Abstract
Studies of the budding yeast Saccharomyces cerevisiae have provided many of the most important insights into the mechanisms of autophagy, which are common to all eukaryotes. However, investigation of yeast self-destruction pathways, including autophagy and programmed cell death, has been almost exclusively restricted to cells undergoing vegetative growth, leaving very little exploration of their functions during developmental transitions in the yeast life cycle. We have recently discovered that whole nuclei are subject to programmed destruction during yeast gametogenesis. Programmed nuclear destruction (PND) possesses characteristics of apoptosis in the form of DNA cleavage by endonuclease G, and involves bulk protein turnover through an unusual autophagic pathway involving lysis of the vacuole rather than delivery of components to it through macroautophagy. We thus illuminate an example of developmentally programmed cellular “self-eating” in yeast, which is associated with the rupture of a lytic organelle, reminiscent of programmed cell death mechanisms in plants and animals.
Keywords: yeast, gametogenesis, sporulation, nucleus, vacuole, endonuclease G
Gametogenesis, also known as sporulation, in the unicellular yeast S. cerevisiae is a developmental response to starvation. Upon withdrawal of environmental nitrogen, while in the presence of nonfermentable carbon, diploid yeast cells exit the mitotic cycle, execute meiosis, and package the haploid meiotic products into quiescent gametes called spores. Following meiosis the mother cell remnant matures into a sac-like structure called the ascus, which contains the mature spores. Sporulation is dependent on the canonical macroautophagic machinery, which comes as little surprise given that it involves substantial cellular remodeling in the absence of external nutrients. In fact, some of the most nascent observations concerning autophagy mutants was their inability to sporulate, providing the first hint of the critical role that autophagy plays in development and cellular remodeling in all eukaryotes.
While laboratory sporulation protocols have been optimized to produce asci containing four spores (tetrads), more than 30 years ago, it was shown that when cells are sporulated under suboptimal carbon availability, they principally produce dyads. This phenomenon is caused by the selective cellularization of only two of the four meiotic products. Using a wild yeast strain that sporulates efficiently following colony growth arrest, we found that spore number reduction occurred predominantly, arguing that it is an often-utilized aspect of the yeast life cycle. Consistent with this conclusion, it has been demonstrated that spore number reduction is indeed a highly regulated developmental decision, and is controlled by metabolic flux. We initially sought to determine the fate of meiotic products that are aborted during spore number reduction, hypothesizing that they are subject to active, autophagic destruction.
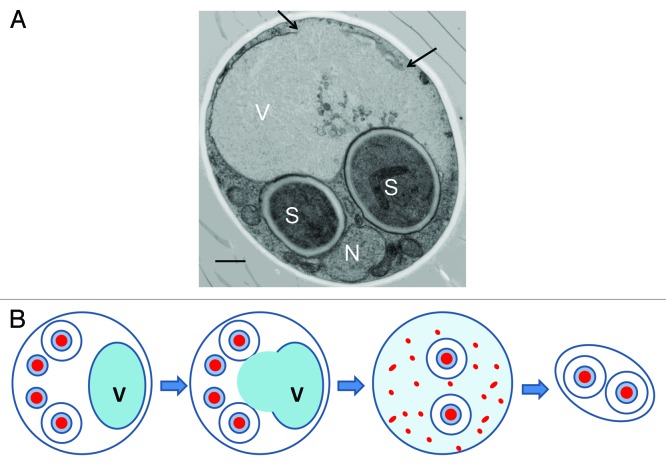
Our imaging experiments revealed that while their sister nuclei are engaged in spore morphogenesis, these aborted nuclei undergo robust degradation. As PND bears conceptual similarity to the apoptotic elimination of unneeded cells during metazoan development, we became curious as to whether the purging of unpackaged nuclei exhibited any hallmarks of programmed cell death. We found PND to be associated with the production of nucleosomal ladders, a process that has long been known to occur in apoptotic mammalian cells. Intriguingly, we found the production of ladders requires Nuc1, the yeast ortholog of endonuclease G (ENDOG), an ancient family of mitochondrial proteins that causes the fragmentation of nuclear genomes into nucleosomal ladders during apoptosis upon release from mitochondria in animals. As nuclei contain a large protein component, yeast PND presented us with an interesting problem—how does a cell go about breaking down an organelle as large as the nucleus? Using genetic and chemical approaches, we found that