Abstract
Introduction
The aim of this study was to evaluate the role of bedside ultrasonography (US) in early diagnosis of musculoskeletal complications (MSC) of acquired brain injuries, to describe its incidence and US features in a neurorehabilitation setting.
Materials and methods
All 163 patients admitted in tertiary-level neurorehabilitation unit with diagnosis of stroke or severe brain injury (SBI), with symptoms or signs of musculoskeletal pathology, underwent bedside US.
Results
MSC were diagnosed in 51.5%. In 86.9% US clarified diagnosis and/or modified therapeutic approach. Shoulder pain was observed in 27.6%. US showed a shoulder subluxation in 73.3% and a frozen shoulder in 8.8% of painful shoulders. In all the cases rotator cuff abnormalities were noted. Wrist-hand syndrome was observed in 29.4%. US showed mild effusion in wrist joints and tendon sheaths and subcutaneous edema without significant vascularity.
Neurogenic heterotopic ossification was observed in 1.8%. US demonstrated the “zone phenomenon” or heterogeneously hypoechoic mass with low resistance vessels within the lesions.
Contractures and spasticity were observed in 18.4%. US allowed reliable guidance for Botulinum toxin A injection. Relapsing osteoarthritis and acute arthritis were diagnosed in 15.3% and 7.3% respectively. Patients with MSC had lower Functional Independence Measurement (FIM) and Katz index scores in discharge (p < 0.04 and p < 0.0294 respectively) and more length of hospital stay (p = 0.0024).
Discussion
Musculoskeletal pathology frequently complicates the course of acquired brain injuries and it delays functional recovery. Bedside US is a cheap and sensitive diagnostic tool and it can aid clinicians to define diagnosis and to choose therapeutic approach.
Keywords: Power Doppler ultrasonography, Musculoskeletal pathology, Stroke, Acquired brain injury
Sommario
Introduzione
Lo scopo di questo studio era quello di valutare il ruolo dell’ecografia (US) eseguita al letto del paziente nella diagnosi precoce delle complicazioni muscolo-scheletriche (MSC) delle cerebrolesioni acquisite, di descriverne incidenza e caratteristiche ecografiche, in una neuroriabilitazione.
Materiali e metodi
Tutti i 163 pazienti ricoverati nella unità operativa di terzo livello di neuroriabilitazione con diagnosi di stroke o grave cerebrolesione acquisista (SBI), con sintomi o segni di patologia muscolo-scheletrica, furono sottoposti ad esame US.
Risultati
MSC furono diagnosticate nel 51,5% dei pazienti. Nel 86,9% US ha chiarito la diagnosi e/o modificato l’approccio terapeutico. La spalla dolorosa fu rilevata nel 27,6%. US dimostrò una sublussazione di spalla nel 73,3% e una spalla congelata nel 8,8% delle spalle dolorose. In tutti i casi furono osservate alterazioni della cuffia dei rotatori. La sindrome polso-mano fu osservata nel 29,4%. US dimostrava modesto versamento nelle articolazioni e nelle guaine tendinee del polso con edema sottocutaneo senza significativa vascolarizzazione.
Le ossificazioni eterotopiche neurogeniche furono osservate nel 1,8%.US dimostrò il cosiddetto “zone phenomenon” oppure una massa eterogeneamente ipoecogena con vasi a bassa resistenza all’interno della lesione. Contratture e spasticità furono osservate nel 18,4%. US ha permesso di effettuare infiltrazioni eco-guidate con tossina Botulinica A in maniera affidabile. Riacutizzazioni di artrosi e artriti acute furono diagnosticate nel 15,3% e 7,3% rispettivamente. I pazienti con MSC avevano più bassi punteggi di Functional Independence Measurement (FIM) e dell’indice di Katz alla dimissione (p < 0,04 and p < 0,0294 rispettivamente) e maggiore durata del ricovero (p = 0,0024).
Conclusioni
La patologia muscolo-scheletrica complica frequentemente il decorso delle cerebrolesioni acquisite e ritarda il recupero funzionale. L’US eseguita al letto del paziente è una metodica diagnostica economica e sensibile e può aiutare il clinico nella definizione della diagnosi e nella scelta dell’approccio terapeutico.
Introduction
Most of acquired brain injuries can result in secondary musculoskeletal complications that delay functional recovery and affect overall outcome [1–4]. Shoulder pain, shoulder-hand syndrome (SHS), wrist-hand syndrome, neurogenic heterotopic ossifications (NHO), contractures and spasticity are the most common musculoskeletal complications described in hemiplegia due to stroke or traumatic brain injury [2–5]. These secondary complications can develop at any stage after the onset of a brain injury, and the rapidity of clinicians to recognize and treat these complications will improve patient functioning. Diagnostic radiographic and laboratory findings may not be helpful in early diagnosis, and three-phase 99m Technetium bone scanning and magnetic resonance imaging (MRI) have shown variable specificity and they are time consuming and high cost techniques [5,6].
