There are a very few drug candidates that either extend lifespan or prevent a broad spectrum of age-related diseases. Of the few drug candidates that have exhibited preclinical success, rapamycin is the best characterized and perhaps shows the most promise for use in a clinical setting. Rapamycin inhibits the mTOR pathway, which integrates signals about nutrient availability, oxygen tension, ATP levels and mitogens to regulate protein synthesis, ribosome biogenesis, cell proliferation, angiogenesis and survival in response to stress.1 Multiple studies conducted using mouse models have demonstrated that rapamycin can extend murine lifespan by upwards of 15%,2,3 and additional studies have indicated that rapamycin can delay or mitigate the effects of a range of age-related pathologies. Currently, a large body of research is dedicated toward understanding precisely how rapamycin extends lifespan, and whether the drug may be clinically relevant in the treatment of one of the most prevalent and deadly age-related diseases, cancer. Two new studies from the Gudkov and the Antoch laboratories and their collaborators provide insight into the molecular mechanisms of rapamycin's effect on lifespan and its anti-tumorigenic potential.4,5 A large-scale 2009 study from The National Institute on Aging Interventions Testing Program demonstrated that rapamycin extended lifespan in mice;2 intriguingly, the rapamycin-fed cohort experienced the same number of cancer-related mortalities as the control group; however, cancer-related deaths were postponed by rapamycin treatment.2,3
The notion that rapamycin may be useful in the context of cancer therapy stems from the observation that the mTOR pathway integrates signaling from several proto-oncogenes, such as PI3K, Akt and eIF4E. Moreover, mTOR signling is often hyperactivated in a broad range of cancers. While these observations seem to suggest that mTOR signaling would be a prime target for cancer therapy, studies in mice and human patients have had mixed success, suggesting that our understanding of the mTOR pathway and the molecular mechanism of rapamycin-based therapies is incomplete. In two new studies, rapamycin was applied to highly tumor-prone p53+/− and p53−/− mice. Interestingly, rapamycin extended the lifespan of these tumor-prone mice and delayed tumorigenesis. A question that comes to mind is why did not rapamycin work well as an anticancer treatment?
Komarova et al. fed rapamycin to heterozygous p53+/− mice, and found that while rapamycin treatment extended lifespan, it appeared to only postpone carcinogenesis. Interestingly, the authors observed that mice that began rapamycin treatment early in life (before 5 mo of age) lived longer and were able to delay tumor formation until later in life than mice that did not begin rapamycin treatment until late in life (after 5 mo of age).4 One interpretation of these results is that rapamycin may function to prevent tumor initiation, but may have little effect on established tumor bodies.
Comas et al. pursued this hypothesis, that inhibition of mTOR signaling might delay oncogenesis.5 The authors synthesized highly soluble, nanoformulated micelles of rapamycin, dubbed Rapatar, for oral delivery to the highly tumorigenic homozygous p53−/− mice. Rapatar demonstrated increased bioavailabilty over conventional rapamycin treatment and displayed no additional toxicity. Rapatar treatment extended the lifespan of the p53−/− mice by 30% compared with control animals. Consistent with previous studies, however, the Rapatar-treated mice developed a similar tumor spectrum as control animals; carcinogenesis was merely delayed until later in life.
A critical question regarding rapamycin is the mechanism by which treatment extends lifespan in mice. There are at least two possibilities, (1) mice are tumor-prone animals, and rapamycin is toxic to cancer cells and can therefore extend murine lifespan, or (2) rapamycin slows aging through other processes and, as a result, cancer develops later in life (Fig. 1). The clinical implications of these two models are quite different: the first model suggests that rapamycin would be an effective anti-tumor therapy and could be prescribed acutely to treat neoplasms; in contrast, the second model suggests that rapamycin prevents tumor initiation and, therefore, that rapamycin needs to be taken before tumor development to prevent carcinogenesis. In these newest studies, it is interesting to note that the survival curves of the rapamycin-treated mice were shifted to the right, but ran parallel to the survival curves of the control animals. This favors the second possibility, and suggests that intervention with rapamycin delayed the onset of aging. If rapamycin were acting by inhibiting carcinogenesis, the rapamycin-treated mice would likely exhibit different aging kinetics, and the shoulder of their survival curve would be steeper, indicating a longer healthspan.
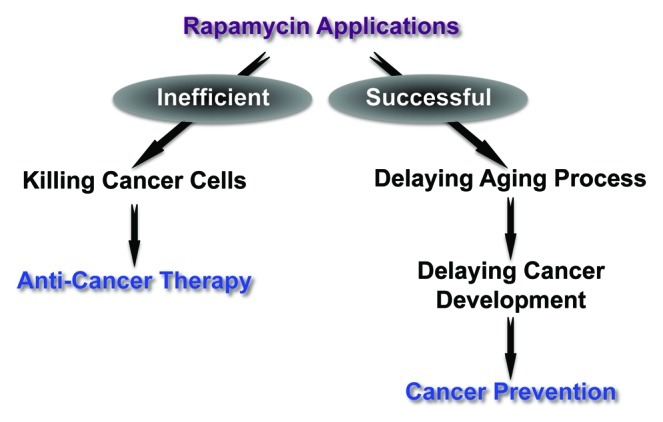
Figure 1. Two possible ways by which rapamycin affects tumorigenesis.
While more research is required to fully differentiate between these possibilities, evidence from the clinic also lends credence to the second possibility. To date, most clinical trials utilizing rapamycin as an anti-tumor therapy have disappointed clinicians; the most successful results came in patients who presented with tumors that were addicted to mTOR signaling, suggesting that rapamycin may only have narrow applications for the treatment of developed tumors.6-8 While there have not yet been clinical trials to assess the efficacy of rapamycin as a tumor-preventative agent, evidence from the early 2000s suggests that rapamycin may have this effect: in 1999, the FDA approved rapamycin for use as an immunosuppressant to promote renal engraftment after transplantation. Patients who received cyclosporine as the primary means of immunosuppression developed malignancies at a high rate due to poor immunosurveillance; in contrast, patients taking rapamycin experienced a lower rate of lymphoproliferative disorders post-transplant.9 This suggests that tumor initiation was delayed in these patients receiving rapamycin, underscoring the drug’s potential as a tumor-preventative medicine. These newest studies4,5 represent important steps toward understanding the mechanism by which rapamycin impacts on aging and age-related diseases. While more work is needed to fully understand the mechanism by which rapamycin works, as well as its clinical potential, these studies underscore the potential of the drug and provide hope that we will one day be able to develop a successful anti-aging medication.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/22868
References
- 1.Zoncu R, et al. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison DE, et al. Nature. 2009;460:392–5. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller RA, et al. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komarova EA, et al. Aging (Albany NY) 2012;4:719–27. [Google Scholar]
- 5.Comas M, et al. Aging (Albany NY) 2012;4:728–35. [Google Scholar]
- 6.Efeyan A, et al. Curr Opin Cell Biol. 2010;22:169–76. doi: 10.1016/j.ceb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosley JD, et al. Mol Cancer Ther. 2007;6:2188–97. doi: 10.1158/1535-7163.MCT-07-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cloughesy TF, et al. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathew T, et al. Clin Transplant. 2004;18:446–9. doi: 10.1111/j.1399-0012.2004.00188.x. [DOI] [PubMed] [Google Scholar]


