention. 2002;3:319–324. [PubMed] [Google Scholar]
- Ramachandran C, Fonseca HB, Jhabvala P, Escalon EA, Melnick SJ. Curcumin inhibits telomerase activity through human telomerase reverse transcritpase in MCF-7 breast cancer cell line. Cancer Letter. 2004;184:1–6. doi: 10.1016/s0304-3835(02)00192-1. [DOI] [PubMed] [Google Scholar]
- Eitsuka T, Nakagawa K, Igarashi M, Miyazawa T. Telomerase inhibition by sulfoquinovosyldiacylglycerol from edible purple laver (Porphyra yezoensis) Cancer Letter. 2004;212:15–20. doi: 10.1016/j.canlet.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Doi K, Kurabe S, Shimazu N. Systemic histopathology of rats with CCl4-induced hepatic cirrhosis. Laboratory Animals. 1991;25:21–25. doi: 10.1258/002367791780808121. [DOI] [PubMed] [Google Scholar]