For these reasons previous studies describe incidences of various complications of acquired brain injuries, but scanty studies provide confirmation of diagnosis by imaging methods [6–9].
Gray-scale ultrasonography (US) is proved to be a sensitive imaging method in soft tissues lesions, calcifications and cortical fractures [10–14]. Moreover US has high specificity in early diagnosis and follow-up of arthritis, tendonopathy and NHO, allowing differentiation from soft tissues tears and tumour, haematoma, infection and deep venous thrombosis (DVT) [10–13,15,16]. US has the advantage of the possibility of bedside application, it is easy to perform, repeatable and inexpensive and requires no radiation. Doppler US has proved to be a reliable tool for semi-quantitative assessment of the vascularity of the soft tissue [10,12]. Only recently gray-scale US has been applied to the study of musculoskeletal complications of stroke with encouraging results [18–20].
The aim of this study was to evaluate the role of bedside US and Power Doppler US (PDUS) in early diagnosis of musculoskeletal complications of acquired brain injuries, to define the incidence and US features of these complications in a tertiary-level neurorehabilitation setting.
Materials and methods
A 16-bed neurorehabilitation unit in a tertiary-level hospital was the setting for the study. All 163 patients admitted from October 2008 to October 2009 with diagnosis of stroke or severe brain injury (SBI) were included in the study. SBI group comprised patients affected by traumatic head injury, post-hypoxic encephalopathy, intracranial subarachnoid hemorrage, subdural hemorrage or intracerebral hemorrage, with a Glascow Coma Scale score of 8 or less at first admission in intensive care unit.
On admission and on discharge each patient underwent neurological and functional assessment, even with compilation of Functional Independence Measurement (FIM) and Katz index.
The demographic and clinical characteristics of the study sample are shown in Table 1. The presence of specific complications was clinically suspected by clinicians and discussed during the interdisciplinary weekly team conference for each patient. The determination was made by the attending physiatrist or rheumatologist, with input from other members of the interdisciplinary team when it was appropriate. Clinical suspect of musculoskeletal complications derived from reduction of the range of motion (ROM) of a joint, with or without local swelling, or evidence for local (spontaneous or provoked) pain. All the patients with clinical suspect of musculoskeletal complications underwent bedside US examination. Other imaging techniques were used when they were necessary to confirm diagnosis.
Table 1.
Demographic and clinical characteristics of patients.
| Overall study population | Patients without musculoskeletal complications | Patients with musculoskeletal complications | p value (statistic test) | |
|---|---|---|---|---|
| Number of subjects (%) | 163 | 79 (48.4%) | 84 (51.5%) | ns (Chi-squared) |
| Disease: stroke/severe brain injury | 120/43 | 58/21 | 62/22 | |
| Female/Male (n) | 70/93 | 26/53 | 44/40 | p = 0.0173 (Fisher) |
| Mean age (range; SD) | 71.3 (23–93; 13.5) | 73.9 (23–88; 11) | 69 (26–93; 15.2) | p = 0.032 (Mann–Whitney) |
| Days from acute event at admission | 25.5 | 23.7 | 27.4 | ns (Mann–Whitney) |
| Length of stay (range; SD) | 32.5(15–120; 19.7) | 30.4(15–120; 20.4) | 34.5(18–90; 19) | p = 0.0024 (Mann–Whitney) |
| Serum urate mg/dL [nv 2.6–7] (SD) | 4.5 (1.9) | 4.6 (1.9) | 4.5 (1.9) | ns (Mann–Whitney) |
| Alkaline phosphatase (nv values 40–129 mg/dL for female, 35–104 mg/dL for male) | 92.6 (39.6) | 96.6 (49) | 89 (29.1) | ns (Mann–Whitney) |
| Hemoglobine g/dL (nv 11.7–16 g/dL for females, 13.2–17.3 g/dL for males) | 12.4 (1.4) | 12.8 (1.3) | 12.1 (1.4) | p = 0.0204 (Mann–Whitney) |
| ESR mm/hour [nv 2–25] (SD) | 42.2 (27.2) | 33.4 (25) | 49.8 (27) | p = 0.0014 (Mann–Whitney) |
| CRP mg/dL[nv<0.5] (SD) | 3.3 (5.7) | 3 (5.3) | 3.6 (6) | ns (Mann–Whitney) |
| Type of recent stroke | ||||
| • Ischemic | 102 (85%) | 52 (50.9%) | 50 (49%) | ns (Fisher) |
| • Hemorrhagic | 18 (15%) | 6 (33.3%) | 12 (66.6%) | |
| Type of severe brain injury: | ||||
| • Traumatic head injury | 13 (30.2%) | 3 | 10 | p = 0.0452 (Fisher) |
| • Post-hypoxic encephalopathy | 8 (18.6%) | 4 | 4 | ns (Fisher) |
| • Subarachnoid hemorrage | 10 (23.2%) | 7 | 3 | ns (Fisher) |
| • Subdural hemorrage | 4 (2.3%) | 1 | 3 | ns (Fisher) |
| • Intracerebral hemorrage | 8 (18.6%) | 6 | 2 | ns (Fisher) |
| FIM (range; SD) | ||||
| • at admission | 39.9(18–89; 19.8) | 41.9(18–85; 20.8) | 38.1(18–89; 18.7) | ns (Mann-Whitney) |
| • at discharge | 54.6(18–101; 24) | 58.6(18–101; 25.9) | 50.9(18–95; 21.6) | p < 0.048 (Mann–Whitney) |
| Katz index (range; SD) | ||||
| • at admission | 19(10–65; 13.7) | 20.8(10–65; 14.9) | 17.4(10–55;12.5) | ns (Mann-Whitney) |
| • at discharge | 33.4(10–75; 19.7) | 37.6(10–35; 20.9) | 29.8(10–30; 17.9) | p < 0.0294 (Mann-Whitney) |
CRP, C-reactive protein. ESR, erythrocyte sedimentation rate. FIM, Functional Independence Measurement. ns, not significant. SD, Standard Deviation.
