Abstract
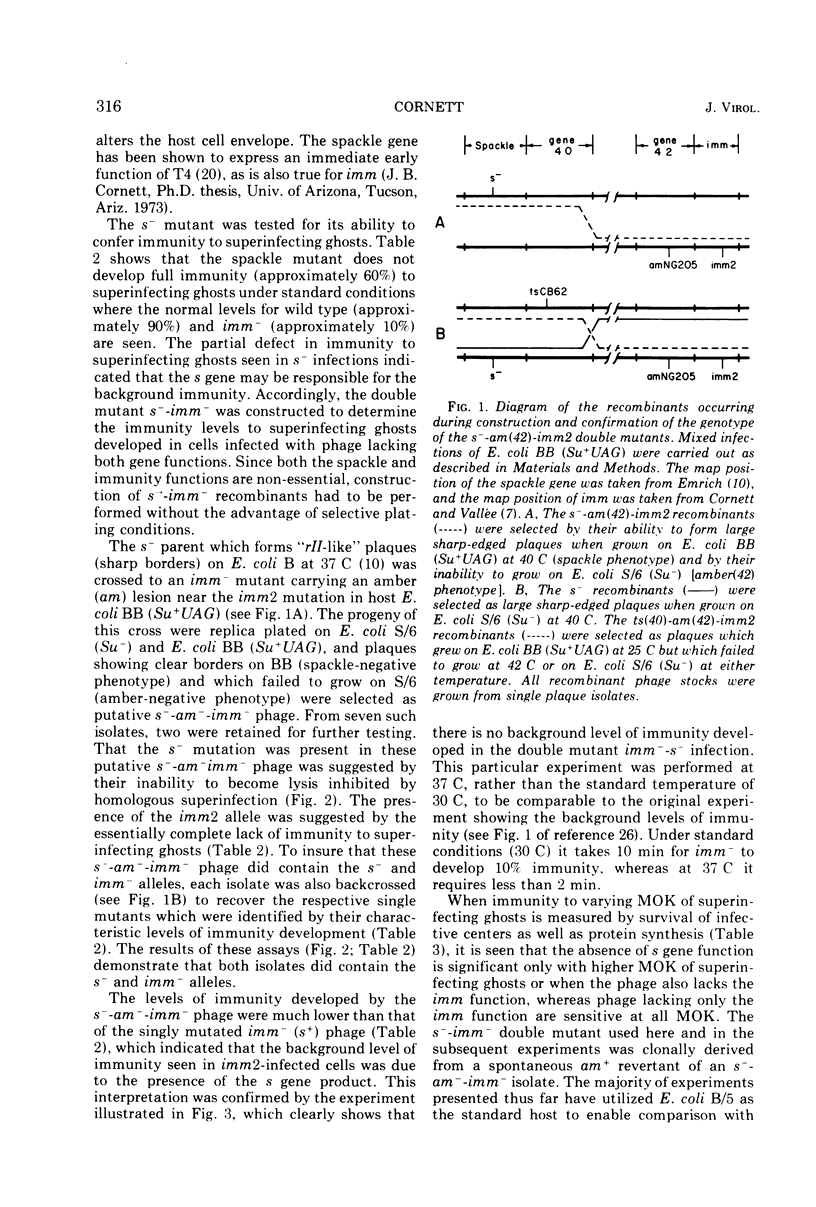
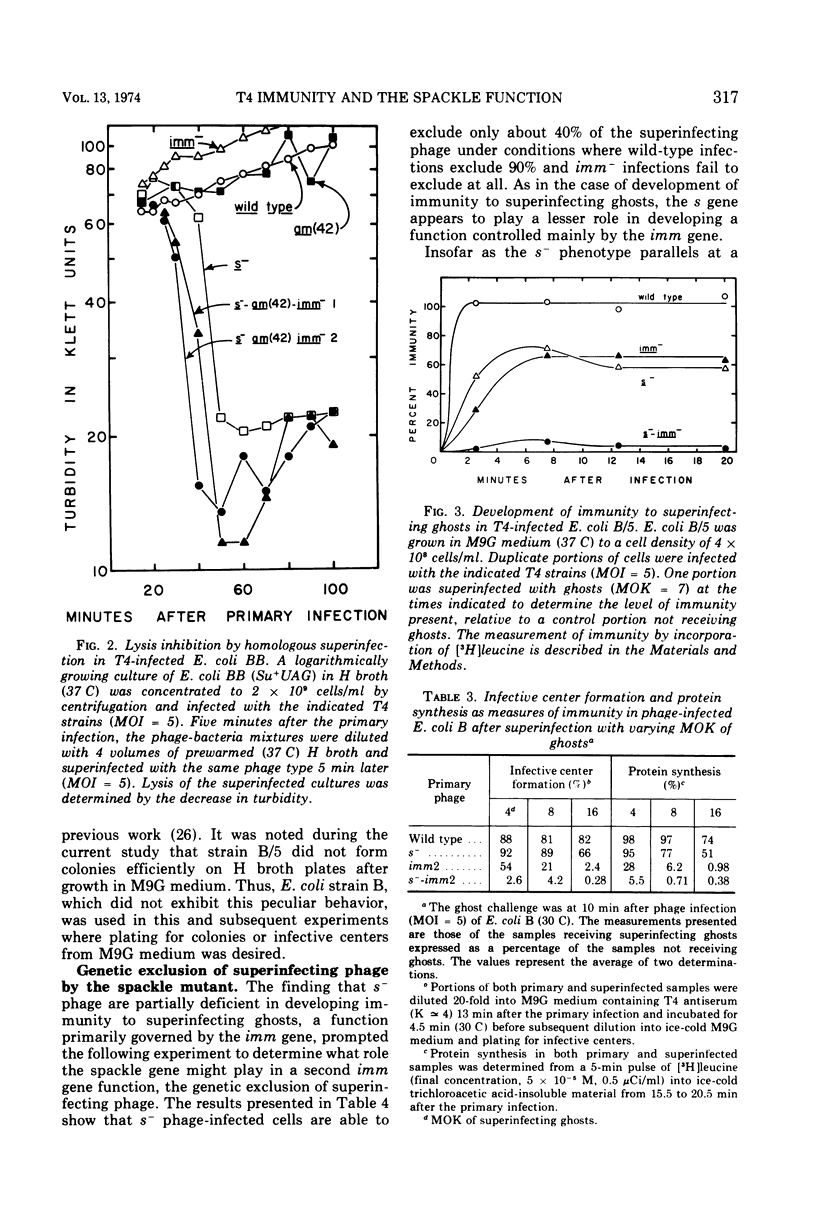
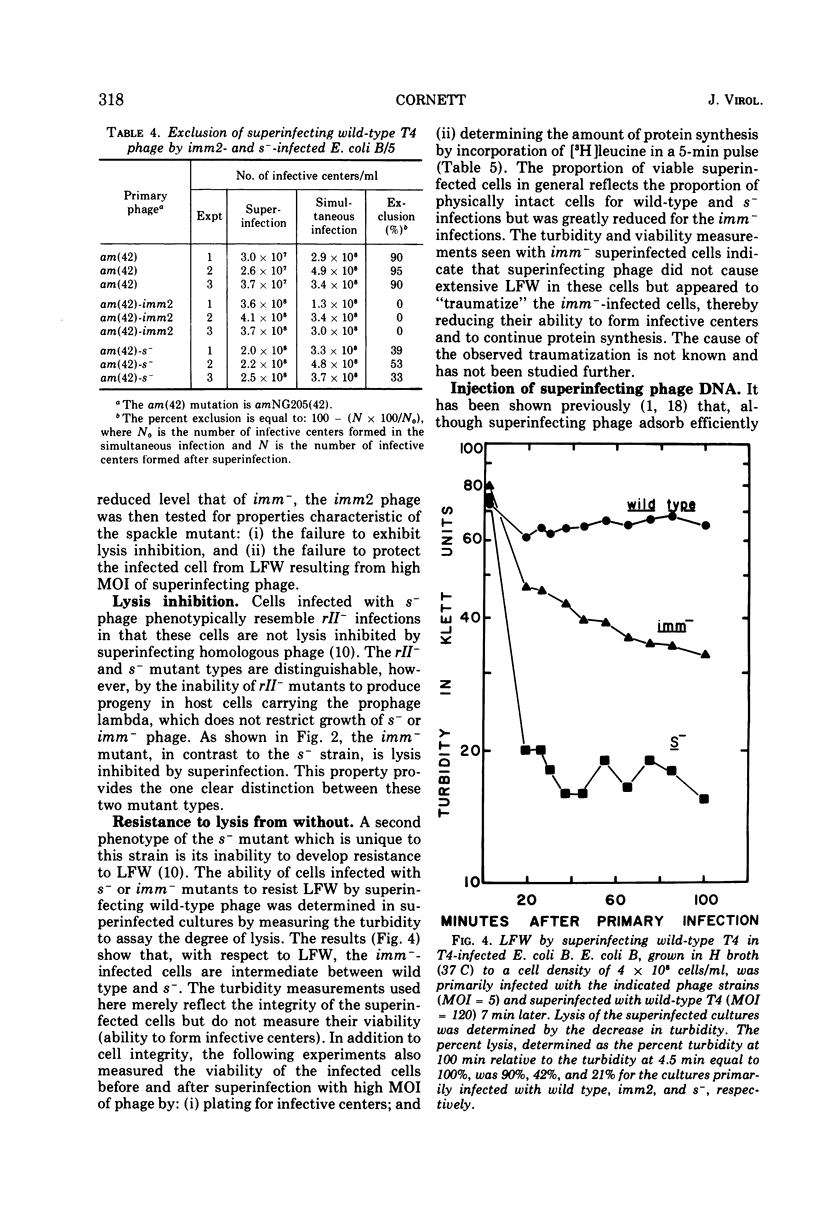
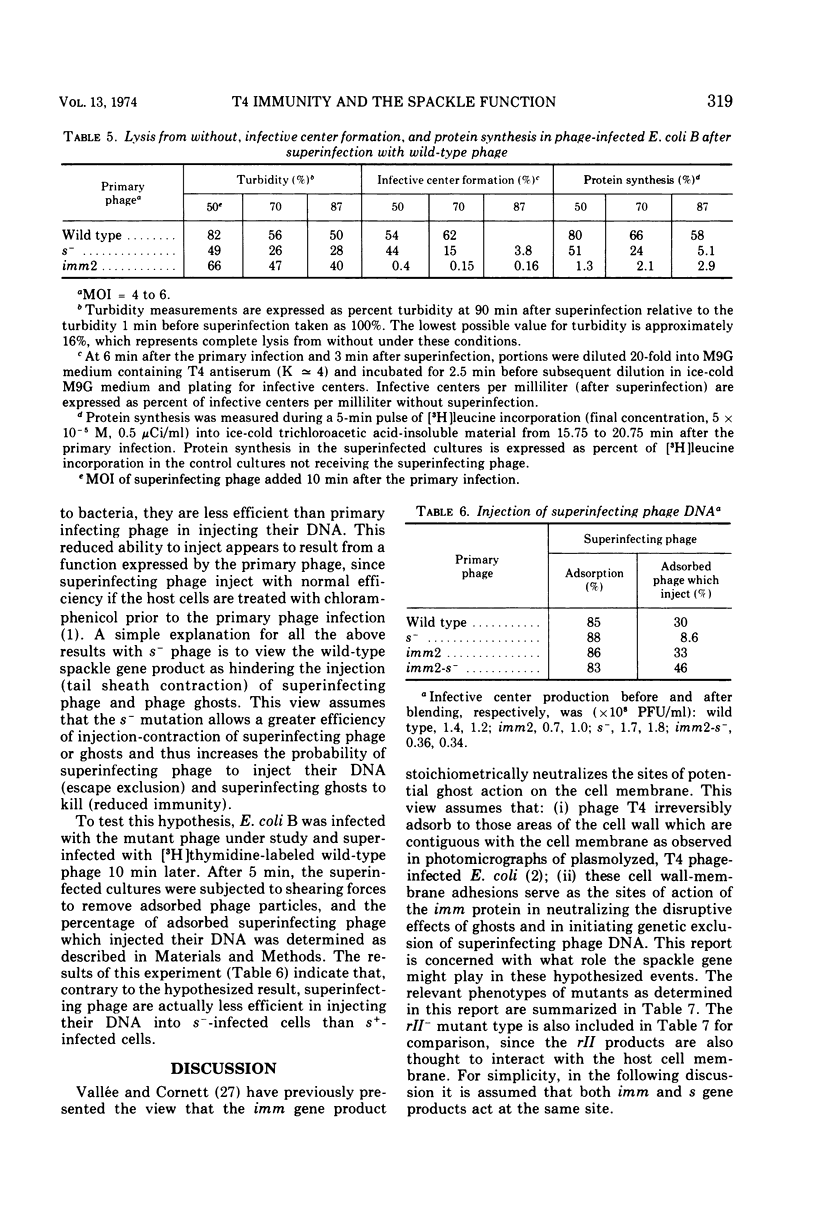
Cells of Escherichia coli B infected with the immunity-negative (imm2) mutant of bacteriophage T4 are able to develop a substantial level of immunity to superinfecting phage ghosts if the ghost challenge is made late in infection. This background immunity is not seen in infections with phage carrying the spackle (s) mutation in addition to the imm2 lesion. The level of immunity in s− infections is intermediate between that of imm− and wild-type infections under standard assay conditions. With respect to genetic exclusion of superinfecting phage, cells infected with imm− phage are completely deficient, whereas infections with the s− phage are only partially deficient compared to wild-type infections. Whereas s−-infected cells are unable to resist lysis from without by a high multiplicity of infection (MOI) of superinfecting phage, cells infected with imm− phage show less than wild-type levels of resistance and the majority of cells remaining intact are unable to incorporate leucine or form infective centers. Under conditions of superinfection by low MOI of homologous phage, imm−-infected cells are lysis inhibited, whereas s−-infected cells do not show this property. Superinfecting phage inject their DNA into imm−-infected cells with the same efficiency as seen in wild-type infections, but this efficiency is reduced when the cells are first infected with s− phage. The s function of T4 appears not only to affect the host cell wall as previously postulated by Emrich, but may also affect the junctures of cell wall and membrane with consequences similar to those of the imm function.