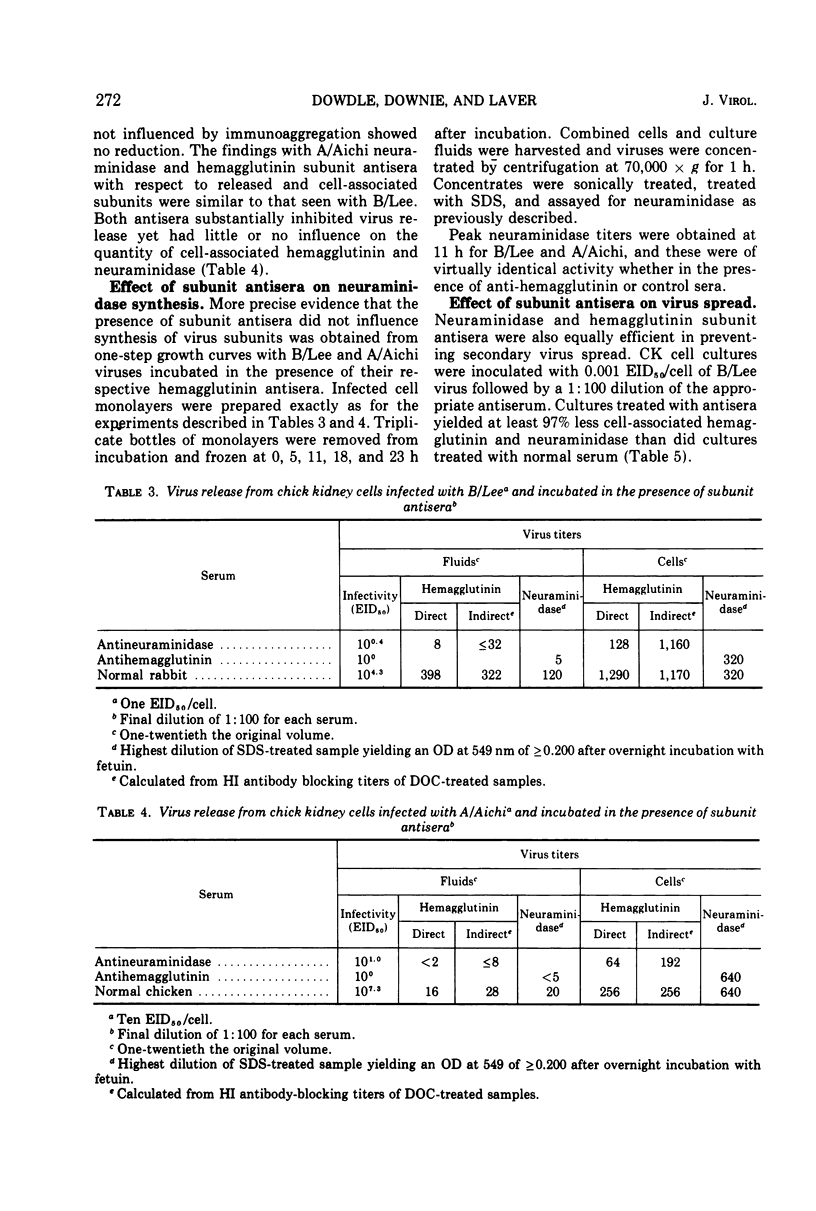
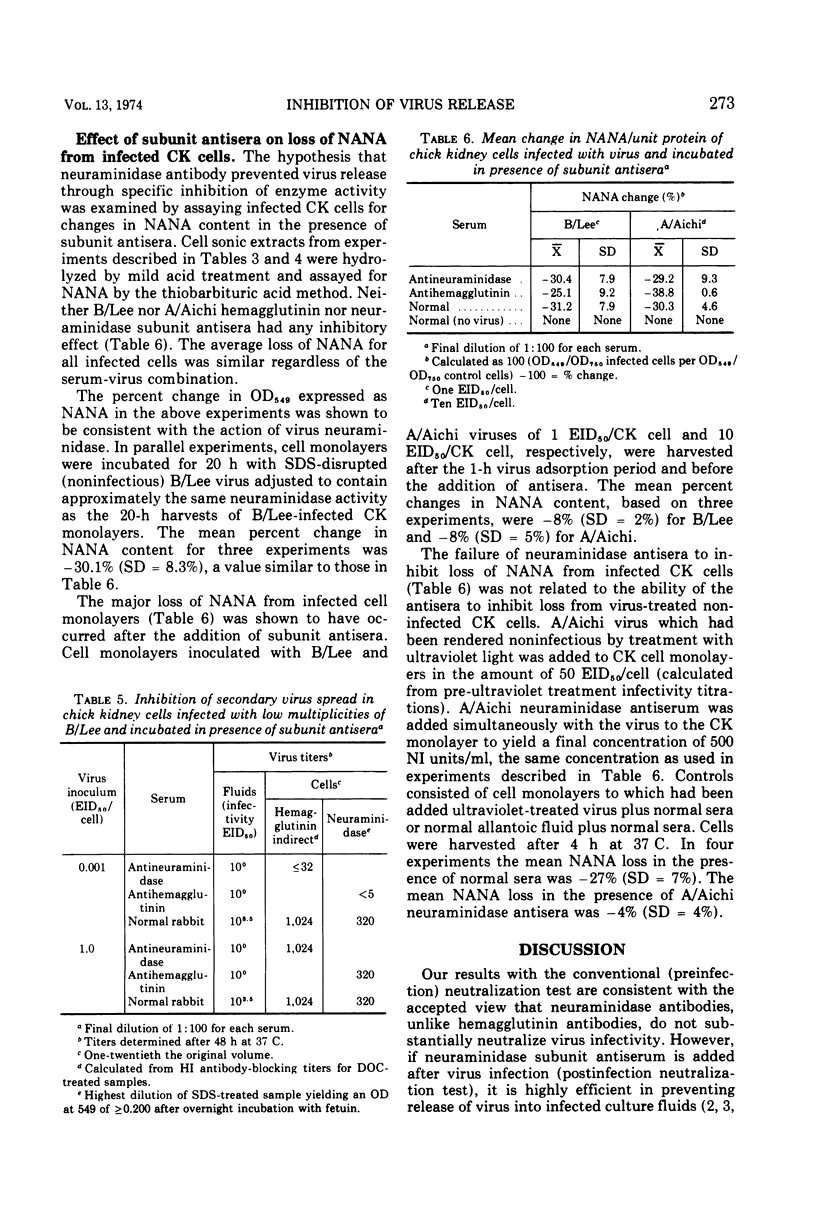
Abstract
When influenza virus was mixed with antisera to its surface subunits before inoculation of cell cultures, anti-hemagglutinin antibodies neutralized infectivity but anti-neuraminidase did not. When the antisera were added after infection of cell cultures, anti-hemagglutinin and anti-neuraminidase antibodies were equally effective in reducing virus titers in culture fluids. Decreased virus titers were not due to interference of antibody with assay and were not accompanied by a reduction in the synthesis of hemagglutinin and neuraminidase subunits. Both antisera also effectively prevented in vitro virus spread. Inhibition of virus release by neuraminidase antibody appeared unrelated to its antienzyme property. Hydrolysis of N-acetyl neuraminic acid residues of infected host cells proceeded unimpaired in the presence of subunit antisera. Anti-hemagglutinin and anti-neuraminidase antibodies may act to prevent virus release by binding newly formed virus subunits to each other and to anti-genically altered cell membranes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aymard-Henry M., Coleman M. T., Dowdle W. R., Laver W. G., Schild G. C., Webster R. G. Influenzavirus neuraminidase and neuraminidase-inhibition test procedures. Bull World Health Organ. 1973;48(2):199–202. [PMC free article] [PubMed] [Google Scholar]
- Brecht H., Hämmerling U., Rott R. Undisturbed release of influenza virus in the presence of univalent antineuraminidase antibodies. Virology. 1971 Nov;46(2):337–343. doi: 10.1016/0042-6822(71)90035-3. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Dimmock N. J., Meier-Ewert H. Effect of antibody to neuraminidase on the maturation and hemagglutinating activity of an influenza A2 virus. J Virol. 1969 Oct;4(4):528–534. doi: 10.1128/jvi.4.4.528-534.