US diagnostic criteria of these lesions were detailed in previous works [10–17,20,21].
Bedside US examination was carried out using a SonoSite (Bothell, USA) Titan with a 5–10 MHz linear transducer (frequency variations depending on site and depth of the lesion). Each US examination was carried out with both longitudinal and transverse scans by the same operator (P.F.), with the same machine setting [11,12]. The technical parameters of power Doppler US were: lowest pulse repetition frequency (PFR) avoiding flash artifact, highest gain level without background noise and low filter [11,12]. The intensity of vascularity at PDUS analysis of the NHO lesions was rated on a semi-quantitative 4-point scale as follows: grade 0 = no flow signal; grade 1 = 1–2 flow signals (only as coloured spot); grade 2 = 2–5 flow signals (both coloured spot and/or definite vessels without secondary branching); grade 3 = >5 flow signals or vascular tree or diffuse blush with vessel boundaries not distinguishable (modified from Falsetti et al) [12]. A spectral Doppler tracing was obtained to confirm that each color signal represented true arterial flow and spectral wave analysis (SWA) was performed in order to define two indices: Pulsatility Index (PI) and Resistance index (RI). In particular the latter, defined as (maximum systolic velocity- end diastolic velocity)/maximum velocity, was recorded as it correlates with increasing peripheral resistance.
Statistical analysis was performed by GraphPad InStat 3 (La Jolla, CA, USA) software. The chi-square test was used for an overall approach to compare the percentages among groups. Fisher’s exact test was used to compare the percentages between two groups. Pearson’s statistics and Spearman’s Rank test were applied to correlate variables (respectively with normal distribution or not). The level of statistical significance was set at a P level of 0.05.
Results
MSC were clinically diagnosed in 84/163 (51.5%) and all underwent US. 16/84 (19%) patients with MSC underwent other imaging modalities to confirm diagnosis (radiographic assessment for 9 patients, three-phase 99m Technetium bone scanning for 4 patients, computed tomography for 1 patient, MRI for 2 patients).
In 73/84 (86.9%) US examination clarified diagnosis and/or modified therapeutic approach.
Shoulder pain was observed in 45/163 patients (27.6%), in 40/120 (33.3%) with stroke and 5/43 (11.6%) with SBI.
Long head of biceps tendon sheath effusion (30/45; 66.6%) was the most common abnormality observed with US. Subacromial-subdeltoid (SA-SD) bursal effusion (12/45; 26.6%), tendinosis of the supraspinatus tendon (19/45; 42.2%) and subscapularis tendon (17/45; 37.7%), partial-thickness tear of the rotator cuff (17/45; 37.7%) and full-thickness tear of the rotator cuff (8/45; 17.7%) were also noted. Gleno-humeral effusion was observed in 12/45 (26.6%). No normal shoulders can be observed with US.
In only 4 cases (2 with stroke and 2 with SBI) a diagnosis of frozen shoulder could be made (8.8% of painful shoulders). The two cases of frozen shoulder in SBI could be related to barbiturates therapy. In these cases US showed gleno-humeral effusion and mild synovitis of the long head of biceps tendon (LHBT) in the intracapsular tract, with no significant abnormalities of rotator cuff. Moreover PDUS showed scanty signals in the rotator cuff outlet. Diagnosis was supported by three-phase 99m Technetium bone scanning and/or MRI.
In 33 patients (32 with stroke and 1 with SBI, 73.3% of painful shoulders) a variable grade of shoulder subluxation could be observed (Fig. 1).
Fig. 1.