the execution of PND requires the activity of the vacuolar (lysosomal) protease Prb1, supporting the view that it occurs through an autophagic pathway. When we next visualized autophagosomes using GFP-Atg8, however, we found that cells ceased the production of GFP-Atg8-positive vesicles following meiosis, suggesting that macroautophagy was not directly responsible for PND. Critically, we never observed colocalization of unpackaged nuclei with autophagic vesicles. We thus entertained alternative hypotheses as to how PND could occur through a vacuolar destruction pathway independent of autophagosomes. The vacuole is not segregated into daughter spores following meiosis, and remains in the mother cell. We imaged the vacuole in live cells that had completed meiosis and were engaged in PND. As cells progressed through sporulation, mother cell vacuoles become increasingly discontinuous, and eventually degrade. We observed the breakage of the vacuole in electron micrographs (Fig. 1), and detected the accumulation of vacuolar contents in the mother cell cytoplasm. The occurrence of ruptured vacuoles shows a striking temporal coincidence with PND and we therefore hypothesize that PND is driven by the post-meiotic release of the lytic contents of the vacuole (Fig. 1). Vacuolar permeabilization allows cells to physically segregate their vital cellular contents into protective spores following meiosis and subsequently destroy the remaining mother cell contents to generate nutrients for the spores and/or other cells in the colony. While our results are consistent with programmed vacuolar permeability, we cannot rule out the possibility that lytic activity initially accumulates in the cytoplasm due to alternative trafficking of proteases that are normally confined to the vacuole. Nevertheless, permeabilization of the mother cell vacuole appears to be an intrinsic aspect of yeast gametogenesis, which accomplishes dramatic differentiation-associated cellular remodeling.
Figure 1. Vacuolar rupture during the final stages of yeast sporulation. (A) Transmission electron micrograph of a cell executing PND. Black arrows indicate loss of vacuolar membrane continuity in the mother cell during spore development. Spores (S), uncellularized nucleus (N) and vacuole (V) indicated. Scale bar: 500 nm, adapted from Eastwood et al., (2012). (B) Summary of our model. The mother cell vacuole (V) undergoes disintegration after the encapsulation of nuclei (red) for spore packaging. Nuclei not selected as spores are subject to destruction upon the permeabilization of the vacuole and release of its lytic contents. We depicted an ascus forming two spores while destroying the remaining two, but we find the disintegration of the vacuole always occurs at this stage of sporulation, irrespective of spore number. Yeast sporulation thus invokes mega-autophagy, or the rupture of the vacuole, as part of a developmental program dedicated to breaking down the contents of the mother cell.
Our observation of vacuolar membrane rupture during yeast sporulation resonates with other examples of programmed cell death that utilize permeability of the lysosome/vacuole. Lysosomal membrane permeabilization functions in certain forms of metazoan programmed cell death and in axon pruning, an example of a localized cell death-like destructive event in neurons. Indeed, vacuolar rupture, sometimes referred to as mega-autophagy, has been observed during developmentally programmed cell death in plants. The programmed permeabilization of these lytic organelles could thus represent an energetically efficient means to break down cellular contents en masse during development. While autophagy is typically described as selectively moving cellular contents to the vacuole for destruction, our study illuminates an interesting and contrasting counter-example of selectively releasing the vacuoleʼs activity into a portion of the cell to promote self-eating.
Glossary
Abbreviations:
- PND
programmed nuclear destruction
- ENDOG
endonuclease G
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/22881