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Eigner J. Breakdown and exclusion of superinfecting T-even bacteriophage in Escherichia coli. J Virol. 1971 Dec;8(6):869–886. doi: 10.1128/jvi.8.6.869-886.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz W. C., Goldberg E. B. Interactions between modified phage T4 particles and spheroplasts. Virology. 1973 May;53(1):225–235. doi: 10.1016/0042-6822(73)90481-9. [DOI] [PubMed] [Google Scholar]
- Bernstein C. A comparison of the number of nucleotides per unit length in Escherichia coli and phage T4 chromosomes. Biophys J. 1970 Dec;10(12):1154–1172. doi: 10.1016/S0006-3495(70)86362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buller C. S., Astrachan L. Replication of T4rII bacteriophage in Escherichia coli K-12 (lambda). J Virol. 1968 Apr;2(4):298–307. doi: 10.1128/jvi.2.4.298-307.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs J. D. Superinfection exclusion by incomplete genomes of bacteriophage T4. J Virol. 1973 Jan;11(1):1–8. doi: 10.1128/jvi.11.1.1-8.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Vallée M. The map position of the immunity (imm) gene of bacteriophage T4. Virology. 1973 Feb;51(2):506–508. doi: 10.1016/0042-6822(73)90452-2. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H. Inhibition of T4 bacteriophage multiplication by superinfecting ghosts and the development of tolerance after bacteriophage infection. J Virol. 1971 Jan;7(1):8–14. doi: 10.1128/jvi.7.1.8-14.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth D. H. The metabolism of T4 phage ghost-infected cells. I. Macromolecular synthesis and ransport of nucleic acid and protein precursors. Virology. 1970 Mar;40(3):673–684. doi: 10.1016/0042-6822(70)90212-6. [DOI] [PubMed] [Google Scholar]
- Emrich J. Lysis of T4-infected bacteria in the absence of lysozyme. Virology. 1968 May;35(1):158–165. doi: 10.1016/0042-6822(68)90315-2. [DOI] [PubMed] [Google Scholar]
- Ennis H. L., Kievitt K. D. Association of the rIIA protein with the bacterial membrane. Proc Natl Acad Sci U S A. 1973 May;70(5):1468–1472. doi: 10.1073/pnas.70.5.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH R. C., SIMINOVITCH L. The action of T2 bacteriophage ghosts on Escherichia coli B. Can J Microbiol. 1955 Dec;1(9):757–774. doi: 10.1139/m55-090. [DOI] [PubMed] [Google Scholar]
- Fabricant R., Kennell D. Exclusion of bacteriophages by T2 ghosts. J Virol. 1972 Oct;10(4):872–874. doi: 10.1128/jvi.10.4.872-874.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricant R., Kennell D. Inhibition of host protein synthesis during infection of Escherichi coli by bacteriophage T4. 3. Inhibition by ghosts. J Virol. 1970 Dec;6(6):772–781. doi: 10.1128/jvi.6.6.772-781.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furrow M. H., Pizer L. I. Phospholipid synthesis in Escherichia coli infected with T4 bacteriophages. J Virol. 