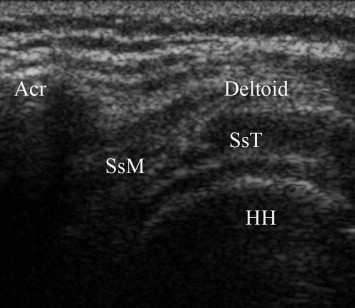
Coronal longitudinal scan over left shoulder in patient with left hemiplegia and shoulder subluxation. The entire superior part of humeral head (HH) and its cartilage is visible under Deltoid muscle, supraspinatus tendon (SsT) and its mio-tendinous junction (SsM). Acr, acromion.
Moreover, in 2 cases of shoulders with subluxation a cortical fractures of greater tuberosity (Hill-Sachs lesion) (Fig. 2) and lesser tuberosity (McLaughlin lesion) (Fig. 3) were respectively noted.
Fig. 2.
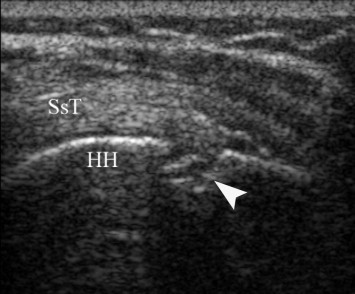
Coronal longitudinal scan over left shoulder in patient with left hemiplegia and shoulder subluxation. The humeral head (HH) shows a cortical defect (arrowhead) under the pre-insertional portion of supraspinatus tendon (SsT), compatible with Hill-Sachs lesion.
Fig. 3.
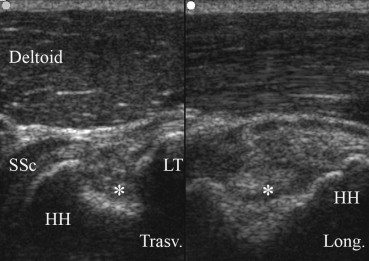
Anterior scans over left shoulder in patient with left hemiplegia, severe hemispatial neglect and shoulder subluxation. On the left side (transverse scan) a large cortical defect (asterisk), compatible with McLaughlin lesion (reverse Hill-Sachs) is visible on humeral head (HH) and its lesser tuberosity (LT). Tendinosis of subscapularis tendon (SSc). On the right side is shown the longitudinal axis of the large cortical defect.
The 8 cases without subluxation or frozen shoulder, were classified as generic painful shoulder (even if there were rotator cuff abnormalities). No NHO could be observed in shoulders.
Wrist-hand syndrome was observed in 48/163 (29.4%) but only in patient with stroke. US showed mild to moderate effusion in radio-carpal and mid-carpal joint and in both extensor and flexor tendon sheaths, with rare vascular signals on PDUS into the radio-carpal and mid-carpal joints. A subcutaneous edema with extensive dilatation of lymphatics was always observed without significant vascularity at this level.
NHO were observed in 3/163 (1.8%) patients, only in patients with severe brain injuries (13.6% in this subgroup) but not in stroke patients. In the case with NHO of the hip US demonstrated the classical pattern of zone phenomenon [15,16] (Fig. 4), and PDUS demonstrated vascular signal within mineralized NHO and in outer hypoechoic area. No vascular signal was observed in the central hypoechoic core. In NHO of knee and elbow only a heterogeneously hypoechoic mass or hyperechoic mineralized mass were respectively evident, with vascular signals within the lesions at PDUS (Fig.5a,b). Spectral wave analysis (SWA) always demonstrates low resistance vessels in NHO (Fig. 5c).
Fig. 4.
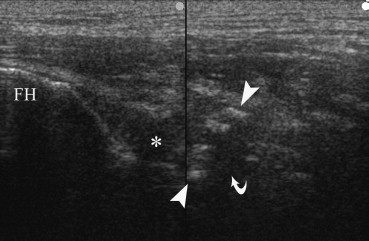
Anterior longitudinal scan of left hip in patient with left hemiplegia and spasticity consequent to intracerebral hemorrage. The classical aspect of the “zone phenomenon” of heterotopic ossification is evident in iliopsoas muscle, with a inner hypoechoic core (curved arrow) surrounded by a ring of hyperechoic mineralized islands (between arrowhead), and an outer hypoechoic zone adjacent to normal muscle. Hypoechoic effusion (asterisk) is visible within coxo-femural joint capsule. FH, femoral head.
Fig. 5.
