Transforming growth factor-β (TGF-β) never ceases to fascinate cancer researchers due to its pleiotropic effects and significant clinical relevance to human diseases. Over the past few decades, TGF-β has been the focus of considerable research efforts, but we still do not fully understand the complex mechanism(s) by which this cytokine influences tumorigenesis. It has become evident that TGF-β modulates carcinoma cell behavior in a cell context-dependent fashion during the early and late stages of tumorigenesis.1 TGF-β is well-known to play tumor-suppressive roles that inhibit tumor cell proliferation and induce apoptosis in premalignant cells. In contrast, this cytokine often provides malignant cells harboring cancer-driving genetic mutations with the hallmarks of cancer-aggressive traits. The latter is exemplified by epithelial-mesenchymal transition and cancer stem cell phenotypes that promote tumor invasion and metastasis.1,2 Cell-autonomous oncogenic signaling conferred upon carcinoma cells often abolishes their tumor-suppressive responsiveness to TGF-β during late stages of tumorigenesis. Interestingly, such paradoxical TGF-β-induced cellular responses may also depend on complex regulation by the tumor microenvironment.3
Carcinoma-associated fibroblasts (CAFs), which consist of fibroblasts and myofibroblasts, are a predominant cell type within the tumor-associated stroma. Carcinoma cell-secreted TGF-β appears to initiate, in a paracrine fashion, the conversion of resident fibroblasts to CAF myofibroblasts within the tumor stroma. During the course of tumor progression, such myofibroblasts markedly increase the level of TGF-β production, which, in turn, enables these cells to activate TGF-β signaling in an autocrine fashion, thereby constitutively driving their myofibroblastic, tumor-promoting property.4
Caveolin-1 (Cav-1) is proposed to be essential for achieving the myofibroblastic state in CAFs and is a potential clinical biomarker for human breast cancers.5 The Cav-1 expression level is inversely correlated with TGF-β signaling in stromal fibroblasts. Downregulation of Cav-1 expression also increases TGF-β signaling in these cells, whereas upregulation of TGF-β signaling suppresses Cav-1 expression.
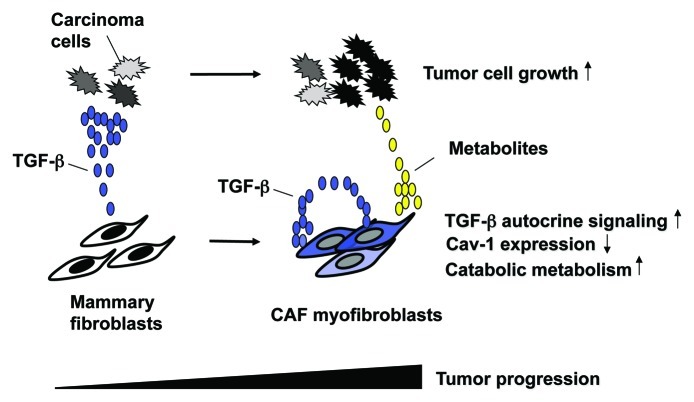
In the August 15, 2012 issue of Cell Cycle, Guido et al. provided evidence supporting a critical role of TGF-β signaling in metabolic reprogramming via Cav-1 in CAFs.6 Metabolism in cancer cells had long been considered to merely be an indirect secondary phenomenon that is simply associated with, i.e., does not cause, tumor progression. However, reprogrammed cancer metabolism now serves as one of the hallmarks of human cancers and not simply as a passive readout.7 Guido and colleagues previously proposed the concept of “two-compartment tumor metabolism,” wherein stromal Cav-1 loss induces a “Warburg effect” in tumor-associated stromal cells, thereby leading to energy-rich metabolites that fuel neighboring cancer cells.5 In the 2012 study, they have indicated that activation of TGF-β signaling in fibroblasts leads to an attenuation of Cav-1 expression that increases oxidative stress, induces autophagy/mitophagy, elevates aerobic glycolysis and, thus, stimulates mammary tumor growth (Fig. 1).6
Figure 1. TGF-β-signaling causes metabolic reprogramming in CAFs to promote tumorigenesis. Cancer cells secrete TGF-β that initiates the conversion of mammary stromal fibroblasts to myofibroblasts in a paracrine fashion. During the series of tumor progression, myofibroblasts increase their TGF-β production and conversely decrease Cav-1 expression. The resulting myofibroblasts activate TGF-β signaling in an autocrine fashion, which leads to increased oxidative stress, induction of autophagy/mitophagy and subsequently aerobic glycolysis (Warburg effect), thereby generating metabolites (lactate, pyruvate, glutamine, ketone bodies, etc.). These metabolites, which are routed to the adjacent cancer cells, boost their anabolic metabolism and growth.
This work also shows that TGF-β released from either carcinoma cells or CAFs drives the canonical Smad2/3 signaling in CAFs via a paracrine or an autocrine mechanism, respectively (Fig. 1). The resulting decrease in Cav-1 expression is a prerequisite for the generation of energy-rich metabolites, thereby promoting apposed cancer cell growth. Collectively, activation of TGF-β signaling in CAFs is elucidated as being the force that drives catabolic metabolic reprogramming via Cav-1 downregulation in these cells, thereby stimulating tumorigenesis in human breast carcinoma cells.
Notably, pharmacological inhibitors and neutralizing antibodies targeting TGF-β signaling, potentially in both tumor and stromal compartments, have indeed been reported to enhance the efficacy of conventional chemotherapies attenuating tumor growth in xenograft tumor models.8,9 These effects presumably involved modulation of vascular permeability, ECM production and recruitment and activation of tumor-promoting stromal cells within tumors.
In summary, the recent study by Guido et al. has demonstrated the importance of TGF-β autocrine signaling and the concomitant Cav-1 downregulation in CAFs, which can promote catabolic metabolism in these cells and, consequently, lead to enhanced tumorigenesis in adjacent human breast carcinoma cells.6 This work represents a step forward in our quest to understand the molecular mechanism(s) underlying CAF-promoted tumorigenesis and the development of novel therapeutic approaches.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/22811
References
- 1.Massagué J. Nat Rev Mol Cell Biol. 2012;13:616–30. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikushima H, et al. Nat Rev Cancer. 2010;10:415–24. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 3.Bierie B, et al. Nat Rev Cancer. 2006;6:506–20. doi: 10.1038/nrc1926. [DOI] [PubMed] [Google Scholar]
- 4.Kojima Y, et al. Proc Natl Acad Sci USA. 2010;107:20009–14. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sotgia F, et al. Breast Cancer Res. 2011;13:213. doi: 10.1186/bcr2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guido C, et al. Cell Cycle. 2012;11:3019–35. doi: 10.4161/cc.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward PS, et al. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, et al. Proc Natl Acad Sci USA. 2012;109:16618–23. doi: 10.1073/pnas.1117610109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kano MR, et al. Proc Natl Acad Sci USA. 2007;104:3460–5. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]