1968 Jun;2(6):594–605. doi: 10.1128/jvi.2.6.594-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A. Physiological effects of rII mutations in bacteriophage T4. Virology. 1961 Jun;14:151–163. doi: 10.1016/0042-6822(61)90190-8. [DOI] [PubMed] [Google Scholar]
- HERRIOTT R. M., BARLOW J. L. The protein coats or ghosts of coli phage T2. II. The biological functions. J Gen Physiol. 1957 Nov 20;41(2):307–331. doi: 10.1085/jgp.41.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufti S. A bacteriophage T4 mutant defective in protection against superinfecting phage. J Gen Virol. 1972 Oct;17(1):119–123. doi: 10.1099/0022-1317-17-1-119. [DOI] [PubMed] [Google Scholar]
- Peterson R. F., Cohen P. S., Ennis H. L. Properties of phage T4 messenger RNA synthesized in the absence of protein synthesis. Virology. 1972 Apr;48(1):201–206. doi: 10.1016/0042-6822(72)90127-4. [DOI] [PubMed] [Google Scholar]
- Peterson R. F., Kievitt K. D., Ennis H. L. Membrane protein synthesis after infection of Escherichia coli B with phage T4: the rIIB protein. Virology. 1972 Nov;50(2):520–527. doi: 10.1016/0042-6822(72)90403-5. [DOI] [PubMed] [Google Scholar]
- REVEL H. R., LURIA S. E., ROTMAN B. Biosynthesis of B-D-galactosidase controlled by phage-carried genes. I. Induced beta-D-galactosidase biosynthesis after transduction of gene z-plus by phage. Proc Natl Acad Sci U S A. 1961 Dec 15;47:1956–1967. doi: 10.1073/pnas.47.12.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG C. M., EDGAR R. S. A critical test of a current theory of genetic recombination in bacteriophage. Genetics. 