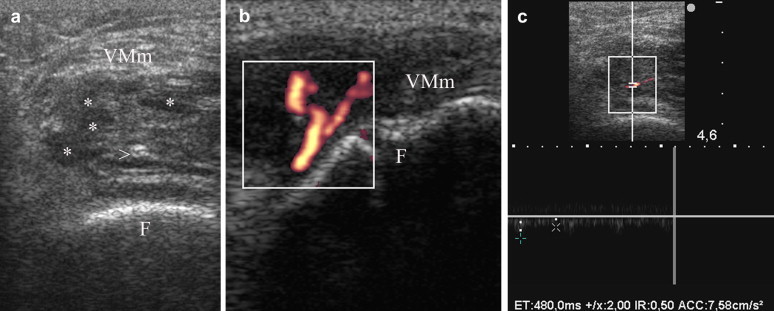
Coronal transverse scans of left knee over vastus medialis muscle in patient with severe bilateral spasticity consequent to post-anoxia encephalopathy. (a) An early heterotopic ossification is evident within vastus medialis muscle (VMm) as a heterogeneously hypoechoic mass, with both hypoechoic (asterisks) and hyperechoic foci (arrowhead). The cortical profile of femur (F) appears regular. (b) PDUS demonstrates a vascular tree within the hypoechoic mass of heterotopic ossification. (c) Spectral wave analysis demonstrates low resistance flow into the vessels of heterotopic hypoechoic mass, with a resistance index (RI) of 0.50.
Contractures and spasticity were observed in 18.4% (30/163) of patients. (24/120; 20% patients with stroke and 6/43, 13.9% patients with SBI). These findings were most common in the upper arm (19/120 in stroke and 6/43 in SBI) than in lower extremities (9/120 in stroke). Eleven patients with spasticity were treated with US-guided injections of Botulinum toxin A (BTX-A) (Dysport, Ipsen SA, France). We used a 23-gauge needle to inoculate 100–250 units of BTX-A with a single injection. US allowed easy identification of target muscle and reliable guidance for needle insertion and BTX-A injection (Fig. 6a–d).
Fig. 6.
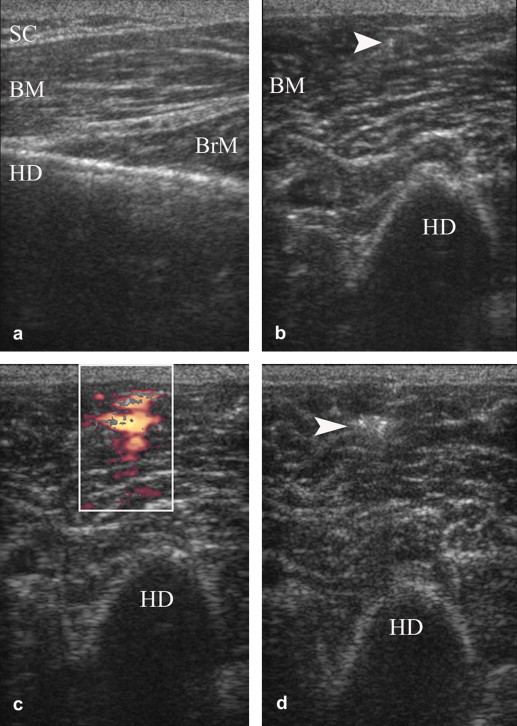
Free hand US-guided Botulinum toxin A injection in biceps brachii muscle of a patient with spasticity consequent to stroke. (a) Anterior longitudinal scan over humeral diaphysis (HD) in order to evaluate the thickness of subcutaneous tissue (SC) and biceps (BM) and brachialis (BrM) muscles, and to exclude muscle fibrosis. (b) Anterior transverse scan during needle (arrowhead) insertion. (c) PDUS examination during fluid injection depicts the flow into the muscle. (d) The hyperechoic drug deposit remains evident within biceps muscle after removal of needle.
Relapsing osteoarthritis was diagnosed in 21/120 (17.5%) patients with stroke (16 with knee osteoarthritis, 2 with hip osteoarthritis, 3 with low back pain in spondylosis) and in 4/43 (9.3%) patients with SBI (2 with knee osteoarthritis and 2 with low back pain in spondylosis).
Acute arthritis was diagnosed in 9/120 (7.5%) patients with stroke (3 knee synovitis and 2 wrist synovitis due to a relapsing calcium pyrophosphate deposition disease, CPDD; 2 knee synovitis and 2 elbow synovitis due to relapsing gout) and in 3/43 (6.9%) patients with SBI (2 metatarsal-phalangeal synovitis due to relapsing gout and CPDD and 1 knee synovitis in CPDD). In these cases US showed hypoechoic effusion and synovial proliferation into the joint space. In all the cases hyperechoic deposits (crystals) can be detected in the fibrocartilaginous menisci or insertional tract of tendons (in CPDD), within the hyaline cartilage of femur (in CPDD), over the hyaline cartilage of femur and into the joint effusion (in gout). PDUS and SWA revealed vascular low resistance vessels within synovial proliferation and/or along the capsular layer (Fig. 7a,b).
Fig. 7.