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierholzer J. C., Suggs M. T., Hall E. C. Standardized viral hemagglutination and hemagglutination-inhibition tests. II. Description and statistical evaluation. Appl Microbiol. 1969 Nov;18(5):824–833. doi: 10.1128/am.18.5.824-833.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holston J. L., Jr, Dowdle W. R. Influenza A neuraminidase antibody assay with sensitized erythrocytes. Appl Microbiol. 1973 Jan;25(1):97–102. doi: 10.1128/am.25.1.97-102.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendal A. P., Madeley C. R. Flocculation of influenza virus by specific anti-neuraminidase antibody. Arch Gesamte Virusforsch. 1970;31(3):219–229. doi: 10.1007/BF01253756. [DOI] [PubMed] [Google Scholar]
- Kilbourne E. D., Laver W. G., Schulman J. L., Webster R. G. Antiviral activity of antiserum specific for an influenza virus neuraminidase. J Virol. 1968 Apr;2(4):281–288. doi: 10.1128/jvi.2.4.281-288.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Compans R. W., Choppin W. P. An electron microscopic study of the presence or absence of neuraminic acid in enveloped viruses. Virology. 1970 Dec;42(4):1158–1162. doi: 10.1016/0042-6822(70)90368-5. [DOI] [PubMed] [Google Scholar]
- LAVER W. G. STRUCTURAL STUDIES ON THE PROTEIN SUBUNITS FROM THREE STRAINS OF INFLUENZA VIRUS. J Mol Biol. 1964 Jul;9:109–124. doi: 10.1016/s0022-2836(64)80094-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Seto J. T., Chang F. S. Functional significance of sialidase during influenza virus multiplication: an electron microscope study. J Virol. 1969 Jul;4(1):58–66. doi: 10.1128/jvi.4.1.58-66.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto J. T., Rott R. Functional significance of sialidose during influenza virus multiplication. Virology. 1966 Dec;30(4):731–737. doi: 10.1016/0042-6822(66)90178-4. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Kilbourne E. D. Reactions of antibodies with surface antigens of influenza virus. J Gen Virol. 1968 Dec;3(3):315–326. doi: 10.1099/0022-1317-3-3-315. [DOI] [PubMed] [Google Scholar]