1962 Feb;47:187–208. doi: 10.1093/genetics/47.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D. A., Mazaitis A. J., Kasai T., Bautz E. K. Involvement of a phage T4 sigma factor and an anti-terminator protein in the transcription of early T4 genes in vivo. Nature. 1970 Mar 14;225(5237):1012–1016. doi: 10.1038/2251012a0. [DOI] [PubMed] [Google Scholar]
- Sekiguchi M. Studies on the physiological defect in rII mutants of bacteriophage T4. J Mol Biol. 1966 Apr;16(2):503–522. doi: 10.1016/s0022-2836(66)80188-2. [DOI] [PubMed] [Google Scholar]
- Vallée M., Cornett J. B. A new gene of bacteriophage T4 determining immunity against superinfecting ghosts and phage in T4-infected Escherichia coli. Virology. 1972 Jun;48(3):777–784. doi: 10.1016/0042-6822(72)90161-4. [DOI] [PubMed] [Google Scholar]
- Vallée M., Cornett J. B., Bernstein H. The action of bacteriophage T4 ghosts on Escherichia coli and the immunity to this action developed in cells preinfected with T4. Virology. 1972 Jun;48(3):766–776. doi: 10.1016/0042-6822(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Vallée M., Cornett J. B. The immunity reaction of bacteriophage T4: a noncatalytic reaction. Virology. 1973 Jun;53(2):441–447. doi: 10.1016/0042-6822(73)90223-7. [DOI] [PubMed] [Google Scholar]
- Weintraub S. B., Frankel F. R. Identification of the T4rIIB gene product as a membrane protein. J Mol Biol. 1972 Oct 14;70(3):589–615. doi: 10.1016/0022-2836(72)90561-x. [DOI] [PubMed] [Google Scholar]