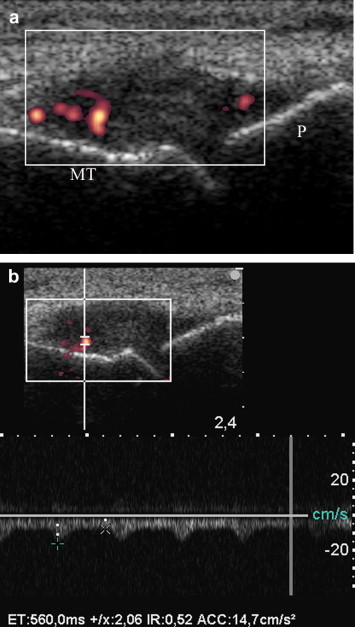
Acute arthritis of first metatarsal-phalangeal (MT-P) of right foot in hyperuricemic patient with post-stroke right hemiplegia. (a) Sagittal longitudinal scan over first MT-P. Hypoechoic distension of joint, with intracapsular vascular signals at PDUS (grade 2), indicating synovitis. (b) Spectral wave analysis demonstrates a low resistance inflammatory flow in MT-P synovitis, with a resistance index (RI) of 0.52.
Only one patient with SBI was treated with a steroid course because of a polymyalgic syndrome. US showed mild bilateral SA-SD bursitis and tenosynovitis of LHBT, associated to tendinosis of rotator cuff, without significant hypervascularity on PDUS.
For laboratory variables significant differences among groups occurred for erythrocyte sedimentation rate (ESR) which was increased in patients with MSC (p = 0.0014), and hemoglobine, which was reduced in patients with MSC (p = 0.0204)(Table 1).
Spearman analysis did not show any significant correlation between laboratory variables and single MSC. Alkaline phosphatase was elevated in all the 3 patients with NHO without statistical significance.
Patients with MSC had lower FIM in discharge (p < 0.048), lower Katz index score in discharge (p < 0.0294), they had more length of hospital stay (p = 0.0024), and they were more frequent females (p = 0.0173). Traumatic brain injuries were more frequently associated with MSC (p < 0.0452) with respect to other SBI.
Discussion
MSC were clinically and US diagnosed in 51.5% of patients with acquired brain injuries admitted to rehabilitation. This data is comparable with those reported in previous clinical studies on patients with stroke [1–4,22].
Wrist-hand syndrome and shoulder pain were the most frequently observed MSC with a prevalence of 29.4% and 27.6% respectively, followed by contractures and spasticity (18.4%), relapsing osteoarthritis (15.3%), acute arthritis (7.3%) and NHO (1.8%). In 86.9% US examination clarified diagnosis and/or modified therapeutic approach.
In wrist-hand syndrome following stroke US can define essentially three patterns. The first pattern is a subcutaneous edema with extensive dilatation of lymphatics with moderate effusion in radio-carpal and mid-carpal joint and in both extensor and flexor tendon sheaths, without vascular signals on PDUS, that could represent the entity defined as simple post-stroke hand edema (PHSE), the most frequent edema of hand and wrist observed in hemiplegic patients [9]. The absence of inflammatory hypervascularization is the most prominent aspect of this pattern and this is compatible with the relative absence of pain. This data supports the role of loss of muscle tone and pump activity and increased venous and lymphatic congestion as etiology of PHSE [9].
The second pattern is the acute arthritis of radio-carpal and mid-carpal joints, in particular due to crystal-related arthritis. In this pattern a significant synovial hypervascularization is detectable with PDUS into the swollen joint spaces. Pain and tenderness are always relevant. A relevant hypervascularization into the tendon sheaths should be differentiate with remitting seronegative symmetrical synovitis with pitting edema (RS3PE) syndrome [23].
The third pattern is a subcutaneous edema, with moderate effusion in joint spaces and in tendon sheaths, associated to mild synovial inflammatory hypervascularization. This pattern could represent the reflex sympathetic dystrophy (RSD) syndrome of wrist and hand of hemiplegic, where mild inflammation and hypervascularization determine moderate pain and tenderness [9].
The PDUS findings should modified therapeutic approach. The patients with the second pattern should be promptly treated with corticosteroid, colchicine or local steroids injection. In third pattern a therapy with corticosteroid and/or bysphosphonates could be attempted [7].
Shoulder pain were observed in 45/163 patients (27.6%), in 40/120 (33.3%) with stroke and 5/43 (11.6%) with SBI. In 33 patients (32 with stroke and 1 with SBI, 73.3% of painful shoulders) a variable grade of shoulder subluxation could be observed.
US seems to have a prominent role in the diagnosis of subluxation of hemiplegics’ shoulder, as reported in recent works [18–20]. US can easily performed at the bedside, can measure inferior and anterior shoulder subluxation with high reproducibility and correlation with clinical scores and it can be used for serial evaluations without exposure to ionizing radiations [20]. In our series US two cortical fractures could be diagnosed. In both cases routine plans radiographs fail to reveal these abnormalities.
Moreover in all painful shoulders US revealed rotator cuff pathology. In our experience, when US showed prevalent inflammatory abnormalities, a short course of corticosteroids or a local corticosteroid injection was associated to physical therapy.
Results of our series show that US is a valuable imaging technique in the study of hemiplegics’ shoulder and it should be associated to clinical examination to support therapeutic approach.
The clinical evidence of restriction of shoulder passive motion in all planes in a patient with acquired brain injuries should induce clinicians to suspect a frozen shoulder. In our series this condition was diagnosed in 8.8% of painful shoulders. In half of cases a barbiturates therapy was associated to this condition. US gave useful information showing gleno-humeral effusion and mild synovitis of the LHBT in the intracapsular tract, and scanty vascular signals in the rotator cuff outlet at PDUS. The absence of abnormalities of rotator cuff was indicative of a primary capsular inflammatory involvement.
Contractures and spasticity were observed in 18.4% of patients. In these conditions US can be useful to guide needle placement of BTX-A injections [24]. Moreover, US provides information about muscle size and fibrosis (fibro-adipose degeneration), factors that can be important in decision making.
NHO can be a cause of reduction of ROM and secondary disability following acquired brain injuries. In our series this condition has been observed only in SBI (13.6%) but not in stroke. Our data confirm that hypervascularization of mineralizing areas is a relevant diagnostic aspect of NHO lesions, as previously observed with angiographic studies [5]. As early detection of NHO and prompt starting of therapy are essential to contain related disability, bedside PDUS seems a useful first-step diagnostic tool when clinical and/or laboratory findings induce suspicion of NHO [15,16].
A higher ESR and a lower concentration of hemoglobine resulted in our patients with MSC. These data support the usefulness of laboratory investigation in early diagnosis of MSC in acquired brain injury [5]. However, low specificity of these laboratory findings should be considered, in particular in critical ill patients (concomitant infection, tracheostomy, recent neurosurgery, pressure sores). The evidence of reduced FIM and Katz scores at discharge and more length of hospital stay supports that MSC determine a delay in functional recovery and affect overall outcome of patients with acquired brain injuries [1,2].
In conclusion, US has proved to be an excellent diagnostic technique also in this field of application and we recommend the use of bedside US and PDUS in the evaluation of every suspected MSC in patients with acquired brain injuries, even before conventional radiography.
Conflict of interest statement
The authors have no conflict of interest.
References
- 1.Sackley C., Brittle N., Patel S., Ellins J., Scott M., Wright C. The prevalence of joint contractures, pressure sores, other pain, and depression in the year after a severely disabling stroke. Stroke. 2008;39:3329–3334. doi: 10.1161/STROKEAHA.108.518563. [DOI] [PubMed] [Google Scholar]
- 2.Mc Lean D.E. Medical complications experienced by a cohort of stroke survivors during inpatient, tertiary-level stroke rehabilitation. Arch Phys Med Rehabil. 2004;85:466–469. doi: 10.1016/s0003-9993(03)00484-2. [DOI] [PubMed] [Google Scholar]
- 3.Kuptniratsaicul V., Kovindha A., Suethanapornkul S., Manimmanakorn N., Archongka Y. Complications during the rehabilitation period in Thai patients with stroke: a multicenter prospective study. Am J Phys Med Rehabil. 2009;88:92–99. doi: 10.1097/PHM.0b013e3181909d5f. [DOI] [PubMed] [Google Scholar]
- 4.Pinedo S., De la Villa F.M. Complications in the hemiplegic patient in the first year after stroke. Rev Neurol. 2001;32:206–209. [PubMed] [Google Scholar]
- 5.Vanden Bossche L., Vanderstraeten G. Heterotopic ossification: a review. J Rehabil Med. 2005;37:129–136. doi: 10.1080/16501970510027628. [DOI] [PubMed] [Google Scholar]
- 6.Geurts A.C., Visschers B.A., van Limbeek J., Ribbers G.M. Scand J Rehabil Med. 2000;32:4–10. doi: 10.1080/003655000750045668. [DOI] [PubMed] [Google Scholar]
- 7.Kondo I., Hosokawa K., Soma M., Iwata M., Maltais D. Protocol to prevent shoulder-hand syndrome after stroke. Arch Phys Med Rehabil. 2001;82:1619–1623. doi: 10.1053/apmr.2001.25975. [DOI] [PubMed] [Google Scholar]
- 8.De Santis A., Ceccarelli G., Cesana B.M., Bello L., Spagnoli D., Villani R.M. Shoulder-hand sindrome in neurosurgical patients treated with barbiturates. A long term evaluation. J Neurosurg Sci. 2000;44:69–75. [PubMed] [Google Scholar]
- 9.Leibovitz A., Baumoehl Y., Roginsky Y., Glick Z., Habot B., Segal R. Edema of the paretic hand in elderly post-stroke nursing patients. Arch Gerontol Geriatr. 2007;44:37–42. doi: 10.1016/j.archger.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Falsetti P., Frediani B., Acciai C., Baldi F., Filippou G., Parra Prada E. Ultrasonographic study of Achilles tendon and plantar fascia in chondrocalcinosis. J Rheumatol. 2004;31:2242–2250. [PubMed] [Google Scholar]
- 11.Falsetti P., Acciai C., Frediani B., Lenzi L. Ultrasound of enthesopathy in rheumatic diseases. Mod Rheumatol. 2009;19:103–113. doi: 10.1007/s10165-008-0129-x. [DOI] [PubMed] [Google Scholar]
- 12.Falsetti P., Frediani B., Acciai C., Baldi F., Filippou G., Galeazzi M. Ultrasonography and magnetic resonance imaging of heel fat pad inflammatory-oedematous lesions in rheumatoid arthritis. Scand J Rheumatol. 2006;35:454–458. doi: 10.1080/03009740600905398. [DOI] [PubMed] [Google Scholar]
- 13.Falsetti P., Frediani B., Storri L., Bisogno S., Baldi F., Campanella V. Evidence for synovitis in active polymyalgia reumatica: sonographic study in a large series of patients. J Rheumatol. 2002;29:123–130. Erratum in: 2005;29:644. [PubMed] [Google Scholar]
- 14.Farin P.U., Kaukanen E., Jaroma H., Harju A., Vaatainen U. Hill-Sachs lesion: sonographic detection. Skeletal Radiol. 1996;25:559–562. doi: 10.1007/s002560050135. [DOI] [PubMed] [Google Scholar]
- 15.Bodley R., Jamous A., Short D. Ultrasound in the early diagnosis of heterotopic ossification in patients with spinal injuries. Paraplegia. 1993;31:500–506. doi: 10.1038/sc.1993.81. [DOI] [PubMed] [Google Scholar]
- 16.Thomas E.A., Cassar-Pullicino V.N., McCall I.W. The role of ultrasound in the early diagnosis and management of heterotopic bone formation. Clin Radiol. 1991;43:190–196. doi: 10.1016/s0009-9260(05)80478-7. [DOI] [PubMed] [Google Scholar]
- 17.Frediani B., Filippou G., Falsetti P., Lorenzini S., Baldi F., Acciai C. Diagnosis of calcium pyrophosphate dihydrate crystal deposition disease: ultrasonographic criteria proposed. Ann Rheum Dis. 2005;64:638–640. doi: 10.1136/ard.2004.024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pong Y.P., Wang L.Y., Wang L., Leong C.P., Huang Y.C., Chen Y.K. Sonography of the shoulder in hemiplegic patients undergoing rehabilitation after a recent stroke. J Clin Ultrasound. 2009;37:199–205. doi: 10.1002/jcu.20573. [DOI] [PubMed] [Google Scholar]
- 19.Lee I.S., Shin Y.B., Moon T.Y., Jeong Y.J., Song J.W., Kim D.H. Sonography of patients with hemiplegic shoulder pain after stroke: correlation with motor recovery stage. Am J Roentgenol. 2009;192:W40–W44. doi: 10.2214/AJR.07.3978. [DOI] [PubMed] [Google Scholar]
- 20.Park G.Y., Kim J.M., Sohn S.I., Shin I.H., Lee M.Y. Ultrasonographic measurement of shoulder subluxation in patients with post-stroke hemiplegia. J Rehabil Med. 2007;39:526–530. doi: 10.2340/16501977-0099. [DOI] [PubMed] [Google Scholar]
- 21.Wakefield R.J., Balint P.V., Szkudlarek M., Filippucci E., Backhaus M., D’Agostino M.A. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J Rheumatol. 2005;32:2485–2487. [PubMed] [Google Scholar]
- 22.Langhorne P., Stott D.J., Robertson L., MacDonald J., Jones L., McAlpine C. Medical complications after stroke. A multicenter study. Stroke. 2000;31:1223–1229. doi: 10.1161/01.str.31.6.1223. [DOI] [PubMed] [Google Scholar]
- 23.Klauser A., Frauscher F., Halpern H.J., Mur E., Springer P., Judmaier W. Remitting seronegative symmetrical synovitis with pitting edema of the hands: ultrasound, color doppler ultrasound, and magnetic resonance imaging findings. Arthritis Rheum. 2005;53:226–233. doi: 10.1002/art.21067. [DOI] [PubMed] [Google Scholar]
- 24.Sconfienza L.M., Perrone N., Lacelli F., Lentino C., Serafini G. Ultrasound-guided injection of botulinum toxin A in the treatment of iliopsoas spasticity. J Ultrasound. 2008;11:113–117. doi: 10.1016/j.jus.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


